Properties of Fluorescent Far-Red Anti-TNF Nanobodies
Abstract
1. Introduction
2. Materials and Methods
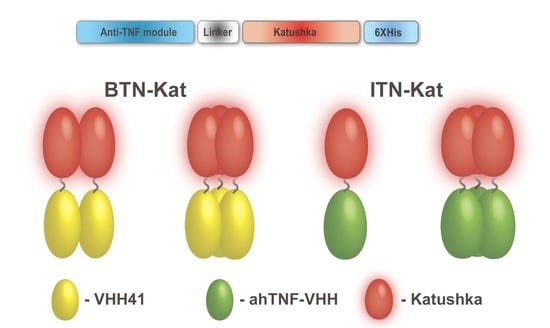
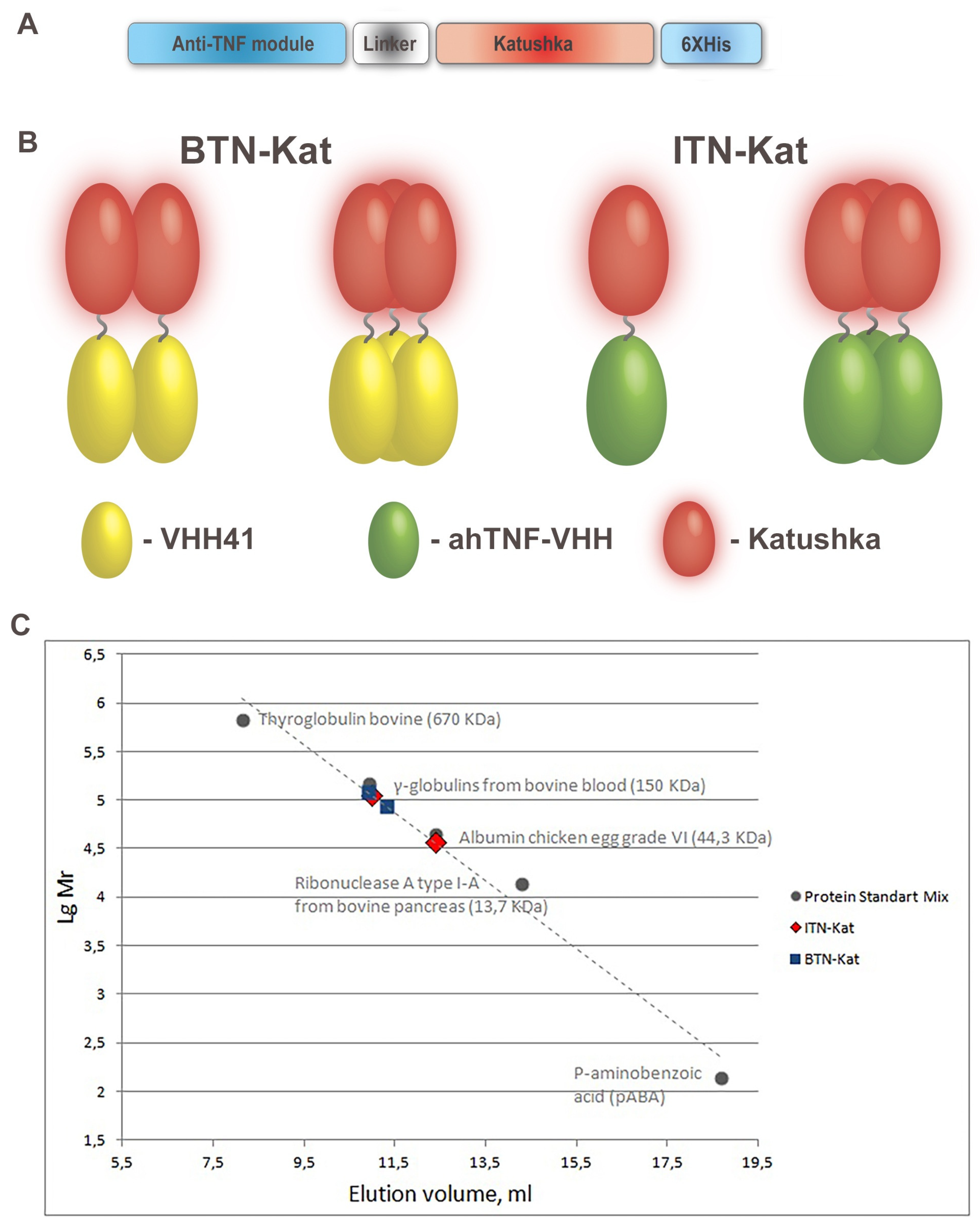
2.1. Design, Expression, and Purification of BTN-Kat and ITN-Kat Recombinant Proteins
2.2. Size Exclusion Chromatography
2.3. Mice
2.4. ELISA Measurement of the TNF Concentration in Murine Blood
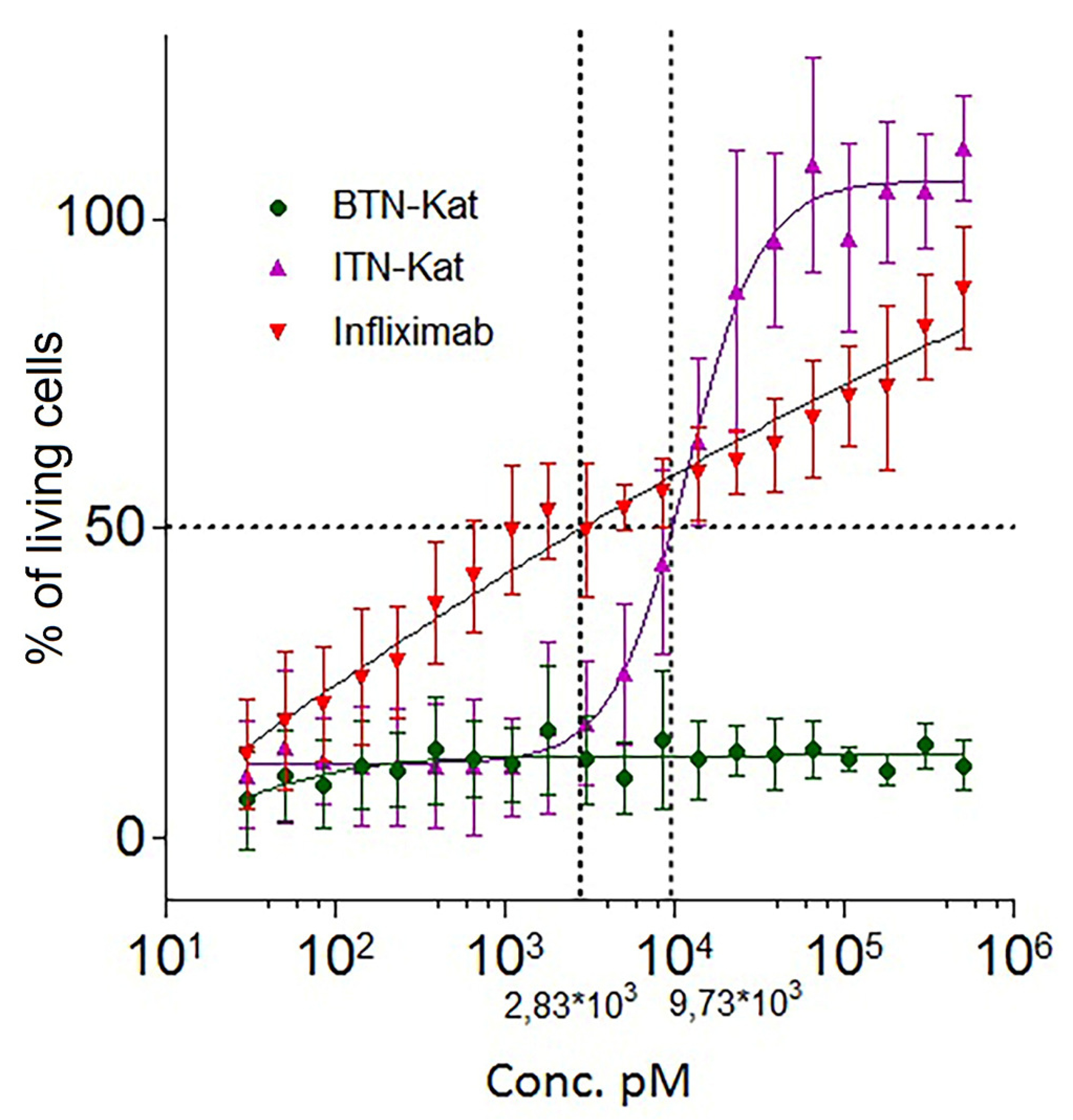
2.5. Cytotoxic Assay
2.6. LPS/d-Galactosamine-Induced Acute Hepatotoxicity Model
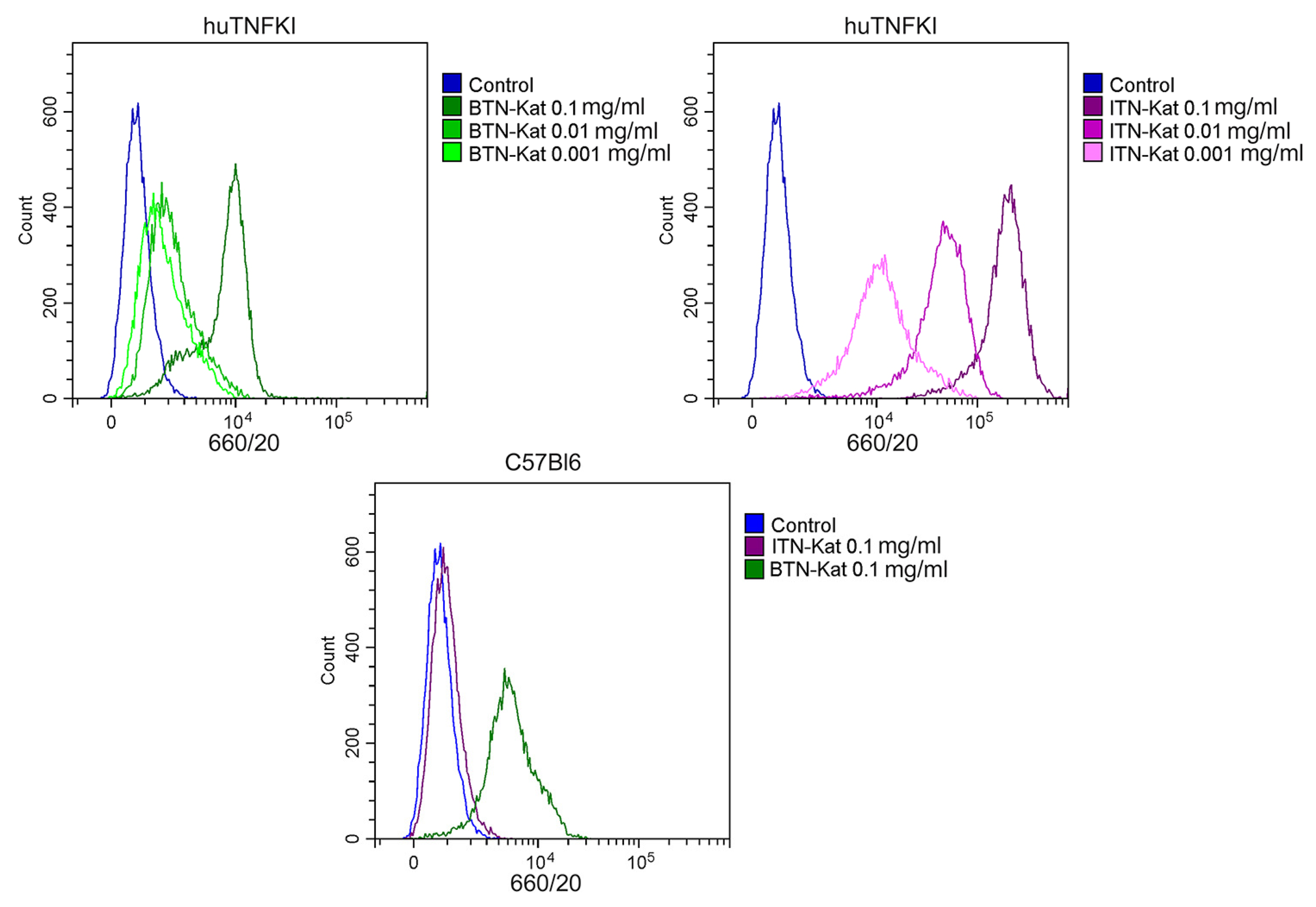
2.7. Flow Cytometry Analysis of Bone Marrow-Derived Macrophages
2.8. Flow Cytometry Analysis of Murine Blood
2.9. Fluorescence Whole-Body Imaging
2.10. Statistical Analysis
3. Results
3.1. Anti-TNF Antibodies Fused to Far-Red Katushka Protein Form Oligomers
3.2. Fluorescent Nanobodies Interact with TNF In Vitro
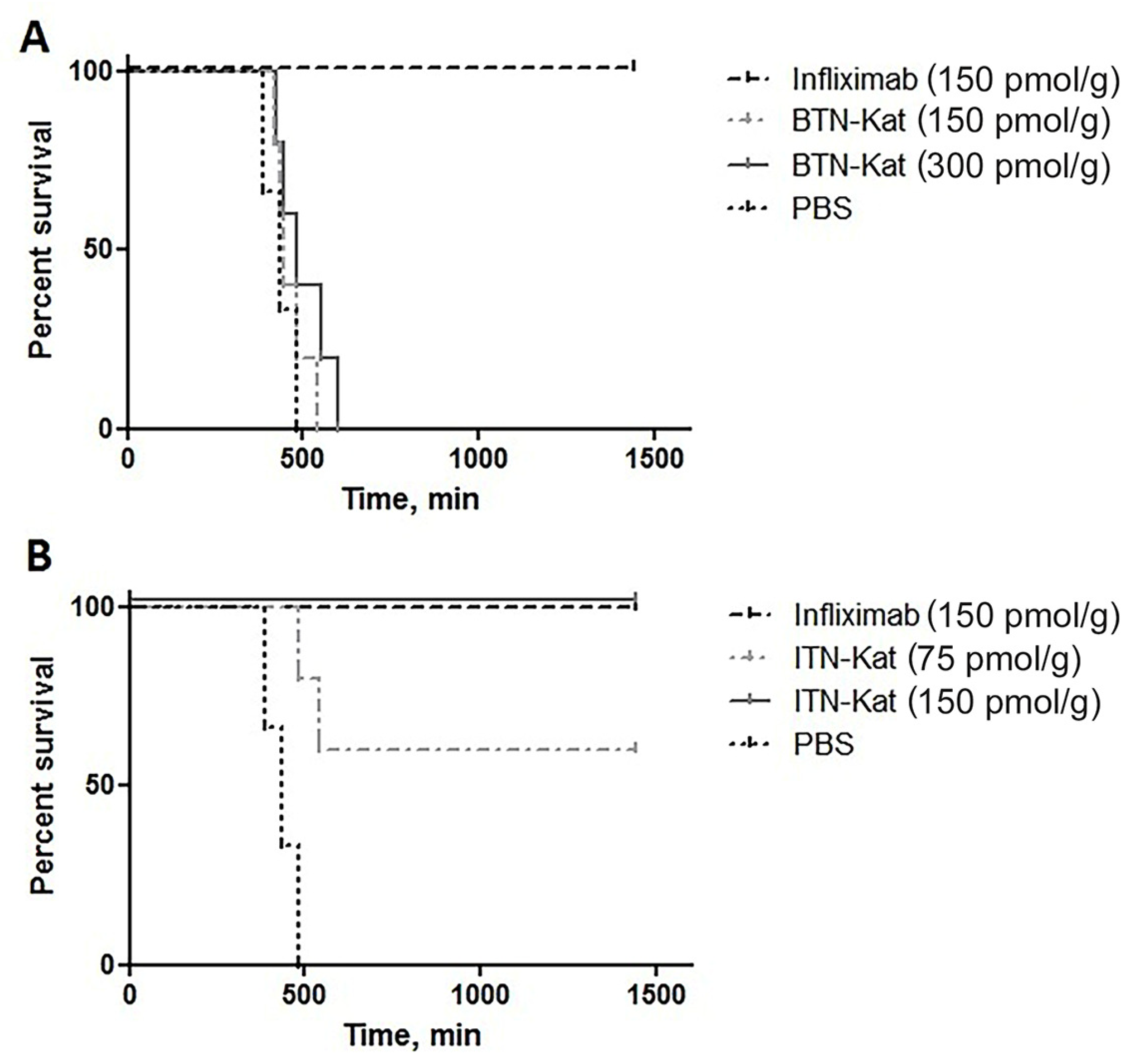
3.3. ITN-Kat Showed TNF Neutralizing Activity In Vivo, while BTN-Kat Did not
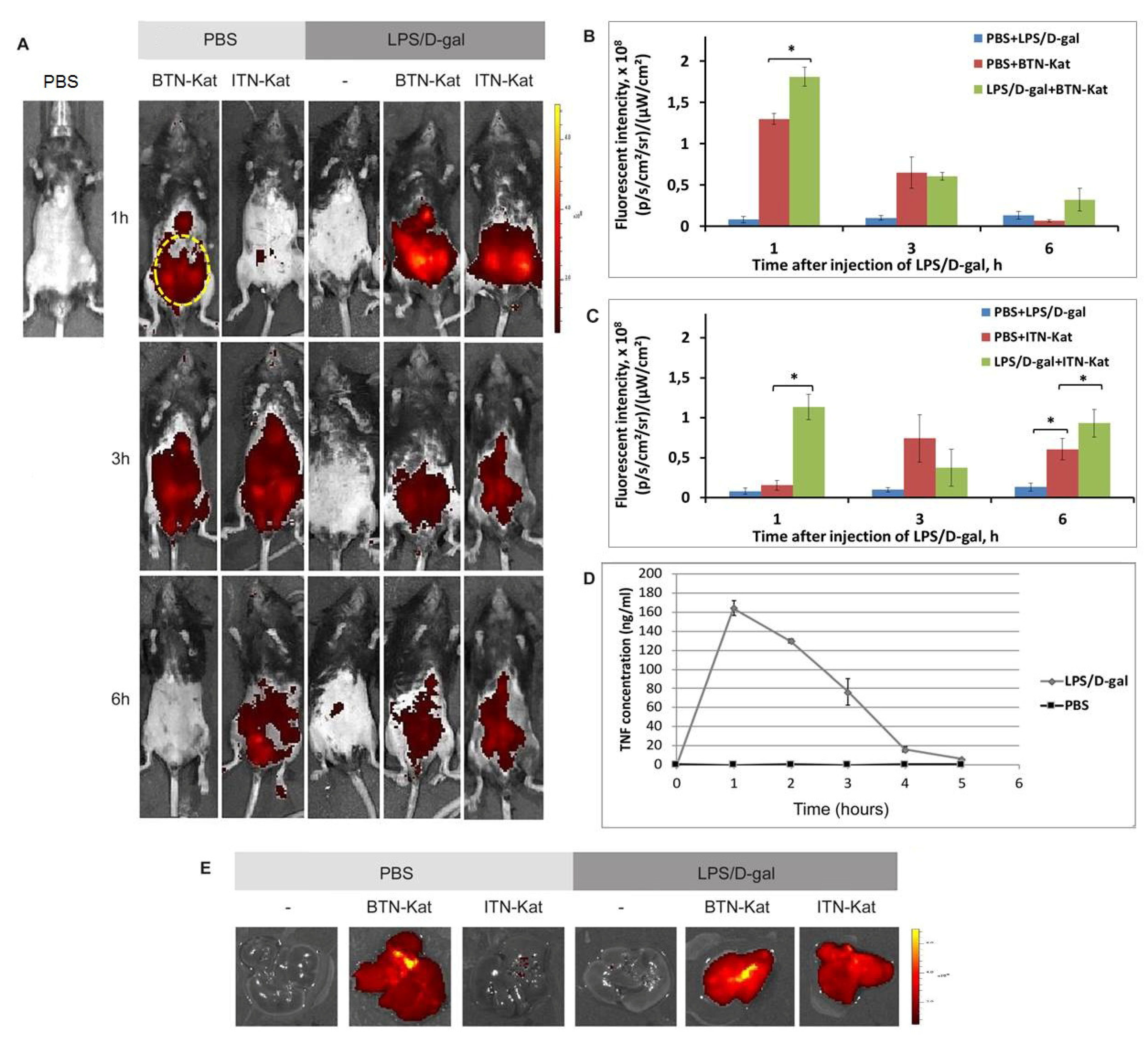
3.4. The In Vivo Fluorescence of Anti-TNF Nanobodies Correlates with TNF Levels in Mice
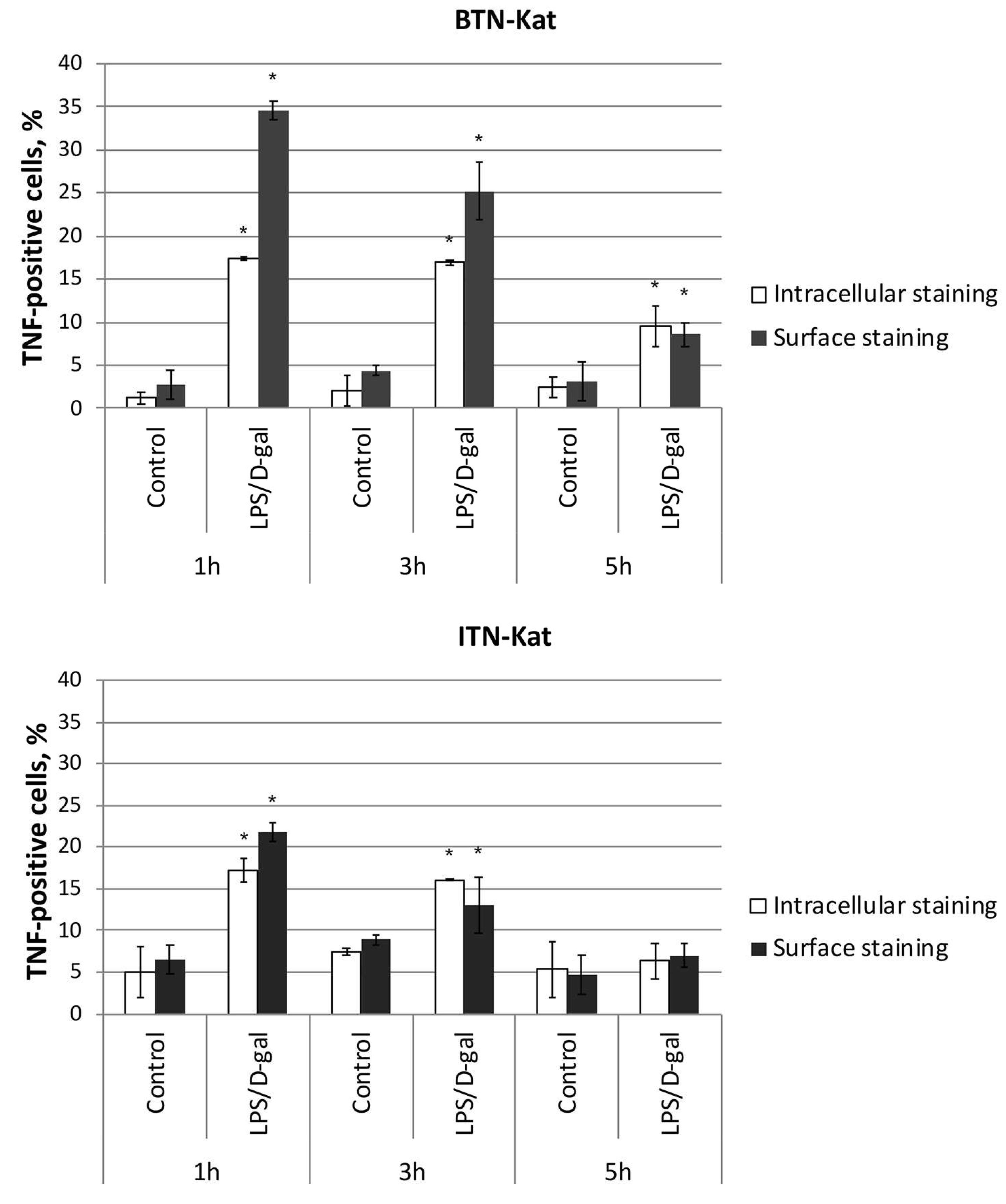
3.5. LPS/d-Galactosamine-Induced Acute Hepatotoxicity Depends on TNF Expression by Monocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, J.; van der Heijde, D.M.; Braun, J.; Dougados, M.; Cush, J.; Clegg, D.; Inman, R.D.; Kivitz, A.; Zhou, L.; Solinger, A.; et al. Sustained durability and tolerability of etanercept in ankylosing spondylitis for 96 weeks. Ann. Rheum. Dis. 2005, 64, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijde, D.; Dijkmans, B.; Geusens, P.; Sieper, J.; DeWoody, K.; Williamson, P.; Braun, J.; Ankylosing Spondylitis Study for the Evaluation of Recombinant Infliximab Therapy Study Group. Efficacy and safety of infliximab in patients with ankylosing spondylitis: Results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum. 2005, 52, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijde, D.; Kivitz, A.; Schiff, M.H.; Sieper, J.; Dijkmans, B.A.; Braun, J.; Dougados, M.; Reveille, J.D.; Wong, R.L.; Kupper, H.; Davis, J.C., Jr.; ATLAS Study Group. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: Results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006, 54, 2136–2146. [Google Scholar] [CrossRef] [PubMed]
- Inman, R.D.; Davis, J.C., Jr.; Heijde, Dv.; Diekman, L.; Sieper, J.; Kim, S.I.; Mack, M.; Han, J.; Visvanathan, S.; Xu, Z.; Hsu, B.; et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: Results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008, 58, 3402–3412. [Google Scholar] [CrossRef] [PubMed]
- Kuchmiy, A.A.; Efimov, G.A.; Nedospasov, S.A. Methods for in vivo molecular imaging. Biochemistry 2012, 77, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Palframan, R.; Airey, M.; Moore, A.; Vugler, A.; Nesbitt, A. Use of biofluorescence imaging to compare the distribution of certolizumab pegol, adalimumab, and infliximab in the inflamed paws of mice with collagen-induced arthritis. J. Immunol. Methods 2009, 348, 36–41. [Google Scholar] [CrossRef]
- Malviya, G.; Conti, F.; Chianelli, M.; Scopinaro, F.; Dierckx, R.A.; Signore, A. Molecular imaging of rheumatoid arthritis by radiolabelled monoclonal antibodies: new imaging strategies to guide molecular therapies. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 386–398. [Google Scholar] [CrossRef]
- Lambert, B.; Carron, P.; D’Asseler, Y.; Bacher, K.; van den Bosch, F.; Elewaut, D.; Verbruggen, G.; Beyaert, R.; Dumolyn, C.; De Vos, F. (99m)Tc-labelled S-HYNIC certolizumab pegol in rheumatoid arthritis and spondyloarthritis patients: a biodistribution and dosimetry study. EJNMMI Res. 2016, 6, 88. [Google Scholar] [CrossRef]
- Carron, P.; Lambert, B.; Van Praet, L.; De Vos, F.; Varkas, G.; Jans, L.; Elewaut, D.; Van den Bosch, F. Scintigraphic detection of TNF-driven inflammation by radiolabelled certolizumab pegol in patients with rheumatoid arthritis and spondyloarthritis. RMD Open 2016, 2, e000265. [Google Scholar] [CrossRef]
- Put, S.; Westhovens, R.; Lahoutte, T.; Matthys, P. Molecular imaging of rheumatoid arthritis: emerging markers, tools, and techniques. Arthritis Res. Ther. 2014, 16, 208. [Google Scholar] [CrossRef]
- Hoffman, R.M. Use of fluorescent proteins and color-coded imaging to visualize cancer cells with different genetic properties. Cancer Metast. Rev. 2016, 35, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Shcherbo, D.; Merzlyak, E.M.; Chepurnykh, T.V.; Fradkov, A.F.; Ermakova, G.V.; Solovieva, E.A.; Lukyanov, K.A.; Bogdanova, E.A.; Zaraisky, A.G.; Lukyanov, S.; et al. Bright far-red fluorescent protein for whole-body imaging. Nat. Methods 2007, 4, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.D.; Maier, J.; Traenkle, B.; Emele, F.; Rothbauer, U. Recent progress in generating intracellular functional antibody fragments to target and trace cellular components in living cells. Biochim. Biophys. Acta 2014, 1844, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446. [Google Scholar] [CrossRef] [PubMed]
- Olichon, A.; Surrey, T. Selection of Genetically Encoded Fluorescent Single Domain Antibodies Engineered for Efficient Expression in Escherichia coli. J. Biol. Chem. 2007, 282, 36314–36320. [Google Scholar] [CrossRef] [PubMed]
- Desmyter, A.; Transue, T.R.; Ghahroudi, M.A.; Thi, M.H.; Poortmans, F.; Hamers, R.; Muyldermans, S.; Wyns, L. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nat. Struct. Biol. 1996, 3, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S.; Cambillau, C.; Wyns, L. Recognition of antigens by single-domain antibody fragments: the superfluous luxury of paired domains. Trends Biochem. Sci. 2001, 26, 230–235. [Google Scholar] [CrossRef]
- van der Linden, R.H.J.; Frenken, L.G.; de Geus, B.; Harmsen, M.M.; Ruuls, R.C.; Stok, W.; de Ron, L.; Wilson, S.; Davis, P.; Verrips, C.T. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim. Biophys. Acta 1999, 1431, 37–46. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Cai, W. Nanobody: The “Magic Bullet” for Molecular Imaging? Theranostics 2014, 4, 386–398. [Google Scholar] [CrossRef]
- Rashidian, M.; Keliher, E.J.; Bilate, A.M.; Duarte, J.N.; Wojtkiewicz, G.R.; Jacobsen, J.T.; Cragnolini, J.; Swee, L.K.; Victora, G.D.; Weissleder, R.; Ploegh, H.L. Noninvasive imaging of immune responses. Proc. Natl. Acad. Sci. USA 2015, 112, 6146–6151. [Google Scholar] [CrossRef]
- Rothbauer, U.; Zolghadr, K.; Tillib, S.; Nowak, D.; Schermelleh, L.; Gahl, A.; Backmann, N.; Conrath, K.; Muyldermans, S.; Cardoso, M.C.; et al. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat. Methods 2006, 3, 887. [Google Scholar] [CrossRef]
- Tillib, S.V.; Vyatchanin, A.S.; Muyldermans, S. Molecular analysis of heavy chain-only antibodies of Camelus bactrianus. Biochemistry 2014, 79, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Efimov, G.A.; Khlopchatnikova, Z.V.; Sazykin, A.Yu.; Drutskaya, M.C.; Kruglov, A.A.; Shilov, E.C.; Kuchmii, A.A.; Nedospasov, S.A.; Tillib, S.V. Isolation and characteristics of a new recombinant single domain antibody that specifically binds to human TNF. Russ. J. Immunol. 2012, 6, 337–345. (In Russian) [Google Scholar]
- Coppieters, K.; Dreier, T.; Silence, K.; de Haard, H.; Lauwereys, M.; Casteels, P.; Beirnaert, E.; Jonckheere, H.; Van de Wiele, C.; Staelens, L.; Hostens, J. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006, 54, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Plagmann, I.; Chalaris, A.; Kruglov, A.A.; Nedospasov, S.; Rosenstiel, P.; Rose-John, S.; Scheller, J. Transglutaminase-catalyzed covalent multimerization of Camelidae anti-human TNF single domain antibodies improves neutralizing activity. J. Biotechnol. 2009, 142, 170–178. [Google Scholar] [CrossRef]
- Olleros, M.L.; Chavez-Galan, L.; Segueni, N.; Bourigault, M.L.; Vesin, D.; Kruglov, A.A.; Drutskaya, M.S.; Bisig, R.; Ehlers, S.; Aly, S.; Walter, K.; et al. Control of Mycobacterial Infections in Mice Expressing Human Tumor Necrosis Factor (TNF) but Not Mouse TNF. Infect Immun. 2015, 83, 3612–3623. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, A.A.; Tumanov, A.V.; Grivennikov, S.I.; Shebzukhov, Y.V.; Kuchmiy, A.A.; Efimov, G.A.; Drutskaya, M.S.; Scheller, J.; Kuprash, D.V.; Nedospasov, S.A. Modalities of Experimental TNF Blockade In Vivo: Mouse Models; Spring: New York, NY, USA, 2011. [Google Scholar]
- Espevik, T.; Nissen-Meyer, J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J. Immunol. Methods 1986, 95, 99–105. [Google Scholar] [CrossRef]
- Yuzhakova, D.V.; Shirmanova, M.V.; Bocharov, A.A.; Astrakhantseva, I.V.; Vasilenko, E.A.; Gorshkova, E.N.; Drutskaya, M.S.; Zagaynova, E.V.; Nedospasov, S.A.; Kruglov, A.A. Microbiota Induces Expression of Tumor Necrosis Factor in Postnatal Mouse Skin. Biochemistry 2016, 81, 1303–1308. [Google Scholar] [CrossRef]
- Pletneva, N.V.; Pletnev, V.Z.; Shemiakina, I.I.; Chudakov, D.M.; Artemyev, I.; Wlodawer, A.; Dauter, Z.; Pletnev, S. Crystallographic study of red fluorescent protein eqFP578 and its far-red variant Katushka reveals opposite pH-induced isomerization of chromophore. Protein Sci. 2011, 20, 1265–1274. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef]
- Albert, S.; Arndt, C.; Koristka, S.; Berndt, N.; Bergmann, R.; Feldmann, A.; Schmitz, M.; Pietzsch, J.; Steinbach, J.; Bachmann, M. From mono- to bivalent: improving theranostic properties of target modules for redirection of UniCAR T cells against EGFR-expressing tumor cells in vitro and in vivo. Oncotarget 2018, 9, 25597–25616. [Google Scholar] [CrossRef] [PubMed]
- Van Brussel, A.S.A.; Adams, A.; Oliveira, S.; Dorresteijn, B.; El Khattabi, M.; Vermeulen, J.F.; van der Wall, E.; Mali, W.P.; Derksen, P.W.; van Diest, P.J.; van Bergen En Henegouwen, P.M. Hypoxia-Targeting Fluorescent Nanobodies for Optical Molecular Imaging of Pre-Invasive Breast Cancer. Mol. Imaging Biol. 2016, 18, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Ann. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Deliolanis, N.C.; Kasmieh, R.; Wurdinger, T.; Tannous, B.A.; Shah, K.; Ntziachristos, V. Performance of the Red-shifted Fluorescent Proteins in deep-tissue molecular imaging applications. J. Biomed. Opt. 2008, 13, 044008. [Google Scholar] [CrossRef] [PubMed]
- Gurskaya, N.G.; Fradkov, A.F.; Terskikh, A.; Matz, M.V.; Labas, Y.A.; Martynov, V.I.; Yanushevich, Y.G.; Lukyanov, K.A.; Lukyanov, S.A. GFP-like chromoproteins as a source of far-red fluorescent proteins. FEBS Lett. 2001, 507, 16–20. [Google Scholar] [CrossRef]
- Wang, L.; Jackson, W.C.; Steinbach, P.A.; Tsien, R.Y. Evolution of new nonantibody proteins via iterative somatic hypermutation. Proc. Natl. Acad. Sci. USA 2004, 101, 16745–16749. [Google Scholar] [CrossRef] [PubMed]
- Olleros, M.L.; Vesin, D.; Fotio, A.L.; Santiago-Raber, M.L.; Tauzin, S.; Szymkowski, D.W.; Garcia, I. Soluble TNF, but not membrane TNF, is critical in LPS-induced hepatitis. J. Hepatol. 2010, 53, 1059–1068. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Tumanov, A.V.; Liepinsh, D.J.; Kruglov, A.A.; Marakusha, B.I.; Shakhov, A.N.; Murakami, T.; Drutskaya, L.N.; Förster, I.; Clausen, B.E.; et al. Distinct and nonredundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity 2005, 22, 93–104. [Google Scholar] [CrossRef]
- Drutskaya, M.S.; Efimov, G.A.; Zvartsev, R.V.; Chashchina, A.A.; Chudakov, D.M.; Tillib, S.V.; Kruglov, A.A.; Nedospasov, S.A. Experimental models of arthritis in which pathogenesis is dependent on tnf expression. Biochemistry 2014, 79, 1650–1658. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorshkova, E.N.; Efimov, G.A.; Ermakova, K.D.; Vasilenko, E.A.; Yuzhakova, D.V.; Shirmanova, M.V.; Mokhonov, V.V.; Tillib, S.V.; Nedospasov, S.A.; Astrakhantseva, I.V. Properties of Fluorescent Far-Red Anti-TNF Nanobodies. Antibodies 2018, 7, 43. https://doi.org/10.3390/antib7040043
Gorshkova EN, Efimov GA, Ermakova KD, Vasilenko EA, Yuzhakova DV, Shirmanova MV, Mokhonov VV, Tillib SV, Nedospasov SA, Astrakhantseva IV. Properties of Fluorescent Far-Red Anti-TNF Nanobodies. Antibodies. 2018; 7(4):43. https://doi.org/10.3390/antib7040043
Chicago/Turabian StyleGorshkova, Ekaterina N., Grigory A. Efimov, Ksenia D. Ermakova, Ekaterina A. Vasilenko, Diana V. Yuzhakova, Marina V. Shirmanova, Vladislav V. Mokhonov, Sergei V. Tillib, Sergei A. Nedospasov, and Irina V. Astrakhantseva. 2018. "Properties of Fluorescent Far-Red Anti-TNF Nanobodies" Antibodies 7, no. 4: 43. https://doi.org/10.3390/antib7040043
APA StyleGorshkova, E. N., Efimov, G. A., Ermakova, K. D., Vasilenko, E. A., Yuzhakova, D. V., Shirmanova, M. V., Mokhonov, V. V., Tillib, S. V., Nedospasov, S. A., & Astrakhantseva, I. V. (2018). Properties of Fluorescent Far-Red Anti-TNF Nanobodies. Antibodies, 7(4), 43. https://doi.org/10.3390/antib7040043