Fluctuating Asymmetry in Ground Beetles (Coleoptera, Carabidae) and Conditions of Its Manifestation
Abstract
1. Introduction
- (i)
- Is FA species-specific in related species of ground beetles;
- (ii)
- Is FA sex-biased;
- (iii)
- How environmental factors affect FA in taken separately species.
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anciães, M.; Marini, M.Â. The effects of fragmentation on fluctuating asymmetry in passerine birds of Brazilian tropical forests. J. Appl. Ecol. 2000, 37, 1013–1028. [Google Scholar] [CrossRef]
- Lens, L.; Van Dongen, S.; Kark, S.; Matthysen, E. Fluctuating asymmetry as an indicator of fitness: Can we bridge the gap between studies? Biol. Rev. 2002, 77, 27–38. [Google Scholar] [CrossRef]
- Schmeller, D.S.; Dolek, M.; Geyer, A.; Settele, J.; Brandl, R. The effect of conservation efforts on morphological asymmetry in a butterfly population. J. Nat. Conserv. 2011, 19, 161–165. [Google Scholar] [CrossRef]
- Sherman, E.; Tock, K.; Clarke, C. Fluctuating asymmetry in Ichthyophonus-sp. infected newts, Notophthalmus viridescens, from Vermont. Appl. Herpetol. 2009, 6, 369–378. [Google Scholar] [CrossRef]
- Weller, B.; Ganzhorn, J.U. Carabid beetle community composition, body size, and fluctuating asymmetry along an urban-rural gradient. Basic Appl. Ecol. 2004, 5, 193–201. [Google Scholar] [CrossRef]
- Rott, H.; Polak, M. Developmental Instability: Causes and Consequences; Oxford University Press: Oxford, UK, 2003; p. 459. [Google Scholar]
- Møller, A.P.; Swaddle, J.P. Asymmetry, Developmental Stability, and Evolution; Oxford University Press: Oxford, UK, 1997; p. 291. [Google Scholar]
- Helle, S.; Huhta, E.; Suorsa, P.; Hakkarainen, H. Fluctuating asymmetry as a biomarker of habitat fragmentation in an area-sensitive passerine, the Eurasian treecreeper (Certhia familiaris). Ecol. Indic. 2011, 11, 861–867. [Google Scholar] [CrossRef]
- Vangestel, C.; Lens, L. Does fluctuating asymmetry constitute a sensitive biomarker of nutritional stress in house sparrows (Passer domesticus)? Ecol. Indic. 2011, 11, 389–394. [Google Scholar] [CrossRef]
- Vangestel, C.; Mergeay, J.; Dawson, D.A.; Vandomme, V.; Lens, L. Developmental stability covaries with genome-wide and single-locus heterozygosity in house sparrows. PLoS ONE 2011, 6, e21569. [Google Scholar] [CrossRef][Green Version]
- Lens, L.; Van Dongen, S.; Matthysen, E. Fluctuating asymmetry as an early warning system in the critically endangered Taita thrush. Conserv. Boil. 2002, 16, 479–487. [Google Scholar] [CrossRef]
- Van Dongen, S. Fluctuating asymmetry and developmental instability in evolutionary biology: Past, present and future. J. Evol. Biol. 2006, 19, 1727–1743. [Google Scholar] [CrossRef]
- De Coster, G.; Van Dongen, S.; Malaki, P.; Muchane, M.; Alca’ntara-Exposito, A.; Matheve, H.; Lens, L. Fluctuating asymmetry and environmental stress: Understanding the role of trait history. PLoS ONE 2013, 8, e0057966. [Google Scholar] [CrossRef]
- Milankov, V.; Francuski, L.; Ludoški1, J.; Stehls, G.; Vuji, A. Genetic structure and phenotypic diversity of two northern populations of Cheilosia aff. longula (Diptera: Syrphidae) has implications for evolution and conservation. Eur. J. Entomol. 2010, 107, 305–315. [Google Scholar] [CrossRef]
- Palmer, A.R.; Strobeck, C. Fluctuating asymmetry as a measure of developmental stability: Implications of nonnormal distributions and power statistical tests. Acta Zool. Fenn. 1992, 191, 57–72. [Google Scholar]
- Willmore, K.E.; Young, N.M.; Richtsmeier, J.T. Phenotypic variability: Its components, measurement and underlying developmental processes. Evol. Biol. 2007, 34, 99–120. [Google Scholar] [CrossRef]
- Clarke, G.M. Relationships between developmental stability and fitness: Application for conservation biology. Conserv. Biol. 1995, 9, 18–24. [Google Scholar] [CrossRef]
- Clarke, G.M. Developmental stability and fitness: The evidence is not quite so clear. Am. Nat. 1998, 152, 762–766. [Google Scholar] [CrossRef]
- Leamy, L.J.; Klingenberg, C.P. The genetics and evolution of fluctuating asymmetry. Annu. Rev. Ecol. Evol. Syst. 2005, 14, 1–21. [Google Scholar] [CrossRef]
- Trotta, V.; Calboli, C.; Garoia, F.; Grifoni, D.; Cavicchi, S. Fluctuating asymmetry as a measure of ecological stress in Drosophila melanogaster (Diptera: Drosophilidae). Eur. J. Entomol. 2005, 102, 195–200. [Google Scholar] [CrossRef]
- Van Dongen, S.; Lens, L.; Pape, E.; Volckaert, F.A.; Raeymaekers, J.A. Evolutionary history shapes the association between developmental instability and population level genetic variation in three-spined sticklebacks. J. Evol. Biol. 2009, 22, 1695–1707. [Google Scholar] [CrossRef]
- Clarke, G.M. Fluctuating asymmetry of invertebrate populations as biological control of environmental quality. Environ. Pollut. 1993, 82, 207–211. [Google Scholar] [CrossRef]
- Thiele, H.U. Carabid beetles in their environments: A study on habitat selection by adaptations in physiology and behavior. In Zoophysiology and Ecology; Springer: Berlin/Heidelberg, Germany, 1977; p. 372. [Google Scholar] [CrossRef]
- Wallin, H. Spatial and temporal distribution of some abundant carabid beetles (Coleoptera: Carabidae) in cereal fields and adjacent habitats. Pedobiologia 1985, 28, 19–34. [Google Scholar]
- Lövei, G.L.; Sunderland, K.D. Ecology and behavior of ground beetles (Coleoptera: Carabidae). Annu. Rev. Entomol. 1996, 41, 231–256. [Google Scholar] [CrossRef]
- Burel, F.; Baudry, J.; Butet, A.; Clergeau, P.; Delettre, Y.; Le Coeur, D.; Dubs, F.; Morvan, N.; Paillat, G.; Petit, S.; et al. Comparative biodiversity along a gradient of agricultural landscapes. Acta Oecol. 1998, 19, 47–60. [Google Scholar] [CrossRef]
- Avgın, S.S.; Luff, M.L. Ground beetles (Coleoptera: Carabidae) as bioindicators of human impact. Munis Entomol. Zool. 2015, 5, 209–215. [Google Scholar]
- Kotze, D.J.; Brandmayr, P.; Casale, A.; Dauffy-Richard, E.; Dekoninck, W.; Koivula, M.J.; Lövei, G.L.; Mossakowski, D.; Noordijk, J.; Paarmann, W.; et al. Forty years of carabid beetle research in Europe e from taxonomy, biology, ecology and population studies to bioindication, habitat assessment and conservation. ZooKeys 2011, 100, 55–148. [Google Scholar] [CrossRef]
- Magura, T.; Tóthmérész, B.; Gábor, L.; Lövei, G.L. Body size inequality of carabids along an urbanisation gradient. Basic Appl. Ecol. 2006, 7, 472–482. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.A. Intraspecific body size variation in ground beetles (Coleoptera, Carabidae) in urban-suburban-rural-natural gradient. Acta Biol. Univ. Daugavp. 2013, 13, 121–128. [Google Scholar]
- Baranovská, E.; Knapp, M. Small-scale spatiotemporal variability in body size of two common carabid beetles. Cent. Eur. J. Biol. 2014, 9, 476–494. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.A.; Saveliev, A.A. Effects of Ecological factors on size related traits in the ground beetle Carabus granulatus L. (Coleoptera, Carabidae). Russ. J. Ecol. 2014, 45, 414–420. [Google Scholar] [CrossRef]
- Sukhodolskaya, R.; Saveliev, A. Body size variation of ground beetles (Coleoptera: Carabidae) in latitude gradient. Period. Biol. 2016, 2016. 118, 273–278. [Google Scholar] [CrossRef]
- Giglio, A.; Cavaliere, F.; Giulianini, P.G.; Mazzeia, A.; Talarico, F.; Vommaro, M.L.; Brandmayr, P. Impact of agrochemicals on non-target species: Calathus fuscipes Goeze 1777 (Coleoptera: Carabidae) as model. Ecotoxicol. Environ. Saf. 2017, 142, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Pizzolotto, R.; Mazzei, A.; Bonacci, T.; Scalercio, S.; Iannotta, N.; Brandmayr, P. Ground beetles in Mediterranean olive agroecosystems: Their significance and functional role as bioindicators (Coleoptera, Carabidae). PLoS ONE 2018, 13, e0194551. [Google Scholar] [CrossRef] [PubMed]
- Sukhodolskaya, R.A.; Avtaeva, T.A.; Brigadirenko, V.V.; Antsiferov, A.L.; Kushaliev, S.A. Tendencies of Poecilus cupreus morphometric alteration depending on habitation region. Adv. Eng. Res. 2018, 177, 10–15. [Google Scholar] [CrossRef]
- Labrie, G.; Prince, C.; Bergeron, J.-M. Abundance and developmental stability of Pterostichus melanarius (Coleoptera: Carabidae) in organic and integrated pest management orchards of Québec, Canada. Environ. Entomol. 2003, 32, 123–132. [Google Scholar] [CrossRef]
- Elek, Z.; Lövei, G.L.; Bátki, M. No increase in fluctuating asymmetry in ground beetles (Carabidae) as urbanisation progresses. Community Ecol. 2014, 15, 131–138. [Google Scholar] [CrossRef]
- Elek, Z.; Lövei, G.L.; Bátki, M. Sex-specific interaction of body condition and asymmetry in carabids in distinct urbanisation stages. Community Ecol. 2017, 18, 253–259. [Google Scholar] [CrossRef]
- Coda, J.; Gomez, D.; Martínez, J.J.; Steinmann, A.; Priotto, J. The use of fluctuating asymmetry as a measure of farming practice effects in rodents: A species-specific response. Ecol. Indic. 2016, 70, 269–275. [Google Scholar] [CrossRef]
- Benítez, H.; Lemic, D.; Püschel, T.A.; Gašparić, H.V.; Kos, T.; Barić, B.; Bažok, R.; Živković, I.P. Fluctuating asymmetry indicates levels of disturbance between agricultural productions: An example in Croatian population of Pterostichus melas melas (Coleptera: Carabidae). Zool. Anz. 2018, 276, 42–49. [Google Scholar] [CrossRef]
- Benítez, H.; Briones, R.; Jerez, V. Fluctuating asymmetry in two populations of Ceroglossus chilensis (Eschscholtz, 1829) (Coleoptera: Carabidae) in agroecosystem of Pinus radiata D. don agroecosystem, Bio-Bio Region, Chile. Gayana 2008, 72, 131–139. [Google Scholar]
- Sukhodolskaya, R.A.; Saveliev, A.A. Crop impact on body size variation in carabid beetle Poecilus cupreus Linnaeus (Coleoptera, Carabidae). In Proceedings of the I (IV) International Scientific and Practical Meeting «Problems of Modern Entomology», Uzhgorod, Ukraine, 15–17 September 2016. [Google Scholar]
- Benítez, H. Assessment of patterns of fluctuating asymmetry and sexual dimorphism in carabid body shape. Neotrop. Entomol. 2013, 42, 164–169. [Google Scholar] [CrossRef]
- Garnier, S.; Gidaszewski, N.; Charlot, M.; Rasplus, J.; Alibert, P. Hybridization, developmental stability, and functionality of morphological traits in the ground beetle Carabus solieri (Coleoptera, Carabidae). Biol. J. Linn. Soc. 2006, 89, 151–158. [Google Scholar] [CrossRef]
- Henríquez, P.; Donoso, D.; Grez, A. Population density, sex ratio, body size and fluctuating asymmetry of Ceroglossus chilensis (Carabidae) in the fragmented Maulino forest and surrounding pine plantations. Acta Oecol. 2009, 35, 811–818. [Google Scholar] [CrossRef]
- Costa, M.; Mateusa, R.P.; Moura, M.O. Constant fluctuating asymmetry but not directional asymmetry along the geographic distribution of Drosophila antonietae (Diptera, Drosophilidae). Rev. Bras. Entomol. 2015, 59, 337–342. [Google Scholar] [CrossRef][Green Version]
- Maryański, M.; Kramarz, P.; Laskowski, R.; Niklinska, M. Decreased energetic reserves, morphological changes and accumulation of metals in carabid beetles (Poecilus cupreus L.) exposed to zinc- or cadmium-contaminated food. Ecotoxicology 2002, 11, 127–139. [Google Scholar] [CrossRef]
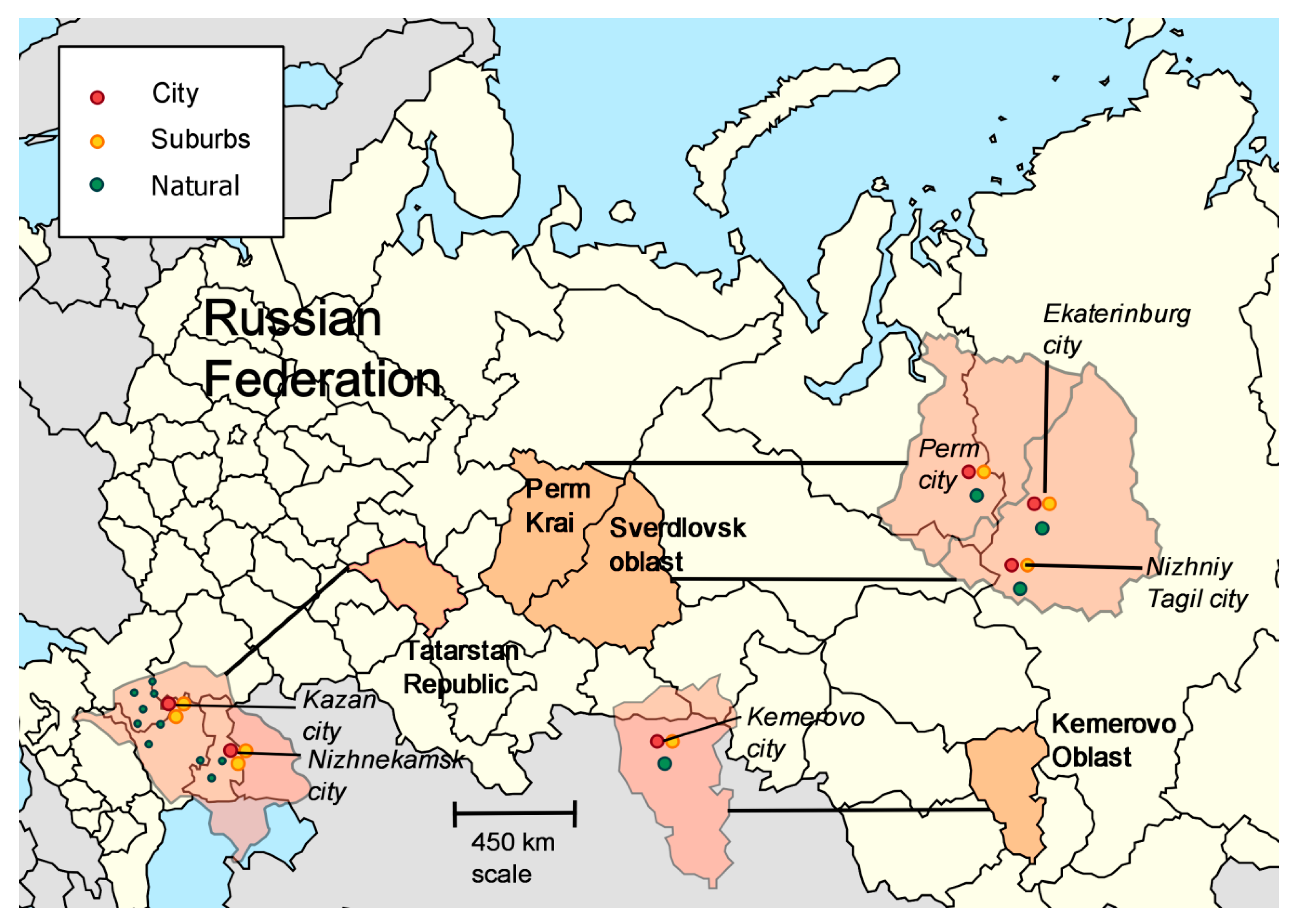
| Region | Latitude, °N | Longitude, °E | |
|---|---|---|---|
| 1 | Kemerovo region | 54°56′ | 87°14′ |
| 2 | Tatarstan Republic | 55°47′ | 49°06′ |
| 3 | Perm kray | 57° 01′ | 57°9′ |
| 4 | Sverdlovsk region | 58°42′ | 61°20′ |
| Species | Number of Sites | Sample Size | |
|---|---|---|---|
| 1 | C. aeruginosus | 3 | 528 |
| 2 | C. cancellatus | 4 | 774 |
| 3 | C. granulatus | 4 | 865 |
| 4 | P. melanarius | 5 | 470 |
| 5 | P. niger | 2 | 59 |
| 6 | P. oblongopunctatus | 2 | 305 |
| 7 | Poec. cupreus | 10 | 1672 |
| Species | Kemerovo | Tatarstan | Perm | Sverdlovsk | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| City | Suburbs | Natural | City | Suburbs | Natural | Agro | City | Suburbs | Natural | City | Suburbs | Natural | |
| C. aeruginosus | 210 | 80 | 238 | - | - | - | - | - | - | - | - | - | - |
| C. cancellatus | - | - | - | 121 | 48 | 205 | - | 30 | 81 | 79 | 35 | 84 | 89 |
| C. granulatus | 85 | 73 | 119 | 79 | 75 | 186 | - | 33 | 31 | 54 | 48 | 37 | 40 |
| P. melanarius | - | - | - | 49 | 32 | 51 | - | 44 | 52 | 51 | 30 | 68 | 93 |
| P. niger | 30 | - | - | 29 | - | - | - | - | - | - | - | - | - |
| P. oblongopunctatus | - | - | - | 29 | 30 | 49 | - | 31 | 32 | 30 | - | - | - |
| Poec. cupreus | 48 | 67 | 70 | 38 | 161 | 517 | 515 | - | - | - | 65 | 92 | 104 |
| Source | D f | Sum_ of Sq | R_S_S | A_I_C | F-value | Pr_(>F) | |
|---|---|---|---|---|---|---|---|
| <none> | 8.036 | −18194 | |||||
| Species | 5 | 0.093 | 8.129 | −18169 | 7.055 | 0.000 | *** |
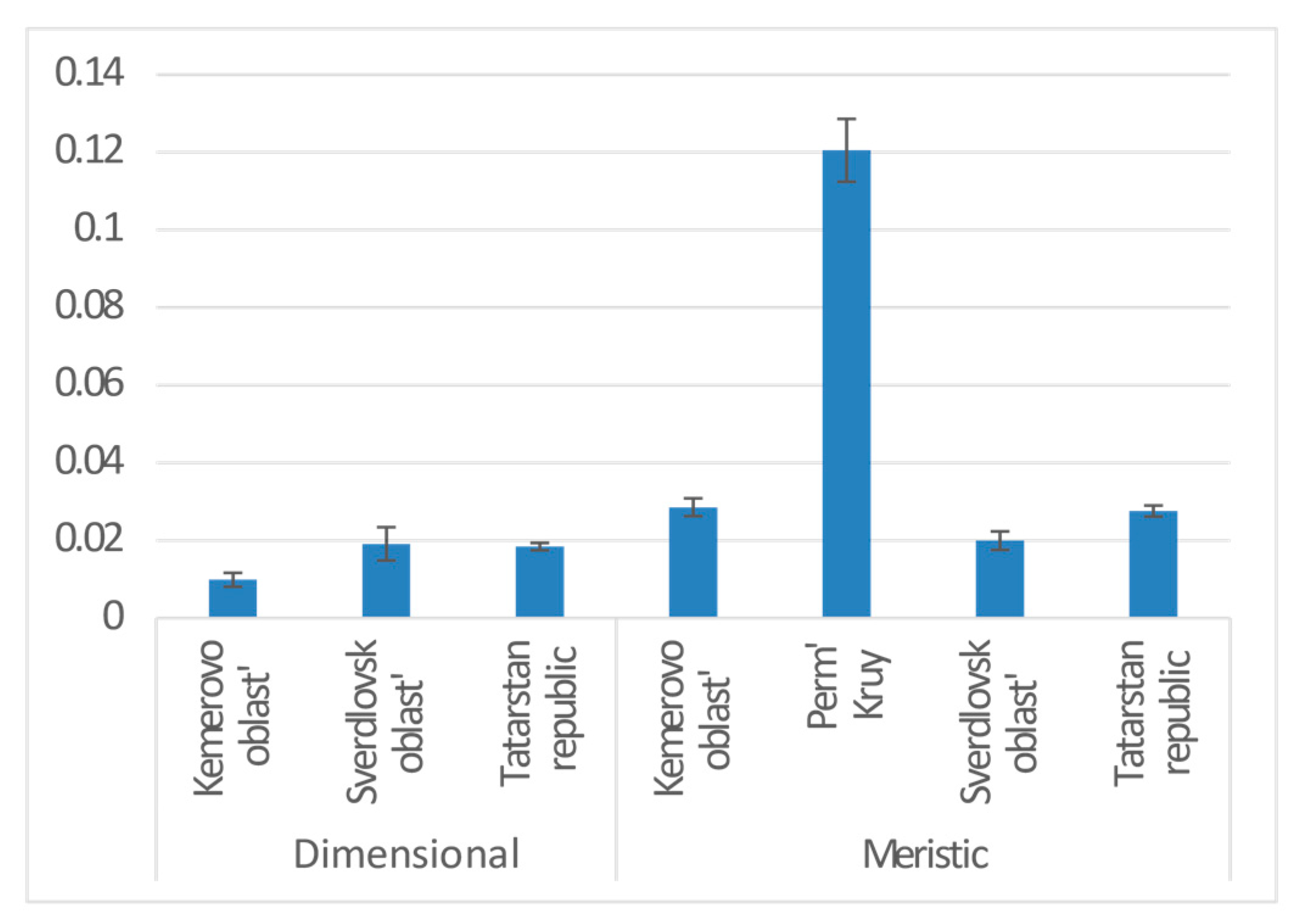
| Province | 2 | 0.020 | 8.056 | −18190 | 3.822 | 0.022 | * |
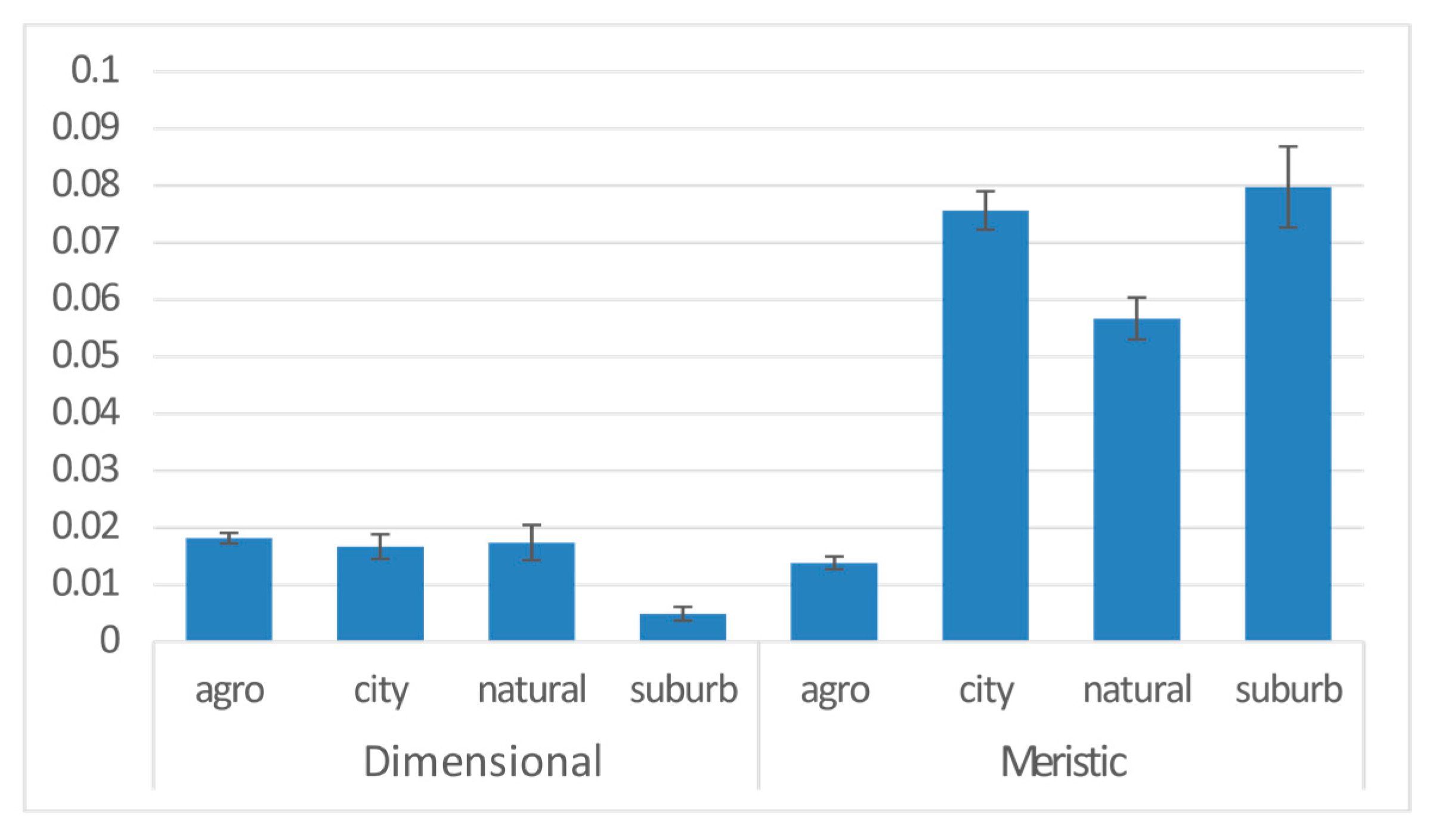
| Anthropogene | 3 | 0.046 | 8.082 | −18183 | 5.856 | 0.001 | *** |
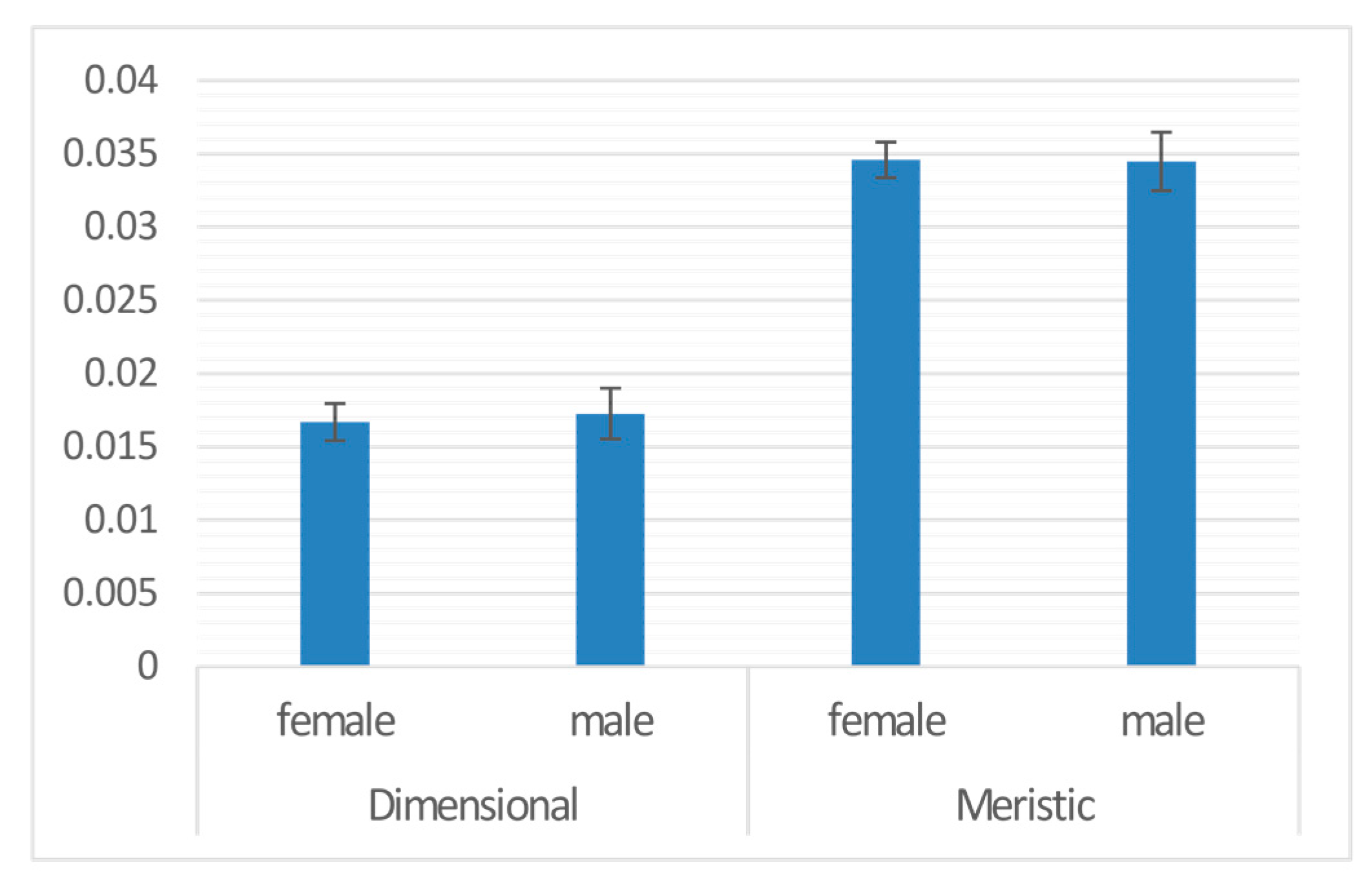
| Sex | 1 | 0.000 | 8.036 | −18196 | 0.001 | 0.973 |
| Source | ESTIMATE | STD. Error | t-value | Pr (>|t|) | ||
|---|---|---|---|---|---|---|
| (Intercept) | −0.010 | 0.006 | −1.841 | 0.066 | . | |
| Species | C. cancellatus | 0.024 | 0.009 | 2.566 | 0.010 | * |
| C. granulatus | 0.014 | 0.011 | 1.217 | 0.224 | ||
| P. cupreus | 0.032 | 0.008 | 3.814 | 0.000 | *** | |
| P. melanarius | 0.003 | 0.011 | 0.323 | 0.747 | ||
| P. niger | −0.012 | 0.013 | −0.938 | 0.348 | ||
| Province | Sverdlovsk oblast | 0.020 | 0.012 | 1.581 | 0.114 | |
| Tatarstan republic | −0.003 | 0.009 | −0.298 | 0.766 | ||
| Anthropogene | city | 0.019 | 0.005 | 3.852 | 0.000 | *** |
| natural | 0.008 | 0.010 | 0.840 | 0.401 | ||
| suburb | 0.013 | 0.007 | 1.865 | 0.062 | . | |
| Sex | males | 0.000 | 0.002 | −0.034 | 0.973 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raisa, S.; Anatoliy, S.; Timur, M.; Natalia, E. Fluctuating Asymmetry in Ground Beetles (Coleoptera, Carabidae) and Conditions of Its Manifestation. Symmetry 2019, 11, 1475. https://doi.org/10.3390/sym11121475
Raisa S, Anatoliy S, Timur M, Natalia E. Fluctuating Asymmetry in Ground Beetles (Coleoptera, Carabidae) and Conditions of Its Manifestation. Symmetry. 2019; 11(12):1475. https://doi.org/10.3390/sym11121475
Chicago/Turabian StyleRaisa, Sukhodolskaya, Saveliev Anatoliy, Mukhametnabiev Timur, and Eremeeva Natalia. 2019. "Fluctuating Asymmetry in Ground Beetles (Coleoptera, Carabidae) and Conditions of Its Manifestation" Symmetry 11, no. 12: 1475. https://doi.org/10.3390/sym11121475
APA StyleRaisa, S., Anatoliy, S., Timur, M., & Natalia, E. (2019). Fluctuating Asymmetry in Ground Beetles (Coleoptera, Carabidae) and Conditions of Its Manifestation. Symmetry, 11(12), 1475. https://doi.org/10.3390/sym11121475





