Optical Transparency and Local Electronic Structure of Yb-Doped Y2O3 Ceramics with Tetravalent Additives
Abstract
1. Introduction
2. Materials and Methods
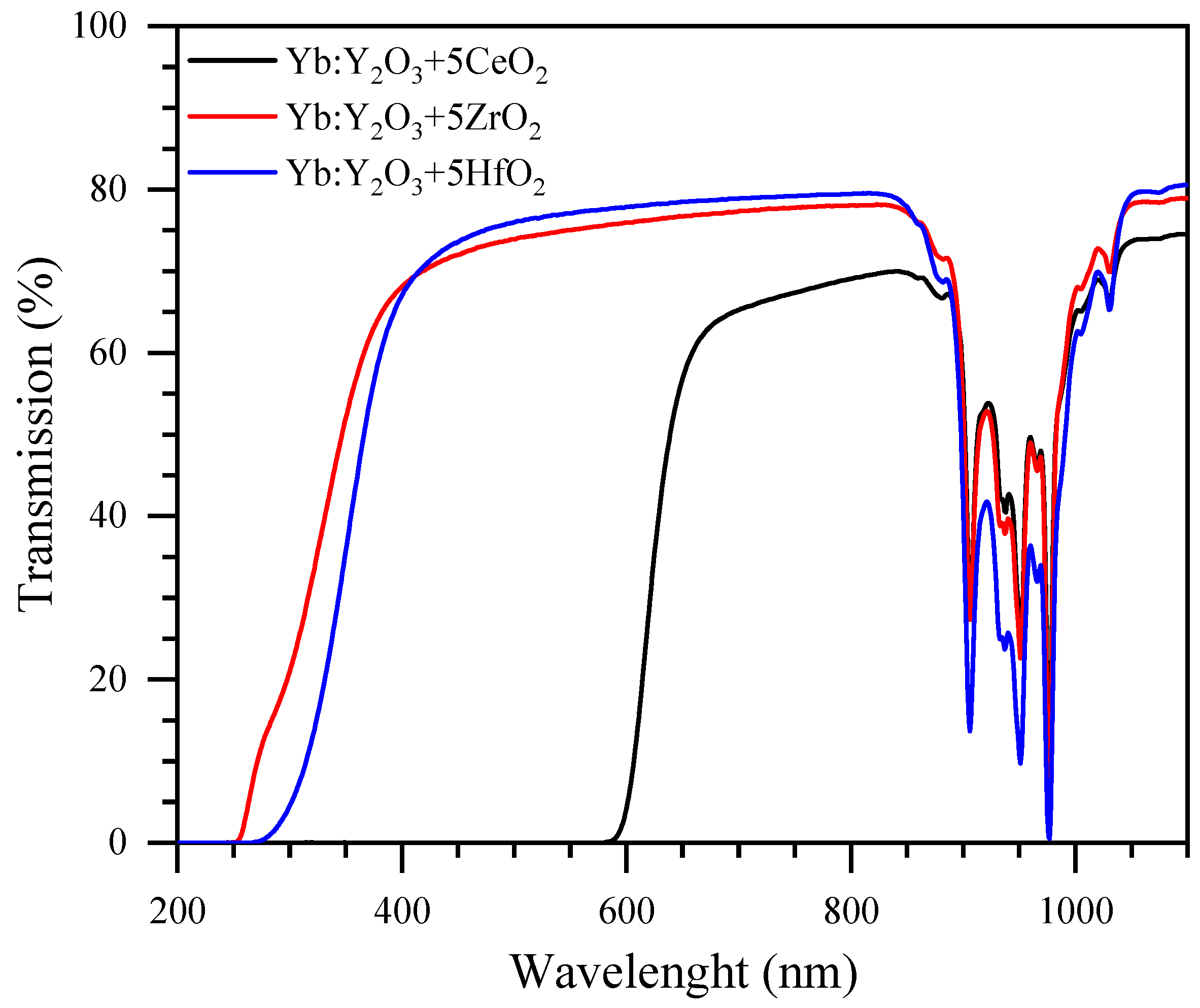
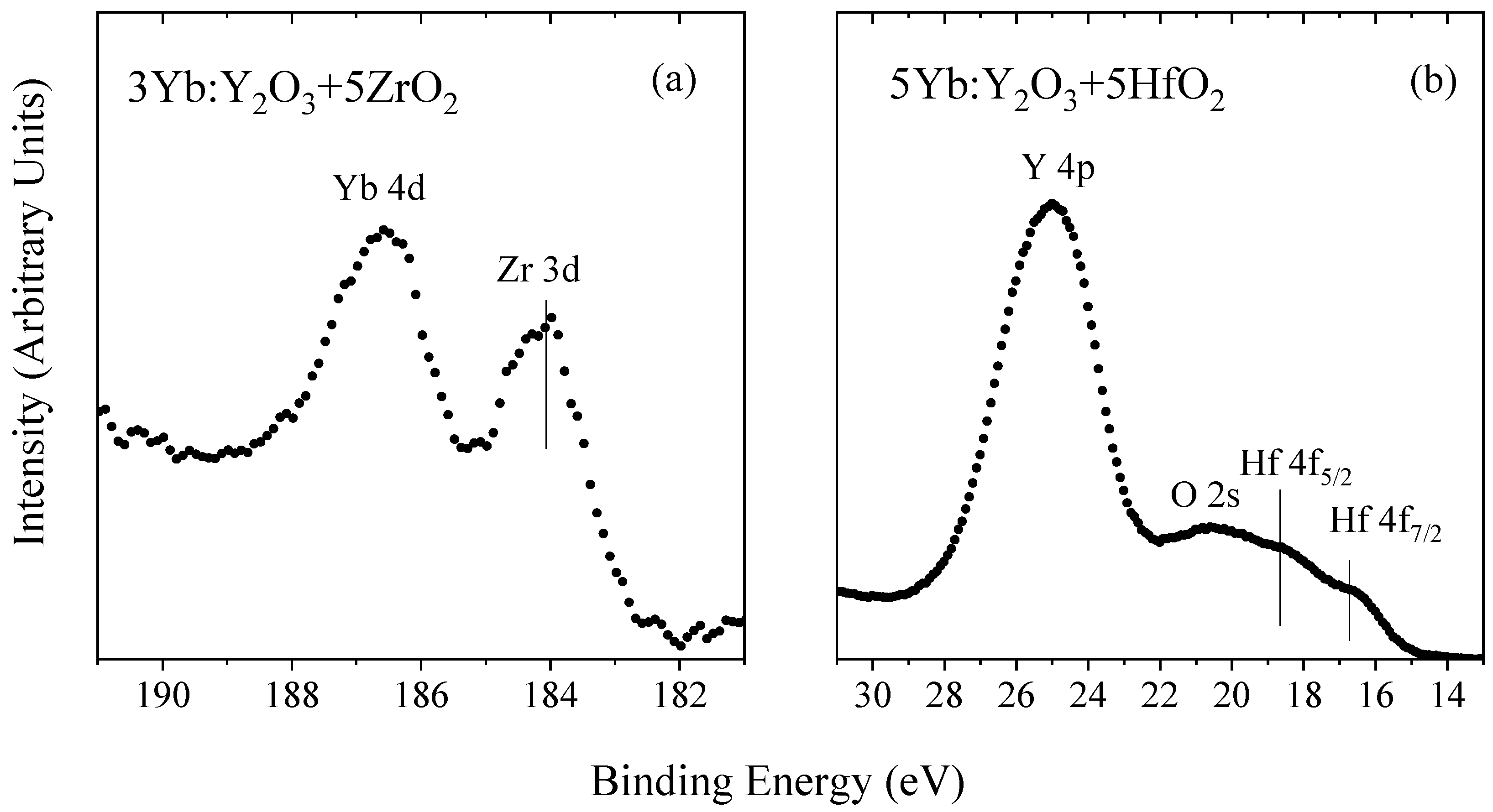
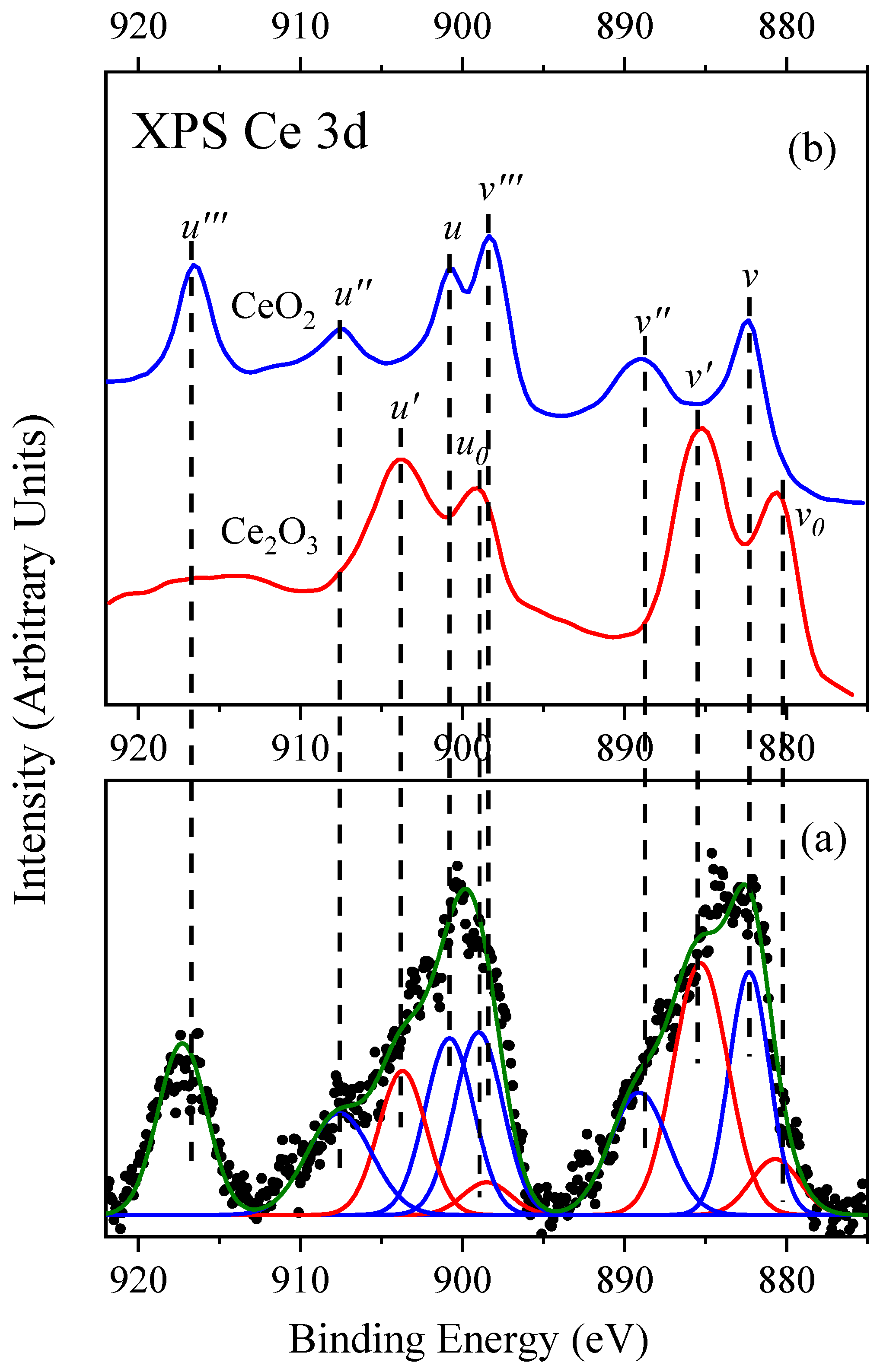
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Pan, Y.-B.; Zeng, Y.-P.; Liu, W.-B.; Jiang, B.-X.; Guo, J.-K. The history, development, and future prospects for laser ceramics: a review. Int. J. Refract. Met. Hard Mater. 2013, 39, 44–52. [Google Scholar] [CrossRef]
- Zhu, L.-L.; Park, Y.-J.; Gan, L.; Kim, H.-N.; Ko, J.-W.; Kim, H.-D. Fabrication of transparent Y2O3 ceramics with record-high thermal shock resistance. J. Eur. Ceram. Soc. 2018, 38, 4050–4056. [Google Scholar] [CrossRef]
- Lu, J.-R.; Takaichi, K.; Uematsu, T.; Shirakawa, A.; Musha, M.; Ueda, K.; Yagi, H.; Yanagitani, T.; Kaminskii, A.A. Yb3+: Y2O3 ceramics – a novel solid-state laser material. Jpn J. Appl. Phys. 2012, 41, L1373–L1375. [Google Scholar] [CrossRef]
- Cheng, X.; Yuan, C.; Green, N.R.; Withey, P.A. Sintering mechanisms of yttria with different additives. Ceram. Int. 2013, 39, 4791–4799. [Google Scholar]
- Osipov, V.V.; Shitov, V.A.; Maksimov, R.N.; Solomonov, V.I. Properties of transparent Re3+: Y2O3 ceramics doped with tetravalent additives. Opt. Mater. 2015, 50, 65–70. [Google Scholar] [CrossRef]
- Tsukuda, Y. Properties of black Y2O3 sintered bodies. Mater. Res. Bull. 1981, 16, 453–459. [Google Scholar] [CrossRef]
- Li, X.; Mao, X.; Feng, M.; Xie, J.; Jiang, B.; Zhang, L. Optical absorption and mechanism of vacuum-sintered ZrO2-doped Y2O3 ceramics. J. Eur. Ceram. Soc. 2016, 36, 4181–4184. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, T.; Wei, N.; Ma, B.; Li, F.; Lu, Z.; Qi, J. Effect of annealing on the optical properties of Nd:YAG transparent ceramics. Opt. Mater. 2012, 34, 685–690. [Google Scholar] [CrossRef]
- An, L.; Ito, A.; Goto, T. Effects of Sintering and Annealing Temperature on Fabrication of Transparent Lu2Ti2O7 by Spark Plasma Sintering. J. Am. Ceram. Soc. 2011, 94, 3851–3855. [Google Scholar] [CrossRef]
- Gan, L.; Park, Y.-J.; Kim, H.-N.; Kim, J.-M.; Ko, J.-W.; Lee, J.-W. Effects of presintering and annealing on the optical transmittance of Zr-doped Y2O3 transparent ceramics fabricated by vacuum sintering conjugated with post-hot-isostatic pressing. Ceram. Int. 2015, 41, 9622–9627. [Google Scholar]
- Zhidkov, I.S.; Maksimov, R.N.; Kukharenko, A.I.; Finkelstein, L.D.; Cholakh, S.O.; Osipov, V.V.; Kurmaev, E.Z. Influence of post-annealing in air on optical and XPS spectra of Y2O3 ceramics doped with CeO2. Mendeleev Commun. 2019, 29, 102–104. [Google Scholar] [CrossRef]
- Osipov, V.V.; Kotov, Yu.A.; Ivanov, M.G.; Samatov, O.M.; Lisenkov, V.V.; Platonov, V.V.; Murzakaev, A.M.; Medvedev, A.I.; Azarkevich, E.I. Laser synthesis of nanopowders. Las. Phys. 2006, 16, 116–125. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.F.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Physical Electronic Division: Eden Praire, MN, USA, 1995. [Google Scholar]
- Hu, Y.; Li, Z.; Pan, W. Sandwich-like transparent ceramic demonstrates ultraviolet and visible broadband downconversion luminescence. RSC Adv. 2018, 8, 13200–13204. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Kukk, E.; Hägerth, T.; Matinlinna, J.P. Surface modification of silica-coated zirconia by chemical treatments. Appl. Surf. Sci. 2010, 257, 1228–1235. [Google Scholar] [CrossRef]
- Mullins, D.R.; Overbury, S.H.; Huntley, D.R. Electron spectroscopy of single crystal and polycrystalline cerium oxide surfaces. Surf. Sci. 1998, 409, 307–319. [Google Scholar] [CrossRef]
- Kotani, A.; Jo, T.; Parlebas, J.C. Many-body effects in core-level spectroscopy of rare-earth compounds. Adv. Phys. 1998, 37, 37–85. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.N.T.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113–133115. [Google Scholar] [CrossRef]
- Jung, W.K.; Ma, H.J.; Park, Y.; Kim, D.K. A robust approach for highly transparent Y2O3 ceramics by stabilizing oxygen defects. Scripta Mater. 2017, 137, 1–4. [Google Scholar] [CrossRef]
- Osipov, V.V.; Solomonov, V.I.; Konev, S.F.; Cholakh, S.O. Trivalent Zirconium and Hafnium Ions in Yttria-Based Transparent Ceramics. Tech. Phys. Lett. 2013, 39, 377–379. [Google Scholar] [CrossRef]



| Sample | C | O | Y | Yb | Hf | Zr | Ce | O/Y |
|---|---|---|---|---|---|---|---|---|
| 3Yb:Y2O3 + 5CeO2 | 39.1 | 38.9 | 20.6 | 1.6 | – | – | 0.2 | 1.88 |
| 5Yb:Y2O3 + 5HfO2 | 44.9 | 35.1 | 18.1 | 1.3 | 0.6 | – | – | 1.93 |
| 3Yb:Y2O3 + 5ZrO2 | 42.4 | 37.0 | 18.8 | 1.4 | – | 0.4 | – | 1.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhidkov, I.S.; Kukharenko, A.I.; Maksimov, R.N.; Finkelstein, L.D.; Cholakh, S.O.; Osipov, V.V.; Kurmaev, E.Z. Optical Transparency and Local Electronic Structure of Yb-Doped Y2O3 Ceramics with Tetravalent Additives. Symmetry 2019, 11, 243. https://doi.org/10.3390/sym11020243
Zhidkov IS, Kukharenko AI, Maksimov RN, Finkelstein LD, Cholakh SO, Osipov VV, Kurmaev EZ. Optical Transparency and Local Electronic Structure of Yb-Doped Y2O3 Ceramics with Tetravalent Additives. Symmetry. 2019; 11(2):243. https://doi.org/10.3390/sym11020243
Chicago/Turabian StyleZhidkov, Ivan S., Andrey I. Kukharenko, Roman N. Maksimov, Larisa D. Finkelstein, Seif O. Cholakh, Vladimir V. Osipov, and Ernst Z. Kurmaev. 2019. "Optical Transparency and Local Electronic Structure of Yb-Doped Y2O3 Ceramics with Tetravalent Additives" Symmetry 11, no. 2: 243. https://doi.org/10.3390/sym11020243
APA StyleZhidkov, I. S., Kukharenko, A. I., Maksimov, R. N., Finkelstein, L. D., Cholakh, S. O., Osipov, V. V., & Kurmaev, E. Z. (2019). Optical Transparency and Local Electronic Structure of Yb-Doped Y2O3 Ceramics with Tetravalent Additives. Symmetry, 11(2), 243. https://doi.org/10.3390/sym11020243







