A Cancer-Specific Anti-Podocalyxin Monoclonal Antibody (humPcMab-60) Demonstrated Antitumor Efficacy in Pancreatic and Colorectal Cancer Xenograft Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Recombinant Antibody Production
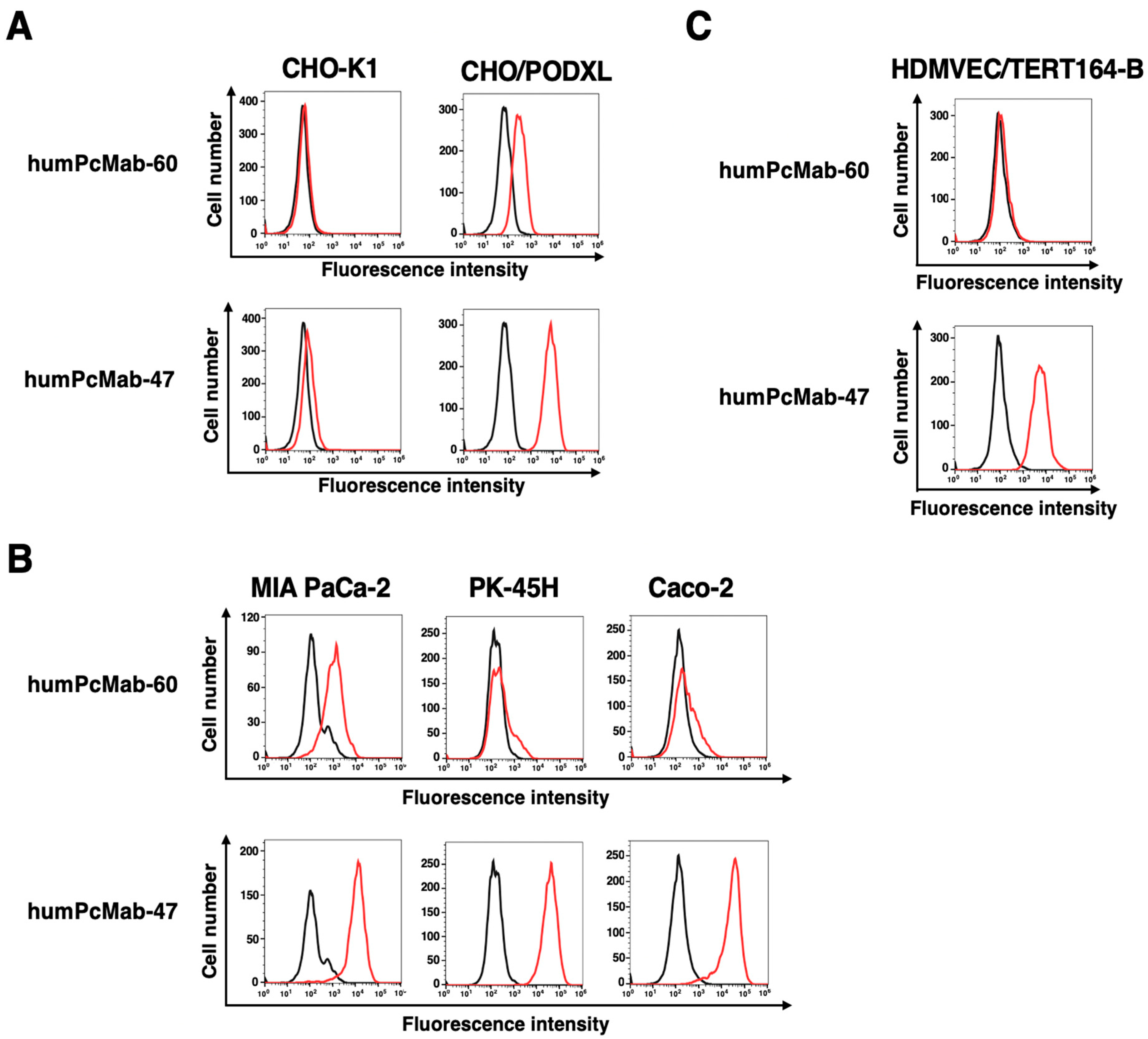
2.3. Flow Cytometry
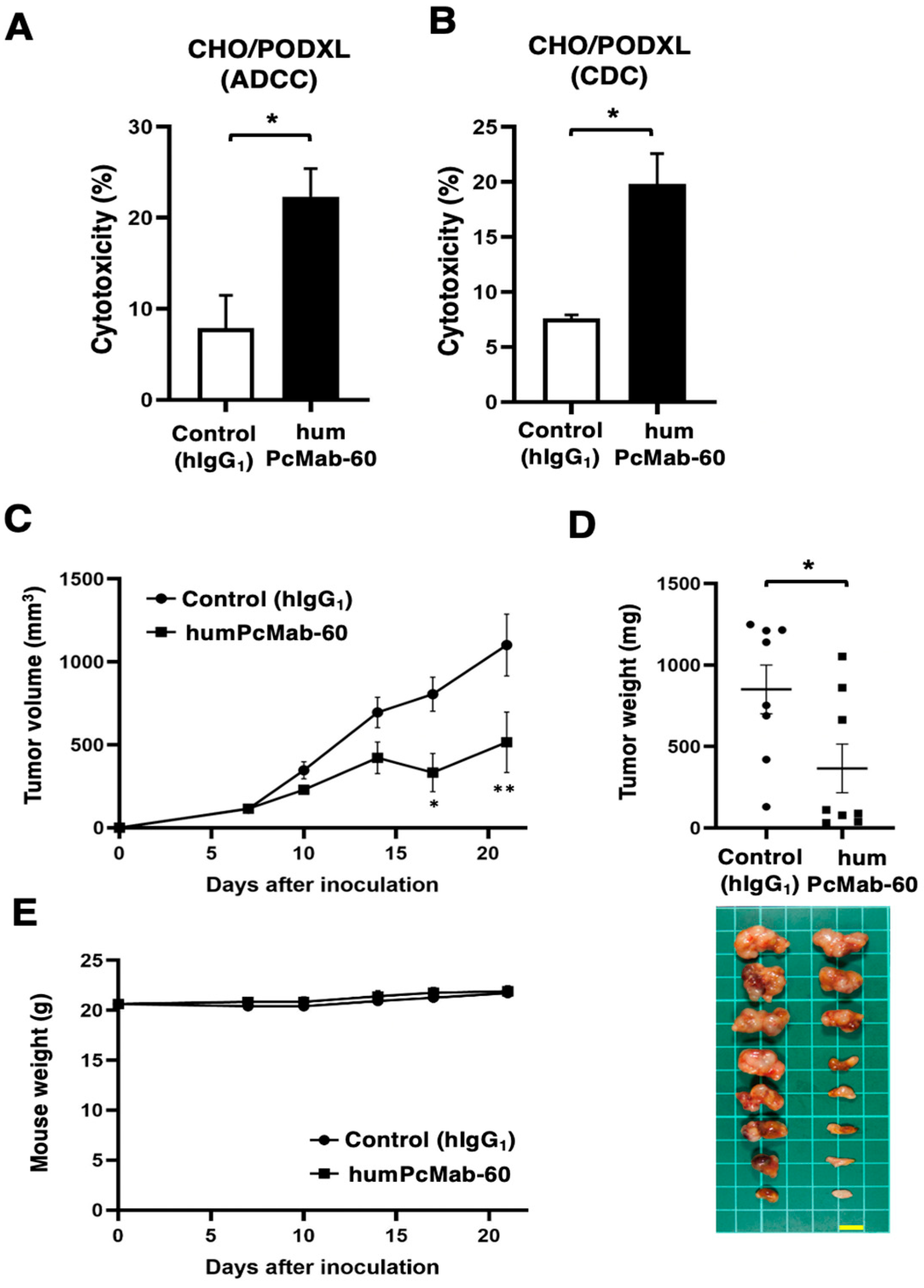
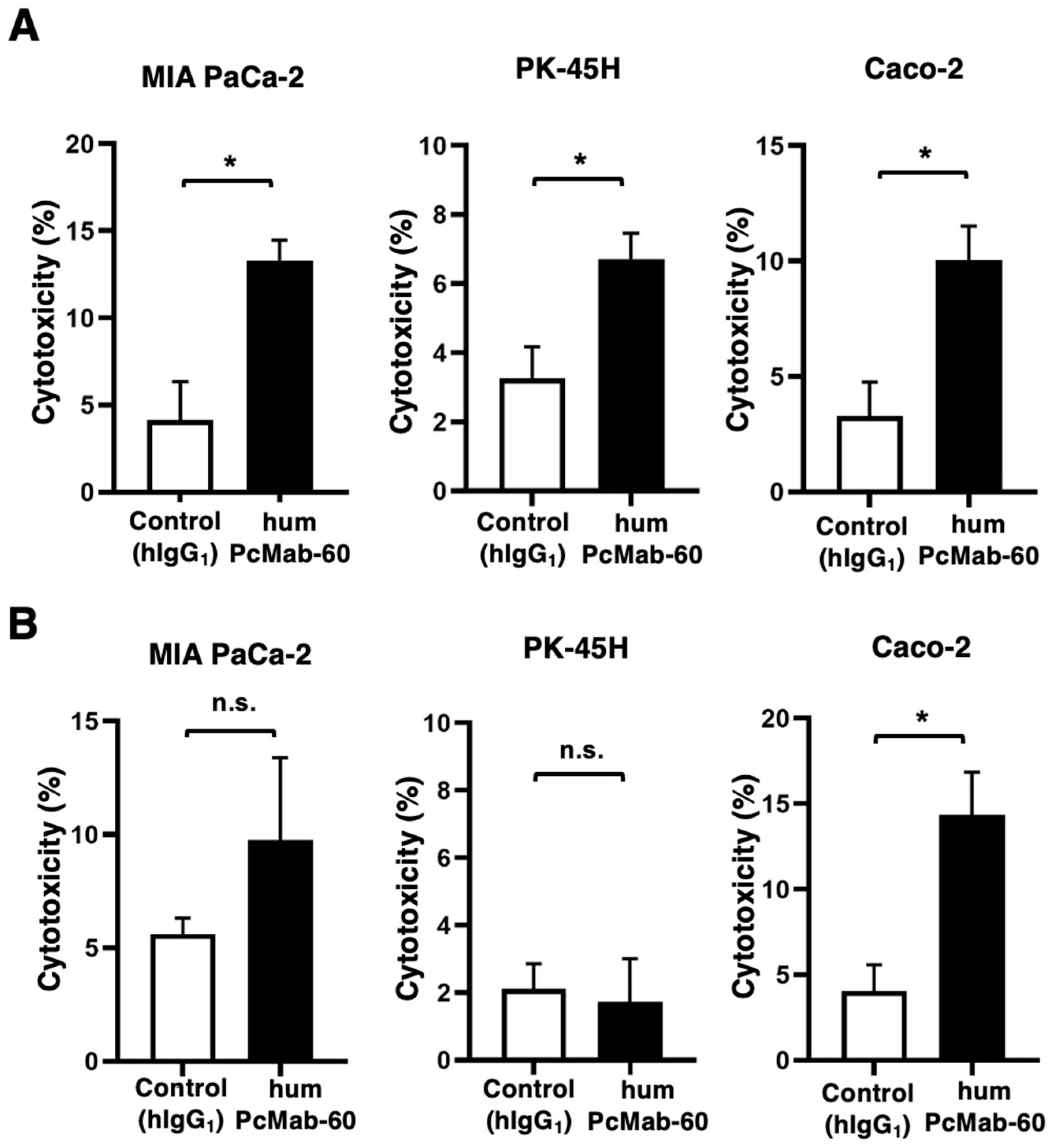
2.4. Antibody-Dependent Cellular Cytotoxicity
2.5. Complement-Dependent Cytotoxicity
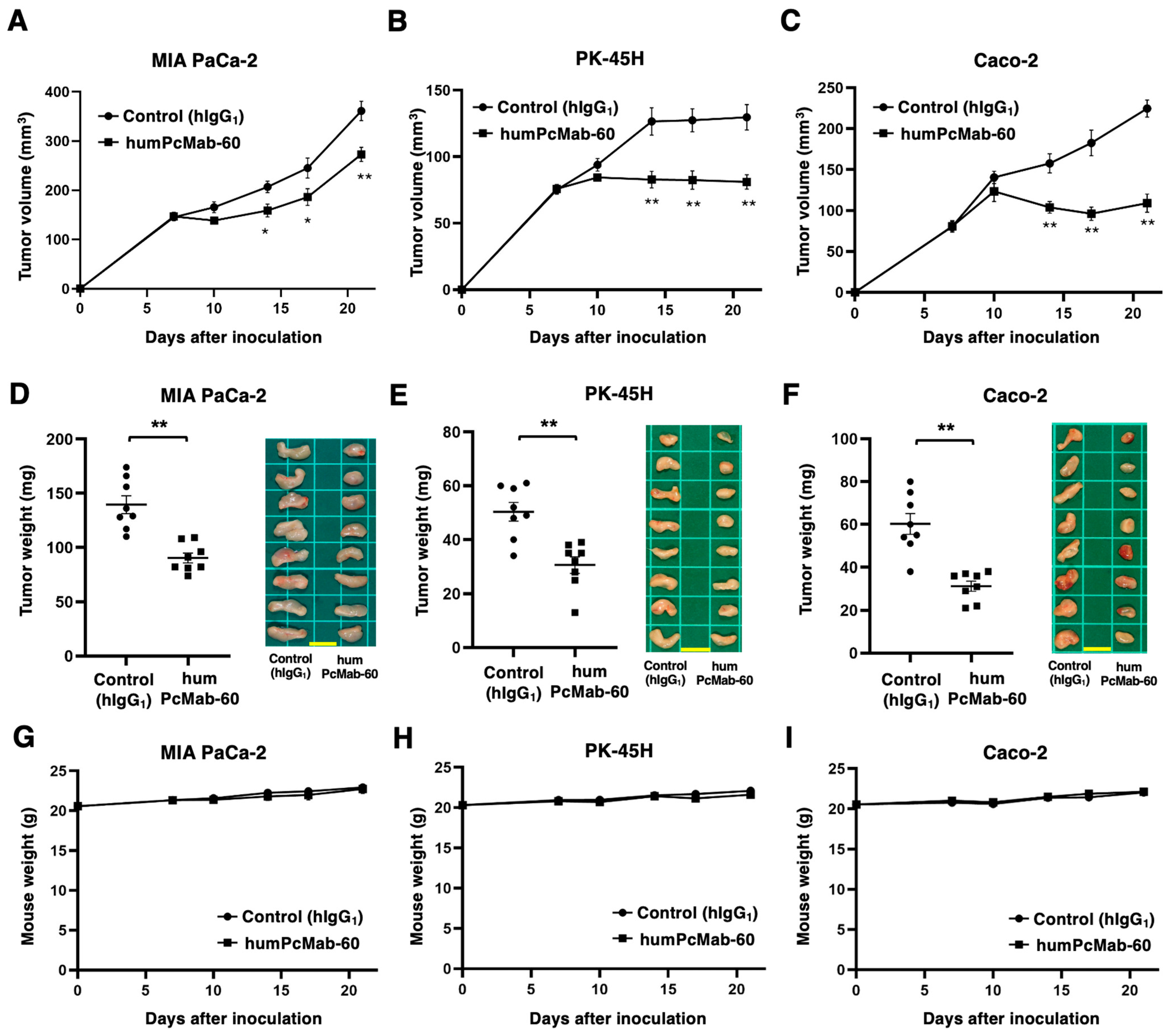
2.6. Antitumor Activity of humPcMab-60
3. Results
3.1. Production of a Humanized Anti-PODXL CasMab, humPcMab-60
3.2. ADCC, CDC, and Antitumor Effect by humPcMab-60 Against CHO/PODXL
3.3. ADCC and CDC by humPcMab-60 Against Human Cancer Cells
3.4. Antitumor Effect of humPcMab-60 Against Human Cancer Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doyonnas, R.; Nielsen, J.S.; Chelliah, S.; Drew, E.; Hara, T.; Miyajima, A.; McNagny, K.M. Podocalyxin is a CD34-related marker of murine hematopoietic stem cells and embryonic erythroid cells. Blood 2005, 105, 4170–4178. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.S.; McNagny, K.M. Novel functions of the CD34 family. J. Cell Sci. 2008, 121 Pt 22, 3683–3692. [Google Scholar] [CrossRef]
- McNagny, K.M.; Pettersson, I.; Rossi, F.; Flamme, I.; Shevchenko, A.; Mann, M.; Graf, T. Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J. Cell Biol. 1997, 138, 1395–1407. [Google Scholar] [CrossRef]
- Kerjaschki, D.; Sharkey, D.J.; Farquhar, M.G. Identification and characterization of podocalyxin--the major sialoprotein of the renal glomerular epithelial cell. J. Cell Biol. 1984, 98, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Kriehuber, E.; Breiteneder-Geleff, S.; Groeger, M.; Soleiman, A.; Schoppmann, S.F.; Stingl, G.; Kerjaschki, D.; Maurer, D. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 2001, 194, 797–808. [Google Scholar] [CrossRef]
- Doyonnas, R.; Kershaw, D.B.; Duhme, C.; Merkens, H.; Chelliah, S.; Graf, T.; McNagny, K.M. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J. Exp. Med. 2001, 194, 13–27. [Google Scholar] [CrossRef]
- Ney, J.T.; Zhou, H.; Sipos, B.; Büttner, R.; Chen, X.; Klöppel, G.; Gütgemann, I. Podocalyxin-like protein 1 expression is useful to differentiate pancreatic ductal adenocarcinomas from adenocarcinomas of the biliary and gastrointestinal tracts. Hum. Pathol. 2007, 38, 359–364. [Google Scholar] [CrossRef]
- Hsu, Y.-H.; Lin, W.-L.; Hou, Y.-T.; Pu, Y.-S.; Shun, C.-T.; Chen, C.-L.; Wu, Y.-Y.; Chen, J.-Y.; Chen, T.-H.; Jou, T.-S. Podocalyxin EBP50 ezrin molecular complex enhances the metastatic potential of renal cell carcinoma through recruiting Rac1 guanine nucleotide exchange factor ARHGEF7. Am. J. Pathol. 2010, 176, 3050–3061. [Google Scholar] [CrossRef]
- Larsson, A.; Johansson, M.E.; Wangefjord, S.; Gaber, A.; Nodin, B.; Kucharzewska, P.; Welinder, C.; Belting, M.; Eberhard, J.; Johnsson, A.; et al. Overexpression of podocalyxin-like protein is an independent factor of poor prognosis in colorectal cancer. Br. J. Cancer 2011, 105, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Forse, C.L.; Yilmaz, Y.E.; Pinnaduwage, D.; O’Malley, F.P.; Mulligan, A.M.; Bull, S.B.; Andrulis, I.L. Elevated expression of podocalyxin is associated with lymphatic invasion, basal-like phenotype, and clinical outcome in axillary lymph node-negative breast cancer. Breast Cancer Res. Treat. 2013, 137, 709–719. [Google Scholar] [CrossRef]
- Lin, C.-W.; Sun, M.-S.; Wu, H.-C. Podocalyxin-like 1 is associated with tumor aggressiveness and metastatic gene expression in human oral squamous cell carcinoma. Int. J. Oncol. 2014, 45, 710–718. [Google Scholar] [CrossRef][Green Version]
- He, S.; Du, W.; Li, M.; Yan, M.; Zheng, F. PODXL might be a new prognostic biomarker in various cancers: A meta-analysis and sequential verification with TCGA datasets. BMC Cancer 2020, 20, 620. [Google Scholar] [CrossRef]
- Chen, M.-J.; Gao, X.-J.; Xu, L.-N.; Liu, T.-F.; Liu, X.-H.; Liu, L.-X. Ezrin is required for epithelial-mesenchymal transition induced by TGF-β1 in A549 cells. Int. J. Oncol. 2014, 45, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Fröse, J.; Chen, M.B.; Hebron, K.E.; Reinhardt, F.; Hajal, C.; Zijlstra, A.; Kamm, R.D.; Weinberg, R.A. Epithelial-Mesenchymal Transition Induces Podocalyxin to Promote Extravasation via Ezrin Signaling. Cell Rep. 2018, 24, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Le Tran, N.; Wang, Y.; Nie, G. Podocalyxin in Normal Tissue and Epithelial Cancer. Cancers 2021, 13, 2863. [Google Scholar] [CrossRef]
- Dallas, M.R.; Chen, S.-H.; Streppel, M.M.; Sharma, S.; Maitra, A.; Konstantopoulos, K. Sialofucosylated podocalyxin is a functional E- and L-selectin ligand expressed by metastatic pancreatic cancer cells. Am. J. Physiol. Physiol. 2012, 303, C616–C624. [Google Scholar] [CrossRef]
- Tamayo-Orbegozo, E.; Amo, L.; Díez-García, J.; Amutio, E.; Riñón, M.; Alonso, M.; Arana, P.; Maruri, N.; Larrucea, S. Emerging Role of Podocalyxin in the Progression of Mature B-Cell Non-Hodgkin Lymphoma. Cancers 2020, 12, 396. [Google Scholar] [CrossRef]
- Snyder, K.A.; Hughes, M.R.; Hedberg, B.; Brandon, J.; Hernaez, D.C.; Bergqvist, P.; Cruz, F.; Po, K.; Graves, M.L.; Turvey, M.E.; et al. Podocalyxin enhances breast tumor growth and metastasis and is a target for monoclonal antibody therapy. Breast Cancer Res. 2015, 17, 46. [Google Scholar] [CrossRef]
- Ogasawara, S.; Kaneko, M.K.; Yamada, S.; Honma, R.; Nakamura, T.; Saidoh, N.; Yanaka, M.; Yoshida, K.; Fujii, Y.; Kato, Y. PcMab-47: Novel Antihuman Podocalyxin Monoclonal Antibody for Immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2017, 36, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Itai, S.; Ohishi, T.; Kaneko, M.K.; Yamada, S.; Abe, S.; Nakamura, T.; Yanaka, M.; Chang, Y.-W.; Ohba, S.-I.; Nishioka, Y.; et al. Anti-podocalyxin antibody exerts antitumor effects via antibody-dependent cellular cytotoxicity in mouse xenograft models of oral squamous cell carcinoma. Oncotarget 2018, 9, 22480–22497. [Google Scholar] [CrossRef][Green Version]
- Kaneko, M.K.; Suzuki, H.; Kato, Y. Establishment of a Novel Cancer-Specific Anti-HER2 Monoclonal Antibody H(2)Mab-250/H(2)CasMab-2 for Breast Cancers. Monoclon. Antibodies Immunodiagn. Immunother. 2024, 43, 35–43. [Google Scholar] [CrossRef]
- Suzuki, H.; Ohishi, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Anti-HER2 Cancer-Specific mAb, H2Mab-250-hG1, Possesses Higher Complement-Dependent Cytotoxicity than Trastuzumab. Int. J. Mol. Sci. 2024, 25, 8386. [Google Scholar] [CrossRef] [PubMed]
- Hosking, M.P.; Shirinbak, S.; Omilusik, K.; Chandra, S.; Kaneko, M.K.; Gentile, A.; Yamamoto, S.; Shrestha, B.; Grant, J.; Boyett, M.; et al. Preferential tumor targeting of HER2 by iPSC-derived CAR T cells engineered to overcome multiple barriers to solid tumor efficacy. Cell Stem Cell 2025, 32, 1087–1101.e4. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.K.; Ohishi, T.; Kawada, M.; Kato, Y. A cancer-specific anti-podocalyxin monoclonal antibody (60-mG2a-f) exerts antitumor effects in mouse xenograft models of pancreatic carcinoma. Biochem. Biophys. Rep. 2020, 24. [Google Scholar] [CrossRef]
- Asano, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Epitope Mapping of a Cancer-Specific Anti-Podocalyxin Monoclonal Antibody (PcMab-60) Using Enzyme-Linked Immunosorbent Assay and Surface Plasmon Resonance. Monoclon. Antibodies Immunodiagn. Immunother. 2021, 40, 227–232. [Google Scholar] [CrossRef]
- Suzuki, H.; Ohishi, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. A Cancer-Specific Monoclonal Antibody against Podocalyxin Exerted Antitumor Activities in Pancreatic Cancer Xenografts. Int. J. Mol. Sci. 2023, 25, 161. [Google Scholar] [CrossRef]
- Taniuchi, K.; Tsuboi, M.; Sakaguchi, M.; Saibara, T. Measurement of serum PODXL concentration for detection of pancreatic cancer. OncoTargets Ther. 2018, 11, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Waddell, N.; Pajic, M.; Patch, A.-M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; Quek, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 2015, 518, 495–501. [Google Scholar] [CrossRef]
- Jones, S.; Zhang, X.; Parsons, D.W.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Kamiyama, H.; Jimeno, A.; et al. core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 2008, 321, 1801–1806. [Google Scholar] [CrossRef]
- Taherian, M.; Wang, H.; Wang, H. Pancreatic Ductal Adenocarcinoma: Molecular Pathology and Predictive Biomarkers. Cells 2022, 11, 3068. [Google Scholar] [CrossRef]
- Bailey, P.; Chang, D.K.; Nones, K.; Johns, A.L.; Patch, A.-M.; Gingras, M.-C.; Miller, D.K.; Christ, A.N.; Bruxner, T.J.C.; Quinn, M.C.; et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016, 531, 47–52. [Google Scholar] [CrossRef]
- Naishiro, Y.; Yamada, T.; Idogawa, M.; Honda, K.; Takada, M.; Kondo, T.; Imai, K.; Hirohashi, S. Morphological and transcriptional responses of untransformed intestinal epithelial cells to an oncogenic beta-catenin protein. Oncogene 2005, 24, 3141–3153. [Google Scholar] [CrossRef][Green Version]
- Lee, H.; Kong, J.-S.; Lee, S.-S.; Kim, A. Radiation-Induced Overexpression of TGFβ and PODXL Contributes to Colorectal Cancer Cell Radioresistance through Enhanced Motility. Cells 2021, 10, 2087. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrot, C.; Ultsch, M.; Dubnovitsky, A.; Abrahmsén, L.; Härd, T. Structural basis for high-affinity HER2 receptor binding by an engineered protein. Proc. Natl. Acad. Sci. USA 2010, 107, 15039–15044. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W., Jr.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Canals Hernaez, D.; Hughes, M.R.; Dean, P.; Bergqvist, P.; Samudio, I.; Blixt, O.; Wiedemeyer, K.; Li, Y.; Bond, C.; Cruz, E.; et al. PODO447: A novel antibody to a tumor-restricted epitope on the cancer antigen podocalyxin. J. Immunother. Cancer 2020, 8, e001128. [Google Scholar] [CrossRef]
- Ring, A.; Nguyen-Sträuli, B.D.; Wicki, A.; Aceto, N. Biology, vulnerabilities and clinical applications of circulating tumour cells. Nat. Rev. Cancer 2023, 23, 95–111. [Google Scholar] [CrossRef]
- Dashzeveg, N.K.; Jia, Y.; Zhang, Y.; Gerratana, L.; Patel, P.; Shajahan, A.; Dandar, T.; Ramos, E.K.; Almubarak, H.F.; Adorno-Cruz, V.; et al. Dynamic Glycoprotein Hyposialylation Promotes Chemotherapy Evasion and Metastatic Seeding of Quiescent Circulating Tumor Cell Clusters in Breast Cancer. Cancer Discov. 2023, 13, 2050–2071. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Liu, H.; El-Shennawy, L. Multi-omic features and clustering phenotypes of circulating tumor cells associated with metastasis and clinical outcomes. Int. Rev. Cell Mol. Biol. 2025, 392, 67–100. [Google Scholar]
- Li, F.; Ding, J. Sialylation is involved in cell fate decision during development, reprogramming and cancer progression. Protein Cell 2019, 10, 550–565. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, H.; Ohishi, T.; Nakamura, T.; Yanaka, M.; Handa, S.; Tanaka, T.; Kaneko, M.K.; Kato, Y. A Cancer-Specific Anti-Podocalyxin Monoclonal Antibody (humPcMab-60) Demonstrated Antitumor Efficacy in Pancreatic and Colorectal Cancer Xenograft Models. Antibodies 2025, 14, 67. https://doi.org/10.3390/antib14030067
Suzuki H, Ohishi T, Nakamura T, Yanaka M, Handa S, Tanaka T, Kaneko MK, Kato Y. A Cancer-Specific Anti-Podocalyxin Monoclonal Antibody (humPcMab-60) Demonstrated Antitumor Efficacy in Pancreatic and Colorectal Cancer Xenograft Models. Antibodies. 2025; 14(3):67. https://doi.org/10.3390/antib14030067
Chicago/Turabian StyleSuzuki, Hiroyuki, Tomokazu Ohishi, Takuro Nakamura, Miyuki Yanaka, Saori Handa, Tomohiro Tanaka, Mika K. Kaneko, and Yukinari Kato. 2025. "A Cancer-Specific Anti-Podocalyxin Monoclonal Antibody (humPcMab-60) Demonstrated Antitumor Efficacy in Pancreatic and Colorectal Cancer Xenograft Models" Antibodies 14, no. 3: 67. https://doi.org/10.3390/antib14030067
APA StyleSuzuki, H., Ohishi, T., Nakamura, T., Yanaka, M., Handa, S., Tanaka, T., Kaneko, M. K., & Kato, Y. (2025). A Cancer-Specific Anti-Podocalyxin Monoclonal Antibody (humPcMab-60) Demonstrated Antitumor Efficacy in Pancreatic and Colorectal Cancer Xenograft Models. Antibodies, 14(3), 67. https://doi.org/10.3390/antib14030067







