A Clinical Approach for Defining the Threshold between Low and Medium Anti-Cardiolipin Antibody Levels for QUANTA Flash Assays
Abstract
:1. Introduction
2. Results
2.1. Analytical Performance
2.2. Threshold between Low and Medium Antibody Levels
2.3. Qualitative Agreement and Quantitative Correlation between Methods
3. Discussion
4. Materials and Methods
4.1. Samples
4.2. QUANTA Flash® Methods
4.3. QUANTA Lite® Methods
4.4. Analytical Performance Assessment of QUANTA Flash Methods
4.5. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts Disclosure Statement
References
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.; DE Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, E.J.; Silvestrini, R. Assessing the usefulness of anticardiolipin antibody assays: a cautious approach is suggested by high variation and limited consensus in multilaboratory testing. Am. J. Clin. Pathol. 2002, 118, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Favaloro, E.; Pollock, W.; Wilson, R.; Hendle, M.; Adelstein, S.; Baumgart, K.; Homes, P.; Smith, S.; Steele, R.; et al. A multi-centre evaluation of the intra-assay and inter-assay variation of commercial and in-house anti-cardiolipin antibody assays. Pathology 2004, 36, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Reber, G.; Tincani, A.; Sanmarco, M.; de Moerloose, P.; Boffa, M.C. Variability of anti-beta2 glycoprotein I antibodies measurement by commercial assays. Thromb. Haemost. 2005, 94, 665–672. [Google Scholar] [PubMed]
- Ruffatti, A.; Olivieri, S.; Tonello, M.; Bortolati, M.; Bison, E.; Salvan, E.; Facchinetti, M.; Pengo, V. Influence of different IgG anticardiolipin antibody cut-off values on antiphospholipid syndrome classification. J. Thromb. Haemost. 2008, 6, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Montaruli, B.; De Luna, E.; Erroi, L.; Marchese, C.; Mengozzi, G.; Napoli, P.; Nicolo’, C.; Romito, A.; Bertero, M.T.; Sivera, P.; et al. Analytical and clinical comparison of different immunoassay systems for the detection of antiphospholipid antibodies. Int. J. Lab. Hematol. 2016, 38, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Lococo, E.; Grasso, M.; Longo, A.; Garofalo, T.; Misasi, R.; Sorice, M. Detection of antiphospholipid antibodies by automated chemiluminescence assay. J. Immunol. Methods 2012, 379, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Persijn, L.; Decavele, A.S.; Schouwers, S.; Devreese, K. Evaluation of a new set of automated chemiluminescense assays for anticardiolipin and anti-beta2-glycoprotein I antibodies in the laboratory diagnosis of the antiphospholipid syndrome. Thromb. Res. 2011, 128, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Bertolaccini, M.L.; Amengual, O.; Andreoli, L.; Atsumi, T.; Chighizola, C.B.; Forastiero, R.; Lakos, G.; Lambert, M.; Meroni, P.; Ortel, T.L.; et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun. Rev. 2014, 13, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.R. Hughes syndrome/APS. 30 years on, what have we learnt? Opening talk at the 14th International Congress on antiphospholipid antibodies Rio de Janiero, October 2013. Lupus 2014, 23, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Willis, R.; Lakos, G.; Harris, E.N. Standardization of antiphospholipid antibody testing—Historical perspectives and ongoing initiatives. Semin. Thromb. Hemost. 2014, 40, 172–177. [Google Scholar] [PubMed]
- Willis, R.; Harris, E.N.; Pierangeli, S.S. Current international initiatives in antiphospholipid antibody testing. Semin. Thromb. Hemost. 2012, 38, 360–374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, Z.; Li, P.; Bai, Y.; Zhang, F.; Li, Y. Evaluation of the Clinical Performance of a Novel Chemiluminescent Immunoassay for Detection of Anticardiolipin and Anti-Beta2-Glycoprotein 1 Antibodies in the Diagnosis of Antiphospholipid Syndrome. Medicine (Baltim.) 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Van Hoecke, F.; Persijn, L.; Decavele, A.S.; Devreese, K. Performance of two new, automated chemiluminescence assay panels for anticardiolipin and anti-beta2-glycoprotein I antibodies in the laboratory diagnosis of the antiphospholipid syndrome. Int. J. Lab. Hematol. 2012, 34, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Meneghel, L.; Ruffatti, A.; Gavasso, S.; Tonello, M.; Mattia, E.; Spiezia, L.; Campello, E.; Hoxha, A.; Fedrigo, M.; Punzi, L.; et al. The clinical performance of a chemiluminescent immunoassay in detecting anti-cardiolipin and anti-beta2 glycoprotein I antibodies. A comparison with a homemade ELISA method. Clin. Chem. Lab. Med. 2015, 53, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Iwaniec, T.; Kaczor, M.P.; Celinska-Lowenhoff, M.; Polanski, S.; Musial, J. Identification of patients with triple antiphospholipid antibody positivity is platform and method independent. Polskie Arch. Med. Wewn. 2016, 126, 19–24. [Google Scholar] [CrossRef]
- Devreese, K.M.; Van, H.F. Anticardiolipin and anti-beta2glycoprotein-I antibody cut-off values in the diagnosis of antiphospholipid syndrome: More than calculating the in-house 99th percentiles, even for new automated assays. Thromb. Res. 2011, 128, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Lakos, G. Analytical Detection Capabilities of Immunoassay-Based Antiphospholipid Antibody Tests: Do They Matter? Drug Dev. Res. 2013, 74, 575–581. [Google Scholar] [CrossRef]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; McShane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Fritzler, M.J.; Bluthner, M. Identification of a SmD3 epitope with a single symmetrical dimethylation of an arginine residue as a specific target of a subpopulation of anti-Sm antibodies. Arthritis Res. Ther. 2005, 7, R19–R29. [Google Scholar] [CrossRef] [PubMed]
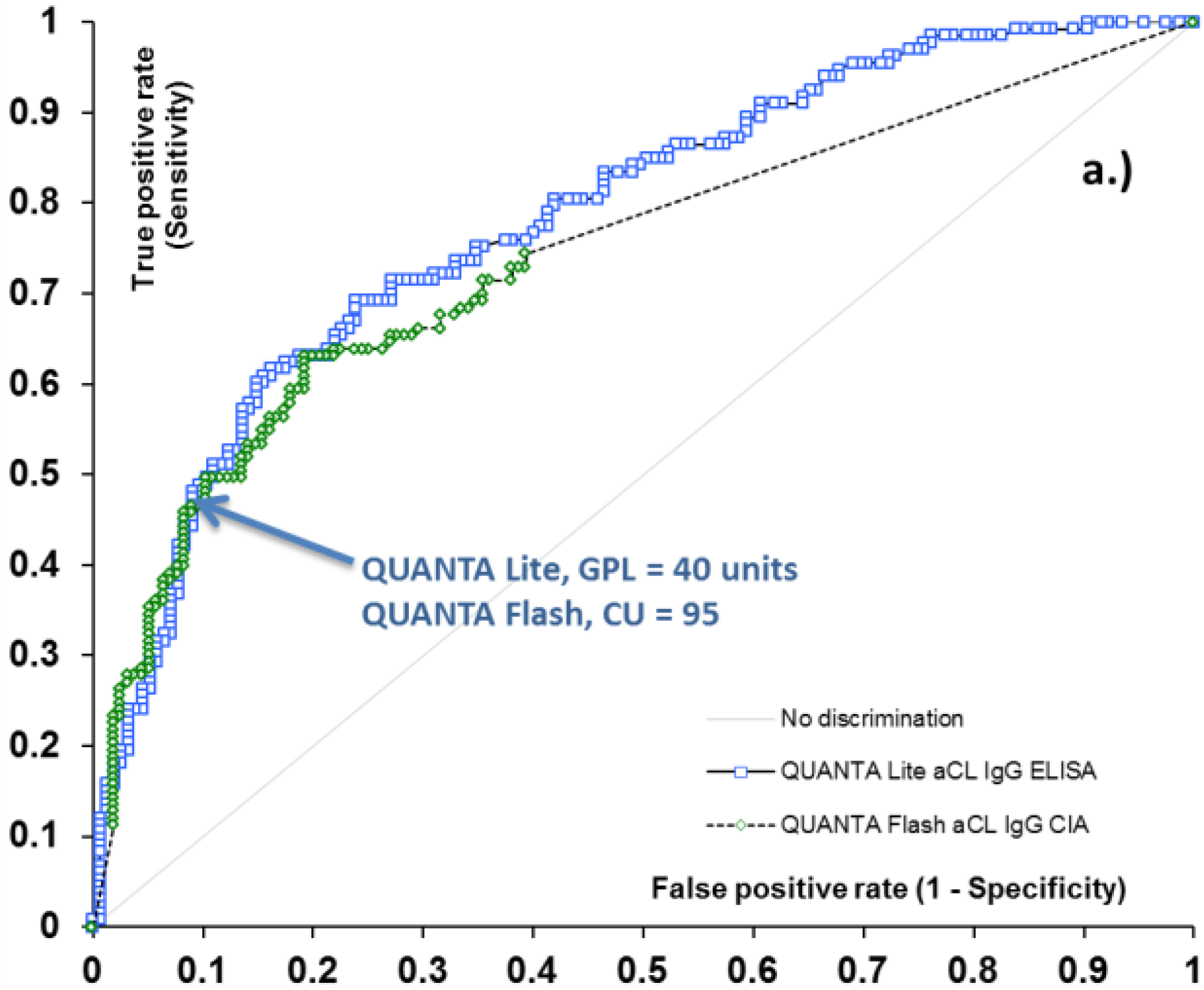

| Assay Characteristic | QUANTA Lite aCL IgG | QUANTA Flash aCL IgG | QUANTA Lite aCL IgM | QUANTA Flash aCL IgM |
|---|---|---|---|---|
| Low/Medium Threshold Unit | 40 GPL | 95 CU | 40 MPL | 31 CU |
| Sensitivity, % (95% CI) at Threshold | 48.1 (39.4–56.9) | 48.1 (39.4–56.9) | 25.0 (17.9–33.3) | 25.0 (17.9–33.3) |
| Specificity, % (95% CI) at Threshold | 91.0 (85.3–95.0) | 89.7 (83.8–94.0) | 92.4 (86.8–96.2) | 92.4 (86.8–96.2) |
| QUANTA Flash CIA vs. QUANTA Lite ELISA | ||||
|---|---|---|---|---|
| Assay | IgG | IgM | ||
| aCL | at the cut-off | Total % agreement (95% CI) | 93.1 (89.5–95.7) | 84.9 (80.1–88.9) |
| Cohen’s kappa coefficient (95% CI) | 0.85 (0.78–0.91) | 0.59 (0.48–0.70) | ||
| Spearman’s rho (p) | 0.83 (p < 0.0001) | 0.74 (p < 0.0001) | ||
| At low/medium threshold | Total % agreement (95% CI) | 96.5 (93.7–98.3) | 93.5 (89.9–96.1) | |
| Cohen’s kappa coefficient (95% CI) | 0.91 (0.86–0.97) | 0.75 (0.64–0.86) | ||
| Assay | Antigen | Units of Measurement | Analytical Measuring Range | Cut-Off Value (Reference Ranges) |
|---|---|---|---|---|
| QF aCL IgG | Cardiolipin and human β2GPI | CU | 2.6–2024 CU | ≥20 Positive |
| QF aCL IgM | Cardiolipin and human β2GPI | CU | 1.0–774 CU | ≥20 Positive |
| QL aCL IgG | Cardiolipin and bovine β2GPI | GPL | 0.0–150.0 GPL | <15 Negative |
| 15–20 Indeterminate | ||||
| >20 Positive | ||||
| QL aCL IgM | Cardiolipin and bovine β2GPI | MPL | 0.0–150.0 MPL | <12.5 Negative |
| 12.5–20 Indeterminate | ||||
| >20 Positive |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakos, G.; Bentow, C.; Mahler, M. A Clinical Approach for Defining the Threshold between Low and Medium Anti-Cardiolipin Antibody Levels for QUANTA Flash Assays. Antibodies 2016, 5, 14. https://doi.org/10.3390/antib5020014
Lakos G, Bentow C, Mahler M. A Clinical Approach for Defining the Threshold between Low and Medium Anti-Cardiolipin Antibody Levels for QUANTA Flash Assays. Antibodies. 2016; 5(2):14. https://doi.org/10.3390/antib5020014
Chicago/Turabian StyleLakos, Gabriella, Chelsea Bentow, and Michael Mahler. 2016. "A Clinical Approach for Defining the Threshold between Low and Medium Anti-Cardiolipin Antibody Levels for QUANTA Flash Assays" Antibodies 5, no. 2: 14. https://doi.org/10.3390/antib5020014
APA StyleLakos, G., Bentow, C., & Mahler, M. (2016). A Clinical Approach for Defining the Threshold between Low and Medium Anti-Cardiolipin Antibody Levels for QUANTA Flash Assays. Antibodies, 5(2), 14. https://doi.org/10.3390/antib5020014





