Monoclonal Antibodies and Small-Molecule Therapies for Lichen Planus: Targeted Immunomodulation and Emerging Evidence
Abstract
1. Introduction
2. Materials and Methods
| Citation | Study Design | LP Subtype/Population | Sample Size | Intervention(s) vs. Comparator(s) | Primary Endpoint(s) | Level of Evidence |
|---|---|---|---|---|---|---|
| Lodi, 2020 (Cochrane) [12] | Systematic review & meta-analysis of RCTs | Adults with symptomatic oral lichen planus (OLP) | 35 RCTs; 1474 participants | Topical corticosteroids (various) vs. placebo or active comparators; some trials TAC vs. clobetasol, TAC vs. triamcinolone, etc. | Pain reduction; clinical resolution; adverse effects | 1a |
| Serafini, 2023 (IJERPH) [11] | Systematic review of RCTs (no meta-analysis) | Adults with symptomatic OLP | 15 RCTs (total N varies) | Topical corticosteroids; calcineurin inhibitors; phytomedicines; PDT/LLLT; ozone; cryotherapy | Pain reduction; clinical resolution; adverse effects | 1a |
| Vinay, 2024 (JAMA Dermatology) [18] | Randomized, double-blind, placebo-controlled RCT | Adults with symptomatic OLP, single center (India) | 64 randomized | Oral acitretin (25–35 mg/day) + topical triamcinolone 0.1% vs. topical triamcinolone + oral placebo | Proportion achieving ODSS-75 at week 28 (and week 36) | 1b |
| Passeron, 2024 (Br J Dermatol)—PRELUDE [19] | Randomized, double-blind, placebo-controlled phase II “basket” RCT | Adults with refractory CLP, MLP or LPP | 111 randomized (37 per cohort) | Secukinumab 300 mg q4w × 32 wks vs. placebo × 16 wks (then secukinumab q2w) | IGA ≤ 2 at week 16 (by cohort) | 1b |
| Hwang, 2025 (J Clin Invest) [20] | Phase II, single-arm, open-label | Adults with cutaneous LP (CLP) | 12 | Baricitinib 2 mg daily for 16 weeks | Clinical response by week 16; translational endpoints | 2b |
| Solimani, 2019 (Front Immunol) [10] | Compassionate-use case series | Recalcitrant mucosal and/or cutaneous LP | 5 | Secukinumab (n = 3), Ustekinumab (n = 1), Guselkumab (n = 1) | Clinical improvement (investigator-assessed) | 4 |
| Asarch, 2009 (JAAD) [21] | Case series + literature review (pharmacovigilance) | Patients on TNF-α antagonists developing LP/lichenoid eruptions | 13 cases (2 new + 11 literature) | Exposure to infliximab or adalimumab (no therapeutic intervention for LP tested) | Occurrence of LP/lichenoid eruption; clinical characterization | 4 |
| Cheng, 2012 (Cochrane) [22] | Systematic review of RCTs/controlled trials | Erosive LP (oral, anogenital, oesophageal) | 15 studies; 473 participants | Topical agents (incl. pimecrolimus, aloe vera), others vs. placebo/vehicle or active | Pain improvement; global clinical improvement; adverse events | 1a |
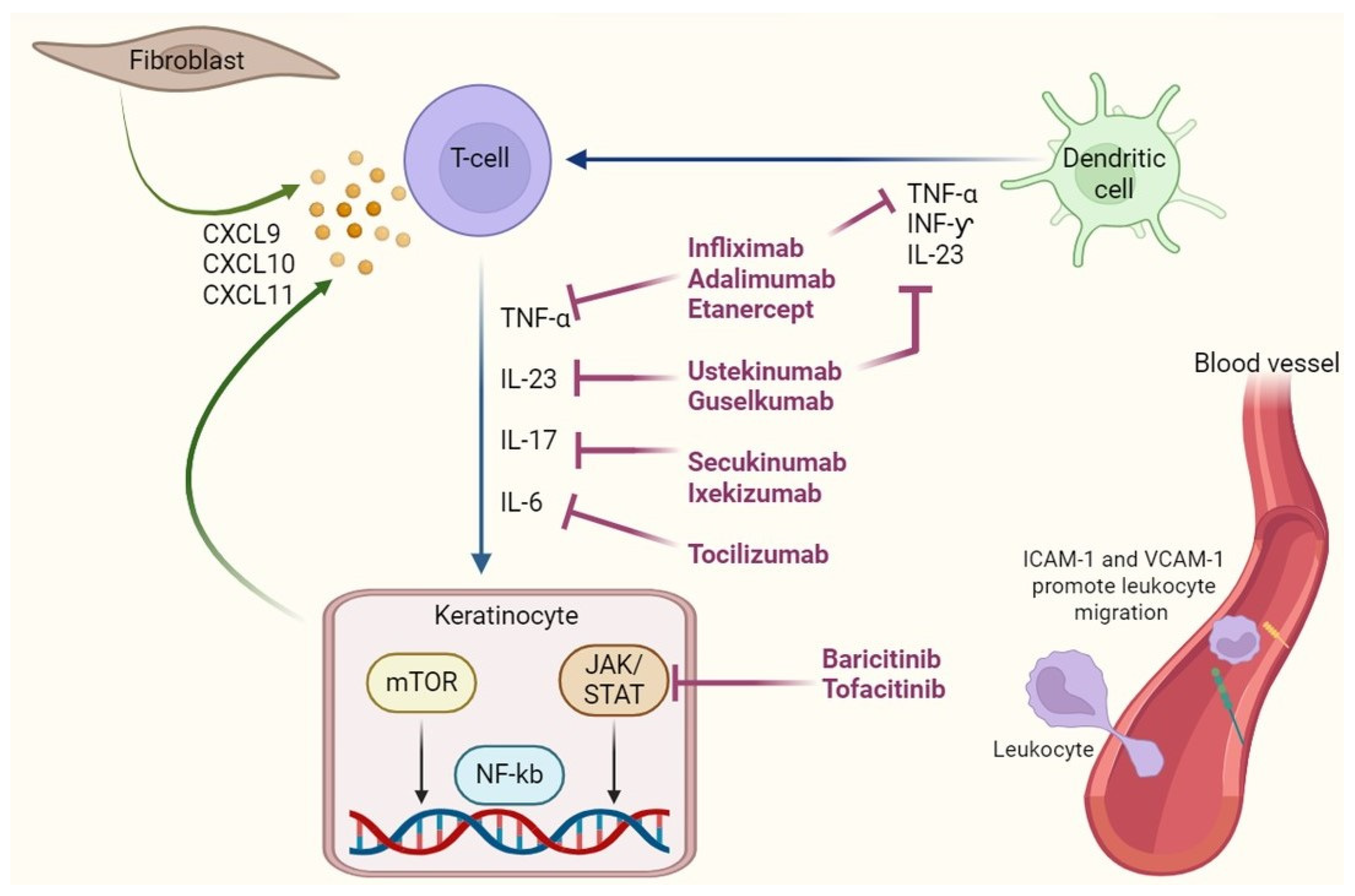
3. Immunopathogenesis of Lichen Planus: Therapeutic Target
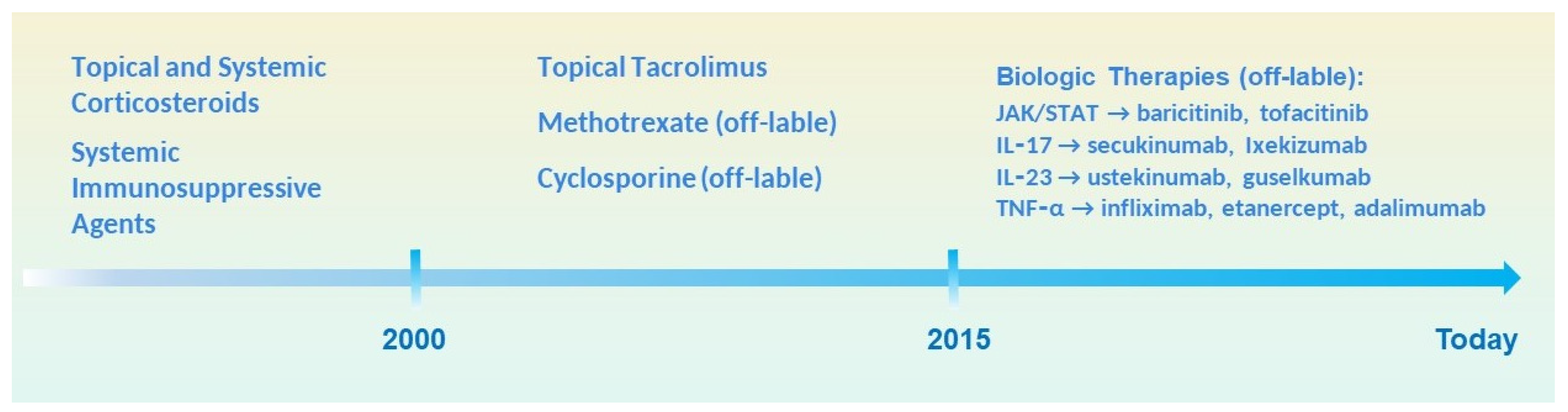
4. Overview of Traditional Therapies and Their Limitations
4.1. Topical and Systemic Corticosteroids
4.2. Systemic Immunosuppressive Agents
- Cyclosporine, a calcineurin inhibitor, suppresses IL-2–mediated T-cell activation. It has shown efficacy in oral and genital LP, particularly in erosive forms. However, its clinical use is hindered by significant toxicity, including nephrotoxicity, hypertension, gingival hyperplasia, and complex pharmacokinetic interactions [5]. Topical cyclosporine has also been used for oral LP, with inconsistent results [11].
- Methotrexate, a folate pathway antagonist with both anti-proliferative and anti-inflammatory properties, is used in generalized and erosive LP [24]. While effective in selected cases, methotrexate carries risks of hepatotoxicity, bone marrow suppression, and gastrointestinal adverse effects, requiring regular laboratory monitoring [24].
- Azathioprine, a purine analog that interferes with DNA synthesis in proliferating immune cells, has been utilized in erosive oral LP and LP associated with autoimmune overlap syndromes [16,23]. Its use is complicated by variable metabolism (requiring TPMT genotyping) and risks such as myelosuppression, gastrointestinal toxicity, and infection susceptibility.
- Acitretin, a systemic retinoid and derivative of vitamin A, has shown utility particularly in hypertrophic and cutaneous LP [18]. It promotes keratinocyte differentiation and modulates epidermal proliferation while exerting mild immunomodulatory effects. Acitretin may be preferred in patients where immunosuppression is contraindicated (e.g., history of infection or malignancy) [18]. However, it is teratogenic, causes mucocutaneous dryness, hyperlipidemia, and requires strict contraceptive measures in women of childbearing potential [15].
4.3. Limitations and Unmet Needs
5. Biologic Therapies in Lichen Planus
5.1. Anti-TNF-α Agents: Infliximab, Adalimumab, Etanercept
5.2. Anti-IL-17 and Anti-IL-23 Agents: Secukinumab, Ixekizumab, Guselkumab (Table 2)
| Drug Name | Type | Target | Indication in LP | Comments |
|---|---|---|---|---|
| Infliximab | Monoclonal Antibody | TNF-α | Refractory oral LP | Infusion reactions, paradoxical LP |
| Adalimumab | Monoclonal Antibody | TNF-α | Cutaneous and oral LP | Subcutaneous use, favorable safety |
| Etanercept | Fusion Protein | TNF-α | Variable efficacy | Relapses post-treatment |
| Secukinumab | Monoclonal Antibody | IL-17A | Cutaneous and mucosal LP | Effective in neutrophilic inflammation |
| Ixekizumab | Monoclonal Antibody | IL-17A | Generalized and erosive oral LP | High binding affinity, rapid action |
| Guselkumab | Monoclonal Antibody | IL-23p19 | Erosive oral LP | Reduces IL-17 indirectly |
| Tocilizumab | Monoclonal Antibody | IL-6 receptor | Anecdotal use in erosive forms | Pleiotropic cytokine role |
| Anakinra | Recombinant Protein | IL-1 receptor | Limited data; theoretical use | Limited clinical evidence |
| Tofacitinib | Small Molecule (JAK Inhibitor) | JAK1/3 | Refractory oral/genital LP | Broad cytokine suppression |
| Baricitinib | Small Molecule (JAK Inhibitor) | JAK1/2 | Exploratory use in inflammatory LP | Inhibits Th1/Th17 axis |
5.3. Anti-IL-6 and IL-1 Inhibitors: Tocilizumab, Anakinra
5.4. JAK Inhibitors and Targeted Small Molecules: Tofacitinib, Baricitinib (Table 2)
5.5. Emerging Targets: PD-1/PD-L1, BTK Inhibitors, Anti-IFN-γ
5.6. IL-4/IL-13 Inhibition: The Case of Dupilumab
6. Research Gaps and Future Directions
6.1. Lack of High-Quality Randomized Controlled Trials
6.2. Phenotypic Heterogeneity and Stratification Needs
6.3. Need for Personalized and Precision Medicine Approaches
6.4. Proposals for Future Studies
- Multicenter, randomized controlled trials assessing specific agents across LP subtypes
- Development and validation of LP-specific clinical scores and patient-reported outcome measures (PROMs)
- Longitudinal registries to monitor real-world effectiveness and long-term safety
- Integration of biomarker discovery and immune profiling into clinical study design
- Exploration of combination therapies, such as biologics with low-dose systemic immunomodulators or topical agents
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boch, K.; Langan, E.A.; Kridin, K.; Zillikens, D.; Ludwig, R.J.; Bieber, K. Lichen Planus. Front. Med. 2021, 8, 737813. [Google Scholar] [CrossRef]
- Gorouhi, F.; Davari, P.; Fazel, N. Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci. World J. 2014, 2014, 742826. [Google Scholar] [CrossRef] [PubMed]
- Lukács, J.; Schliemann, S.; Elsner, P. Lichen planus and lichenoid reactions as a systemic disease. Clin. Dermatol. 2015, 33, 512–519. [Google Scholar] [CrossRef]
- Li, C.; Tang, X.; Zheng, X.; Ge, S.; Wen, H.; Lin, X.; Chen, Z.; Lu, L. Global Prevalence and Incidence Estimates of Oral Lichen Planus: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 172–181. [Google Scholar] [CrossRef]
- Louisy, A.; Humbert, E.; Samimi, M. Oral Lichen Planus: An Update on Diagnosis and Management. Am. J. Clin. Dermatol. 2024, 25, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Vičić, M.; Hlača, N.; Kaštelan, M.; Brajac, I.; Sotošek, V.; Prpić Massari, L. Comprehensive Insight into Lichen Planus Immunopathogenesis. Int. J. Mol. Sci. 2023, 24, 3038. [Google Scholar] [CrossRef] [PubMed]
- El-Howati, A.; Thornhill, M.H.; Colley, H.E.; Murdoch, C. Immune mechanisms in oral lichen planus. Oral Dis. 2023, 29, 1400–1415. [Google Scholar] [CrossRef]
- Shao, S.; Tsoi, L.C.; Sarkar, M.K.; Xing, X.; Xue, K.; Uppala, R.; Berthier, C.C.; Zheng, C.; Patrick, M.; Billi, A.C.; et al. IFN-γ enhances cell-mediated cytotoxicity against keratinocytes via JAK2/STAT1 in lichen planus. Sci. Transl. Med. 2019, 11, eaav7561. [Google Scholar] [CrossRef]
- Pietschke, K.; Holstein, J.; Meier, K.; Schäfer, I.; Müller-Hermelink, E.; Gonzalez-Menendez, I.; Quintanilla-Martinez, L.; Ghoreschi, F.C.; Solimani, F.; Ghoreschi, K. The inflammation in cutaneous lichen planus is dominated by IFN-ϒ and IL-21—A basis for therapeutic JAK1 inhibition. Exp. Dermatol. 2021, 30, 262–270. [Google Scholar] [CrossRef]
- Solimani, F.; Pollmann, R.; Schmidt, T.; Schmidt, A.; Zheng, X.; Savai, R.; Mühlenbein, S.; Pickert, J.; Eubel, V.; Möbs, C.; et al. Therapeutic Targeting of Th17/Tc17 Cells Leads to Clinical Improvement of Lichen Planus. Front. Immunol. 2019, 10, 1808. [Google Scholar] [CrossRef]
- Serafini, G.; De Biase, A.; Lamazza, L.; Mazzucchi, G.; Lollobrigida, M. Efficacy of Topical Treatments for the Management of Symptomatic Oral Lichen Planus: A Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 1202. [Google Scholar] [CrossRef]
- Lodi, G.; Manfredi, M.; Mercadante, V.; Murphy, R.; Carrozzo, M. Interventions for treating oral lichen planus: Corticosteroid therapies. Cochrane Database Syst. Rev. 2020, 2020, CD001168. [Google Scholar] [CrossRef]
- Wu, T.; Bai, Y.; Jing, Y.; Chen, F. What can we learn from treatments of oral lichen planus? Front. Cell. Infect. Microbiol. 2024, 14, 1279220. [Google Scholar] [CrossRef]
- Tekin, B.; Xie, F.; Lehman, J.S. Lichen Planus: What is New in Diagnosis and Treatment? Am. J. Clin. Dermatol. 2024, 25, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Nukaly, H.Y.; Halawani, I.R.; Alghamdi, S.M.S.; Alruwaili, A.G.; Binhezaim, A.; Algahamdi, R.A.A.; Alzahrani, R.A.J.; Alharamlah, F.S.S.; Aldumkh, S.H.S.; Alasqah, H.M.A.; et al. Oral Lichen Planus: A Narrative Review Navigating Etiologies, Clinical Manifestations, Diagnostics, and Therapeutic Approaches. J. Clin. Med. 2024, 13, 5280. [Google Scholar] [CrossRef]
- Didona, D.; Caposiena Caro, R.D.; Sequeira Santos, A.M.; Solimani, F.; Hertl, M. Therapeutic strategies for oral lichen planus: State of the art and new insights. Front. Med. 2022, 9, 997190. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Vinay, K.; Kumar, S.; Dev, A.; Cazzaniga, S.; Borradori, L.; Thakur, V.; Dogra, S. Oral Acitretin Plus Topical Triamcinolone vs Topical Triamcinolone Monotherapy in Patients with Symptomatic Oral Lichen Planus: A Randomized Clinical Trial. JAMA Dermatol. 2024, 160, 80–87. [Google Scholar] [CrossRef]
- Passeron, T.; Reinhardt, M.; Ehst, B.; Weiss, J.; Sluzevich, J.; Sticherling, M.; Reygagne, P.; Wohlrab, J.; Hertl, M.; Fazel, N.; et al. Secukinumab in adult patients with lichen planus: Efficacy and safety results from the randomized placebo-controlled proof-of-concept PRELUDE study. Br. J. Dermatol. 2024, 191, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Hwang, A.S.; Kechter, J.A.; Do, T.H.; Hughes, A.N.; Zhang, N.; Li, X.; Bogle, R.; Brumfiel, C.M.; Patel, M.H.; Boudreaux, B.; et al. Rapid response of lichen planus to baricitinib associated with suppression of cytotoxic CXCL13+CD8+ T cells. J. Clin. Investig. 2025, 135, e179436. [Google Scholar] [CrossRef]
- Asarch, A.; Gottlieb, A.B.; Lee, J.; Masterpol, K.S.; Scheinman, P.L.; Stadecker, M.J.; Massarotti, E.M.; Bush, M.L. Lichen planus-like eruptions: An emerging side effect of tumor necrosis factor-α antagonists. J. Am. Acad. Dermatol. 2009, 61, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Kirtschig, G.; Cooper, S.; Thornhill, M.; Leonardi-Bee, J.; Murphy, R. Interventions for erosive lichen planus affecting mucosal sites. Cochrane Database Syst. Rev. 2012, 2012, CD008092. [Google Scholar] [CrossRef]
- Manousaridis, I.; Manousaridis, K.; Peitsch, W.K.; Schneider, S.W. Individualisierte behandlung und therapiewahl bei lichen planus: Ein schrittweiser ansatz. JDDG—J. Ger. Soc. Dermatol. 2013, 11, 981–992. [Google Scholar] [CrossRef]
- Husein-ElAhmed, H.; Gieler, U.; Steinhoff, M. Lichen planus: A comprehensive evidence-based analysis of medical treatment. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1847–1862. [Google Scholar] [CrossRef]
- Jairath, V.; Acosta Felquer, M.L.; Jaihyun Cho, R. IL-23 inhibition for chronic inflammatory disease. Lancet 2024, 404, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Ogata, A.; Kishimoto, T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin. Immunol. 2014, 26, 88–96. [Google Scholar] [CrossRef]
- Choong, D.J.; Tan, E. Does tocilizumab have a role in dermatology? A review of clinical applications, its adverse side effects and practical considerations. Dermatol. Ther. 2021, 34, e14990. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Kishimoto, T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int. Immunol. 2015, 27, 21–29. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Anakinra therapy for non-cancer inflammatory diseases. Front. Pharmacol. 2018, 9, 1157. [Google Scholar] [CrossRef]
- Arnold, D.D.; Yalamanoglu, A.; Boyman, O. Systematic Review of Safety and Efficacy of IL-1-Targeted Biologics in Treating Immune-Mediated Disorders. Front. Immunol. 2022, 13, 888392. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Fiocco, Z.; Satoh, T.K.; Peris, K.; French, L.E. Therapeutic potential of targeting interleukin-1 family cytokines in chronic inflammatory skin diseases. Br. J. Dermatol. 2022, 186, 925–941. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Brock, J.; Basu, N.; McInnes, I.B.; Siebert, S. The role for JAK inhibitors in the treatment of immune-mediated rheumatic and related conditions. J. Allergy Clin. Immunol. 2021, 148, 941–952. [Google Scholar] [CrossRef]
- Taylor, P.C.; Choy, E.; Baraliakos, X.; Szekanecz, Z.; Xavier, R.M.; Isaacs, J.D.; Strengholt, S.; Parmentier, J.M.; Lippe, R.; Tanaka, Y. Differential properties of Janus kinase inhibitors in the treatment of immune-mediated inflammatory diseases. Rheumatology 2024, 63, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Janus kinase (JAK) inhibitors in the treatment of neoplastic and inflammatory disorders. Pharmacol. Res. 2022, 183, 106362. [Google Scholar] [CrossRef]
- Traves, P.G.; Murray, B.; Campigotto, F.; Galien, R.; Meng, A.; Di Paolo, J.A. JAK selectivity and the implications for clinical inhibition of pharmacodynamic cytokine signalling by filgotinib, upadacitinib, tofacitinib and baricitinib. Ann. Rheum. Dis. 2021, 80, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, M.; Boffini, N.; Barone, E.; Cincinelli, G.; Gerardi, M.C.; Luciano, N.; Manara, M.; Ughi, N.; Epis, O.M.; Selmi, C. Safety of Janus Kinase Inhibitors: A Real-World Multicenter Retrospective Cohort Study. J. Rheumatol. 2023, 50, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Wang, Y.; Jiang, L.; Li, J.; Chen, Q. Updates on immunological mechanistic insights and targeting of the oral lichen planus microenvironment. Front. Immunol. 2023, 13, 1023213. [Google Scholar] [CrossRef]
- Elmasry, M.F.; Mosaad, R.A.; Azzam, O.A.; Rashed, L.A.; Fahim, A. Assessment of PD-1 and PD-L1 tissue expression levels in lichen planus patients: A case–control study. Arch. Dermatol. Res. 2024, 316, 97. [Google Scholar] [CrossRef]
- Shirouchi, K.; Koshikawa, S.; Shinya, K.; Watanabe, H.; Izumi, M.; Yoshimura, K.; Sueki, H. Reduced expression of programmed cell death 1 and programmed cell death ligand 1 in infiltrating inflammatory cells of lichen planus without administration of immune checkpoint inhibitors. J. Dermatol. 2021, 48, 1428–1432. [Google Scholar] [CrossRef]
- Shi, V.J.; Rodic, N.; Gettinger, S.; Leventhal, J.S.; Neckman, J.P.; Girardi, M.; Bosenberg, M.; Choi, J.N. Clinical and Histologic Features of Lichenoid Mucocutaneous Eruptions Due to Anti–Programmed Cell Death 1 and Anti–Programmed Cell Death Ligand 1 Immunotherapy. JAMA Dermatol. 2016, 152, 1128–1136. [Google Scholar] [CrossRef]
- Shah, R.R.; Bhate, C.; Hernandez, A.; Ho, C.H. Lichen planus pemphigoides: A unique form of bullous and lichenoid eruptions secondary to nivolumab. Dermatol. Ther. 2022, 35, e15432. [Google Scholar] [CrossRef]
- Tetzlaff, M.T.; Nagarajan, P.; Chon, S.; Huen, A.; Diab, A.; Omar, P.; Aung, P.P.; Torres-Cabala, C.A.; Mays, S.R.; Prieto, V.G.; et al. Lichenoid Dermatologic Toxicity from Immune Checkpoint Blockade Therapy: A Detailed Examination of the Clinicopathologic Features. Am. J. Dermatopathol. 2017, 39, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Wat, M.; Mollanazar, N.K.; Ellebrecht, C.T.; Forrestel, A.; Elenitsas, R.; Chu, E.Y. Lichen-planus-pemphigoides-like reaction to PD-1 checkpoint blockade. J. Cutan. Pathol. 2022, 49, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Damo, M.; Hornick, N.I.; Venkat, A.; William, I.; Clulo, K.; Venkatesan, S.; He, J.; Fagerberg, E.; Loza, J.L.; Kwok, D.; et al. PD-1 maintains CD8 T cell tolerance towards cutaneous neoantigens. Nature 2023, 619, 151–159. [Google Scholar] [CrossRef]
- Boyle, M.M.; Ashi, S.; Puiu, T.; Reimer, D.; Sokumbi, O.; Soltani, K.; Onajin, O. Lichen Planus Pemphigoides Associated with PD-1 and PD-L1 Inhibitors: A Case Series and Review of the Literature. Am. J. Dermatopathol. 2022, 44, 360–367. [Google Scholar] [CrossRef]
- Mendes-Bastos, P.; Brasileiro, A.; Kolkhir, P.; Frischbutter, S.; Scheffel, J.; Moñino-Romero, S.; Maurer, M. Bruton’s tyrosine kinase inhibition—An emerging therapeutic strategy in immune-mediated dermatological conditions. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 2355–2366. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Murrell, D.F. The potential of Bruton’s tyrosine kinase (BTK) inhibitors in the pharmacotherapeutic management of immune and dermatological disease. Expert Opin. Pharmacother. 2024, 25, 1657–1665. [Google Scholar] [CrossRef]
- Robak, E.; Robak, T. Bruton’s Kinase Inhibitors for the Treatment of Immunological Diseases: Current Status and Perspectives. J. Clin. Med. 2022, 11, 2807. [Google Scholar] [CrossRef]
- Rozkiewicz, D.; Hermanowicz, J.M.; Kwiatkowska, I.; Krupa, A.; Pawlak, D. Bruton’s Tyrosine Kinase Inhibitors (BTKIs): Review of Preclinical Studies and Evaluation of Clinical Trials. Molecules 2023, 28, 2400. [Google Scholar] [CrossRef]
- Cool, A.; Nong, T.; Montoya, S.; Taylor, J. BTK inhibitors: Past, present, and future. Trends Pharmacol. Sci. 2024, 45, 691–707. [Google Scholar] [CrossRef]
- Neys, S.F.H.; Rip, J.; Hendriks, R.W.; Corneth, O.B.J. Bruton’s Tyrosine Kinase Inhibition as an Emerging Therapy in Systemic Autoimmune Disease. Drugs 2021, 81, 1605–1626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Gong, H.; Meng, F. Recent advances in btk inhibitors for the treatment of inflammatory and autoimmune diseases. Molecules 2021, 26, 4907. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Cantafio Duò, V.L.; Vecco, C.; Gelato, F.; Giordano, S.; Roccuzzo, G.; Cavaliere, G.; Avallone, G.; Ortoncelli, M.; Ribero, S.; et al. Impact of comorbidities in the response of atopic patients treated with dupilumab: A real-life study up to 36 weeks. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e1021–e1023. [Google Scholar] [CrossRef]
- Mastorino, L.; Viola, R.; Panzone, M.; Avallone, G.; Gallo, G.; Ortoncelli, M.; Cavaliere, G.; Quaglino, P.; Ribero, S. Dupilumab induces a rapid decrease of pruritus in adolescents: A pilot real-life study. Dermatol. Ther. 2021, 34, e15115. [Google Scholar] [CrossRef]
- Mastorino, L.; Ortoncelli, M.; Giura, M.T.; Avallone, G.; Viola, R.; Quaglino, P.; Ribero, S. Lichen ruber planus arising during dupilumab treatment for atopic dermatitis. Ital. J. Dermatol. Venereol. 2022, 157, 449–450. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosset, F.; Sciamarrelli, N.; Mastorino, L.; Pala, V.; Boskovic, S.; Bongiovanni, E.; Crespi, O.; Liao, Y.; Ribero, S.; Quaglino, P. Monoclonal Antibodies and Small-Molecule Therapies for Lichen Planus: Targeted Immunomodulation and Emerging Evidence. Antibodies 2025, 14, 79. https://doi.org/10.3390/antib14030079
Rosset F, Sciamarrelli N, Mastorino L, Pala V, Boskovic S, Bongiovanni E, Crespi O, Liao Y, Ribero S, Quaglino P. Monoclonal Antibodies and Small-Molecule Therapies for Lichen Planus: Targeted Immunomodulation and Emerging Evidence. Antibodies. 2025; 14(3):79. https://doi.org/10.3390/antib14030079
Chicago/Turabian StyleRosset, Francois, Nadia Sciamarrelli, Luca Mastorino, Valentina Pala, Sara Boskovic, Eleonora Bongiovanni, Orsola Crespi, Yingying Liao, Simone Ribero, and Pietro Quaglino. 2025. "Monoclonal Antibodies and Small-Molecule Therapies for Lichen Planus: Targeted Immunomodulation and Emerging Evidence" Antibodies 14, no. 3: 79. https://doi.org/10.3390/antib14030079
APA StyleRosset, F., Sciamarrelli, N., Mastorino, L., Pala, V., Boskovic, S., Bongiovanni, E., Crespi, O., Liao, Y., Ribero, S., & Quaglino, P. (2025). Monoclonal Antibodies and Small-Molecule Therapies for Lichen Planus: Targeted Immunomodulation and Emerging Evidence. Antibodies, 14(3), 79. https://doi.org/10.3390/antib14030079








