Factors Affecting the Pharmacology of Antibody–Drug Conjugates
Abstract
:1. Introduction
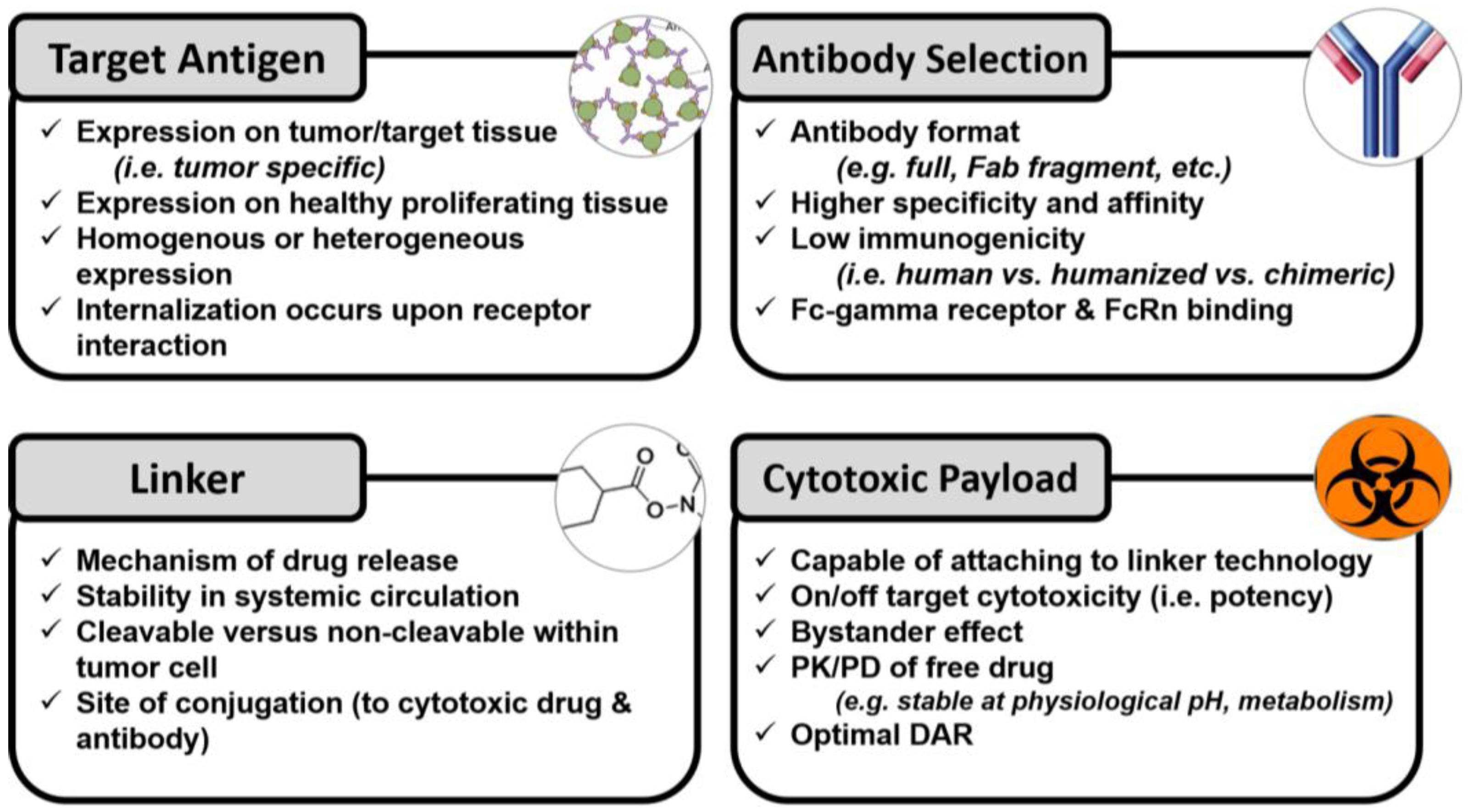
2. Formulation Considerations
2.1. Monoclonal Antibody Selection
2.2. Target Antigen
2.3. Cytotoxic Payload
2.4. Linkers
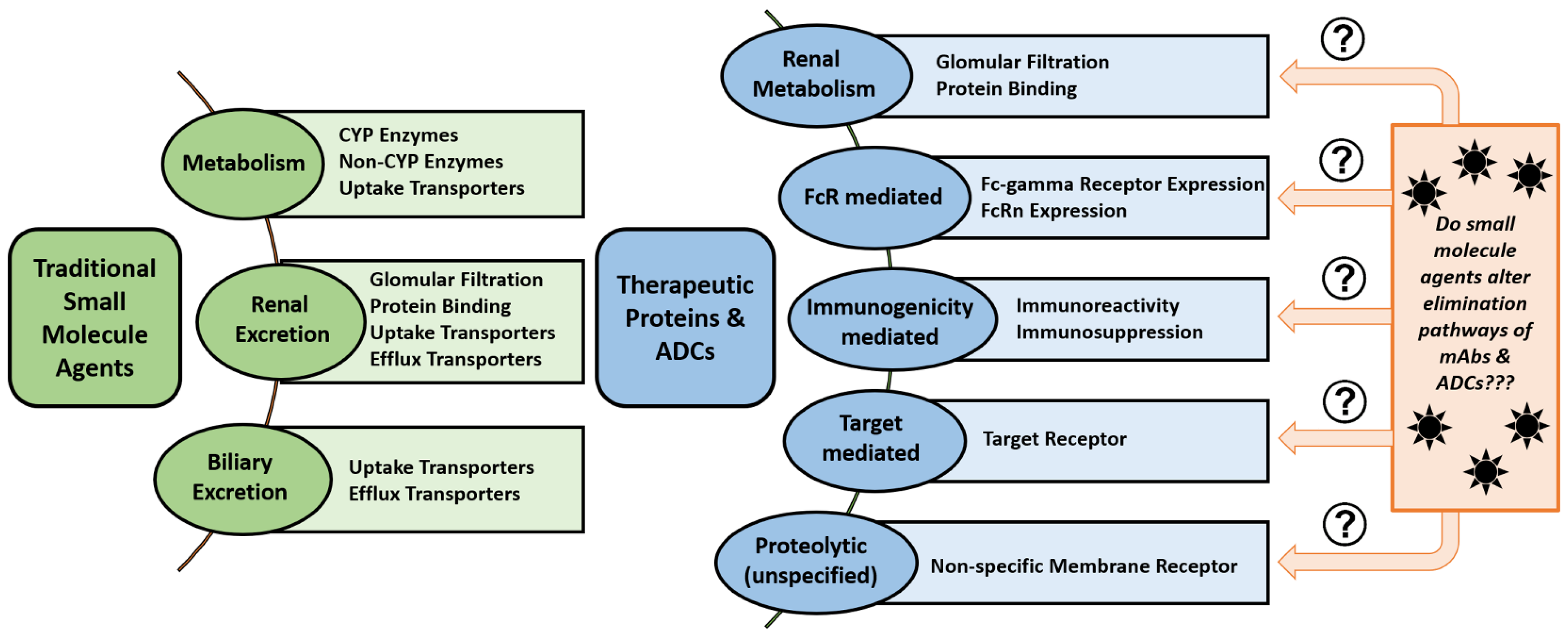
3. Pharmacokinetic Considerations
3.1. Pharmacokinetic Disposition
3.2. Mononuclear Phagocyte System
4. Physical Characteristics of ADCs
4.1. Size
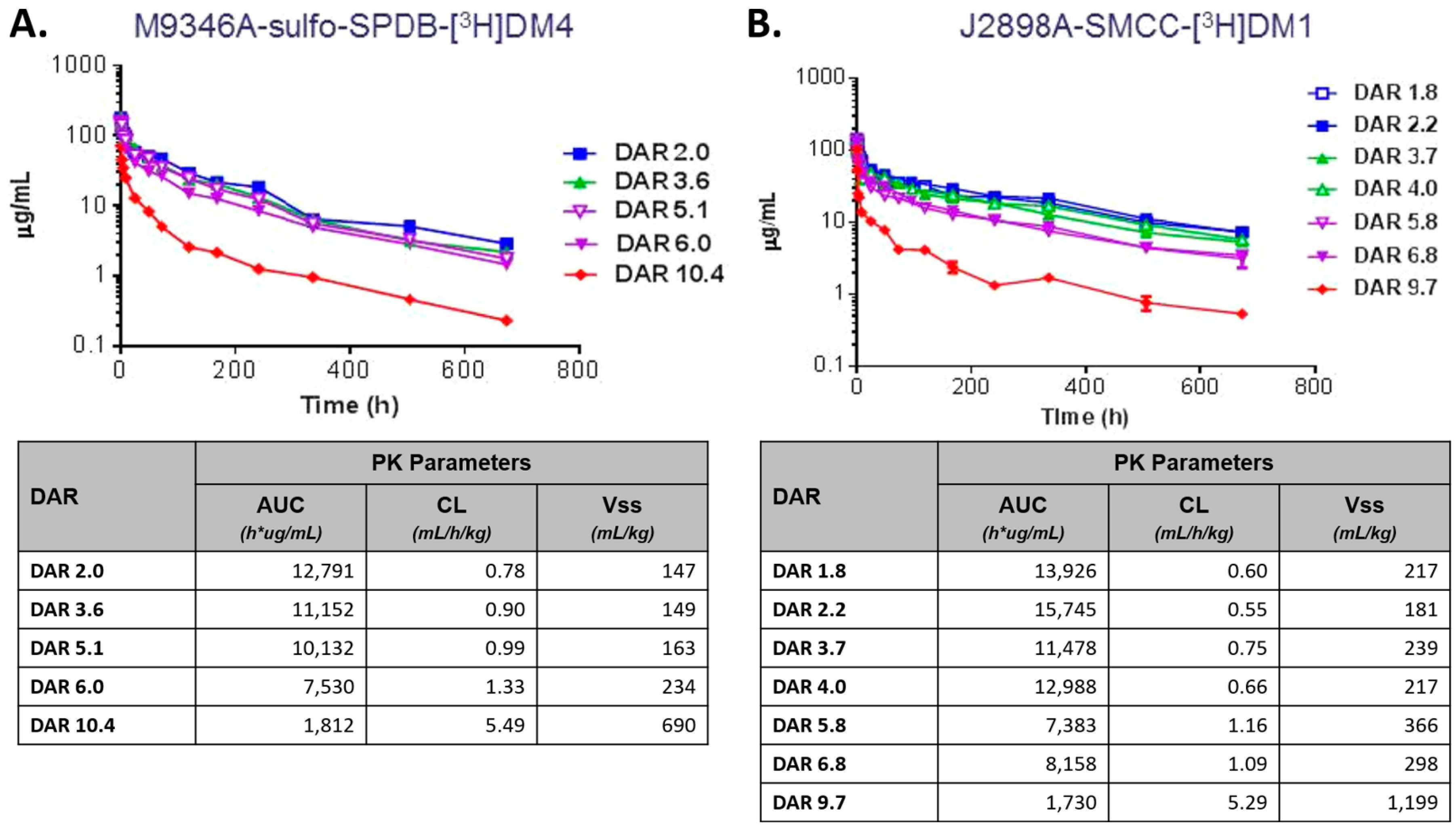
4.2. Drug–Antibody Ratio (DAR)
4.3. Surface Modifications
4.4. Charge and pH Engineering
5. Host-Associated Factors and Disease Status
5.1. Sex and Body Habitus
5.2. Chemical Modulators of Immunity in Blood
5.3. Renal or Hepatic Impairment
5.4. Neonatal Fc Receptor (FcRn)
5.5. Fc-Gamma Receptors (FcɣR)
6. Pharmacologic-Associated Factors
Drug–Drug Interactions (DDIs)
7. The Next Generation of ADCs
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADA | anti-drug antibodies |
| ADC | Antibody–drug conjugate |
| ADCC | antibody-dependent cell-mediated cytotoxicity |
| AML | acute myeloid leukemia |
| BSA | body surface area |
| CDC | complement-dependent cytotoxicity |
| CEA | carcinoembryonic antigen |
| CFDA | Chinese Food & Drug Administration |
| CL | clearance |
| DAR | drug–antibody ratio |
| DDI | drug–drug interaction |
| DLT | dose limiting toxicity |
| ESRD | end stage renal disease |
| Fabs | Fab fragments |
| FcγR | Fc-gamma receptors |
| FcRn | neonatal Fc receptor |
| GEMMs | genetically engineered mouse models |
| HGF | hepatocyte growth factor |
| iv | intravenously |
| KO | knockout |
| mAb | monoclonal antibody |
| MCC | maleimidomethyl cyclohexane-1-carboxylate |
| MMAE | monomethyl auristatin E |
| MPS | mononuclear phagocyte system |
| NHP | non-human primate |
| NP | nanoparticle |
| OS | overall survival |
| PBD | pyrrolobenzodiazepine |
| PD | pharmacodynamic |
| PEG | polyethylene glycol |
| PFS | progression-free survival |
| pI | isoelectric point |
| PK | pharmacokinetic |
| sc | subcutaneously |
| scFv | single chain variable fragment |
| SM | small molecule |
| WT | wild type |
References
- Lucas, A.T.; Price, L.S.; Schorzman, A.; Zamboni, W.C. Complex effects of tumor microenvironment on the tumor disposition of carrier-mediated agents. Nanomedicine 2017, 12, 2021–2042. [Google Scholar] [CrossRef] [PubMed]
- Ordas, I.; Mould, D.R.; Feagan, B.G.; Sandborn, W.J. Anti-tnf monoclonal antibodies in inflammatory bowel disease: Pharmacokinetics-based dosing paradigms. Clin. Pharmacol. Ther. 2012, 91, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Diamantis, N.; Banerji, U. Antibody-drug conjugates—An emerging class of cancer treatment. Br. J. Cancer 2016, 114, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Panowski, S.; Bhakta, S.; Raab, H.; Polakis, P.; Junutula, J.R. Site-specific antibody drug conjugates for cancer therapy. mAbs 2014, 6, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Perez, H.L.; Cardarelli, P.M.; Deshpande, S.; Gangwar, S.; Schroeder, G.M.; Vite, G.D.; Borzilleri, R.M. Antibody-drug conjugates: Current status and future directions. Drug Discov. Today 2014, 19, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Brown, S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci. Rep. 2015, 35, e00225. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, R. Antibody therapeutics: Isotype and glycoform selection. Exp. Opin. Biol. Ther. 2007, 7, 1401–1413. [Google Scholar] [CrossRef] [PubMed]
- Van der Neut Kolfschoten, M.; Schuurman, J.; Losen, M.; Bleeker, W.K.; Martinez-Martinez, P.; Vermeulen, E.; den Bleker, T.H.; Wiegman, L.; Vink, T.; Aarden, L.A.; et al. Anti-inflammatory activity of human igg4 antibodies by dynamic fab arm exchange. Science 2007, 317, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.M.; Wims, L.A.; Chan, L.A.; Morrison, S.L. Human IgG2 can form covalent dimers. J. Immunol. 2003, 170, 3134–3138. [Google Scholar] [CrossRef] [PubMed]
- Arbab, A.S.; Rashid, M.H.; Angara, K.; Borin, T.F.; Lin, P.C.; Jain, M.; Achyut, B.R. Major Challenges and Potential Microenvironment-Targeted Therapies in Glioblastoma. Int. J. Mol. Sci. 2017, 18, pii:E2732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, Y.; Pang, Z. Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery. Front. Pharmacol. 2017, 8, 952. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, G.; Liu, S.; Su, H.; Wang, Y.; Li, J.; Luo, C. Remodeling the Tumor Microenvironment with Emerging Nanotherapeutics. Trends Pharmacol. Sci. 2018, 39, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Lane, L.A.; Nie, S. Stimuli-responsive nanoparticles for targeting the tumor microenvironment. J. Control. Release 2015, 219, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Langer, R. Nanomedicines Targeting the Tumor Microenvironment. Cancer J. 2015, 21, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Polson, A.G.; Williams, M.; Gray, A.M.; Fuji, R.N.; Poon, K.A.; McBride, J.; Raab, H.; Januario, T.; Go, M.; Lau, J.; et al. Anti-cd22-mcc-dm1: An antibody-drug conjugate with a stable linker for the treatment of non-hodgkin’s lymphoma. Leukemia 2010, 24, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Zolot, R.S.; Basu, S.; Million, R.P. Antibody-drug conjugates. Nat. Rev. Drug Discov. 2013, 12, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaia, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Vankemmelbeke, M.; Durrant, L. Third-generation antibody drug conjugates for cancer therapy—A balancing act. Ther. Deliv. 2016, 7, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Gerber, H.P.; Koehn, F.E.; Abraham, R.T. The antibody-drug conjugate: An enabling modality for natural product-based cancer therapeutics. Nat. Prod. Rep. 2013, 30, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Smith, S.W.; Ghone, S.; Tomczuk, B. Current adc linker chemistry. Pharm. Res. 2015, 32, 3526–3540. [Google Scholar] [CrossRef] [PubMed]
- McCombs, J.R.; Owen, S.C. Antibody drug conjugates: Design and selection of linker, payload and conjugation chemistry. AAPS J. 2015, 17, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Ducry, L. Antibody Drug Conjugates; Humana Press: New York, NY, USA, 2013. [Google Scholar]
- Casi, G.; Neri, D. Antibody-drug conjugates: Basic concepts, examples and future perspectives. J. Control. Release 2012, 161, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Schlenk, R.F. Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Exp. Opin. Biol. Ther. 2014, 14, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Ten Cate, B.; Bremer, E.; de Bruyn, M.; Bijma, T.; Samplonius, D.; Schwemmlein, M.; Huls, G.; Fey, G.; Helfrich, W. A novel aml-selective trail fusion protein that is superior to gemtuzumab ozogamicin in terms of in vitro selectivity, activity and stability. Leukemia 2009, 23, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Cianfriglia, M. The biology of mdr1-p-glycoprotein (mdr1-pgp) in designing functional antibody drug conjugates (adcs): The experience of gemtuzumab ozogamicin. Annali Dell’istituto Superiore di Sanita 2013, 49, 150–168. [Google Scholar] [PubMed]
- Castaigne, S.; Pautas, C.; Terre, C.; Raffoux, E.; Bordessoule, D.; Bastie, J.-N.; Legrand, O.; Thomas, X.; Turlure, P.; Reman, O.; et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): A randomized, open-label, phase 3 study. Lancet 2012, 379, 1508–1516. [Google Scholar] [CrossRef]
- Amadori, S.; Suciu, S.; Selleslag, D.; Aversa, F.; Gaidano, G.; Musso, M.; Annino, L.; Venditti, A.; Voso, M.T.; Mazzone, C.; et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: Results of the randomized phase III EORTC-GIMEMA AML-19 trial. J. Clin. Oncol. 2016, 34, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Receives FDA Approval for Mylotarg™ (Gemtuzumab Ozogamicin) Pfizer, Inc. 2017. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_receives_fda_approval_for_mylotarg_gemtuzumab_ozogamicin (accessed on 12 October 2017).
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ji, P.; Li, Z.; Roy, P.; Sahajwalla, C.G. The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. J. Clin. Pharmacol. 2013, 53, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, M.; Bornstein, G.G.; Suria, H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010, 12, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Gkretsi, V.; Stylianou, A.; Papageorgis, P.; Polydorou, C.; Stylianopoulos, T. Remodeling components of the tumor microenvironment to enhance cancer therapy. Front. Oncol. 2015, 5, 214. [Google Scholar] [CrossRef] [PubMed]
- Hendry, S.A.; Farnsworth, R.H.; Solomon, B.; Achen, M.G.; Stacker, S.A.; Fox, S.B. The role of the tumor vasculature in the host immune response: Implications for therapeutic strategies targeting the tumor microenvironment. Front. Immunol. 2016, 7, 621. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, S.H.; Bein, T.; Meiners, S. Medical nanoparticles for next generation drug delivery to the lungs. Eur. Respir. J. 2014, 44, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Tannock, I.F. The distribution of the therapeutic monoclonal antibodies cetuximab and trastuzumab within solid tumors. BMC Cancer 2010, 10, 255. [Google Scholar] [CrossRef] [PubMed]
- Scheife, R.T. Protein binding: What does it mean? DICP Ann. Pharmacother. 1989, 23, S27–S31. [Google Scholar] [CrossRef]
- Roberts, J.A.; Pea, F.; Lipman, J. The clinical relevance of plasma protein binding changes. Clin. Pharmacokinet. 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Breij, E.C.; de Goeij, B.E.; Verploegen, S.; Schuurhuis, D.H.; Amirkhosravi, A.; Francis, J.; Miller, V.B.; Houtkamp, M.; Bleeker, W.K.; Satijn, D.; et al. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014, 74, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Ferri, N.; Bellosta, S.; Baldessin, L.; Boccia, D.; Racagni, G.; Corsini, A. Pharmacokinetics interactions of monoclonal antibodies. Pharmacol. Res. 2016, 111, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Lux, A.; Yu, X.; Scanlan, C.N.; Nimmerjahn, F. Impact of immune complex size and glycosylation on IgG binding to human fcgammars. J. Immunol. 2013, 190, 4315–4323. [Google Scholar] [CrossRef] [PubMed]
- Kasturirangan, S.; Rainey, G.J.; Xu, L.; Wang, X.; Portnoff, A.; Chen, T.; Fazenbaker, C.; Zhong, H.; Bee, J.; Zeng, Z.; et al. Targeted fcgamma receptor (fcgammar)-mediated clearance by a biparatopic bispecific antibody. J. Biol. Chem. 2017, 292, 4361–4370. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.T.; Madden, A.J.; Zamboni, W.C. Formulation and physiologic factors affecting the pharmacology of carrier-mediated anticancer agents. Exp. Opin. Drug Metab. Toxicol. 2015, 11, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Sadauskas, E.; Wallin, H.; Stoltenberg, M.; Vogel, U.; Doering, P.; Larsen, A.; Danscher, G. Kupffer cells are central in the removal of nanoparticles from the organism. Part. Fibre Toxicol. 2007, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Wong, K.J.; Baidoo, K.E.; Nayak, T.K.; Regino, C.A.; Garmestani, K.; Brechbiel, M.W. Targeting HER2: A report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. mAbs 2010, 2, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Cao, H.; Xing, C.G.; Wei, S.H.; Jiang, G.Q.; Liu, Z.L. Visualization and body distribution of [(1)(3)(1)i]-herceptin in nude mice with bt-474 breast carcinoma. Genet. Mol. Res. 2014, 13, 6804–6812. [Google Scholar] [CrossRef] [PubMed]
- Schell, R.F.; Sidone, B.J.; Caron, W.P.; Walsh, M.D.; White, T.F.; Zamboni, B.A.; Ramanathan, R.K.; Zamboni, W.C. Meta-analysis of inter-patient pharmacokinetic variability of liposomal and non-liposomal anticancer agents. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bruno, R.; Washington, C.B.; Lu, J.F.; Lieberman, G.; Banken, L.; Klein, P. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother. Pharmacol. 2005, 56, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.P.; Mager, D.E.; Khan, M.K. Synthesis and biodisposition of dendrimer composite nanoparticles. In Handbook of Materials Nanomedicine; Vladimir, T., Mansoor, M.A., Eds.; Pan Stanford Publishing: Singapore, 2010; pp. 255–290. [Google Scholar]
- Glassman, P.M.; Balthasar, J.P. Mechanistic considerations for the use of monoclonal antibodies for cancer therapy. Cancer Biol. Med. 2014, 11, 20–33. [Google Scholar] [PubMed]
- Gabizon, A.; Horowitz, A.T.; Goren, D.; Tzemach, D.; Shmeeda, H.; Zalipsky, S. In vivo fate of folate-targeted polyethylene-glycol liposomes in tumor-bearing mice. Clin. Cancer Res. 2003, 9, 6551–6559. [Google Scholar] [PubMed]
- Leyland-Jones, B.; Gelmon, K.; Ayoub, J.P.; Arnold, A.; Verma, S.; Dias, R.; Ghahramani, P. Pharmacokinetics, safety, and efficacy of trastuzumab administered every three weeks in combination with paclitaxel. J. Clin. Oncol. 2003, 21, 3965–3971. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Carbonell, X.; Castaneda-Soto, N.J.; Clemens, M.; Green, M.; Harvey, V.; Morales, S.; Barton, C.; Ghahramani, P. Phase ii study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J. Clin. Oncol. 2005, 23, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A., 3rd.; Rugo, H.S.; Vukelja, S.J.; Vogel, C.L.; Borson, R.A.; Limentani, S.; Tan-Chiu, E.; Krop, I.E.; Michaelson, R.A.; Girish, S.; et al. Phase ii study of the antibody drug conjugate trastuzumab-dm1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior her2-directed therapy. J. Clin. Oncol. 2011, 29, 398–405. [Google Scholar] [PubMed]
- Quartino, A.L.; Hillenbach, C.; Li, J.; Li, H.; Wada, R.D.; Visich, J.; Li, C.; Heinzmann, D.; Jin, J.Y.; Lum, B.L. Population pharmacokinetic and exposure-response analysis for trastuzumab administered using a subcutaneous “manual syringe” injection or intravenously in women with HER2-positive early breast cancer. Cancer Chemother. Pharmacol. 2016, 77, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Ramanathan, R.K.; Zamboni, B.A.; Strychor, S.; Ramalingam, S.; Edwards, R.P.; Friedland, D.M.; Stoller, R.G.; Belani, C.P.; Maruca, L.J.; et al. Population pharmacokinetics of pegylated liposomal ckd-602 (s-ckd602) in patients with advanced malignancies. J. Clin. Pharmacol. 2012, 52, 180–194. [Google Scholar] [CrossRef] [PubMed]
- Kai, M.P.; Brighton, H.E.; Fromen, C.A.; Shen, T.W.; Luft, J.C.; Luft, Y.E.; Keeler, A.W.; Robbins, G.R.; Ting, J.P.; Zamboni, W.C.; et al. Tumor presence induces global immune changes and enhances nanoparticle clearance. ACS Nano 2016, 10, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Sabnani, M.K.; Rajan, R.; Rowland, B.; Mavinkurve, V.; Wood, L.M.; Gabizon, A.A.; La-Beck, N.M. Liposome promotion of tumor growth is associated with angiogenesis and inhibition of antitumor immune responses. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Igawa, T.; Tsunoda, H.; Kuramochi, T.; Sampei, Z.; Ishii, S.; Hattori, K. Engineering the variable region of therapeutic igg antibodies. mAbs 2011, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tesar, D.; Boswell, C.A.; Cahaya, H.S.; Wong, A.; Zhang, J.; Meng, Y.G.; Eigenbrot, C.; Pantua, H.; Diao, J.; et al. Framework selection can influence pharmacokinetics of a humanized therapeutic antibody through differences in molecule charge. mAbs 2014, 6, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Vugmeyster, Y.; Allen, S.; Szklut, P.; Bree, A.; Ryan, M.; Ma, M.; Spaulding, V.; Young, D.; Guay, H.; Bloom, L.; et al. Correlation of pharmacodynamic activity, pharmacokinetics, and anti-product antibody responses to anti-il-21r antibody therapeutics following iv administration to cynomolgus monkeys. J. Transl. Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Vugmeyster, Y.; Guay, H.; Szklut, P.; Qian, M.D.; Jin, M.; Widom, A.; Spaulding, V.; Bennett, F.; Lowe, L.; Andreyeva, T.; et al. In vitro potency, pharmacokinetic profiles, and pharmacological activity of optimized anti-il-21r antibodies in a mouse model of lupus. mAbs 2010, 2, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pfarr, D.S.; Johnson, S.; Brewah, Y.A.; Woods, R.M.; Patel, N.K.; White, W.I.; Young, J.F.; Kiener, P.A. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J. Mol. Biol. 2007, 368, 652–665. [Google Scholar] [CrossRef] [PubMed]
- Vugmeyster, Y.; Xu, X.; Theil, F.P.; Khawli, L.A.; Leach, M.W. Pharmacokinetics and toxicology of therapeutic proteins: Advances and challenges. World J. Biol. Chem. 2012, 3, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Beeram, M.; Modi, S.; Jones, S.F.; Holden, S.N.; Yu, W.; Girish, S.; Tibbitts, J.; Yi, J.H.; Sliwkowski, M.X.; et al. Phase i study of trastuzumab-dm1, an her2 antibody-drug conjugate, given every 3 weeks to patients with her2-positive metastatic breast cancer. J. Clin. Oncol. 2010, 28, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Gopal, A.K.; Smith, S.E.; Ansell, S.M.; Rosenblatt, J.D.; Savage, K.J.; Ramchandren, R.; Bartlett, N.L.; Cheson, B.D.; de Vos, S.; et al. Results of a pivotal phase ii study of brentuximab vedotin for patients with relapsed or refractory hodgkin’s lymphoma. J. Clin. Oncol. 2012, 30, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.V.; Iyer, S. Challenges and advances in the assessment of the disposition of antibody-drug conjugates. Biopharm. Drug Dispos. 2016, 37, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Q.; Bumbaca, D.; Saad, O.; Yue, Q.; Pastuskovas, C.V.; Khojasteh, S.C.; Tibbitts, J.; Kaur, S.; Wang, B.; Chu, Y.W.; et al. Catabolic fate and pharmacokinetic characterization of trastuzumab emtansine (t-dm1): An emphasis on preclinical and clinical catabolism. Curr. Drug Metab. 2012, 13, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Alley, S.C.; Zhang, X.; Okeley, N.M.; Anderson, M.; Law, C.L.; Senter, P.D.; Benjamin, D.R. The pharmacologic basis for antibody-auristatin conjugate activity. J. Pharmacol. Exp. Ther. 2009, 330, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Mundo, E.E.; Zhang, C.; Bumbaca, D.; Valle, N.R.; Kozak, K.R.; Fourie, A.; Chuh, J.; Koppada, N.; Saad, O.; et al. Impact of drug conjugation on pharmacokinetics and tissue distribution of anti-steap1 antibody-drug conjugates in rats. Bioconj. Chem. 2011, 22, 1994–2004. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Modi, S.; LoRusso, P.M.; Pegram, M.; Guardino, E.; Althaus, B.; Lu, D.; Strasak, A.; Elias, A. Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res. 2016, 18, 34. [Google Scholar] [CrossRef] [PubMed]
- Blumenschein, G.R.; Hassan, R.; Moore, K.N.; Santin, A.; Kindler, H.L.; Nemunaitis, J.J.; Seward, S.M.; Rajagopalan, P.; Walter, A.; Sarapa, N.; et al. Phase I study of anti-mesothelin antibody drug conjugate anetumab ravtansine (AR): Abstract 2509. In Proceedings of the 2016 ASCO Annual Meeting, Chicago, IL, USA, 3–7 June 2016. [Google Scholar]
- Leelawattanachai, J.; Kwon, K.W.; Michael, P.; Ting, R.; Kim, J.Y.; Jin, M.M. Side-by-side comparison of commonly used biomolecules that differ in size and affinity on tumor uptake and internalization. PLoS ONE 2015, 10, e0124440. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Jianhua, W.; Rong, L.; Sheng, Q.; Yi, C.; Hongcheng, S.; Yushen, G. Pharmacokinetics of 131I-labeled-metuximab and transarterial chemoembolization for treatment of hepatocellular carcinoma. Chin. J. Radiol. 2010, 42, 74–78. [Google Scholar]
- Borghaei, H.; Alpaugh, K.; Hedlund, G.; Forsberg, G.; Langer, C.; Rogatko, A.; Hawkins, R.; Dueland, S.; Lassen, U.; Cohen, R.B. Phase I dose escalation, pharmacokinetic and pharmacodynamic study of naptumomab estafenatox alone in patients with advanced cancer and with docetaxel in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 4116–4123. [Google Scholar] [CrossRef] [PubMed]
- Hamblett, K.J.; Senter, P.D.; Chace, D.F.; Sun, M.M.; Lenox, J.; Cerveny, C.G.; Kissler, K.M.; Bernhardt, S.X.; Kopcha, A.K.; Zabinski, R.F.; et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 2004, 10, 7063–7070. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ponte, J.F.; Yoder, N.C.; Laleau, R.; Coccia, J.; Lanieri, L.; Qiu, Q.; Wu, R.; Hong, E.; Bogalhas, M.; et al. Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-maytansinoid conjugates. Bioconj. Chem. 2017, 28, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Catcott, K.C.; McShea, M.A.; Bialucha, C.U.; Miller, K.L.; Hicks, S.W.; Saxena, P.; Gesner, T.G.; Woldegiorgis, M.; Lewis, M.E.; Bai, C.; et al. Microscale screening of antibody libraries as maytansinoid antibody-drug conjugates. mAbs 2016, 8, 513–523. [Google Scholar] [CrossRef] [PubMed]
- King, H.D.; Dubowchik, G.M.; Mastalerz, H.; Willner, D.; Hofstead, S.J.; Firestone, R.A.; Lasch, S.J.; Trail, P.A. Monoclonal antibody conjugates of doxorubicin prepared with branched peptide linkers: Inhibition of aggregation by methoxytriethyleneglycol chains. J. Med. Chem. 2002, 45, 4336–4343. [Google Scholar] [CrossRef] [PubMed]
- Yurkovetskiy, A.V.; Yin, M.; Bodyak, N.; Stevenson, C.A.; Thomas, J.D.; Hammond, C.E.; Qin, L.; Zhu, B.; Gumerov, D.R.; Ter-Ovanesyan, E.; et al. A polymer-based antibody-vinca drug conjugate platform: Characterization and preclinical efficacy. Cancer Res. 2015, 75, 3365–3372. [Google Scholar] [CrossRef] [PubMed]
- Ab, O.; Whiteman, K.R.; Bartle, L.M.; Sun, X.; Singh, R.; Tavares, D.; LaBelle, A.; Payne, G.; Lutz, R.J.; Pinkas, J.; et al. Imgn853, a folate receptor-alpha (fralpha)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against fralpha-expressing tumors. Mol. Cancer Ther. 2015, 14, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Strop, P.; Liu, S.H.; Dorywalska, M.; Delaria, K.; Dushin, R.G.; Tran, T.T.; Ho, W.H.; Farias, S.; Casas, M.G.; Abdiche, Y.; et al. Location matters: Site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem. Biol. 2013, 20, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tibbitts, J.; Canter, D.; Graff, R.; Smith, A.; Khawli, L.A. Key factors influencing adme properties of therapeutic proteins: A need for adme characterization in drug discovery and development. mAbs 2016, 8, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Shibata-Koyama, M.; Iida, S.; Misaka, H.; Mori, K.; Yano, K.; Shitara, K.; Satoh, M. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for fcgammariiib and mhc class ii expression on the phagocytotic neutrophils. Exp. Hematol. 2009, 37, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Stork, R.; Zettlitz, K.A.; Muller, D.; Rether, M.; Hanisch, F.G.; Kontermann, R.E. N-glycosylation as novel strategy to improve pharmacokinetic properties of bispecific single-chain diabodies. J. Biol. Chem. 2008, 283, 7804–7812. [Google Scholar] [CrossRef] [PubMed]
- Goetze, A.M.; Liu, Y.D.; Zhang, Z.; Shah, B.; Lee, E.; Bondarenko, P.V.; Flynn, G.C. High-mannose glycans on the fc region of therapeutic igg antibodies increase serum clearance in humans. Glycobiology 2011, 21, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.; Morrison, S.L. Effect of altered ch2-associated carbohydrate structure on the functional properties and in vivo fate of chimeric mouse-human immunoglobulin g1. J. Exp. Med. 1994, 180, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Stefano, J.E.; Manning, C.; Kyazike, J.; Chen, B.; Gianolio, D.A.; Park, A.; Busch, M.; Bird, J.; Zheng, X.; et al. Site-specific antibody-drug conjugation through glycoengineering. Bioconj. Chem. 2014, 25, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Gefen, T.; Vaya, J.; Khatib, S.; Harkevich, N.; Artoul, F.; Heller, E.D.; Pitcovski, J.; Aizenshtein, E. The impact of pegylation on protein immunogenicity. Int. Immunopharmacol. 2013, 15, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Mero, A. The impact of pegylation on biological therapies. BioDrugs Clin. Immunother. Biopharm. Gene Ther. 2008, 22, 315–329. [Google Scholar] [CrossRef]
- Mero, A.; Clementi, C.; Veronese, F.M.; Pasut, G. Covalent conjugation of poly(ethylene glycol) to proteins and peptides: Strategies and methods. Methods Mol. Boil. 2011, 751, 95–129. [Google Scholar]
- Caliceti, P.; Veronese, F.M. Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv. Drug Deliv. Rev. 2003, 55, 1261–1277. [Google Scholar] [CrossRef]
- Burke, P.J.; Hamilton, J.Z.; Jeffrey, S.C.; Hunter, J.H.; Doronina, S.O.; Okeley, N.M.; Miyamoto, J.B.; Anderson, M.E.; Stone, I.J.; Ulrich, M.L.; et al. Optimization of a pegylated glucuronide-monomethylauristatin e linker for antibody-drug conjugates. Mol. Cancer Ther. 2017, 16, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.A.; Tesar, D.B.; Mukhyala, K.; Theil, F.P.; Fielder, P.J.; Khawli, L.A. Effects of charge on antibody tissue distribution and pharmacokinetics. Bioconj. Chem. 2010, 21, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, A.; Petrick, A.T.; Thomas, P. Modification of antibody isoelectric point affects biodistribution of 111-indium-labeled antibody. Nucl. Med. Biol. 1996, 23, 257–261. [Google Scholar] [CrossRef]
- ten Kate, C.I.; Fischman, A.J.; Rubin, R.H.; Fucello, A.J.; Riexinger, D.; Wilkinson, R.A.; Du, L.; Khaw, B.A.; Strauss, H.W. Effect of isoelectric point on biodistribution and inflammation: Imaging with indium-111-labelled igg. Eur. J. Nucl. Med. 1990, 17, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Bickel, U.; Lee, V.M.; Pardridge, W.M. Pharmacokinetic differences between 111in-and 125i-labeled cationized monoclonal antibody against β-amyloid in mouse and dog. Drug Deliv. 1995, 2, 128–135. [Google Scholar] [CrossRef]
- Khawli, L.A.; Goswami, S.; Hutchinson, R.; Kwong, Z.W.; Yang, J.; Wang, X.; Yao, Z.; Sreedhara, A.; Cano, T.; Tesar, D.; et al. Charge variants in igg1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. mAbs 2010, 2, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Wall, D.A.; Maack, T. Endocytic uptake, transport, and catabolism of proteins by epithelial cells. Am. J. Physiol. 1985, 248, C12–C20. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Bickel, U.; Buciak, J.; Yang, J.; Diagne, A.; Aepinus, C. Cationization of a monoclonal antibody to the human immunodeficiency virus rev protein enhances cellular uptake but does not impair antigen binding of the antibody. Immunol. Lett. 1994, 42, 191–195. [Google Scholar] [CrossRef]
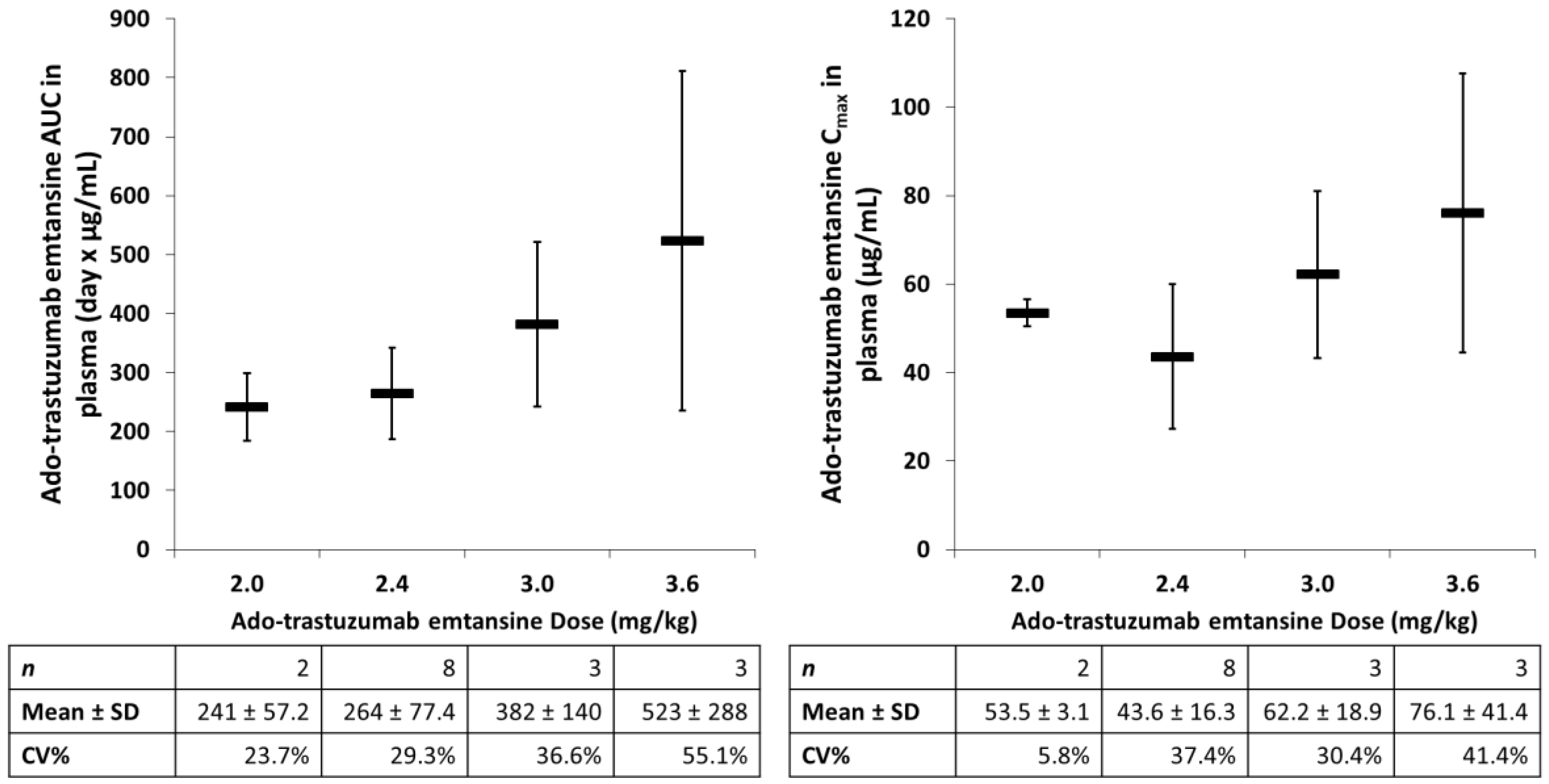
- Lu, D.; Girish, S.; Gao, Y.; Wang, B.; Yi, J.H.; Guardino, E.; Samant, M.; Cobleigh, M.; Rimawi, M.; Conte, P.; et al. Population pharmacokinetics of trastuzumab emtansine (T-DM1), a her2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer: Clinical implications of the effect of covariates. Cancer Chemother. Pharmacol. 2014, 74, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Lorusso, P.M.; Wang, B.; Yi, J.H.; Burris, H.A., 3rd.; Beeram, M.; Modi, S.; Chu, Y.W.; Agresta, S.; Klencke, B.; et al. Clinical implications of pathophysiological and demographic covariates on the population pharmacokinetics of trastuzumab emtansine, a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. J. Clin. Pharmacol. 2012, 52, 691–703. [Google Scholar] [PubMed]
- Li, H.; Han, T.H.; Hunder, N.N.; Jang, G.; Zhao, B. Population pharmacokinetics of brentuximab vedotin in patients with cd30-expressing hematologic malignancies. J. Clin. Pharmacol. 2017, 57, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Tarrant, T.K.; White, T.F.; Barrow, D.A.; Santos, C.M.; Timoshchenko, R.G.; Hanna, S.K.; Ramanathan, R.K.; Lee, C.R.; Bae-Jump, V.L.; et al. Roles of chemokines ccl2 and ccl5 in the pharmacokinetics of pegylated liposomal doxorubicin in vivo and in patients with recurrent epithelial ovarian cancer. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Darr, D.B.; Santos, C.M.; Ross, M.; Valdivia, A.; Jordan, J.L.; Midkiff, B.R.; Cohen, S.; Nikolaishvili-Feinberg, N.; Miller, C.R.; et al. Effects of tumor microenvironment heterogeneity on nanoparticle disposition and efficacy in breast cancer tumor models. Clin. Cancer Res. 2014, 20, 6083–6095. [Google Scholar] [CrossRef] [PubMed]
- Caron, W.P.; Lay, J.C.; Fong, A.M.; La-Beck, N.M.; Kumar, P.; Newman, S.E.; Zhou, H.; Monaco, J.H.; Clarke-Pearson, D.L.; Brewster, W.R.; et al. Translational studies of phenotypic probes for the mononuclear phagocyte system and liposomal pharmacology. J. Pharmacol. Exp. Ther. 2013, 347, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.; Netti, F.; Schreiber, A.D. Effect of estradiol and steroid analogues on the clearance of immunoglobulin g-coated erythrocytes. J. Clin. Investig. 1985, 75, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Holdstock, G.; Chastenay, B.F.; Krawitt, E.L. Effects of testosterone, oestradiol and progesterone on immune regulation. Clin. Exp. Immunol. 1982, 47, 449–456. [Google Scholar] [PubMed]
- Schreiber, A.D.; Nettl, F.M.; Sanders, M.C.; King, M.; Szabolcs, P.; Friedman, D.; Gomez, F. Effect of endogenous and synthetic sex steroids on the clearance of antibody-coated cells. J. Immunol. 1988, 141, 2959–2966. [Google Scholar] [PubMed]
- Werb, Z.; Foley, R.; Munck, A. Interaction of glucocorticoids with macrophages. Identification of glucocorticoid receptors in monocytes and macrophages. J. Exp. Med. 1978, 147, 1684–1694. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.P.; Schreiber, A.D.; Frank, M.M. Effects of corticosteroids and splenectomy on the immune clearance and destruction of erythrocytes. J. Clin. Investig. 1973, 52, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Ruiz, P.; Briceno, F.; Lopez, R.; Michan, A. Treatment with progesterone analogues decreases macrophage fcgamma receptors expression. Clin. Immunol. Immunopathol. 1998, 89, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Gomez, F.; Ruiz, P.; Bernal, J.A.; Escobar, M.; Garcia-Egido, A.; Lopez-Saez, J.J. Enhancement of splenic-macrophage fcgamma receptor expression by treatment with estrogens. Clin. Diagn. Lab. Immunol. 2001, 8, 806–810. [Google Scholar] [PubMed]
- Boltz-Nitulescu, G.; Willheim, M.; Spittler, A.; Leutmezer, F.; Tempfer, C.; Winkler, S. Modulation of igA, igE, and igG Fc receptor expression on human mononuclear phagocytes by 1 alpha,25-dihydroxyvitamin D3 and cytokines. J. Leukoc. Biol. 1995, 58, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Blifeld, C.; Prehn, J.L.; Jordan, S.C. Stimulus-specific 1,25(oh)2d3 modulation of tnf and il-1-beta gene expression in human peripheral blood mononuclear cells and monocytoid cell lines. Transplantation 1991, 51, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Taimi, M.; Defacque, H.; Commes, T.; Favero, J.; Caron, E.; Marti, J.; Dornand, J. Effect of retinoic acid and vitamin d on the expression of interleukin-1 beta, tumour necrosis factor-alpha and interleukin-6 in the human monocytic cell line u937. Immunology 1993, 79, 229–235. [Google Scholar] [PubMed]
- Miyaura, C.; Jin, C.H.; Yamaguchi, Y.; Tomida, M.; Hozumi, M.; Matsuda, T.; Hirano, T.; Kishimoto, T.; Suda, T. Production of interleukin 6 and its relation to the macrophage differentiation of mouse myeloid leukemia cells (m1) treated with differentiation-inducing factor and 1 alpha,25-dihydroxyvitamin d3. Biochem. Biophys. Res. Commun. 1989, 158, 660–666. [Google Scholar] [CrossRef]
- Bhalla, A.K.; Amento, E.P.; Krane, S.M. Differential effects of 1,25-dihydroxyvitamin d3 on human lymphocytes and monocyte/macrophages: Inhibition of interleukin-2 and augmentation of interleukin-1 production. Cell. Immunol. 1986, 98, 311–322. [Google Scholar] [CrossRef]
- Saggese, G.; Federico, G.; Balestri, M.; Toniolo, A. Calcitriol inhibits the pha-induced production of il-2 and ifn-gamma and the proliferation of human peripheral blood leukocytes while enhancing the surface expression of hla class ii molecules. J. Endocrinol. Investig. 1989, 12, 329–335. [Google Scholar] [CrossRef]
- Guidance for Industry: Pharmacokinetics in Patients with Impaired Renal Function—Study Design Data Analysis, and Impact on Dosing and Labeling. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdf (accessed on 3 November 2017).
- Guidance for Industry: Pharmacokinetics in Patients with Impaired Hepatic Function: Study Design Data Analysis, and Impact on Dosing and Labeling. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072123.pdf (accessed on 3 November 2017).
- Aldoss, I.T.; Plumb, T.; Zhen, W.K.; Lydiatt, D.D.; Ganti, A.K. Cetuximab in hemodialysis: A case report. Head Neck 2009, 31, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Doden, K.; Nishida, Y.; Shimizu, S.; Tanaka, N.; Yagi, D.; Asaumi, Y.; Hirano, Y.; Maeda, K.; Miyanaga, T.; et al. Bevacizumab therapy for a colorectal cancer patient or hemodialysis with hepatic metastasis. Gan Kagaku Ryoho Cancer Chemother. 2013, 40, 647–650. [Google Scholar]
- Micallef, R.A.; Barrett-Lee, P.J.; Donovan, K.; Ashraf, M.; Williams, L. Trastuzumab in patients on haemodialysis for renal failure. Clin. Oncol. 2007, 19, 559. [Google Scholar] [CrossRef] [PubMed]
- Shi, S. Biologics: An update and challenge of their pharmacokinetics. Curr. Drug Metab. 2014, 15, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Czock, D.; Keller, F.; Seidling, H.M. Pharmacokinetic predictions for patients with renal impairment: Focus on peptides and protein drugs. Br. J. Clin. Pharmacol. 2012, 74, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sockolosky, J.T.; Szoka, F.C. The neonatal fc receptor, fcrn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 2015, 91, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Brambell, F.W.; Hemmings, W.A.; Morris, I.G. A theoretical model of gamma-globulin catabolism. Nature 1964, 203, 1352–1354. [Google Scholar] [CrossRef] [PubMed]
- Roopenian, D.C.; Akilesh, S. FcRn: The neonatal fc receptor comes of age. Nat. Rev. Immunol. 2007, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG subclasses and allotypes: From structure to effector functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Wang, W.; Fauty, S.; Fang, Y.; Hamuro, L.; Hussain, A.; Prueksaritanont, T. The effect of the neonatal fc receptor on human igg biodistribution in mice. mAbs 2014, 6, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Zalevsky, J.; Chamberlain, A.K.; Horton, H.M.; Karki, S.; Leung, I.W.; Sproule, T.J.; Lazar, G.A.; Roopenian, D.C.; Desjarlais, J.R. Enhanced antibody half-life improves in vivo activity. Nat. Biotechnol. 2010, 28, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.A.; Leabman, M.K.; Marvin, J.S.; Qiu, J.; Adams, C.W.; Lien, S.; Starovasnik, M.A.; Lowman, H.B. Engineering human IgG1 affinity to human neonatal fc receptor: Impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 2009, 182, 7663–7671. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.R.; Xiong, J.M.; Johlfs, M.G.; Tang, M.T.; Keller, S.; Tsurushita, N. An engineered human IgG1 antibody with longer serum half-life. J. Immunol. 2006, 176, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Datta-Mannan, A.; Witcher, D.R.; Tang, Y.; Watkins, J.; Wroblewski, V.J. Monoclonal antibody clearance. Impact of modulating the interaction of igg with the neonatal fc receptor. J. Biol. Chem. 2007, 282, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Datta-Mannan, A.; Witcher, D.R.; Tang, Y.; Watkins, J.; Jiang, W.; Wroblewski, V.J. Humanized IgG1 variants with differential binding properties to the neonatal fc receptor: Relationship to pharmacokinetics in mice and primates. Drug Metab. Dispos. Biol. Fate Chem. 2007, 35, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Tsen, M.F.; Ghetie, V.; Ward, E.S. Identifying amino acid residues that influence plasma clearance of murine igg1 fragments by site-directed mutagenesis. Eur. J. Immunol. 1994, 24, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Hinton, P.R.; Johlfs, M.G.; Xiong, J.M.; Hanestad, K.; Ong, K.C.; Bullock, C.; Keller, S.; Tang, M.T.; Tso, J.Y.; Vasquez, M.; et al. Engineered human igg antibodies with longer serum half-lives in primates. J. Biol. Chem. 2004, 279, 6213–6216. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Park, Y.; Hong, H.J. Antibody engineering for the development of therapeutic antibodies. Mol. Cells 2005, 20, 17–29. [Google Scholar] [PubMed]
- Kuo, T.T.; Aveson, V.G. Neonatal fc receptor and IgG-based therapeutics. mAbs 2011, 3, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.S.; Devanaboyina, S.C.; Ober, R.J. Targeting fcrn for the modulation of antibody dynamics. Mol. Immunol. 2015, 67, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, W.F.; Woods, R.M.; Ward, E.S.; Palaszynski, S.R.; Patel, N.K.; Brewah, Y.A.; Wu, H.; Kiener, P.A.; Langermann, S. Increasing the affinity of a human IgG1 for the neonatal fc receptor: Biological consequences. J. Immunol. 2002, 169, 5171–5180. [Google Scholar] [PubMed]
- Gurbaxani, B.; Dela Cruz, L.L.; Chintalacharuvu, K.; Morrison, S.L. Analysis of a family of antibodies with different half-lives in mice fails to find a correlation between affinity for FcRn and serum half-life. Mol. Immunol. 2006, 43, 1462–1473. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Mateos, F.; Ober, R.J.; Ward, E.S. Conferring the binding properties of the mouse mhc class i-related receptor, fcrn, onto the human ortholog by sequential rounds of site-directed mutagenesis. J. Mol. Biol. 2005, 345, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Buckley, L.A.; Benson, K.; Davis-Bruno, K.; Dempster, M.; Finch, G.L.; Harlow, P.; Haggerty, H.G.; Hart, T.; Kinter, L.; Leighton, J.K.; et al. Nonclinical aspects of biopharmaceutical development: Discussion of case studies at a phrma-fda workshop. Int. J. Toxicol. 2008, 27, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, M.A.; Tseng, C.M.; Roskos, L.K. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov. Today 2006, 11, 81–88. [Google Scholar] [CrossRef]
- Tam, S.H.; McCarthy, S.G.; Brosnan, K.; Goldberg, K.M.; Scallon, B.J. Correlations between pharmacokinetics of igg antibodies in primates vs. Fcrn-transgenic mice reveal a rodent model with predictive capabilities. mAbs 2013, 5, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Petkova, S.B.; Akilesh, S.; Sproule, T.J.; Christianson, G.J.; Al Khabbaz, H.; Brown, A.C.; Presta, L.G.; Meng, Y.G.; Roopenian, D.C. Enhanced half-life of genetically engineered human IgG1 antibodies in a humanized fcrn mouse model: Potential application in humorally mediated autoimmune disease. Int. Immunol. 2006, 18, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.; Brown, D.; Alexander, R.; March, R.; Morgan, P.; Satterthwaite, G.; Pangalos, M.N. Lessons learned from the fate of astrazeneca’s drug pipeline: A five-dimensional framework. Nat. Rev. Drug Discov. 2014, 13, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Bruhns, P.; Saeys, Y.; Hammad, H.; Lambrecht, B.N. The function of fcgamma receptors in dendritic cells and macrophages. Nat. Rev. Immunol. 2014, 14, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.M. Incorporation of fcrn-mediated disposition model to describe the population pharmacokinetics of therapeutic monoclonal igg antibody in clinical patients. Biopharm. Drug Dispos. 2016, 37, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Ternant, D.; Arnoult, C.; Pugniere, M.; Dhommee, C.; Drocourt, D.; Perouzel, E.; Passot, C.; Baroukh, N.; Mulleman, D.; Tiraby, G.; et al. IgG1 allotypes influence the pharmacokinetics of therapeutic monoclonal antibodies through fcrn binding. J. Immunol. 2016, 196, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P. Properties of mouse and human igg receptors and their contribution to disease models. Blood 2012, 119, 5640–5649. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, D.A.; Albanesi, M.; Jonsson, F.; Iannascoli, B.; Van Rooijen, N.; Kang, X.; England, P.; Daeron, M.; Bruhns, P. The high-affinity human igg receptor fcgammari (cd64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood 2013, 121, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, C.E.; Spaapen, R.M.; van de Winkel, J.G.; Leusen, J.H. Functional characteristics of the high affinity igg receptor, fcgammari. J. Immunol. 2011, 186, 2699–2704. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, G.G.; Klakamp, S.L.; Andrews, L.; Boyle, W.J.; Tabrizi, M. Surrogate approaches in development of monoclonal antibodies. Drug Discov. Today 2009, 14, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Ravetch, J.V. Fcgamma receptors: Old friends and new family members. Immunity 2006, 24, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Mechetina, L.V.; Najakshin, A.M.; Alabyev, B.Y.; Chikaev, N.A.; Taranin, A.V. Identification of CD16-2, a novel mouse receptor homologous to CD16/Fc gamma riii. Immunogenetics 2002, 54, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.A.; Scinicariello, F.; Attanasio, R. IgG Fc receptor iii homologues in nonhuman primate species: Genetic characterization and ligand interactions. J. Immunol. 2006, 177, 3848–3856. [Google Scholar] [CrossRef] [PubMed]
- Abuqayyas, L.; Zhang, X.; Balthasar, J.P. Application of knockout mouse models to investigate the influence of fcgammar on the pharmacokinetics and anti-platelet effects of mwreg30, a monoclonal anti-gpiib antibody. Int. J. Pharm. 2013, 444, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Colbern, G.T.; Hiller, A.J.; Musterer, R.S.; Working, P.K.; Henderson, I.C. Antitumor activity of herceptin in combination with STEALTH liposomal cisplatin or nonliposomal cisplatin in a HER2 positive human breast cancer model. J. Inorg. Biochem. 1999, 77, 117–120. [Google Scholar] [CrossRef]
- Yamashita-Kashima, Y.; Iijima, S.; Yorozu, K.; Furugaki, K.; Kurasawa, M.; Ohta, M.; Fujimoto-Ouchi, K. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin. Cancer Res. 2011, 17, 5060–5070. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Pegram, M.; Ngo, D. Application and potential limitations of animal models utilized in the development of trastuzumab (herceptin): A case study. Adv. Drug Deliv. Rev. 2006, 58, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Hammond, S.A.; Oberst, M.; Wilkinson, R.W. The role of fc gamma receptors in the activity of immunomodulatory antibodies for cancer. J. ImmunoTherapy Cancer 2014, 2, 29. [Google Scholar] [CrossRef]
- Kenny, J.R.; Liu, M.M.; Chow, A.T.; Earp, J.C.; Evers, R.; Slatter, J.G.; Wang, D.D.; Zhang, L.; Zhou, H. Therapeutic protein drug-drug interactions: Navigating the knowledge gaps-highlights from the 2012 aaps nbc roundtable and iq consortium/fda workshop. AAPS J. 2013, 15, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Agus, D.B.; Gordon, M.S.; Taylor, C.; Natale, R.B.; Karlan, B.; Mendelson, D.S.; Press, M.F.; Allison, D.E.; Sliwkowski, M.X.; Lieberman, G.; et al. Phase I clinical study of pertuzumab, a novel her dimerization inhibitor, in patients with advanced cancer. J. Clin. Oncol. 2005, 23, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Swain, S.M.; Kudaba, I.; Hauschild, M.; Patel, T.; Grincuka, E.; Masuda, N.; McNally, V.; Ross, G.; Brewster, M.; et al. Absence of pharmacokinetic drug-drug interaction of pertuzumab with trastuzumab and docetaxel. Anti-Cancer Drugs 2013, 24, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Doshi, S.; Zhu, M. Pharmacokinetics and pharmacodynamics of rilotumumab: A decade of experience in preclinical and clinical cancer research. Br. J. Clin. Pharmacol. 2015, 80, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Rosen, P.J.; Sweeney, C.J.; Park, D.J.; Beaupre, D.M.; Deng, H.; Leitch, I.M.; Shubhakar, P.; Zhu, M.; Oliner, K.S.; Anderson, A.; et al. A phase ib study of amg 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin. Cancer Res. 2010, 16, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.J.; Rosenthal, M.; Ng, S.; Alumkal, J.; Picus, J.; Gravis, G.; Fizazi, K.; Forget, F.; Machiels, J.P.; Srinivas, S.; et al. Targeted met inhibition in castration-resistant prostate cancer: A randomized phase ii study and biomarker analysis with rilotumumab plus mitoxantrone and prednisone. Clin. Cancer Res. 2013, 19, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Doshi, S.; Gisleskog, P.O.; Oliner, K.S.; Perez Ruixo, J.J.; Loh, E.; Zhang, Y. Population pharmacokinetics of rilotumumab, a fully human monoclonal antibody against hepatocyte growth factor, in cancer patients. J. Pharm. Sci. 2014, 103, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Lorigan, P.; Soria, J.; Stephenson, J.; Maru, A.; Gervais, R.; Zhu, M.; McCaffery, I.; Jiang, Y.; McGreivy, J.; Glisson, B. Safety and Pharmacokinetics of First-Line Amg 479 (mab to igf1r) or Amg 102 (mab to hgf/sf) with Platinum-Based Chemotherapy in Extensive-Stage Small Cell Lung Cancer (SCLC); Annals of Oncology; Oxford University Press: Oxford, UK, 2010; p. 149. [Google Scholar]
- Kung Sutherland, M.S.; Walter, R.B.; Jeffrey, S.C.; Burke, P.J.; Yu, C.; Kostner, H.; Stone, I.; Ryan, M.C.; Sussman, D.; Lyon, R.P.; et al. Sgn-cd33a: A novel cd33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant aml. Blood 2013, 122, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.A. The development of pyrrolobenzodiazepines as antitumour agents. Exp. Opin. Investig. Drugs 2011, 20, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, S.C.; Burke, P.J.; Lyon, R.P.; Meyer, D.W.; Sussman, D.; Anderson, M.; Hunter, J.H.; Leiske, C.I.; Miyamoto, J.B.; Nicholas, N.D.; et al. A potent anti-cd70 antibody-drug conjugate combining a dimeric pyrrolobenzodiazepine drug with site-specific conjugation technology. Bioconj. Chem. 2013, 24, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- van der Lee, M.M.; Groothuis, P.G.; Ubink, R.; van der Vleuten, M.A.; van Achterberg, T.A.; Loosveld, E.M.; Damming, D.; Jacobs, D.C.; Rouwette, M.; Egging, D.F.; et al. The preclinical profile of the duocarmycin-based her2-targeting adc syd985 predicts for clinical benefit in low her2-expressing breast cancers. Mol. Cancer Ther. 2015, 14, 692–703. [Google Scholar] [CrossRef] [PubMed]
- Elgersma, R.C.; Coumans, R.G.; Huijbregts, T.; Menge, W.M.; Joosten, J.A.; Spijker, H.J.; de Groot, F.M.; van der Lee, M.M.; Ubink, R.; van den Dobbelsteen, D.J.; et al. Design, synthesis, and evaluation of linker-duocarmycin payloads: Toward selection of her2-targeting antibody-drug conjugate syd985. Mol. Pharm. 2015, 12, 1813–1835. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.M.; Albers, A.E.; Baker, J.; Banas, S.; Barfield, R.M.; Bhat, A.S.; de Hart, G.W.; Garofalo, A.W.; Holder, P.; Jones, L.C.; et al. Aldehyde tag coupled with hips chemistry enables the production of adcs conjugated site-specifically to different antibody regions with distinct in vivo efficacy and pk outcomes. Bioconj. Chem. 2014, 25, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Lhospice, F.; Bregeon, D.; Belmant, C.; Dennler, P.; Chiotellis, A.; Fischer, E.; Gauthier, L.; Boedec, A.; Rispaud, H.; Savard-Chambard, S.; et al. Site-specific conjugation of monomethyl auristatin e to anti-cd30 antibodies improves their pharmacokinetics and therapeutic index in rodent models. Mol. Pharm. 2015, 12, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Beerli, R.R.; Hell, T.; Merkel, A.S.; Grawunder, U. Sortase enzyme-mediated generation of site-specifically conjugated antibody drug conjugates with high in vitro and in vivo potency. PLoS ONE 2015, 10, e0131177. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Q.; Xu, K.; Liu, L.; Raab, H.; Bhakta, S.; Kenrick, M.; Parsons-Reponte, K.L.; Tien, J.; Yu, S.F.; Mai, E.; et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 2012, 30, 184–189. [Google Scholar] [CrossRef] [PubMed]
| Generic Name | Brand Name | Manufacturer | Phase/Studies Open | Target Antigen | Linker | Payload | Indications |
| Brentuximab vedotin | Adcetris | Seattle Genetics | Approved | CD30 | Cleavable (protease) | MMAE | Hematological |
| Gemtuzumab ozogamicin | Mylotarg | Pfizer | Approved | CD33 | Cleavable (acid labile) | Calicheamicin | Hematological |
| Inotuzumab ozogamicin | Besponsa | Pfizer | Approved | CD22 | Cleavable (acid labile) | Calicheamicin | Hematological |
| Trastuzumab emtansine | Kadcyla | Genentech | Approved | HER2 | Non-cleavable | DM1 | Solid |
| Generic Name | Investigational Name | Manufacturer | Phase/Studies Open | Target Antigen | Linker | Payload | Indications |
| Mirvetuximab Soravtansine | IMGN-853 | ImmunoGen | I, II, III | FOLRI 1 | Cleavable (disulfide) | DM4 | Solid |
| Polatuzumab vedotin | DCDS-4501A | Genentech | I, II, III | CD79b | Cleavable (protease) | MMAE | Hematological |
| Rovalpituzumab tesirine | SC0001-SCX | Stemcentrx | I, I/II, II, III | DLL3 | Cleavable (protease) | SCX | Solid |
| Sacituzumab govitecan | IMMU-132 | Immunomedics | I/II, II, III | TROP2 EGP1 | Cleavable (acid labile) | SN-38 | Solid |
| - | AGS-16C3F | Agensys | II | AGS-16/ENPP3 | Non-cleavable | MMAF | Solid |
| Denintuzumab mafodotin | SGN-CD19a | Seattle Genetics | II | CD19 | Non-cleavable | MMAF | Hematological |
| PSMA ADC | - | Progenics | II | PSMA | Cleavable (protease) | MMAE | Solid |
| Anetumab Ravtansine | BAY 94-9343 | Bayer Healthcare | I, I/II, II | Mesothelin | Cleavable (disulfide) | DM4 | Solid |
| Depatuxizumab Mafodotin | ABT-414 | Abbvie | I, II | EGFR | Non-cleavable | MMAF | Solid |
| Enfortumab Vedotin | ASG-22CE | Astellas Pharma | I, II | Nectin 4 | Cleavable (protease) | MMAE | Solid |
| Glembatumumab vedotin | CDX-011 | Celldex | I/II, II | gpNMB | Cleavable (protease) | MMAE | Solid |
| Labetuzumab govitecan | IMMU-130 | Immunomedics | I, II | CEACAM5 | Cleavable (acid labile) | SN-38 | Solid |
| Tisotumab Vedotin | HuMax-TF | Genmab Seattle Genetics | I/II, II | Tissue Factor | Cleavable (disulfide) | MMAE | Solid |
| - | CDX-014 | Celldex | I/II | TIM-1 | Cleavable (disulfide) | MMAE | Solid |
| - | CX-2009 | Cytomx | I/II | CD166 | Cleavable (protease) | DM4 | Solid |
| - | DT2219ARL OXS-1550 | GT Biopharma | I/II | CD19 & CD22 | Cleavable (protease) | Modified diphtheria toxin | Hematological |
| - | HuMax-AXL | Genmab | I/II | AXL | Cleavable (protease) | MMAE | Solid |
| Indatuximab ravtansine | BT-062 | Biotest | I/II | CD138 | Cleavable (disulfide) | DM4 | Hematological |
| Pinatuzumab vedotin | DCDT-2980S | Genentech | I/II | CD22 | Cleavable (protease) | MMAE | Hematological |
| Characteristic | Cause | Example | |
|---|---|---|---|
| 1 | High delivery and distribution to MPS organs | MPS cells are involved in the distribution and capture of these agents in liver and spleen. |  |
| 2 | Faster clearance is associated with agents that have a greater number of ligands linked to the carrier. | MPS is able to recognize and take up these “non-self” agents to a greater extent. |  |
| 3 | High interpatient PK and PD variability | MPS function is highly variable in patients | 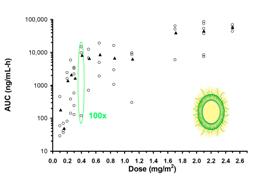 |
| 4 | Non-linear/saturable clearance at high doses. | MPS uptake of particles has a maximum capacity that can be saturated | 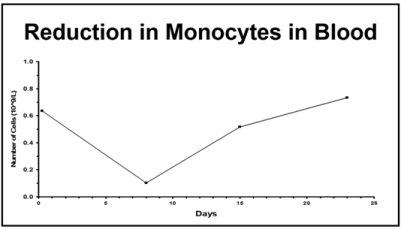 |
| 5 | Body weight, body composition, body habitus are covariates related to clearance. | MPS function is altered in patients with large body mass and weight |  |
| 6 | Tumor burden is a covariate related to clearance | MPS function is increased in patients & animals with large tumor burden, especially when tumors are present in the liver. |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucas, A.T.; Price, L.S.L.; Schorzman, A.N.; Storrie, M.; Piscitelli, J.A.; Razo, J.; Zamboni, W.C. Factors Affecting the Pharmacology of Antibody–Drug Conjugates. Antibodies 2018, 7, 10. https://doi.org/10.3390/antib7010010
Lucas AT, Price LSL, Schorzman AN, Storrie M, Piscitelli JA, Razo J, Zamboni WC. Factors Affecting the Pharmacology of Antibody–Drug Conjugates. Antibodies. 2018; 7(1):10. https://doi.org/10.3390/antib7010010
Chicago/Turabian StyleLucas, Andrew T., Lauren S. L. Price, Allison N. Schorzman, Mallory Storrie, Joseph A. Piscitelli, Juan Razo, and William C. Zamboni. 2018. "Factors Affecting the Pharmacology of Antibody–Drug Conjugates" Antibodies 7, no. 1: 10. https://doi.org/10.3390/antib7010010
APA StyleLucas, A. T., Price, L. S. L., Schorzman, A. N., Storrie, M., Piscitelli, J. A., Razo, J., & Zamboni, W. C. (2018). Factors Affecting the Pharmacology of Antibody–Drug Conjugates. Antibodies, 7(1), 10. https://doi.org/10.3390/antib7010010




