A 3D Surface Plot for the Effective Visualization of Specific Serum Antibody Binding Properties
Abstract
1. Introduction
2. Methods
2.1. Dataset of SARS-CoV-2 Specific Antibody Binding Results
2.2. Visualization Program
3. Results
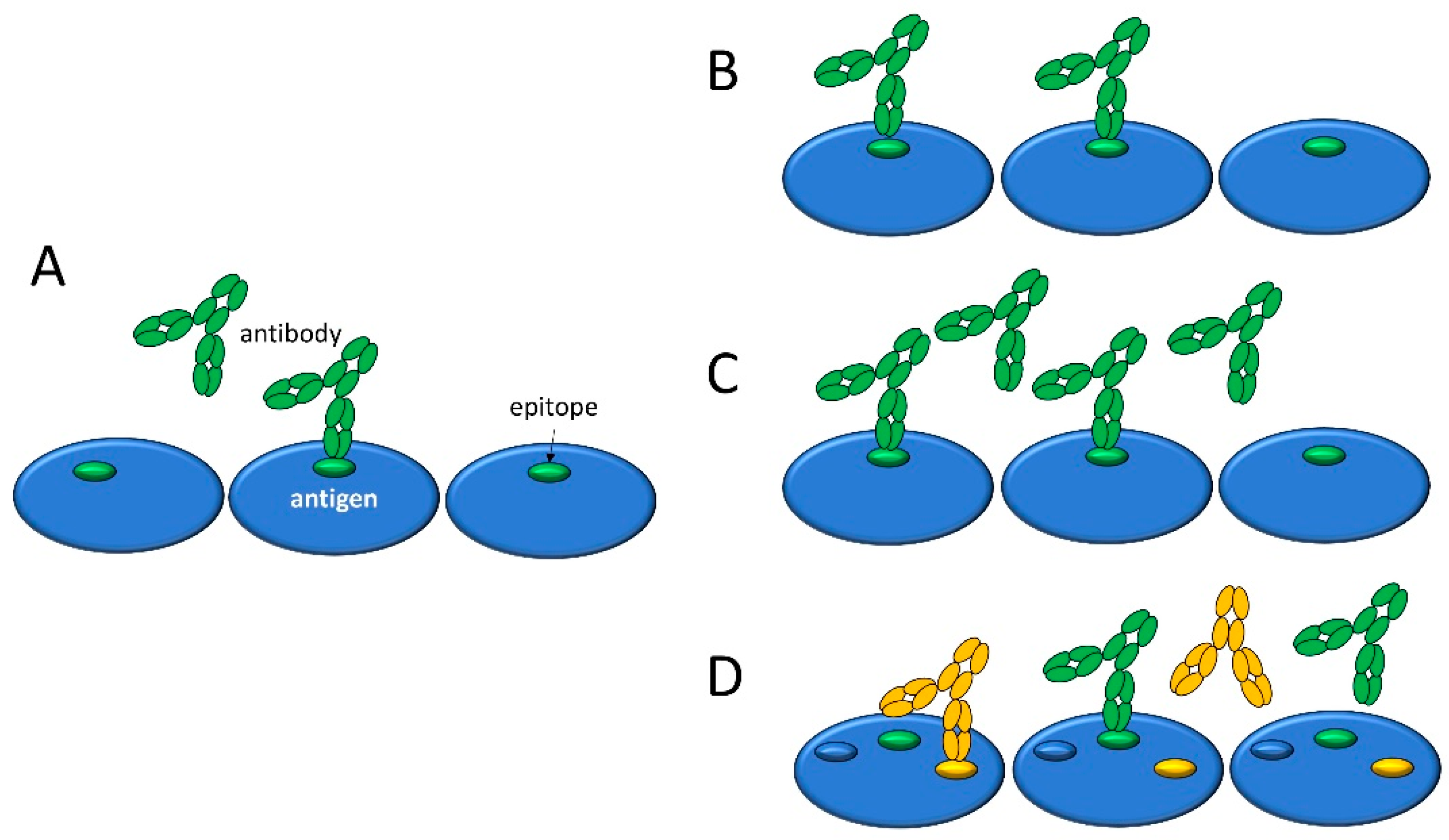
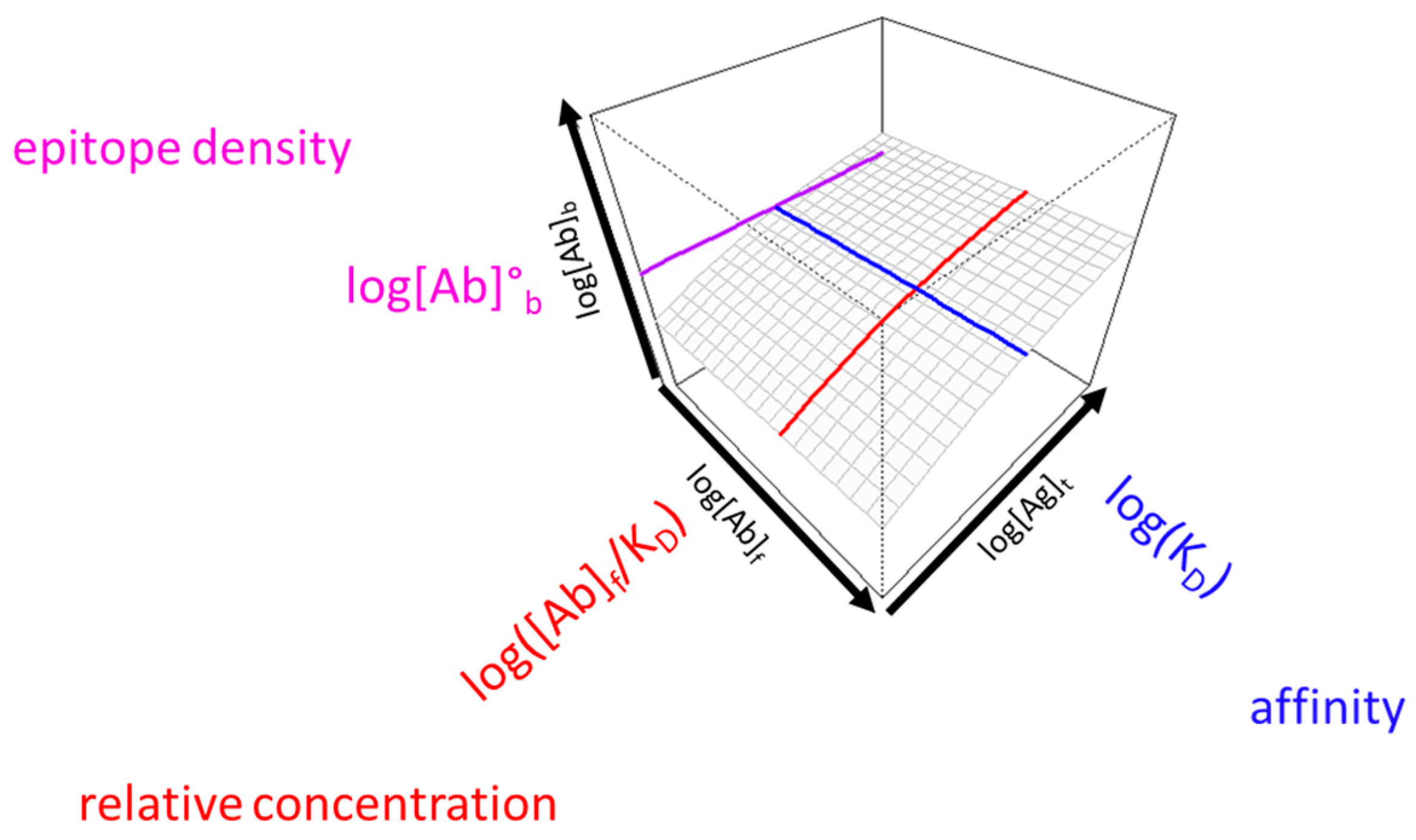
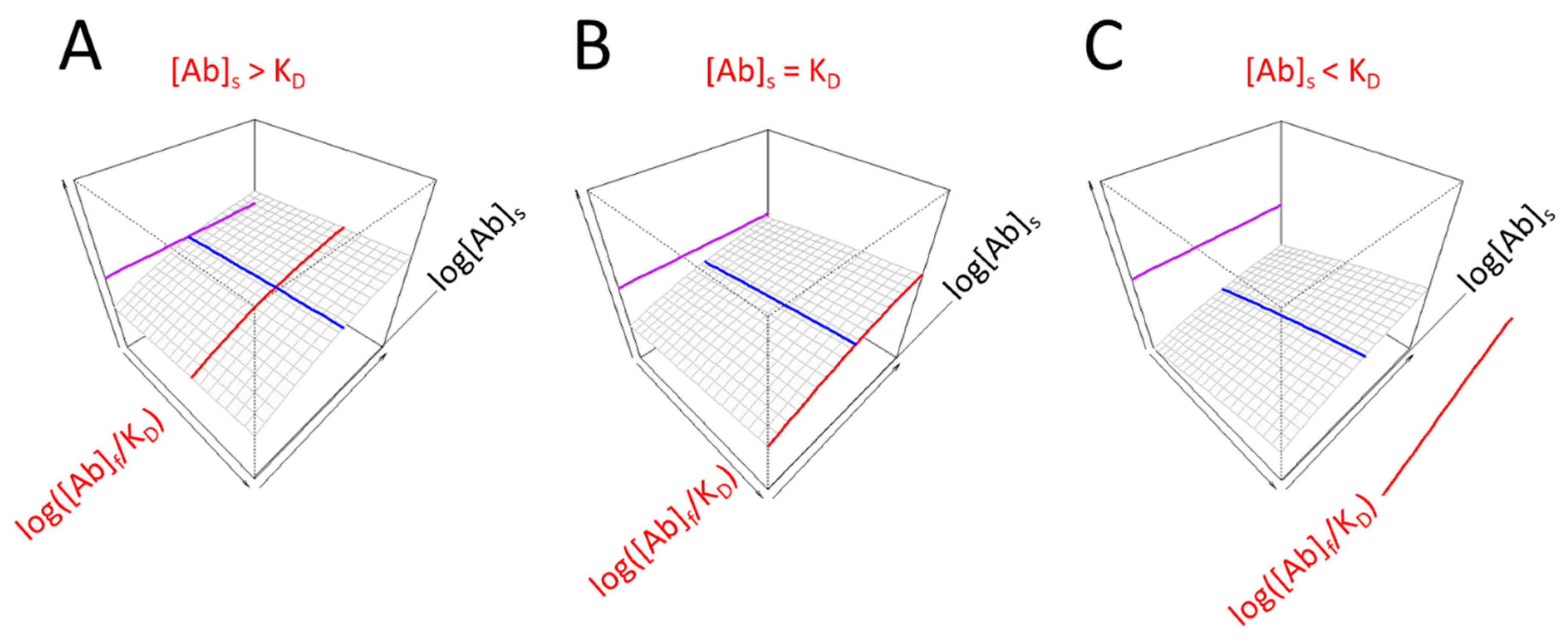
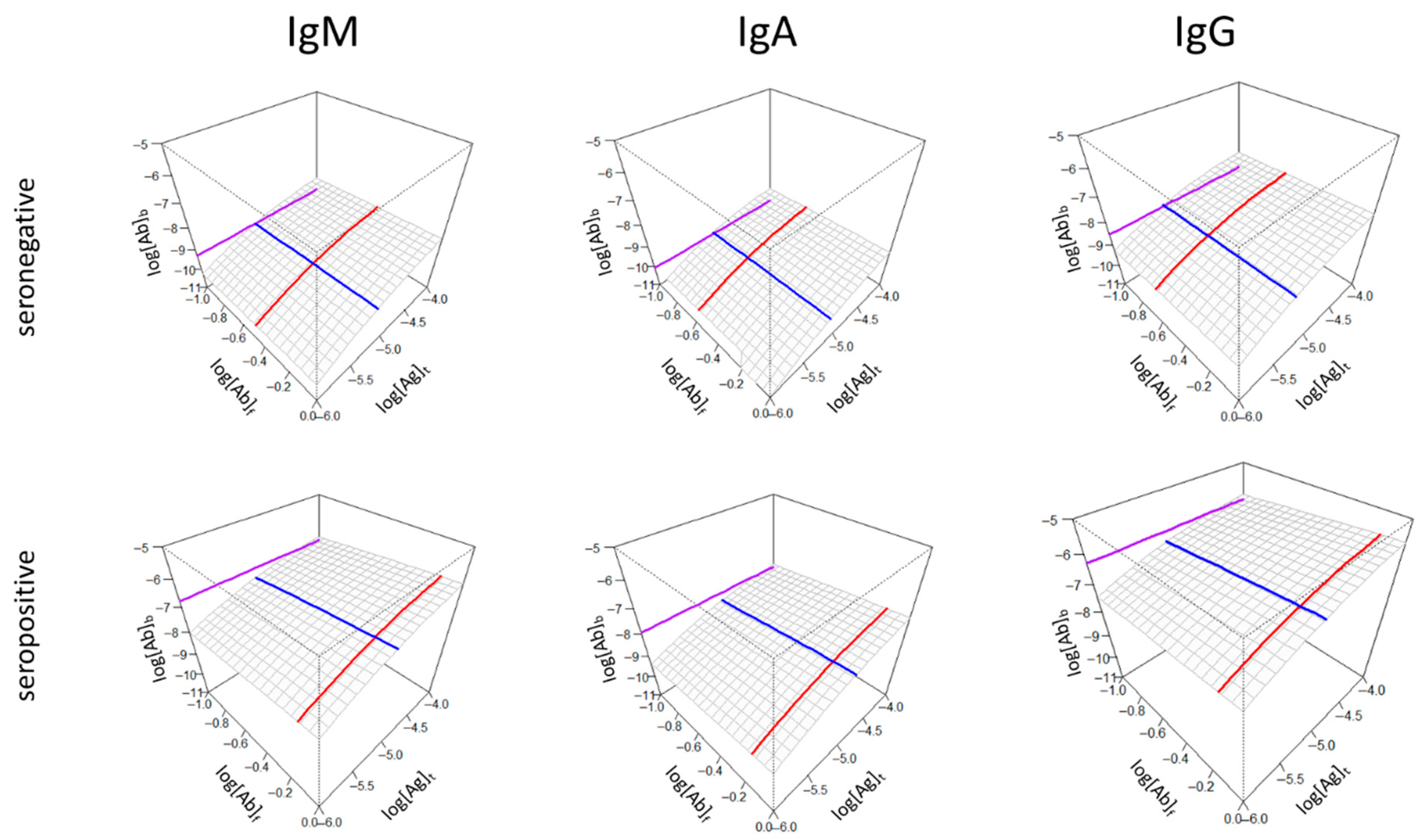
3.1. Visual Interpretation of Key Variables of Serum Antibody Binding
3.2. Application of the Visualization Scheme to Experimental Data
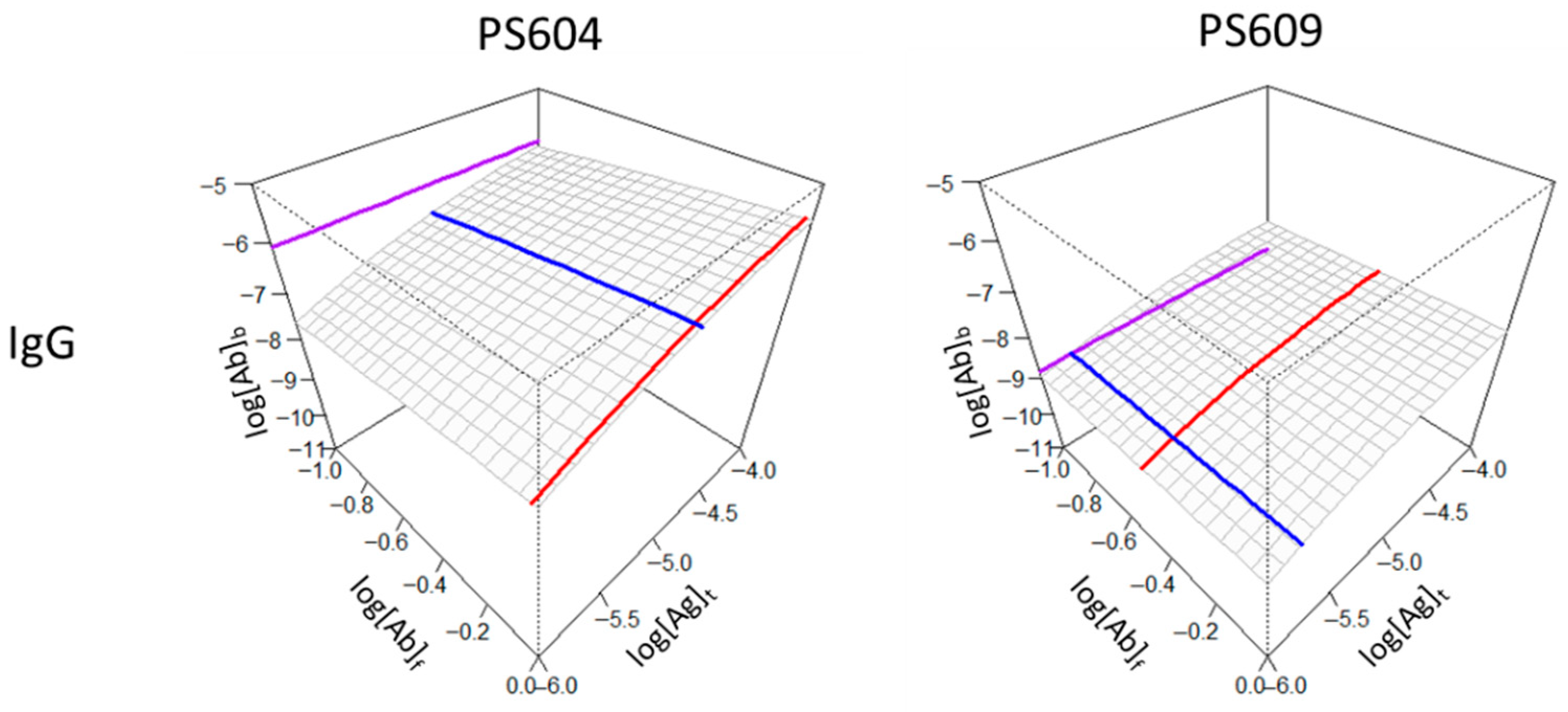
3.2.1. Demonstration of Individual Differences
3.2.2. Demonstration of Group Differences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haselbeck, A.H.; Im, J.; Prifti, K.; Marks, F.; Holm, M.; Zellweger, R.M. Serology as a tool to assess infectious disease landscapes and guide public health policy. Pathogens 2022, 11, 732. [Google Scholar] [CrossRef]
- Agmon-Levin, N.; Damoiseaux, J.; Kallenberg, C.; Sack, U.; Witte, T.; Herold, M.; Bossuyt, X.; Musset, L.; Cervera, R.; Plaza-Lopez, A.; et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 2014, 73, 17–23. [Google Scholar] [CrossRef]
- Ansotegui, I.J.; Melioli, G.; Canonica, G.W.; Caraballo, L.; Villa, E.; Ebisawa, M.; Passalacqua, G.; Savi, E.; Ebo, D.; Gómez, R.M.; et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organiz J. 2020, 13, 100080. [Google Scholar] [CrossRef]
- Morrison, B.J.; Labo, N.; Miley, W.J.; Whitby, D. Serodiagnosis for tumor viruses. Semin. Oncol. 2015, 42, 191–206. [Google Scholar] [CrossRef]
- Ionov, S.; Lee, J. An immunoproteomic survey of the antibody landscape: Insights and opportunities revealed by serological repertoire profiling. Front. Immunol. 2022, 13, 832533. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.E.; Galloway, J.G.; Wolf, C.; Logue, J.K.; Franko, N.; Chu, H.Y.; Matsen, F.A.; Overbaugh, J.M. Comprehensive characterization of the antibody responses to SARS-CoV-2 Spike protein finds additional vaccine-induced epitopes beyond those for mild infection. eLife 2022, 11, e73490. [Google Scholar] [CrossRef]
- Eliyahu, S.; Sharabi, O.; Elmedvi, S.; Timor, R.; Davidovich, A.; Vigneault, F.; Clouser, C.; Hope, R.; Nimer, A.; Braun, M.; et al. Antibody repertoire analysis of hepatitis C virus infections identifies immune signatures associated with spontaneous clearance. Front. Immunol. 2018, 9, 3004. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.M.; van der Maas, L.; Pennings, J.L.A.; Fuursted, K.; Jørgensen, C.S.; van Riet, E.; Metz, B.; Kersten, G.F.A.; Dalby, T. Antibody Specificity Following a Recent Bordetella pertussis Infection in Adolescence Is Correlated with the Pertussis Vaccine Received in Childhood. Front. Immunol. 2019, 10, 1364. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Song, W.; Fang, Y.; Yu, L.; Liu, S.; Churilov, L.P.; Zhang, F. The roles and applications of autoantibodies in progression, diagnosis, treatment and prognosis of human malignant tumours. Autoimmun. Rev. 2017, 16, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Prechl, J. Why current quantitative serology is not quantitative and how systems immunology could provide solutions. Biol. Futur. 2021, 72, 37–44. [Google Scholar] [CrossRef]
- Thomas, A.; Messer, W.B.; Hansel, D.E.; Streblow, D.N.; Kazmierczak, S.C.; Lyski, Z.L.; Lu, Z.; Slifka, M.K. Establishment of Monoclonal Antibody Standards for Quantitative Serological Diagnosis of SARS-CoV-2 in Low-Incidence Settings. Open Forum Infect. Dis. 2021, 8, ofab061. [Google Scholar] [CrossRef]
- Wang, L.; Patrone, P.N.; Kearsley, A.J.; Izac, J.R.; Gaigalas, A.K.; Prostko, J.C.; Kwon, H.J.; Tang, W.; Kosikova, M.; Xie, H.; et al. Monoclonal Antibodies as SARS-CoV-2 Serology Standards: Experimental Validation and Broader Implications for Correlates of Protection. Int. J. Mol. Sci. 2023, 24, 15705. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Verwilligen, A.; Sadoff, J.; Brandenburg, B.; Sneekes-Vriese, E.; van den Kerkhof, T.; Dillen, L.; Rutten, L.; Juraszek, J.; Callewaert, K.; et al. Absolute quantitation of binding antibodies from clinical samples. npj Vaccines 2024, 9, 8. [Google Scholar] [CrossRef]
- Blais, B.W.; Leggate, J.; Bosley, J.; Martinez-Perez, A. Comparison of fluorogenic and chromogenic assay systems in the detection of Escherichia coli O157 by a novel polymyxin-based ELISA. Lett Appl Microbiol. 2004, 39, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.B.; Dikova, E.B.; Sheller, U.; Dikov, M.M.; Gavrilova, E.M.; Egorov, A.M. Evaluation of dissociation constants of antigen-antibody complexes by ELISA. J. Immunol. Methods. 1990, 131, 213–222. [Google Scholar] [CrossRef]
- Lew, A.M. The effect of epitope density and antibody affinity on the ELISA as analysed by monoclonal antibodies. J. Immunol. Methods 1984, 72, 171–176. [Google Scholar] [CrossRef]
- Bobrovnik, S.A.; Demchenko, M.; Komisarenko, S.; Stevens, F. Traditional ELISA methods for antibody affinity determination fail to reveal the presence of low affinity antibodies in antisera: An alternative approach. J. Mol. Recognit. 2010, 23, 448–456. [Google Scholar] [CrossRef]
- Real-Fernández, F.; Rossi, G.; Panza, F.; Pratesi, F.; Migliorini, P.; Rovero, P. Surface Plasmon Resonance Method to Evaluate Anti-citrullinated Protein/Peptide Antibody Affinity to Citrullinated Peptides. Methods Mol Biol. 2015, 1348, 267–274. [Google Scholar]
- Szarka, E.; Aradi, P.; Huber, K.; Pozsgay, J.; Végh, L.; Magyar, A.; Gyulai, G.; Nagy, G.; Rojkovich, B.; Kiss, É.; et al. Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19, 326. [Google Scholar] [CrossRef]
- Lippok, S.; Seidel, S.A.I.; Duhr, S.; Uhland, K.; Holthoff, H.-P.; Jenne, D.; Braun, D. Direct detection of antibody concentration and affinity in human serum using microscale thermophoresis. Anal. Chem. 2012, 84, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.; Piziorska, M.A.; Denninger, V.; Morgunov, A.S.; Ilsley, A.; Malik, A.Y.; Schneider, M.M.; Devenish, S.R.A.; Meisl, G.; Kosmoliaptsis, V.; et al. Antibody Affinity Governs the Inhibition of SARS-CoV-2 Spike/ACE2 Binding in Patient Serum. ACS Infect. Dis. 2021, 7, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Papp, K.; Kovács, Á.; Orosz, A.; Hérincs, Z.; Randek, J.; Liliom, K.; Pfeil, T.; Prechl, J. Absolute quantitation of serum antibody reactivity using the richards growth model for antigen microspot titration. Sensors 2022, 22, 3962. [Google Scholar] [CrossRef]
- Kovács, Á.; Hérincs, Z.; Papp, K.; Kaczmarek, J.Z.; Larsen, D.N.; Stage, P.; Bereczki, L.; Ujhelyi, E.; Pfeil, T.; Prechl, J.; et al. In-depth immunochemical characterization of the serum antibody response using a dual-titration microspot assay. Front. Immunol. 2025, 16, 1494624. [Google Scholar] [CrossRef]
- Prechl, J.; Papp, K.; Kovács, Á.; Pfeil, T. The binding landscape of serum antibodies: How physical and mathematical concepts can advance systems immunology. Antibodies 2022, 11, 43. [Google Scholar] [CrossRef]
- Tian, L.; Elsheikh, E.B.; Patrone, P.N.; Kearsley, A.J.; Gaigalas, A.K.; Inwood, S.; Lin-Gibson, S.; Esposito, D.; Wang, L. Towards Quantitative and Standardized Serological and Neutralization Assays for COVID-19. Int. J. Mol. Sci. 2021, 22, 2723. [Google Scholar] [CrossRef] [PubMed]
- Monogioudi, E.; Martos, G.; Hutu, D.P.; Schimmel, H.; Meroni, P.L.; Sheldon, J.; Zegers, I. Standardization of autoimmune testing—Is it feasible? Clin. Chem. Lab. Med. 2018, 56, 1734–1742. [Google Scholar] [CrossRef]
- Thompson, G.E.; Fussner, L.A.; Hummel, A.M.; Schroeder, D.R.; Silva, F.; Snyder, M.R.; Langford, C.A.; Merkel, P.A.; Monach, P.A.; Seo, P.; et al. Clinical Utility of Serial Measurements of Antineutrophil Cytoplasmic Antibodies Targeting Proteinase 3 in ANCA-Associated Vasculitis. Front. Immunol. 2020, 11, 2053. [Google Scholar] [CrossRef]
- Hardy, M.Y.; Tye-Din, J.A. Coeliac disease: A unique model for investigating broken tolerance in autoimmunity. Clin. Transl. Immunol. 2016, 5, e112. [Google Scholar] [CrossRef]
- Carlé, C.; Fortenfant, F.; Bost, C.; Belliere, J.; Faguer, S.; Chauveau, D.; Huart, A.; Ribes, D.; Alric, L.; Pugnet, G.; et al. The added value of coupling anti-dsDNA and anti-chromatin antibodies in follow-up monitoring of systemic lupus erythematosus patients. J. Transl. Autoimmun. 2025, 10, 100274. [Google Scholar] [CrossRef] [PubMed]
- Kongpachith, S.; Lingampalli, N.; Ju, C.-H.; Blum, L.K.; Lu, D.R.; Elliott, S.E.; Mao, R.; Robinson, W.H. Affinity Maturation of the Anti-Citrullinated Protein Antibody Paratope Drives Epitope Spreading and Polyreactivity in Rheumatoid Arthritis. Arthritis Rheumatol. 2019, 71, 507–517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prechl, J.; Kovács, Á.; Papp, K.; Hérincs, Z.; Pfeil, T. A 3D Surface Plot for the Effective Visualization of Specific Serum Antibody Binding Properties. Antibodies 2025, 14, 68. https://doi.org/10.3390/antib14030068
Prechl J, Kovács Á, Papp K, Hérincs Z, Pfeil T. A 3D Surface Plot for the Effective Visualization of Specific Serum Antibody Binding Properties. Antibodies. 2025; 14(3):68. https://doi.org/10.3390/antib14030068
Chicago/Turabian StylePrechl, József, Ágnes Kovács, Krisztián Papp, Zoltán Hérincs, and Tamás Pfeil. 2025. "A 3D Surface Plot for the Effective Visualization of Specific Serum Antibody Binding Properties" Antibodies 14, no. 3: 68. https://doi.org/10.3390/antib14030068
APA StylePrechl, J., Kovács, Á., Papp, K., Hérincs, Z., & Pfeil, T. (2025). A 3D Surface Plot for the Effective Visualization of Specific Serum Antibody Binding Properties. Antibodies, 14(3), 68. https://doi.org/10.3390/antib14030068







