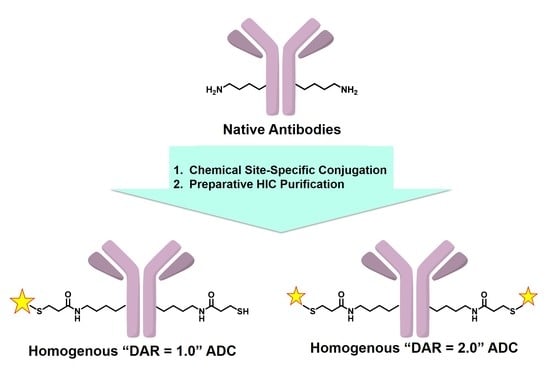
A Purification Strategy Utilizing Hydrophobic Interaction Chromatography to Obtain Homogeneous Species from a Site-Specific Antibody Drug Conjugate Produced by AJICAP™ First Generation
Abstract
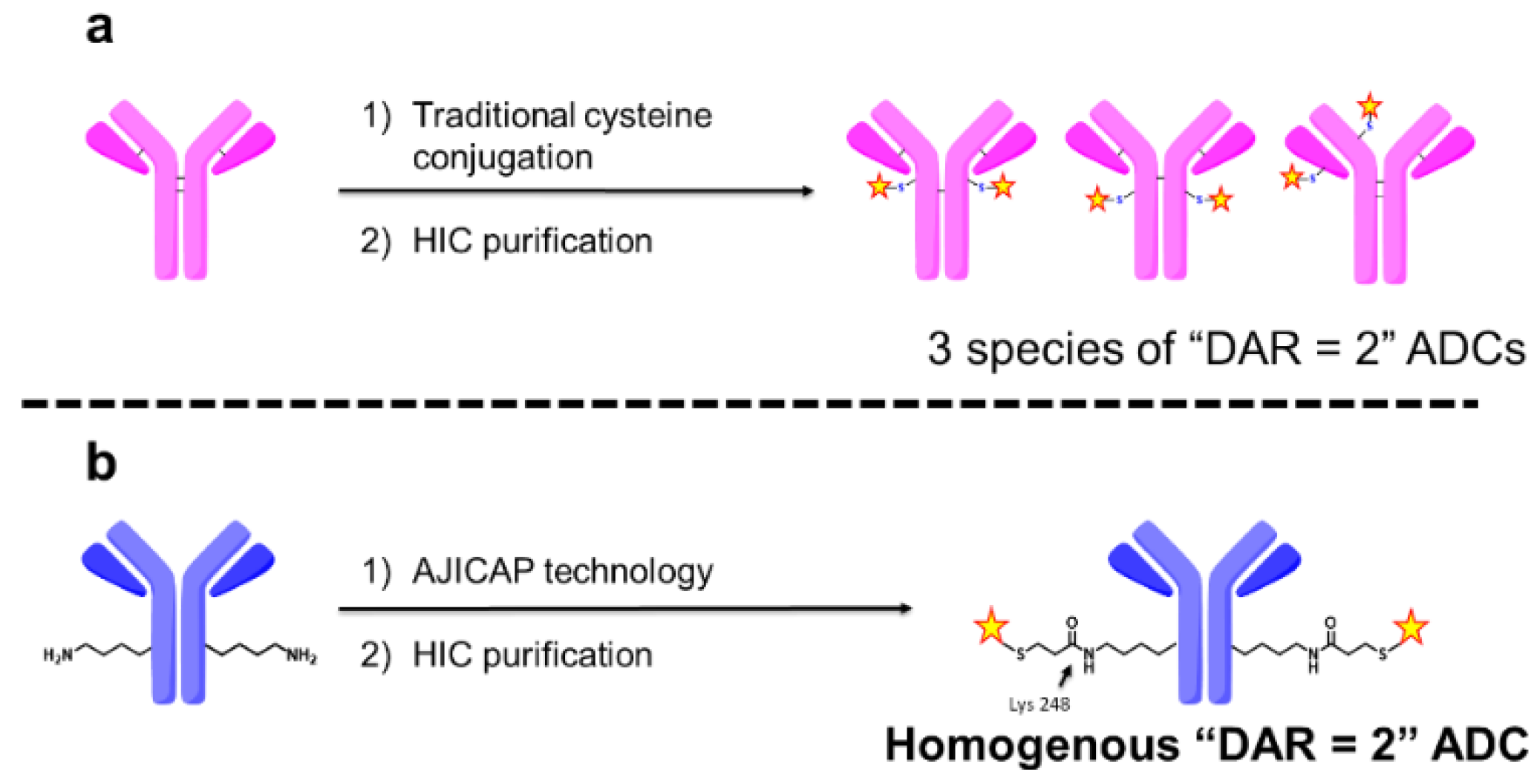
1. Introduction
2. Materials and Methods
2.1. Materials
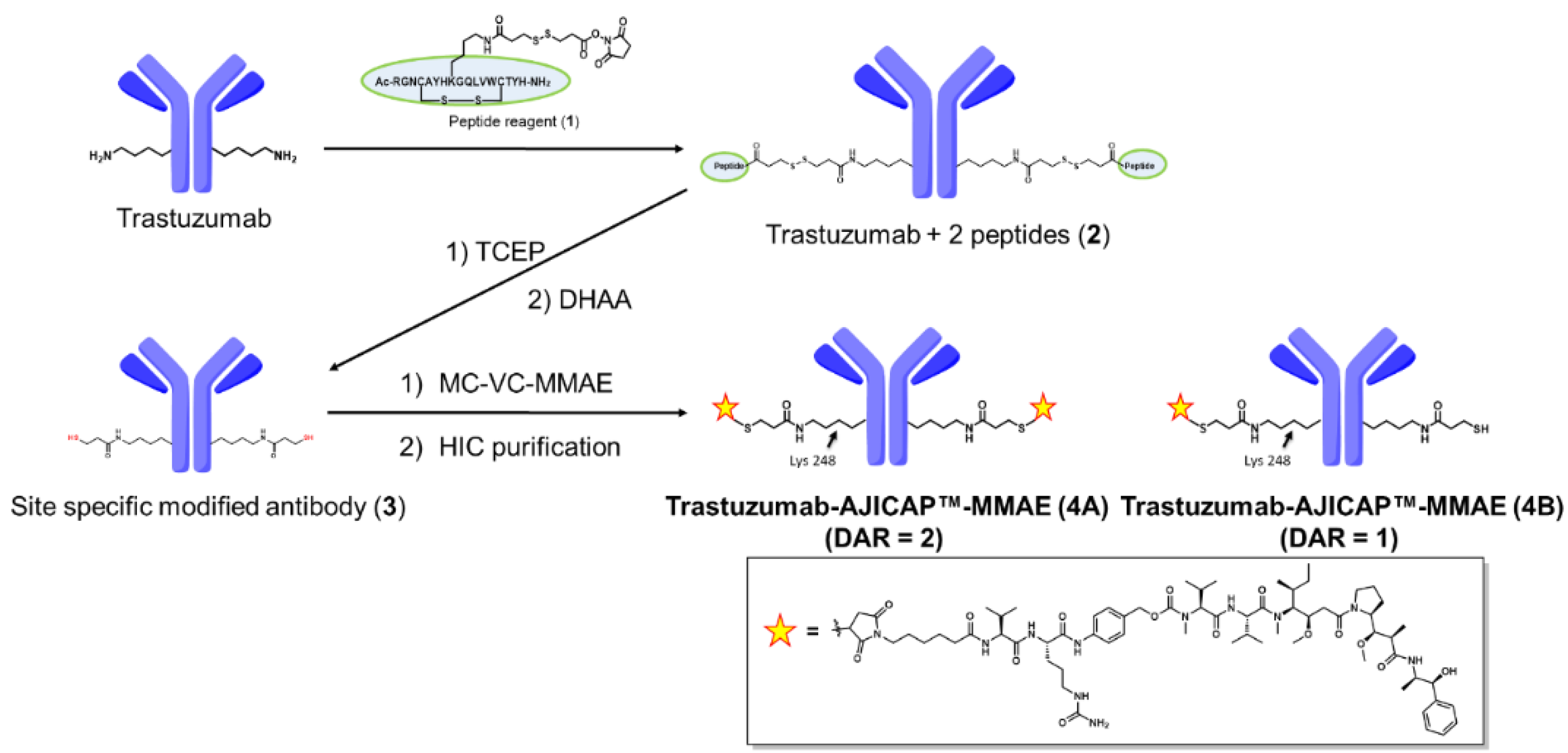
2.2. Synthetic Procedure for Unpurified Trastuzumab-AJICAP™-MMAE
2.3. Preparative HIC
2.4. ADC Concentration
2.5. HIC-HPLC Analysis
2.6. RP-HPLC Analysis
2.7. Q-TOF MS Analysis
2.8. SEC-HPLC Analysis
2.9. In Vitro Cytotoxicity
3. Results and Discussion
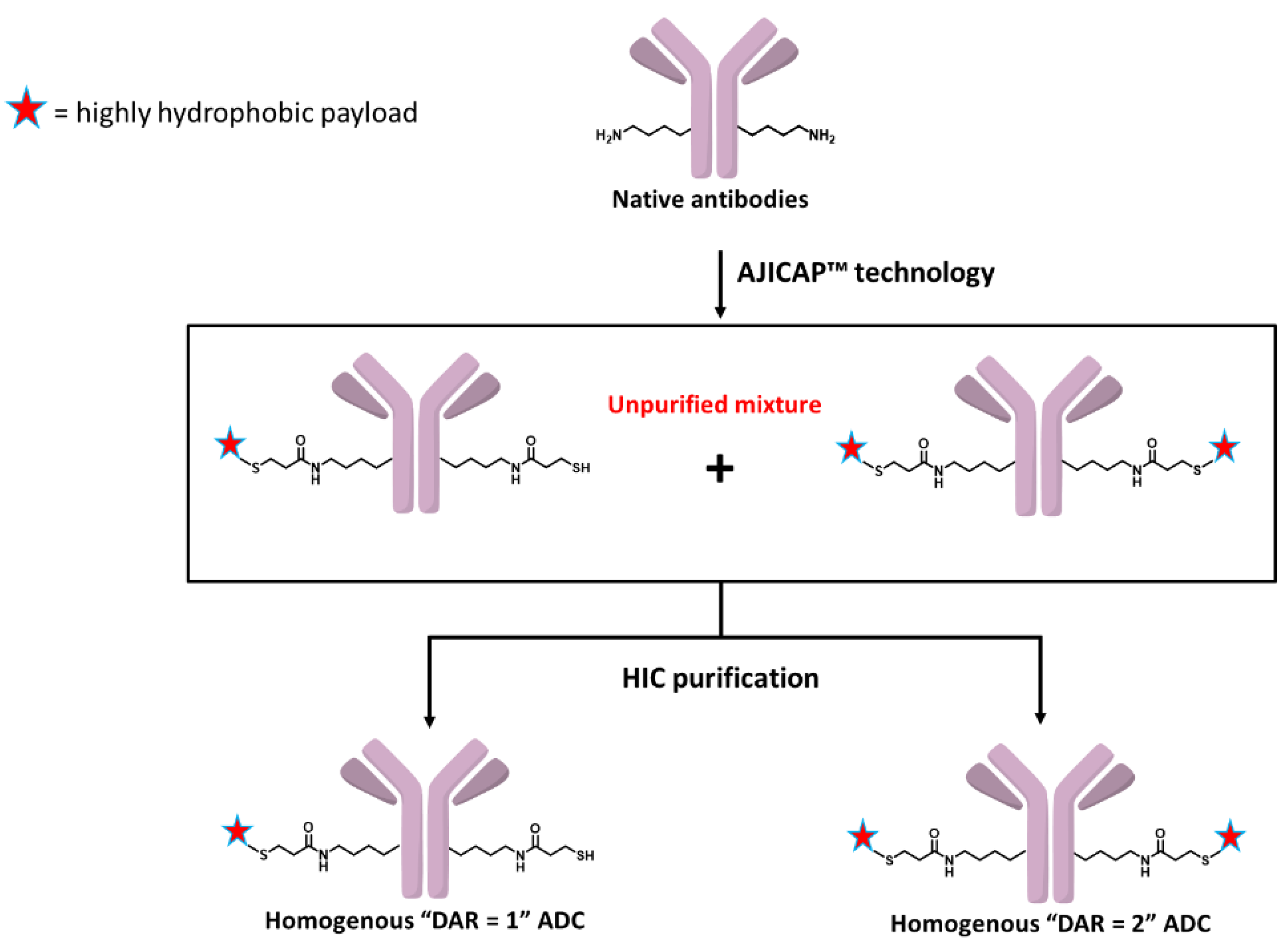
3.1. Site-Specific Conjugation and Purification of Trastuzumab-AJICAP™-MMAE
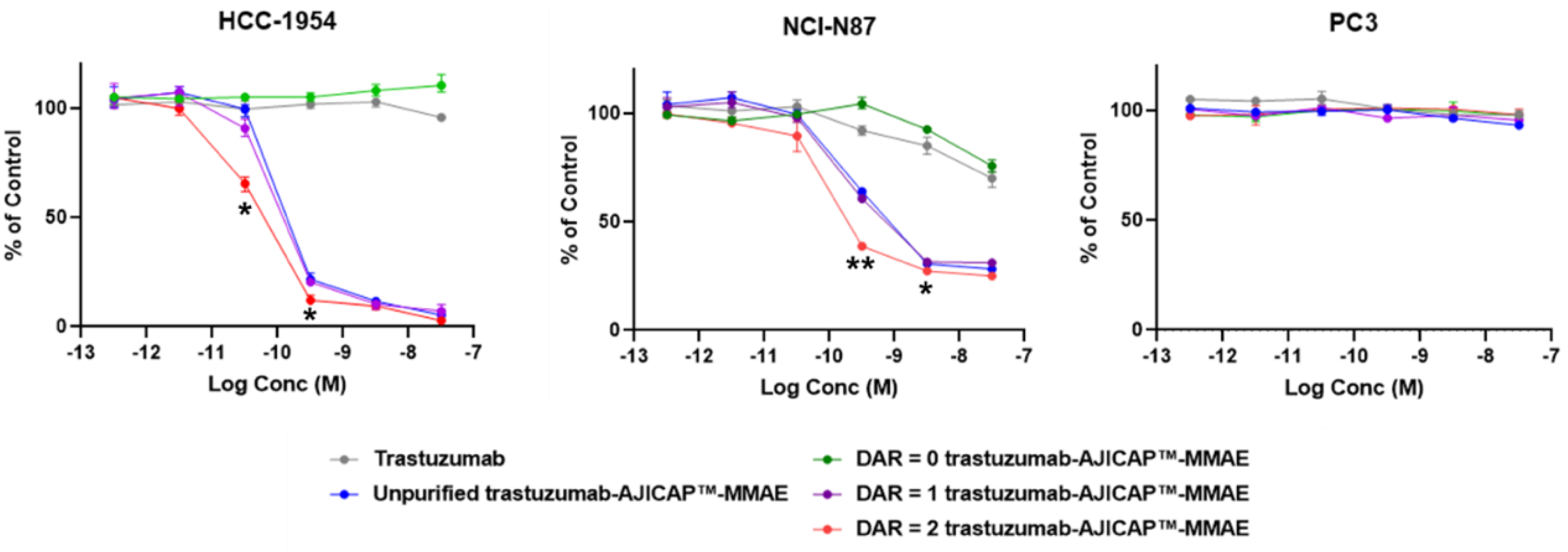
3.2. In Vitro Cell Based Assay of DAR = 1 and DAR = 2 ADCs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdollahpour-Alitappeh, M.; Lotfinia, M.; Gharibi, T.; Mardaneh, J.; Farhadihosseinabadi, B.; Larki, P.; Faghfourian, B.; Sepehr, K.S.; Abbaszadeh-Goudarzi, K.; Abbaszadeh-Goudarzi, G.; et al. Antibody–drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. J. Cell. Physiol. 2019, 234, 5628–5642. [Google Scholar] [CrossRef]
- Leung, D.; Wurst, J.M.; Liu, T.; Martinez, R.M.; Datta-Mannan, A.; Feng, Y. Antibody Conjugates-Recent Advances and Future Innovations. Antibodies 2020, 9, 2. [Google Scholar] [CrossRef]
- Lucas, A.T.; Robinson, R.; Schorzman, A.N.; Piscitelli, J.A.; Razo, J.F.; Zamboni, W.C. Pharmacologic Considerations in the Disposition of Antibodies and Antibody-Drug Conjugates in Preclinical Models and in Patients. Antibodies 2019, 8, 3. [Google Scholar] [CrossRef]
- Senter, P.D.; Sievers, E.L. The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Nat. Biotechnol. 2012, 30, 631–637. [Google Scholar] [CrossRef]
- Junutula, J.R.; Raab, H.; Clark, S.; Bhakta, S.; Leipold, D.D.; Weir, S.; Chen, Y.; Simpson, M.; Tsai, S.P.; Dennis, M.S.; et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 2008, 26, 925–932. [Google Scholar] [CrossRef]
- Harel, E.T.; Drake, P.M.; Barfield, R.M.; Lui, I.; Farr-Jones, S.; Veer, L.V.; Gartner, G.J.; Green, E.M.; Lourenço, A.L.; Cheng, Y.; et al. Antibody-Drug Conjugates Targeting the Urokinase Receptor (uPAR) as a Possible Treatment of Aggressive Breast Cancer. Antibodies 2019, 8, 54. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z. Antibody-drug conjugates: Recent advances in conjugation and linker chemistries. Protein Cell 2018, 9, 33–46. [Google Scholar] [CrossRef]
- Yamada, K.; Shikida, N.; Shimbo, K.; Ito, Y.; Khedri, Z.; Matsuda, Y.; Mendelsohn, B.A. AJICAP: Affinity Peptide Mediated Regiodivergent Functionalization of Native Antibodies. Angew. Chem. Int. Ed. 2019, 58, 5592–5597. [Google Scholar] [CrossRef]
- Matsuda, Y.; Clancy, C.; Tawfiq, Z.; Robles, V.; Mendelsohn, B.A. Good Manufacturing Practice Strategy for Antibody-Drug Conjugate Synthesis Using Site-Specific Chemical Conjugation: First-Generation AJICAP. ACS Omega 2019, 4, 20564–20570. [Google Scholar] [CrossRef]
- Matsuda, Y.; Yamada, K.; Okuzumi, T.; Mendelsohn, B.A. Gram-Scale Antibody-Drug Conjugate Synthesis by Site-Specific Chemical Conjugation: AJICAP first Generation. Org. Process. Res. Dev. 2019, 23, 2647–2654. [Google Scholar] [CrossRef]
- Matsuda, Y.; Malinao, M.-C.; Robles, V.; Song, J.; Yamada, K.; Mendelsohn, B.A. Proof of Site-Specificity of Antibody-Drug Conjugates Produced by Chemical Conjugation Technology: AJICAP First Generation. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1140, 121981. [Google Scholar] [CrossRef]
- Becker, C.L.; Duffy, R.J.; Gandarilla, J.; Richter, S.M. Purification of ADCs by Hydrophobic Interaction Chromatography. Methods Mol. Biol. 2019, 2078, 237–289. [Google Scholar]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.C.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of Drug Loading on the Antitumor Activity of a Monoclonal Antibody Drug Conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef] [PubMed]
- Badescu, G.; Bryant, P.; Bird, M.; Henseleit, K.; Swierkosz, J.; Parekh, V.; Tommasi, R.; Pawlisz, E.; Jurlewicz, K.; Farys, M.; et al. Bridging disulfides for stable and defined antibody drug conjugates. Bioconjug. Chem. 2014, 25, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Bryant, P.; Pabst, M.; Badescu, G.; Bird, M.; McDowell, W.; Jamieson, E.; Swierkosz, J.; Jurlewicz, K.; Tommasi, R.; Henseleit, K.; et al. In Vitro and In Vivo Evaluation of Cysteine Rebridged Trastuzumab-MMAE Antibody Drug Conjugates with Defined Drug-to-Antibody Ratios. Mol. Pharm. 2015, 12, 1872–1879. [Google Scholar] [CrossRef]
- Schumacher, F.F.; Nunes, J.P.M.; Maruani, A.; Chudasama, V.; Smith, M.E.B.; Chester, K.A.; Baker, J.R.; Caddick, S. Next generation maleimides enable the controlled assembly of antibody–drug conjugates via native disulfide bond bridging. Org. Biomol. Chem. 2014, 12, 7261–7269. [Google Scholar] [CrossRef]
- Strop, P.; Liu, S.-H.H.; Dorywalska, M.; Delaria, K.; Dushin, R.G.; Tran, T.-T.T.; Ho, W.-H.H.; Farias, S.; Casas, M.G.; Abdiche, Y.; et al. Location Matters: Site of Conjugation Modulates Stability and Pharmacokinetics of Antibody Drug Conjugates. Chem. Biol. 2013, 20, 161–167. [Google Scholar] [CrossRef]
- Gregson, S.J.; Tiberghien, A.C.; Masterson, L.A.; Howard, P.W. Pyrrolobenzodiazepine Dimers as Antibody–Drug Conjugate (ADC) Payloads. In Cytotoxic Payloads for Antibody–Drug Conjugates; Thurston, D.V., Jackson, P.J.M., Eds.; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Fu, Y.; Ho, M. DNA Damaging Agent Based Antibody-Drug Conjugates for Cancer Therapy. Antib. Ther. 2018, 1, 33–43. [Google Scholar] [CrossRef]
- Matsuda, Y.; Robles, V.; Malinao, M.-C.; James, S.; Mendelsohn, B.A. Comparison of Analytical Methods for Antibody–Drug Conjugates Produced by Chemical Site-Specific Conjugation: First-Generation AJICAP. Anal. Chem. 2019, 91, 12724–12732. [Google Scholar] [CrossRef]
- Tawfiq, Z.; Matsuda, Y.; Alfonso, M.J.; Robles, V.; Leung, M.; Mendelsohn, B.A. Analytical Comparison of Antibody-Drug Conjugates Based on Good Manufacturing Practice Strategies. Anal. Sci. 2020. [Google Scholar] [CrossRef]
- Tawfiq, Z.; Caiazza, N.C.; Kambourakis, S.; Matsuda, Y.; Griffin, B.; Lippmeier, J.C.; Mendelsohn, B.A. Synthesis and Biological Evaluation of Antibody Drug Conjugates Based on an Antibody Expression System: Conamax. ACS Omega 2020, 5, 7193–7200. [Google Scholar] [CrossRef] [PubMed]
- Källsten, M.; Hartmann, R.; Artemenko, K.; Lind, S.B.; Lehmann, F.; Bergquist, B. Qualitative analysis of antibody–drug conjugates (ADCs): An experimental comparison of analytical techniques of cysteine-linked ADCs. Analysis 2018, 143, 5487–5496. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Pell, R.; Clarke, A.; Guillarme, D.; Fekete, S. Is hydrophobic interaction chromatography the most suitable technique to characterize site-specific antibody-drug conjugates? J. Chromatogr. A 2019, 1586, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Kliman, M.; Mendelsohn, B.A. Application of Native Ion Exchange Mass Spectrometry to Intact and Subunit Analysis of Site-Specific Antibody-Drug Conjugates Produced by AJICAP First Generation Technology. J. Am. Soc. Mass Spectrom. 2020. accepted. [Google Scholar]
- Bickel, F.; Herold, E.M.; Signes, A.; Romeijn, S.; Jiskoot, W.; Kiefer, H. Reversible NaCl-induced aggregation of a monoclonal antibody at low pH: Characterization of aggregates and factors affecting aggregation. Eur. J. Pharm. Biopharm. 2016, 107, 310–320. [Google Scholar] [CrossRef]
- Kruse, T.; Schmidt, A.; Kampmann, M.; Strube, J. Integrated Clarification and Purification of Monoclonal Antibodies by Membrane Based Separation of Aqueous Two-Phase Systems. J. Antibodies 2019, 8, 40. [Google Scholar] [CrossRef]
- McDonagh, C.F.; Kim, K.M.; Turcott, E.; Brown, L.L.; Westendorf, L.; Feist, T.; Sussman, D.; Stone, I.; Anderson, M.; Miyamoto, J.; et al. Engineered anti-CD70 antibody-drug conjugate with increased therapeutic index. Mol. Cancer Ther. 2008, 7, 2913–2923. [Google Scholar] [CrossRef]
- Tscheuschner, G.; Schwaar, T.; Weller, M.G. Fast Confirmation of Antibody Identity by MALDI-TOF MS Fingerprints. Antibodies 2020, 9, 8. [Google Scholar] [CrossRef]
- Satomaa, T.; Pynnönen, H.; Vilkman, A.; Kotiranta, T.; Pitkänen, V.; Heiskanen, A.; Herpers, B.; Price, L.S.; Helin, J.; Saarinen, J. Hydrophilic Auristatin Glycoside Payload Enables Improved Antibody-Drug Conjugate Efficacy and Biocompatibility. Antibodies 2018, 7, 15. [Google Scholar] [CrossRef]
- Müller, E.; Sevilla, M.; Endres, P. Evaluation of hydrophobic-interaction-chromatography Resins for purification of antibody-drug conjugates using a mimetic model with adjustable hydrophobicity. J. Sep. Sci. in press. [CrossRef]
- Ghose, S.; Tao, Y.; Conley, L.; Cecchini, D. Purification of monoclonal antibodies by hydrophobic interaction chromatography under no-salt conditions. MAbs 2013, 5, 795–800. [Google Scholar] [CrossRef] [PubMed]
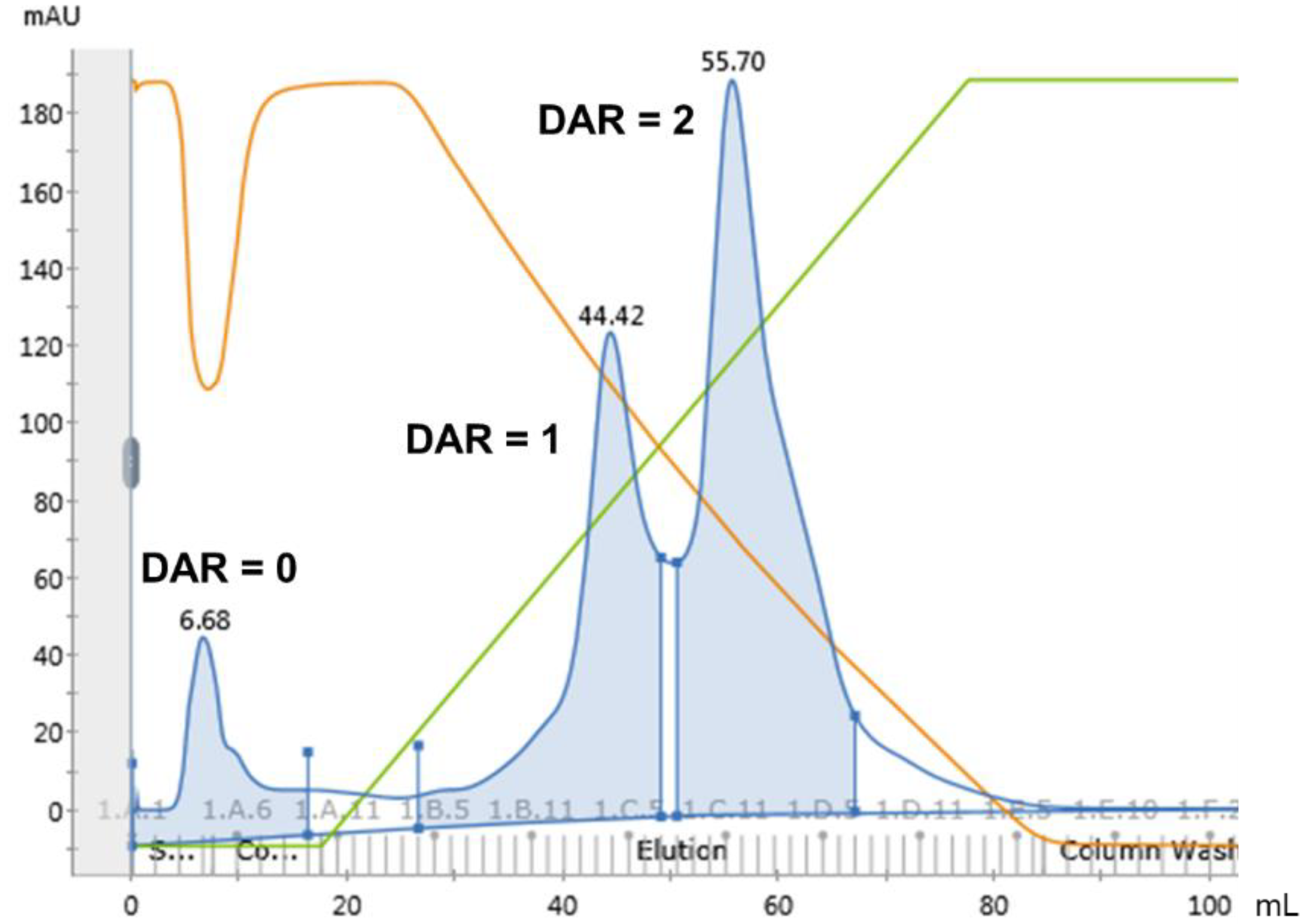

| Entry | Resin | DAR Separation | % Recovery |
|---|---|---|---|
| 1 | Phenyl resin (TOYOPEARL Phenyl-650S) | Separated | 65% |
| 2 | Butyl resin (TOYOPEARL Butyl-650M) | Not eluted | - |
| 3 | PPG resin (TOYOPEARL PPG-600M) | Not separated | 72% |
| Entry | HCC-1954 | NCI-N87 | PC-3 |
|---|---|---|---|
| Trastuzumab | >30 nM | >30 nM | >30 nM |
| Unpurified trastuzumab-AJICAP™-MMAE | 0.21 nM | 0.28 nM | >30 nM |
| Purified site-specifically modified antibody (3, DAR = 0) | >30 nM | >30 nM | >30 nM |
| Purified DAR = 1 trastuzumab-AJICAP™-MMAE | 0.18 nM | 0.24 nM | >30 nM |
| Purified DAR = 2 trastuzumab-AJICAP™-MMAE | 0.12 nM | 0.12 nM | >30 nM |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, Y.; Leung, M.; Okuzumi, T.; Mendelsohn, B. A Purification Strategy Utilizing Hydrophobic Interaction Chromatography to Obtain Homogeneous Species from a Site-Specific Antibody Drug Conjugate Produced by AJICAP™ First Generation. Antibodies 2020, 9, 16. https://doi.org/10.3390/antib9020016
Matsuda Y, Leung M, Okuzumi T, Mendelsohn B. A Purification Strategy Utilizing Hydrophobic Interaction Chromatography to Obtain Homogeneous Species from a Site-Specific Antibody Drug Conjugate Produced by AJICAP™ First Generation. Antibodies. 2020; 9(2):16. https://doi.org/10.3390/antib9020016
Chicago/Turabian StyleMatsuda, Yutaka, Monica Leung, Tatsuya Okuzumi, and Brian Mendelsohn. 2020. "A Purification Strategy Utilizing Hydrophobic Interaction Chromatography to Obtain Homogeneous Species from a Site-Specific Antibody Drug Conjugate Produced by AJICAP™ First Generation" Antibodies 9, no. 2: 16. https://doi.org/10.3390/antib9020016
APA StyleMatsuda, Y., Leung, M., Okuzumi, T., & Mendelsohn, B. (2020). A Purification Strategy Utilizing Hydrophobic Interaction Chromatography to Obtain Homogeneous Species from a Site-Specific Antibody Drug Conjugate Produced by AJICAP™ First Generation. Antibodies, 9(2), 16. https://doi.org/10.3390/antib9020016