Selection of Single-Domain Antibodies towards Western Equine Encephalitis Virus
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
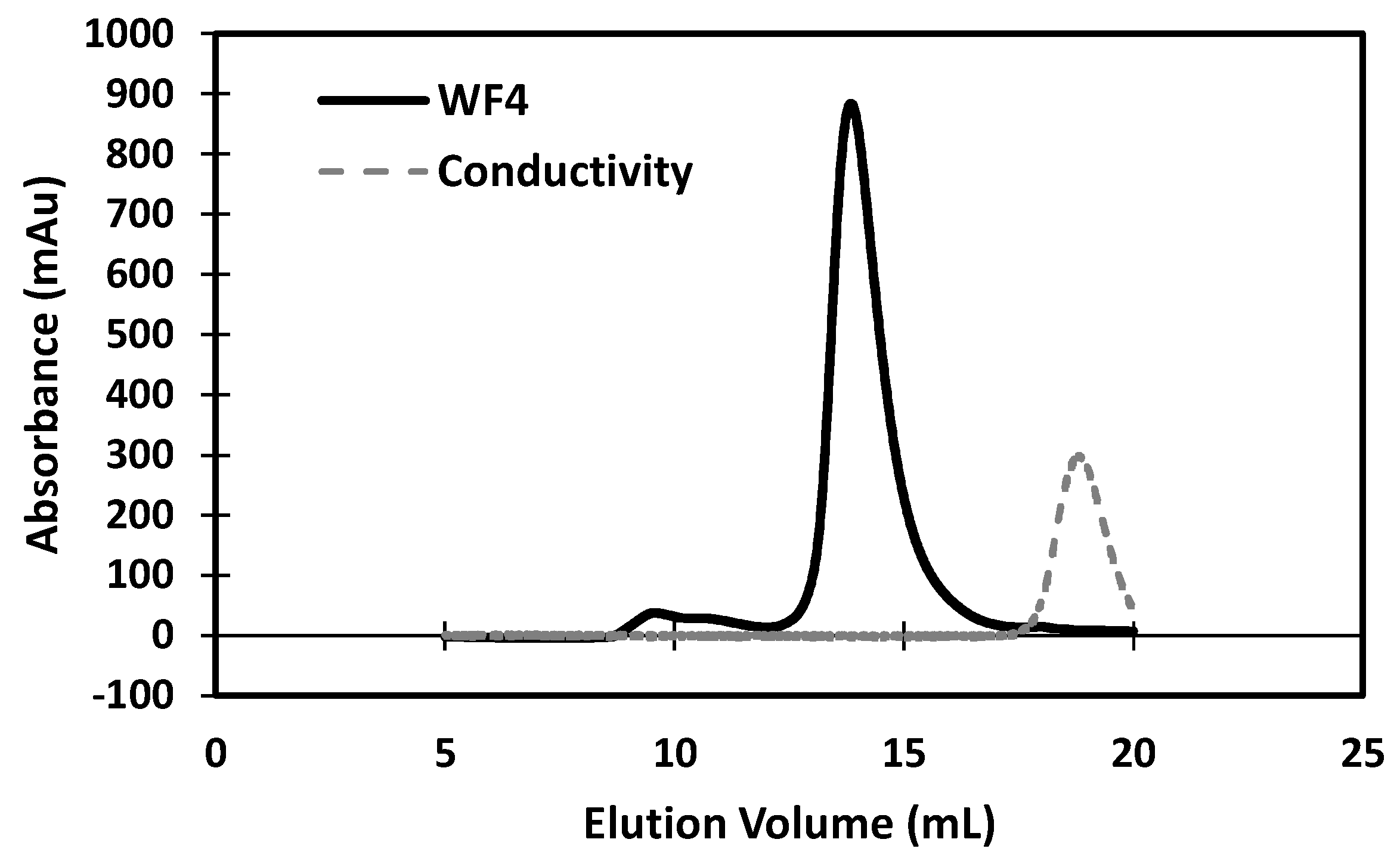
2.2. Library Construction, Panning, and Production of sdAb
2.3. Surface Plasmon Resonance
2.4. Determining Melting Temperature by Fluorescent Dye Melt Assay
2.5. MagPlex Direct Binding and Sandwich Assays
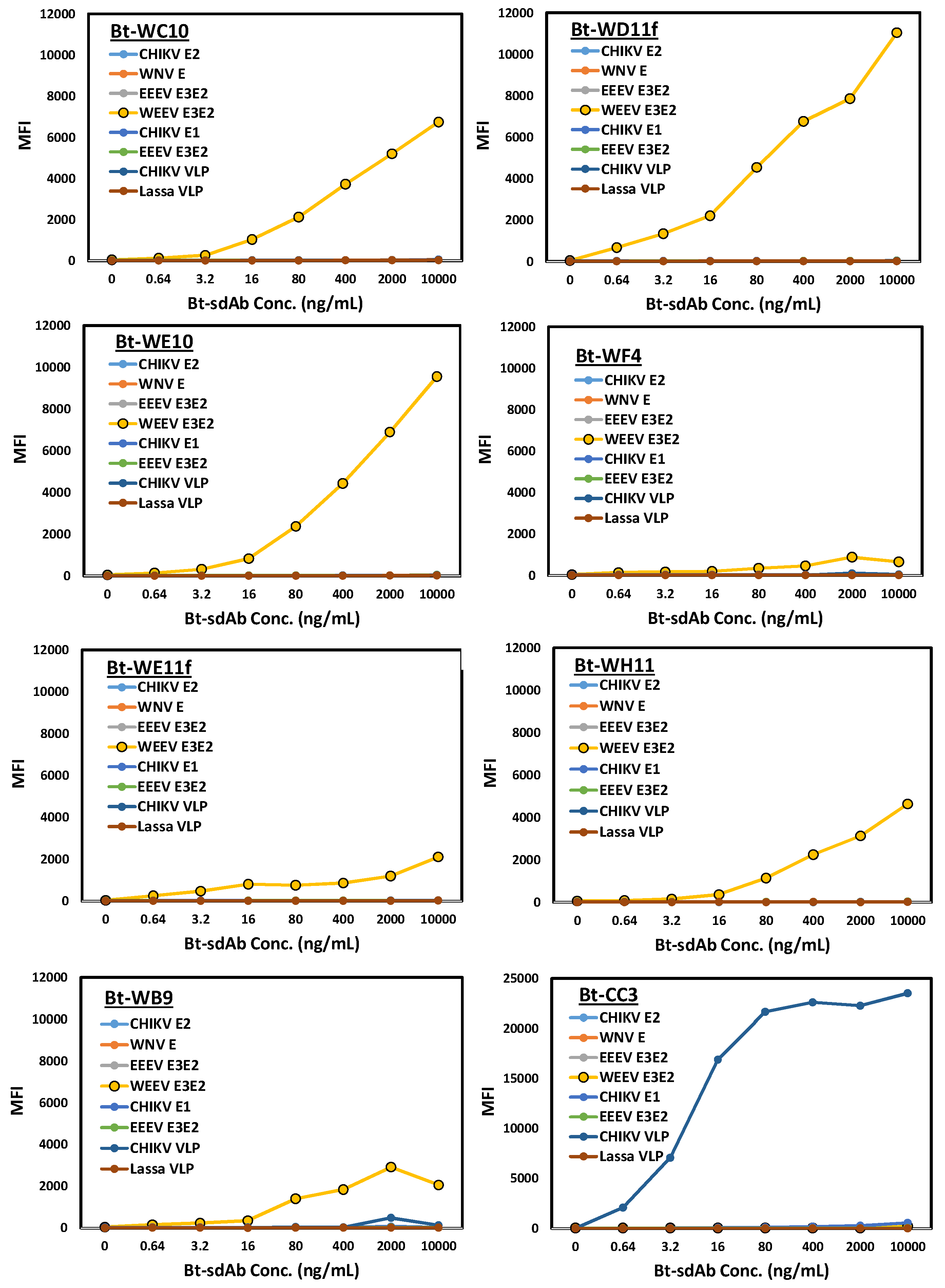
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Hahn, C.S.; Lustig, S.; Strauss, E.G.; Strauss, J.H. Western equine encephalitis virus is a recombinant virus. Proc. Natl. Acad. Sci. USA 1988, 85, 5997–6001. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.M.; Brault, A.C.; Shirako, Y.; Strauss, E.G.; Kang, W.; Strauss, J.H.; Weaver, S.C. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 2001, 75, 10118–10131. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.H.; Strauss, E.G. The alphaviruses: Gene expression, replication, and evolution. Microb. Rev. 1994, 58, 491–562. [Google Scholar]
- Goh, L.Y.; Hobson-Peters, J.; Prow, N.A.; Gardner, J.; Bielefeldt-Ohmann, H.; Pyke, A.T.; Suhrbier, A.; Hall, R.A. Neutralizing monoclonal antibodies to the e2 protein of chikungunya virus protects against disease in a mouse model. Clin. Immunol. 2013, 149, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Eastern, Western and Venezuelan Equine Encephalomyelitis. Available online: http://www.cfsph.iastate.edu/Factsheets/pdfs/easter_wester_venezuelan_equine_encephalomyelitis.pdf (accessed on 25 October 2018).
- Reisen, W.; Monath, T. Western equine encephalitis. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1989; Volume V, pp. 89–137. [Google Scholar]
- Zacks, M.A.; Paessler, S. Encephalitic alphaviruses. Vet. Microbiol. 2010, 140, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.C.; Hutson, G.A.; Bellamy, R.E.; Scrivani, R.P. Chronic latent infections of birds with western equine encephalomyelitis virus. Proc. Soc. Exp. Biol. Med. 1958, 97, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Phillpotts, R.J. Venezuelan equine encephalitis virus complex-specific monoclonal antibody provides broad protection, in murine models, against airborne challenge with viruses from serogroups i, ii and iii. Virus Res. 2006, 120, 107–112. [Google Scholar] [CrossRef]
- Reed, D.S.; Larsen, T.; Sullivan, L.J.; Lind, C.M.; Lackemeyer, M.G.; Pratt, W.D.; Parker, M.D. Aerosol exposure to western equine encephalitis virus causes fever and encephalitis in cynomolgus macaques. J. Infect. Dis. 2005, 192, 1173–1182. [Google Scholar] [CrossRef]
- Hawley, R.J.; Eitzen, E.M., Jr. Biological weapons—A primer for microbiologists. Annu. Rev. Microb. 2001, 55, 235–253. [Google Scholar] [CrossRef]
- Sidwell, R.W.; Smee, D.F. Viruses of the bunya- and togaviridae families: Potential as bioterrorism agents and means of control. Antiv. Res. 2003, 57, 101–111. [Google Scholar] [CrossRef]
- Nagata, L.P.; Wong, J.P.; Hu, W.-G.; Wu, J.Q. Vaccines and therapeutics for the encephalitic alphaviruses. Future Virol. 2013, 8, 661–674. [Google Scholar] [CrossRef]
- Sherman, M.B.; Weaver, S.C. Structure of the recombinant alphavirus western equine encephalitis virus revealed by cryoelectron microscopy. J. Virol. 2010, 84, 9775–9782. [Google Scholar] [CrossRef]
- Uchime, O.; Fields, W.; Kielian, M. The role of e3 in ph protection during alphavirus assembly and exit. J. Virol. 2013, 87, 10255–10262. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Kriangkum, J.; Nagata, L.P.; Fulton, R.E.; Suresh, M.R. Development of a biotin mimic tagged scfv antibody against western equine encephalitis virus: Bacterial expression and refolding. J. Virol. Methods 2004, 117, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kriangkum, J.; Nagata, L.P.; Fulton, R.E.; Suresh, M.R. A single chain fv specific against western equine encephalitis virus. Hybridoma 1999, 18, 315–323. [Google Scholar] [CrossRef]
- Long, M.C.; Jager, S.; Mah, D.C.W.; Jebailey, L.; Mah, M.A.; Masri, S.A.; Nagata, L.P. Construction and characterization of a novel recombinant single-chain variable fragment antibody against western equine encephalitis virus. Hybridoma 2000, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hülseweh, B.; Rülker, T.; Pelat, T.; Langermann, C.; Frenzel, A.; Schirrmann, T.; Dübel, S.; Thullier, P.; Hust, M. Human-like antibodies neutralizing western equine encephalitis virus. mAbs 2014, 6, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Burke, C.W.; Froude, J.W.; Miethe, S.; Hülseweh, B.; Hust, M.; Glass, P.J. Human-like neutralizing antibodies protect mice from aerosol exposure with western equine encephalitis virus. Viruses 2018, 10, 147. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Ghahroudi, M.A.; Desmyter, A.; Wyns, L.; Hamers, R.; Muyldermans, S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar] [CrossRef]
- De Marco, A. Biotechnological applications of recombinant single-domain antibody fragments. Microb. Cell Fact. 2011, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, J.; Alzogaray, V.; Reyelt, J.; Unger, M.; Juarez, K.; Urrutia, M.; Cauerhff, A.; Danquah, W.; Rissiek, B.; Scheuplein, F.; et al. Single domain antibodies: Promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 2009, 198, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Eyer, L.; Hruska, K. Single-domain antibody fragments derived from heavy-chain antibodies: A review. Vet. Med. 2012, 57, 439–513. [Google Scholar] [CrossRef]
- Hussack, G.; Hirama, T.; Ding, W.; MacKenzie, R.; Tanha, J. Engineered single-domain antibodies with high protease resistance and thermal stability. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Walper, S.A.; Turner, K.B.; Brozozog-Lee, A.; Medintz, I.L.; Susumu, K.; Oh, E.; Zabetakis, D.; Goldman, E.R.; Anderson, G.P. Conjugation of biotin-coated luminescent quantum dots with single domain antibody-rhizavidin fusions. Biotechnol. Rep. 2016, 10, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Zabetakis, D.; Brozozog Lee, P.A.; Goldman, E.R.; Anderson, G.P. Single domain antibody alkaline phosphatase fusion proteins for antigen detection—Analysis of affinity and thermal stability of single domain antibody. J. Immunol. Methods 2013, 393, 1–7. [Google Scholar] [CrossRef]
- Pleschberger, M.; Saerens, D.; Weigert, S.; Sleytr, U.B.; Muyldermans, S.; Sara, M.; Egelseer, E.M. An s-layer heavy chain camel antibody fusion protein for generation of a nanopatterned sensing layer to detect the prostate-specific antigen by surface plasmon resonance technology. Bioconjug. Chem. 2004, 15, 664–671. [Google Scholar] [CrossRef]
- Raphael, M.P.; Christodoulides, J.A.; Byers, J.M.; Anderson, G.P.; Liu, J.L.; Turner, K.B.; Goldman, E.R.; Delehanty, J.B. Optimizing nanoplasmonic biosensor sensitivity with orientated single domain antibodies. Plasmonics 2015, 10, 1649–1655. [Google Scholar] [CrossRef]
- Sherwood, L.J.; Hayhurst, A. Hapten mediated display and pairing of recombinant antibodies accelerates assay assembly for biothreat countermeasures. Sci. Rep. 2012, 2, 807. [Google Scholar] [CrossRef]
- Liu, J.L.; Goldman, E.R.; Zabetakis, D.; Walper, S.A.; Turner, K.B.; Shriver-Lake, L.C.; Anderson, G.P. Enhanced production of a single domain antibody with an engineered stabilizing extra disulfide bond. Microb. Cell Fact. 2015, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, Y.; Mine, S.; Uegaki, K. Stabilization of an immunoglobulin fold domain by an engineered disulfide bond at the buried hydrophobic region. J. Biol. Chem. 2007, 282, 36489–36495. [Google Scholar] [CrossRef] [PubMed]
- Saerens, D.; Conrath, K.; Govaert, J.; Muyldermans, S. Disulfide bond introduction for general stabilization of immunoglobulin heavy-chain variable domains. J. Mol. Biol. 2008, 377, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.B.; Liu, J.L.; Zabetakis, D.; Lee, A.B.; Anderson, G.P.; Goldman, E.R. Improving the biophysical properties of anti-ricin single-domain antibodies. Biotechnol. Rep. 2015, 6, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.R.; Liu, J.L.; Zabetakis, D.; Anderson, G.P. Enhancing stability of camelid and shark single domain antibodies: An overview. Front. Immunol. 2017, 8, 865. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, C.; Muyldermans, S. Nanobody-based delivery systems for diagnosis and targeted tumor therapy. Front. Immunol. 2017, 8, 1442. [Google Scholar] [CrossRef] [PubMed]
- Cordy, J.C.; Morley, P.J.; Wright, T.J.; Birchler, M.A.; Lewis, A.P.; Emmins, R.; Chen, Y.Z.; Powley, W.M.; Bareille, P.J.; Wilson, R.; et al. Specificity of human anti-variable heavy (vh) chain autoantibodies and impact on the design and clinical testing of a vh domain antibody antagonist of tumour necrosis factor-alpha receptor 1. Clin. Exp. Immunol. 2015, 182, 139–148. [Google Scholar] [CrossRef]
- Liu, J.L.; Shriver-Lake, L.C.; Anderson, G.P.; Zabetakis, D.; Goldman, E.R. Selection, characterization, and thermal stabilization of llama single domain antibodies towards ebola virus glycoprotein. Microb. Cell Fact. 2017, 16, 223. [Google Scholar] [CrossRef]
- Anderson, G.; Matney, R.; Liu, J.; Hayhurst, A.; Goldman, E. Multiplexed fluid array screening of phage displayed anti-ricin single domain antibodies for rapid assessment of specificity. Biotechniques 2007, 43, 806–811. [Google Scholar] [CrossRef]
- Goldman, E.; Anderson, G.; Liu, J.; Delehanty, J.; Sherwood, L.; Osborn, L.; Cummins, L.; Hayhurst, A. Facile generation of heat-stable antiviral and antitoxin single domain antibodies from a semisynthetic llama library. Analyt. Chem. 2006, 78, 8245–8255. [Google Scholar] [CrossRef]
- Walper, S.A.; Liu, J.L.; Zabetakis, D.; Anderson, G.P.; Goldman, E.R. Development and evaluation of single domain antibodies for vaccinia and the l1 antigen. PLoS ONE 2014, 9, e106263. [Google Scholar] [CrossRef] [PubMed]
- Marcus, W.D.; Lindsay, S.M.; Sierks, M.R. Identification and repair of positive binding antibodies containing randomly generated amber codons from synthetic phage display libraries. Biotechnol. Prog. 2006, 22, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Shriver-Lake, L.C.; Zabetakis, D.; Goldman, E.R.; Anderson, G.P. Evaluation of anti-botulinum neurotoxin single domain antibodies with additional optimization for improved production and stability. Toxicon 2017, 135, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Walper, S.A.; Lee, P.A.B.; Anderson, G.P.; Goldman, E.R. Selection and characterization of single domain antibodies specific for bacillus anthracis spore proteins. Antibodies 2013, 2, 152–167. [Google Scholar] [CrossRef]
- Liu, J.L.; Zabetakis, D.; Goldman, E.R.; Anderson, G.P. Selection and evaluation of single domain antibodies toward ms2 phage and coat protein. Mol. Immunol. 2013, 53, 118–125. [Google Scholar] [CrossRef]
- Corpet, F. Multiple sequence alignment with hierarchical-clustering. Nucleic. Acids Res. 1988, 16, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Shriver-Lake, L.C.; Zabetakis, D.; Anderson, G.P.; Goldman, E.R. Selection and characterization of protective anti-chikungunya virus single domain antibodies. Mol. Immunol. 2019, 105, 190–197. [Google Scholar] [CrossRef]
- Hunt, A.R.; Frederickson, S.; Maruyama, T.; Roehrig, J.T.; Blair, C.D. The first human epitope map of the alphaviral e1 and e2 proteins reveals a new e2 epitope with significant virus neutralizing activity. PLOS Negl. Trop. Dis. 2010, 4, e739. [Google Scholar] [CrossRef]
- Parker, M.D.; Buckley, M.J.; Melanson, V.R.; Glass, P.J.; Norwood, D.; Hart, M.K. Antibody to the e3 glycoprotein protects mice against lethal venezuelan equine encephalitis virus infection. J. Virol. 2010, 84, 12683–12690. [Google Scholar] [CrossRef]
- Liu, J.L.; Zabetakis, D.; Walper, S.A.; Goldman, E.R.; Anderson, G.P. Bioconjugates of rhizavidin with single domain antibodies as bifunctional immunoreagents. J. Immunol. Methods 2014, 411, 37–42. [Google Scholar] [CrossRef]
- Walper, S.A.; Brozozog Lee, P.A.; Goldman, E.R.; Anderson, G.P. Comparison of single domain antibody immobilization strategies evaluated by surface plasmon resonance. J. Immunol. Methods 2013, 388, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tanha, J.; Hirama, T.; Khieu, N.H.; To, R.; Tong-Sevinc, H.; Stone, E.; Brisson, J.-R.; Roger MacKenzie, C. Pentamerization of single-domain antibodies from phage libraries: A novel strategy for the rapid generation of high-avidity antibody reagents. J. Mol. Biol. 2004, 335, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Shriver-Lake, L.; Walper, S.; Ashford, L.; Zabetakis, D.; Liu, J.; Breger, J.; Brozozog Lee, P.; Goldman, E. Genetic fusion of an anti-bcla single-domain antibody with beta galactosidase. Antibodies 2018, 7, 36. [Google Scholar] [CrossRef]
- Vanlandschoot, P.; Stortelers, C.; Beirnaert, E.; Ibanez, L.I.; Schepens, B.; Depla, E.; Saelens, X. Nanobodies(r): New ammunition to battle viruses. Antiviral. Res. 2011, 92, 389–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, S.; Ying, T. Single-domain antibodies as therapeutics against human viral diseases. Front. Immunol. 2017, 8, 1802. [Google Scholar] [CrossRef] [PubMed]
- Boruah, B.M.; Liu, D.; Ye, D.; Gu, T.-J.; Jiang, C.-L.; Qu, M.; Wright, E.; Wang, W.; He, W.; Liu, C.; et al. Single domain antibody multimers confer protection against rabies infection. PLoS ONE 2013, 8, e71383. [Google Scholar] [CrossRef]
- Hultberg, A.; Temperton, N.J.; Rosseels, V.; Koenders, M.; Gonzalez-Pajuelo, M.; Schepens, B.; Ibañez, L.I.; Vanlandschoot, P.; Schillemans, J.; Saunders, M.; et al. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS ONE 2011, 6, e17665. [Google Scholar] [CrossRef] [PubMed]
- Matz, J.; Kessler, P.; Bouchet, J.; Combes, O.; Ramos, O.H.; Barin, F.; Baty, D.; Martin, L.; Benichou, S.; Chames, P. Straightforward selection of broadly neutralizing single-domain antibodies targeting the conserved cd4 and coreceptor binding sites of hiv-1 gp120. J. Virol. 2013, 87, 1137–1149. [Google Scholar] [CrossRef]
- Larios Mora, A.; Detalle, L.; Gallup, J.M.; Van Geelen, A.; Stohr, T.; Duprez, L.; Ackermann, M.R. Delivery of alx-0171 by inhalation greatly reduces respiratory syncytial virus disease in newborn lambs. mAbs 2018, 10, 778–795. [Google Scholar] [CrossRef]

| Clone | Yield (mg/L) | Tm °C | ka (1/Ms) | kd (1/s) | KD (M) |
|---|---|---|---|---|---|
| WC10 | 48 | 51 | 1.0 × 108 | 8.5 × 10−1 | 8.5 ×10−9 |
| WD11f | 18 | 50 | 7.3 × 107 | 5.3 × 10−1 | 7.2 ×10−9 |
| WE10 | 55 | 75 | 7.0 × 104 | 8.3 ×10−4 | 1.2 × 10−8 |
| WE11f | 38 | 60 | nbo | nbo | nbo |
| WH11 | 15 | 72 | 1.5 × 105 | 3.3 × 10−3 | 2.2 × 10−8 |
| WB9 | 21 | 54 | 1.1 × 106 | 2.9 × 10−3 | 2.6 × 10−9 |
| WF4 | 3 | 43 | 6.3 × 104 | 1.5 × 10−3 | 2.4 × 10−8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.L.; Shriver-Lake, L.C.; Zabetakis, D.; Goldman, E.R.; Anderson, G.P. Selection of Single-Domain Antibodies towards Western Equine Encephalitis Virus. Antibodies 2018, 7, 44. https://doi.org/10.3390/antib7040044
Liu JL, Shriver-Lake LC, Zabetakis D, Goldman ER, Anderson GP. Selection of Single-Domain Antibodies towards Western Equine Encephalitis Virus. Antibodies. 2018; 7(4):44. https://doi.org/10.3390/antib7040044
Chicago/Turabian StyleLiu, Jinny L., Lisa C. Shriver-Lake, Dan Zabetakis, Ellen R. Goldman, and George P. Anderson. 2018. "Selection of Single-Domain Antibodies towards Western Equine Encephalitis Virus" Antibodies 7, no. 4: 44. https://doi.org/10.3390/antib7040044
APA StyleLiu, J. L., Shriver-Lake, L. C., Zabetakis, D., Goldman, E. R., & Anderson, G. P. (2018). Selection of Single-Domain Antibodies towards Western Equine Encephalitis Virus. Antibodies, 7(4), 44. https://doi.org/10.3390/antib7040044





