Survival Outcomes and Prognostic Factors in Rheumatoid Arthritis Patients Receiving Biologic or Targeted Synthetic Therapy: Real-World Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.2.1. Sample Selection
2.2.2. Inclusion and Exclusion Criteria
- Demographic data (sex, age, BMI).
- Disease duration and prior therapeutic strategies.
- Radiographic staging of RA, assessed via conventional imaging, based on ACR classification, and extracted from medical records [24].
- Functional class, which describes the degree of functional limitation experienced by the patient, reflecting the overall impact of the disease on the individual’s ability to perform daily activities (I–III) [25].
- Compliance with National Health Insurance Fund (NHIF) criteria for b/tsDMARD Seropositive RA includes radiographic stage II or higher, an inadequate response to at least two conventional DMARDs (one being methotrexate at 20 mg/week), no malignancies, no severe hepatic or renal diseases, heart failure classification of NYHA class III or higher, and exclusion of patients in class IV [26].
2.2.3. Patient Follow-Up
2.3. Assessment of Clinical Parameters
2.4. Mortality Follow-Up
2.5. Definition of Variables
2.6. Data Sources and Measurement Methods
2.7. Selection Bias Evaluation
2.8. Statistical Methods
Survival Analysis (Kaplan-Meier)
3. Results
3.1. Baseline Clinical and Demographic Characteristics
3.2. Patient Characteristics and Survival Outcomes
3.3. Baseline Patient Characteristics and Inter-Group Comparisons
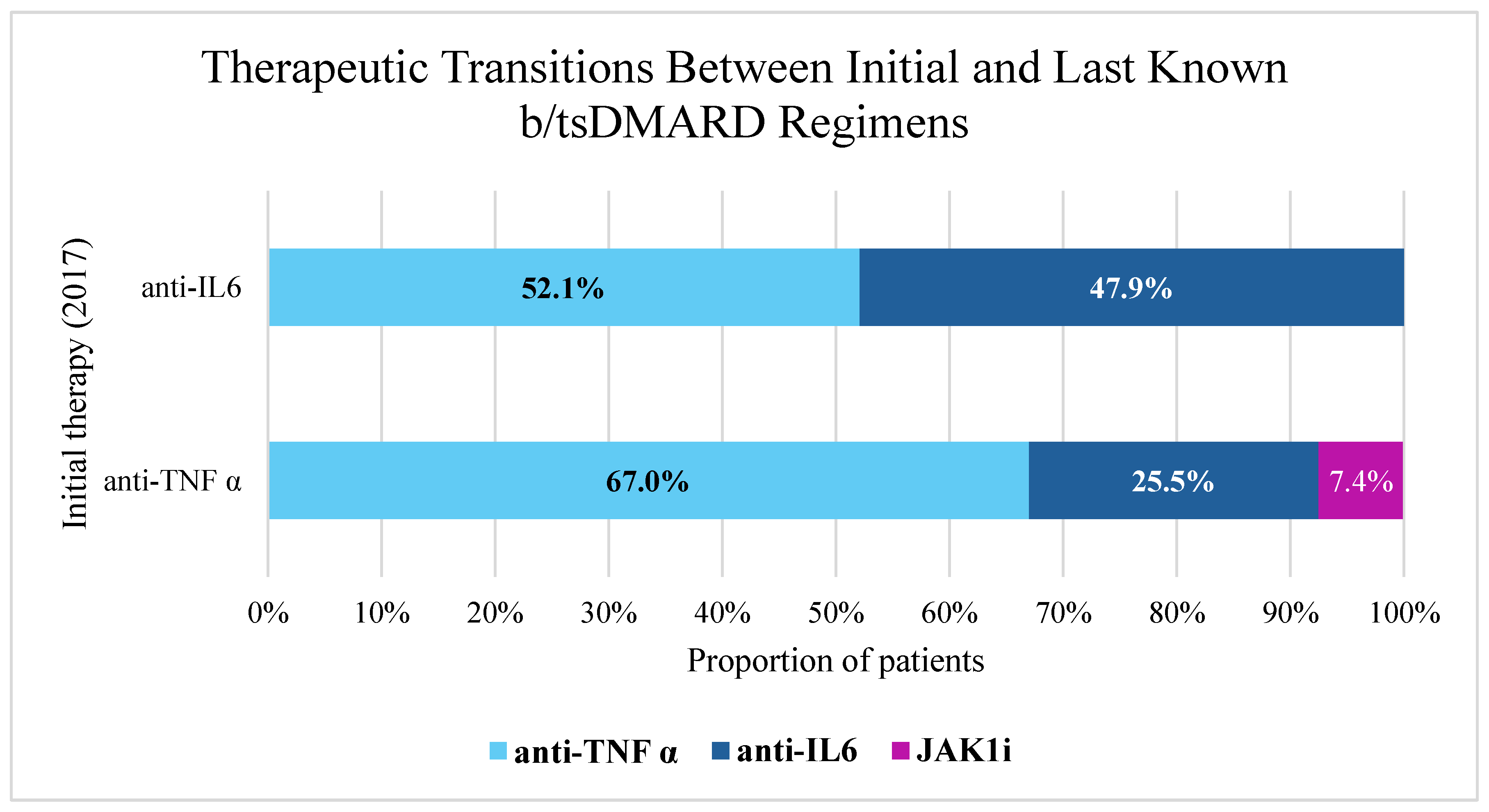
3.4. Therapeutic Transitions Between Initial and Last Known b/tsDMARD Regimens
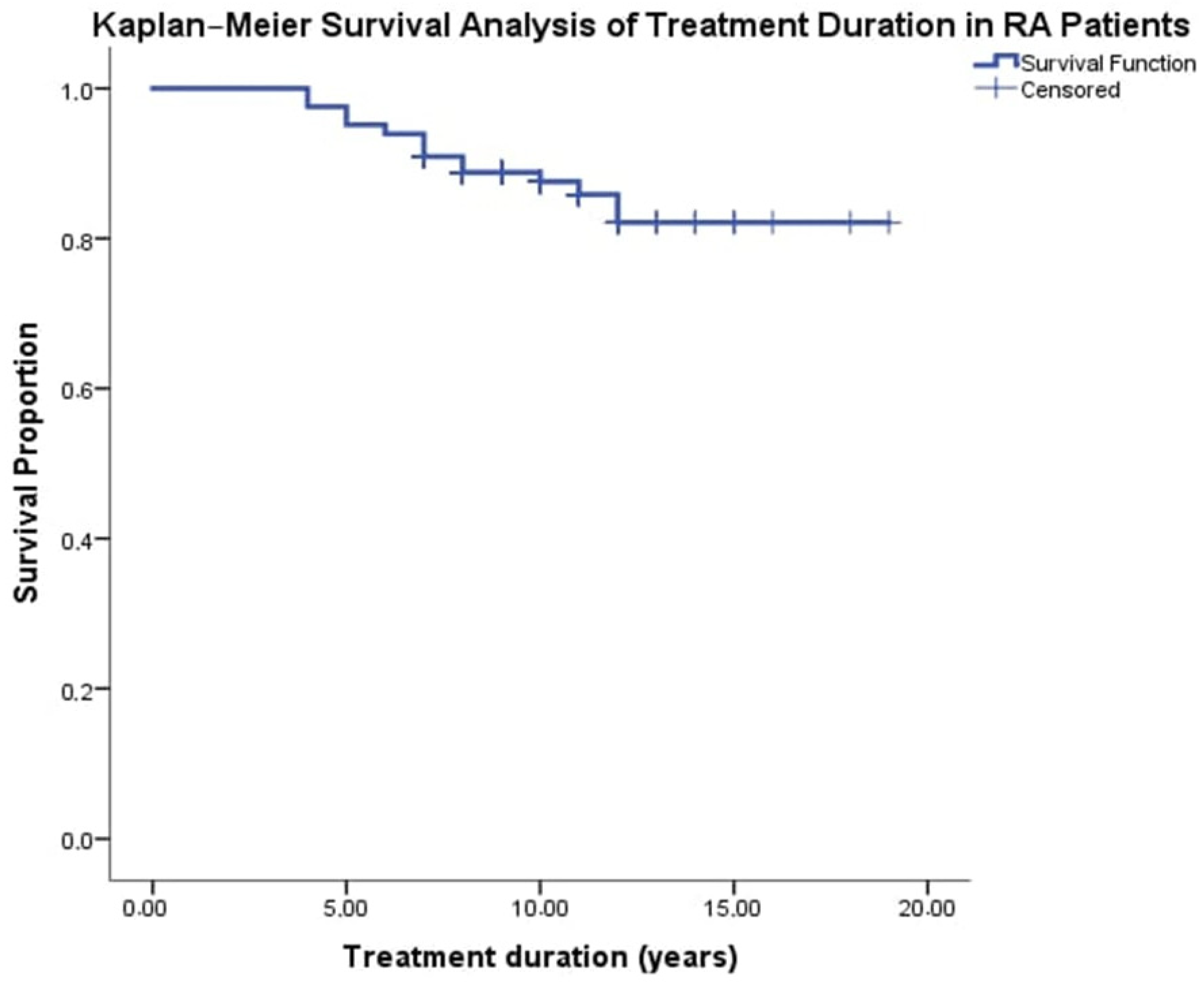
3.5. Kaplan–Meier Analysis of Treatment Duration and Survival Rate in RA Patients
3.6. Multivariate Survival Analysis: Cox Proportional Hazards Model
3.7. Subgroup Analysis of Prognostic Factors by Initial Biologic/tsDMARD Therapy
4. Discussion
4.1. Comparison of Survival Outcomes with Published Literature
4.2. Implications of Baseline Treatment Group Differences
4.3. Prognostic Factors in Specific Treatment Subgroups
4.4. General Risk Factors and the Impact of Long-Term Targeted Therapy
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Heckert, S.L.; Maassen, J.M.; Nevins, I.; Baudoin, P.; Steup-Beekman, G.M.; Huizinga, T.W.J.; Bergstra, S.A.; Allaart, C.F. Long-Term Clinical Outcomes in Early Rheumatoid Arthritis That Was Treated-to-Target in the BeSt and IMPROVED Studies. Rheumatology 2025, 64, 1052–1059. [Google Scholar] [CrossRef]
- Listing, J.; Kekow, J.; Manger, B.; Burmester, G.-R.; Pattloch, D.; Zink, A.; Strangfeld, A. Mortality in Rheumatoid Arthritis: The Impact of Disease Activity, Treatment with Glucocorticoids, TNFα Inhibitors and Rituximab. Ann. Rheum. Dis. 2015, 74, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Løppenthin, K.; Esbensen, B.A.; Østergaard, M.; Ibsen, R.; Kjellberg, J.; Jennum, P. Morbidity and Mortality in Patients with Rheumatoid Arthritis Compared with an Age- and Sex-Matched Control Population: A Nationwide Register Study. J. Comorb. 2019, 9, 2235042X19853484. [Google Scholar] [CrossRef] [PubMed]
- Einarsdottir, M.J.; Ekman, P.; Molin, M.; Trimpou, P.; Olsson, D.S.; Johannsson, G.; Ragnarsson, O. High Mortality Rate in Oral Glucocorticoid Users: A Population-Based Matched Cohort Study. Front. Endocrinol. 2022, 13, 918356. [Google Scholar] [CrossRef]
- Jain, K.; Laila, D.; Nandagudi, A.; Bharadwaj, A. Long-Term Outcomes in Rheumatoid Arthritis: Review of Data from the ‘Basildon Inflammatory Arthritis Cohort’. Rheumatol. Adv. Pract. 2022, 6, rkac075. [Google Scholar] [CrossRef]
- Heckert, S.L.; Maassen, J.M.; le Cessie, S.; Goekoop-Ruiterman, Y.P.M.; Güler-Yüksel, M.; Lems, W.; Huizinga, T.W.; Bergstra, S.A.; Allaart, C.F. Long-Term Mortality in Treated-to-Target RA and UA: Results of the BeSt and IMPROVED Cohort. Ann. Rheum. Dis. 2024, 83, 161–168. [Google Scholar] [CrossRef]
- Frisell, T.; Bower, H.; Morin, M.; Baecklund, E.; Di Giuseppe, D.; Delcoigne, B.; Feltelius, N.; Forsblad-d’Elia, H.; Lindqvist, E.; Lindström, U.; et al. Safety of Biological and Targeted Synthetic Disease-Modifying Antirheumatic Drugs for Rheumatoid Arthritis as Used in Clinical Practice: Results from the ARTIS Programme. Ann. Rheum. Dis. 2023, 82, 601–610. [Google Scholar] [CrossRef]
- Molina-Collada, J.; Alonso, F.; Otero, L.; Bohórquez, C.; Díaz Torné, C.; Pérez García, C.; Blanco Madrigal, J.M.; Vela, P.; Álvaro-Gracia, J.M.; Castrejón, I.; et al. Cancer Risk with Biologic and Targeted Synthetic DMARDs in Patients with Rheumatic Diseases and Previous Malignancies: Results from the BIOBADASER Register. Semin. Arthritis Rheum. 2024, 64, 152341. [Google Scholar] [CrossRef]
- Abhishek, A.; Nakafero, G.; Kuo, C.-F.; Mallen, C.; Zhang, W.; Grainge, M.J.; Doherty, M. Rheumatoid Arthritis and Excess Mortality: Down but Not out. A Primary Care Cohort Study Using Data from Clinical Practice Research Datalink. Rheumatology 2018, 57, 977–981. [Google Scholar] [CrossRef]
- Almutairi, K.B.; Inderjeeth, C.A.; Preen, D.B.; Keen, H.I.; Nossent, J.C. Mortality trends among patients with rheumatoid arthritis in Western Australia. Rheumatol. Ther. 2023, 10, 1021–1037. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, L.; Leon, L.; Ivorra-Cortes, J.; Gómez, A.; Lamas, J.R.; Pato, E.; Jover, J.Á.; Abásolo, L. Treatment in Rheumatoid Arthritis and Mortality Risk in Clinical Practice: The Role of Biologic Agents. Clin. Exp. Rheumatol. 2016, 34, 1026–1032. [Google Scholar] [PubMed]
- Chester Wasko, M.; Dasgupta, A.; Ilse Sears, G.; Fries, J.F.; Ward, M.M. Prednisone use and risk of mortality in patients with rheumatoid arthritis: Moderation by use of disease-modifying antirheumatic drugs. Arthritis Care Res. 2016, 68, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Ocon, A.J.; Reed, G.; Pappas, D.A.; Curtis, J.R.; Kremer, J.M. Short-Term Dose and Duration-Dependent Glucocorticoid Risk for Cardiovascular Events in Glucocorticoid-Naive Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2021, 80, 1522–1529. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, L.; Zhu, J.; Qin, Q.; Fang, Y.; Zhang, J.-A. Methotrexate Use Reduces Mortality Risk in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis of Cohort Studies. Semin. Arthritis Rheum. 2022, 55, 152031. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Symmons, D.P.M.; MacGregor, A.J.; Chipping, J.R.; Marshall, T.; Lunt, M.; Verstappen, S.M.M. Twenty-Year Outcome and Association between Early Treatment and Mortality and Disability in an Inception Cohort of Patients with Rheumatoid Arthritis: Results from the Norfolk Arthritis Register. Arthritis Rheumatol. 2017, 69, 1566–1575. [Google Scholar] [CrossRef]
- Kerola, A.M.; Rollefstad, S.; Semb, A.G. Atherosclerotic Cardiovascular Disease in Rheumatoid Arthritis: Impact of Inflammation and Antirheumatic Treatment. Eur. Cardiol. 2021, 16, e18. [Google Scholar] [CrossRef]
- Burmester, G.R.; Gordon, K.B.; Rosenbaum, J.T.; Arikan, D.; Lau, W.L.; Li, P.; Faccin, F.; Panaccione, R. Long-Term Safety of Adalimumab in 29,967 Adult Patients from Global Clinical Trials across Multiple Indications: An Updated Analysis. Adv. Ther. 2020, 37, 364–380. [Google Scholar] [CrossRef]
- Jarlborg, M.; Gabay, C. Systemic Effects of IL-6 Blockade in Rheumatoid Arthritis beyond the Joints. Cytokine 2022, 149, 155742. [Google Scholar] [CrossRef]
- Matsumori, A. Targeting Inflammation in the Diagnosis, Management, and Prevention of Cardiovascular Diseases. Glob. Heart 2022, 17, 80. [Google Scholar] [CrossRef]
- Charukevič, G.; Miltinienė, D.; Dadonienė, J. Mortality in patients with rheumatoid arthritis: A retrospective cohort study and systematic review. Med. Sci. Forum 2021, 6, 5. [Google Scholar] [CrossRef]
- Black, R.J.; Lester, S.; Tieu, J.; Sinnathurai, P.; Barrett, C.; Buchbinder, R.; Lassere, M.; March, L.; Proudman, S.M.; Hill, C.L. Mortality Estimates and Excess Mortality in Rheumatoid Arthritis. Rheumatology 2023, 62, 3576–3583. [Google Scholar] [CrossRef] [PubMed]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S. The American Rheumatism Association 1987 Revised Criteria for the Classification of Rheumatoid Arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Chauhan, K.; Jandu, J.S.; Brent, L.H.; Al-Dhahir, M.A. Rheumatoid Arthritis. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK441999/ (accessed on 14 May 2025).
- Hochberg, M.; Chang, R.; Chang, R.; Dwosh, I.; Dwosh, I.; Lindsey, S.; Pincus, T.; Pincus, T.; Wolfe, F.; Wolfe, F. The American College of Rheumatology 1991 Revised Criteria for the Classification of Global Functional Status in Rheumatoid Arthritis: The American College of Rheumatology 1991 Revised Criteria for the Classification of Global Functional Status in Rheumatoid Arthritis. Arthritis Rheum. 1992, 35, 498–502. [Google Scholar] [CrossRef]
- National Health Insurance Fund. Treatment of Seropositive Rheumatoid Arthritis—Guidelines for Biologic Therapy. NHIF Website. Available online: https://www.nhif.bg/bg/medical_requirements/definitions (accessed on 7 May 2025).
- Smolen, J.S.; Breedveld, F.C.; Schiff, M.H.; Kalden, J.R.; Emery, P.; Eberl, G.; van Riel, P.L.; Tugwell, P. A Simplified Disease Activity Index for Rheumatoid Arthritis for Use in Clinical Practice. Rheumatology 2003, 42, 244–257. [Google Scholar] [CrossRef]
- Stucki, G.; Stoll, T.; Brühlmann, P.; Michel, B.A. Construct Validation of the ACR 1991 Revised Criteria for Global Functional Status in Rheumatoid Arthritis. Clin. Exp. Rheumatol. 1995, 13, 349–352. [Google Scholar]
- Costello, R.E.; Marsden, A.; Movahedi, M.; Lunt, M.; Humphreys, J.H.; Emsley, R.; Dixon, W.G. The Effect of Glucocorticoid Therapy on Mortality in Patients with Rheumatoid Arthritis and Concomitant Type II Diabetes: A Retrospective Cohort Study. BMC Rheumatol. 2020, 4, 4. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Verstappen, S.M.; Humphreys, J.H. The Impact of Lifestyle Behaviours, Physical Activity and Smoking on Morbidity and Mortality in Patients with Rheumatoid Arthritis. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101562. [Google Scholar] [CrossRef]
- Movahedi, M.; Cesta, A.; Li, X.; Kuriya, B.; Aydin, S.; Keystone, E.; Pope, J.; Bombardier, C. Pos0614 Associations between Disease Activity, Physical Function and Anti-Rheumatic Medications with All-Cause Mortality in Rheumatoid Arthritis (Ra): Data from a Canadian Ra Registry. In Proceedings of the 84th Scientific Sessions, Orlando, FL, USA, 21 June 2024; BMJ Publishing Group Ltd.: London, UK; European League Against Rheumatism: Zurich, Switzerland, 2024; Volume 83, pp. 756–757. [Google Scholar]
- Pombo-Suarez, M.; Sanchez-Piedra, C.; Garcia-Magallón, B.; Pérez-Gómez, A.; Manrique-Arija, S.; Martín-Doménech, R.; Colazo, M.; Campos, C.; Campos, J.; Del Pino-Montes, J.; et al. Factors Associated with Long-Term Retention of Treatment with Golimumab in Rheumatoid Arthritis, Axial Spondyloarthritis, and Psoriatic Arthritis: An Analysis of the Spanish BIOBADASER Registry. Clin. Rheumatol. 2021, 40, 3979–3988. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.; Bijlsma, J.; Burmester, G.; Chatzidionysiou, K.; Dougados, M.; Nam, J.; Ramiro, S.; Voshaar, M.; van Vollenhoven, R.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2016 Update. Ann. Rheum. Dis. 2017, 76, 960–977. [Google Scholar] [CrossRef]
- Ezeanuna, M.N.; Prince, D.K.; Alexander, S.A.; Richards, J.S.; Kerr, G.S.; Jalal, D.; Bansal, N.; Liew, J.W.; Singh, N. Association of Rheumatoid Arthritis with Mortality in Chronic Kidney Disease: A Cohort Study. Clin. Rheumatol. 2022, 41, 2669–2676. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.F.; England, B.R.; George, M.; Cannon, G.; Sauer, B.; Ogdie, A.; Hamilton, B.C.; Hunter, C.; Duryee, M.J.; Thiele, G.; et al. Disease Activity, Cytokines, Chemokines and the Risk of Incident Diabetes in Rheumatoid Arthritis. Ann. Rheum. Dis. 2021, 80, 566–572. [Google Scholar] [CrossRef]
- Yamamoto, K.; Goto, H.; Hirao, K.; Nakajima, A.; Origasa, H.; Tanaka, K.; Tomobe, M.; Totsuka, K. Longterm Safety of Tocilizumab: Results from 3 Years of Followup Postmarketing Surveillance of 5573 Patients with Rheumatoid Arthritis in Japan. J. Rheumatol. 2015, 42, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.-J.; Lee, C.-H.; Tsai, M.-L.; Kao, C.-F.; Lan, W.-C.; Huang, Y.-T.; Tseng, W.-Y.; Wen, M.-S.; Chang, S.-H. Biologic Agents Reduce Cardiovascular Events in Rheumatoid Arthritis Not Responsive to Tumour Necrosis Factor Inhibitors: A National Cohort Study. Can. J. Cardiol. 2020, 36, 1739–1746. [Google Scholar] [CrossRef]
- Curtis, J.R.; Xie, F.; Crowson, C.S.; Sasso, E.H.; Hitraya, E.; Chin, C.L.; Bamford, R.D.; Ben-Shachar, R.; Gutin, A.; Flake, D.D., 2nd; et al. Derivation and Internal Validation of a Multi-Biomarker-Based Cardiovascular Disease Risk Prediction Score for Rheumatoid Arthritis Patients. Arthritis Res. Ther. 2020, 22, 282. [Google Scholar] [CrossRef]
- Jagpal, A.; Navarro-Millán, I. Cardiovascular Co-Morbidity in Patients with Rheumatoid Arthritis: A Narrative Review of Risk Factors, Cardiovascular Risk Assessment and Treatment. BMC Rheumatol. 2018, 2, 10. [Google Scholar] [CrossRef]
- Choy, E.; Ganeshalingam, K.; Semb, A.G.; Szekanecz, Z.; Nurmohamed, M. Cardiovascular Risk in Rheumatoid Arthritis: Recent Advances in the Understanding of the Pivotal Role of Inflammation, Risk Predictors and the Impact of Treatment. Rheumatology 2014, 53, 2143–2154. [Google Scholar] [CrossRef]
- Michaud, K.; Vera-Llonch, M.; Oster, G. Mortality Risk by Functional Status and Health-Related Quality of Life in Patients with Rheumatoid Arthritis. J. Rheumatol. 2012, 39, 54–59. [Google Scholar] [CrossRef]
- Rosa-Gonçalves, D.; Bernardes, M.; Costa, L. Quality of Life and Functional Capacity in Patients with Rheumatoid Arthritis—Cross-Sectional Study. Reumatol. Clín. 2018, 14, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Buttgereit, F.; Combe, B. Glucocorticoids in Rheumatoid Arthritis: Current Status and Future Studies. RMD Open 2020, 6, e000536. [Google Scholar] [CrossRef]
- Nakashima, Y.; Kondo, M.; Fukuda, T.; Harada, H.; Horiuchi, T.; Ishinishi, T.; Jojima, H.; Kuroda, K.; Miyahara, H.; Maekawa, M.; et al. Remission in Patients with Active Rheumatoid Arthritis by Tocilizumab Treatment in Routine Clinical Practice: Results from 3 Years of Prospectively Registered Data. Mod. Rheumatol. 2014, 24, 258–264. [Google Scholar] [CrossRef]

| Parameter | All Included Patients (N = 190) | Analyzed Patients (N = 165) | Patients with Missing Data (N = 25) | p-Value * |
|---|---|---|---|---|
| Demographic Characteristics | ||||
| Sex, n (%) | 0.213 † | |||
| Male | 27 (14.2%) | 26 (15.8%) | 1 (4.0%) | |
| Female | 163 (85.8%) | 139 (84.2%) | 24 (96.0%) | |
| BMI, n (%) | 0.688 † | |||
| <30 kg/m2 | 146 (76.8%) | 126 (76.4%) | 20 (80.0%) | |
| ≥30 kg/m2 | 44 (23.2%) | 39 (23.6%) | 5 (20.0%) | |
| Smoking Status, n (%) | 0.447 † | |||
| No | 132 (69.5%) | 113 (68.5%) | 19 (76.0%) | |
| Yes | 58 (30.5%) | 52 (31.5%) | 6 (24.0%) | |
| RA Subgroups, n (%) | 0.114 † | |||
| <2000 years | 40 (21.1%) | 38 (23.0%) | 2 (8.0%) | |
| >2000 years | 150 (78.9%) | 127 (77.0%) | 23 (92.0%) | |
| Age, years (mean ± SD) | 64.38 ± 11.10 | 64.12 ± 11.40 | 66.12 ± 8.90 | 0.403 |
| Clinical Characteristics at Baseline | ||||
| Duration of Treatment, years (mean ± SD) | N/A | 9.36 ± 2.47 | N/A | N/A |
| Duration of RA, years (mean ± SD) | N/A | 18.07 ± 9.55 | N/A | N/A |
| Type of Biologic Therapy, n (%) | 0.282 | |||
| anti-IL6 | 85 (44.7%) | 71 (43.0%) | 14 (56.0%) | |
| anti-TNF | 105 (55.3%) | 94 (57.0%) | 11 (44.0%) | |
| Methotrexate Use, n (%) | 1.000 | |||
| No | 73 (38.4%) | 63 (38.2%) | 10 (40.0%) | |
| Yes | 117 (61.6%) | 102 (61.8%) | 15 (60.0%) | |
| Methylprednisolone Use, n (%) | 0.132 † | |||
| No | 81 (42.6%) | 74 (44.8%) | 7 (28.0%) | |
| Yes | 109 (57.4%) | 91 (55.2%) | 18 (72.0%) | |
| Radiographic Stage, n (%) | 0.507 | |||
| II | 65 (34.2%) | 55 (33.3%) | 10 (40.0%) | |
| III + IV | 125 (65.8%) | 110 (66.7%) | 15 (60.0%) | |
| Functional Class, n (%) | 0.668 | |||
| II | 103 (54.2%) | 88 (53.3%) | 15 (60.0%) | |
| III | 87 (45.8%) | 77 (46.7%) | 10 (40.0%) | |
| Presence of Comorbidities, n (%) | ||||
| Arterial Hypertension, n (%) | 0.387 † | |||
| No | 78 (41.1%) | 70 (42.4%) | 8 (32.0%) | |
| Yes | 112 (58.9%) | 95 (57.6%) | 17 (68.0%) | |
| Pulmonary Disease, n (%) | 0.387 † | |||
| No | 78 (41.1%) | 70 (42.4%) | 8 (32.0%) | |
| Yes | 112 (58.9%) | 95 (57.6%) | 17 (68.0%) | |
| Ischemic Heart Disease, n (%) | 0.321 † | |||
| No | 167 (87.9%) | 143 (86.7%) | 24 (96.0%) | |
| Yes | 23 (12.1%) | 22 (13.3%) | 1 (4.0%) | |
| Chronic Kidney Disease, n (%) | 1.000 † | |||
| No | 174 (91.6%) | 151 (91.5%) | 23 (92.0%) | |
| Yes | 16 (8.4%) | 14 (8.5%) | 2 (8.0%) | |
| Diabetes Mellitus, n (%) | 0.547 † | |||
| No | 161 (84.7%) | 141 (85.5%) | 20 (80.0%) | |
| Yes | 29 (15.3%) | 24 (14.5%) | 5 (20.0%) | |
| SDAI Low Disease Activity (2017–2018) | ||||
| Six-month LDA, n (%) | 0.659 † | |||
| No | 122 (64.2%) | 107 (64.8%) | 15 (60.0%) | |
| Yes | 68 (35.8%) | 58 (35.2%) | 10 (40.0%) | |
| Twelve-month LDA, n (%) | 0.493 † | |||
| No | 128 (67.4%) | 113 (68.5%) | 15 (60.0%) | |
| Yes | 62 (32.6%) | 52 (31.5%) | (40.0%) | |
| Demographic and Baseline Characteristics | Survivors (N = 144) | Non-Survivors (N = 21) | p-Value |
|---|---|---|---|
| Age at the survival (years, mean ± SD) | 63.8 ± 11.2 | 65.9 ± 12.6 | NS |
| Female sex, n (%) | 122 (87.8) | 17 (81.0) | NS |
| Smokers | 43 (29.9) | 9 (42.9) | NS |
| BMI, mean ± SD | 27.09 ± 4.91 | 27.42 ± 7.06 | NS |
| BMI > 30, n (%) | 33 (22.9) | 6 (28.6) | NS |
| RA Diagnosis Year, n (%) | |||
| Before 2000 | 31 (21.5) | 7 (33.3) | NS |
| 2000 or later | 113 (78.5) | 14 (66.7) | |
| RA Duration (years, mean ± SD) | 18.03 ± 9.23 | 18.11 ± 9.77 | NS |
| Radiographic stage III–IV, n (%) | 93 (64.6) | 17 (81.0) | NS |
| Functional class III, n (%) | 62 (43.1) | 15 (71.4) | 0.015 |
| Parameter | Survivors (N = 144) | Non-Survivors (N = 21) | p-Value |
|---|---|---|---|
| Methotrexate Therapy, n (%) | 91 (63.2) | 11 (52.4) | NS |
| Methylprednisolone Use, n (%) | 73 (50.7) | 18 (85.7) | 0.003 |
| Mean Biologic Therapy Duration (years, mean ± SD) | 9.77 ± 2.21 | 6.66 ± 2.28 | <0.001 |
| b/tsDMARDs at the beginning of the study | |||
| Anti TNFi, n (%) | 87 (60.4) | 7 (33.3) | =0.019 |
| Anti IL6i, n (%) | 57 (39.6) | 14 (66.7) | =0.019 |
| b/tsDMARDs at Mortality Follow-Up | |||
| Anti-TNFi, n (%) | 84 (58.3) | 16 (76.2) | NS |
| Anti-IL6i, n (%) | 53 (36.8) | 5 (23.8) | |
| JAK1i, n (%) | 7 (4.9) | NA | |
| Level of Sustained SDAI LDA (assessed between 2017–2018) | |||
| Twelve-month LDA, n (%) | 49 (34.0) | 3 (14.3) | NS |
| Six-month LDA, n (%) | 26(18.1) | 5 (23.8) | NS |
| One-moment LDA, n (%) | 23 (16.0) | 4 (19.0) | NS |
| No LDA, n (%) | 46 (31.9) | 9 (42.9) | NS |
| Comorbidities at Baseline | |||
| Arterial Hypertension, n (%) | 80 (55.6) | 15 (71.4) | NS |
| Diabetes Mellitus, n (%) | 21 (14.3) | 3 (14.3) | NS |
| Ischemic Heart Disease, n (%) | 18 (12.5) | 4 (19.0) | NS |
| Chronic Kidney Disease, n (%) | 10 (6.9) | 4 (19.0) | NS |
| Pulmonary Disease, n (%) | 10 (6.9) | NA | NA |
| Characteristic | Anti-IL6 (N = 71) Mean ± SD or n (%) | Anti-TNF (N = 94) Mean ± SD or n (%) | p-Value |
|---|---|---|---|
| Age (years) (mean ± SD) | 65.99 ± 10.09 | 62.71 ± 12.15 | 0.061 |
| Age > 55 years, n (%) | 53 (74.6%) | 55 (58.5%) | 0.031 |
| Years from diagnosis to Biologic Treatment (mean ± SD) | 9.45 ± 9.77 | 8.12 ± 8.57 | 0.353 |
| Duration of RA (years) (mean ± SD) | 18.41 ± 10.15 | 17.81 ± 9.12 | 0.691 |
| BMI kg/m2 (mean ± SD) | 26.97 ± 4.87 | 26.79 ± 5.67 | 0.825 |
| BMI > 30, n (%) | 14 (19.7%) | 25 (26.6%) | 0.303 |
| Smoking (yes), n (%) | 18 (25.4%) | 34 (36.2%) | 0.139 |
| X-ray stage III/IV vs II n (%) | 58 (81.7%) | 52 (55.3%) | <0.001 |
| Functional class III vs II, n (%) | 40 (56.3%) | 37 (39.4%) | <0.001 |
| Methylprednisolone Use (yes), n (%) | 47 (66.2%) | 44 (46.8%) | 0.013 |
| Arterial hypertension (yes), n (%) | 46 (64.8%) | 49 (52.1%) | 0.103 |
| Ischemic Heart Disease (yes), n (%) | 12 (16.9%) | 10 (10.6%) | 0.241 |
| Pulmonary diseases (yes), n (%) | 5 (7.0%) | 5 (5.3%) | 0.646 |
| Chronic kidney diseases (yes), n (%) | 7 (9.9%) | 7 (7.4%) | 0.582 |
| Diabetes Mellitus (yes), n (%) | 12 (16.9%) | 12 (12.8%) | 0.582 |
| Sustained 6-month LDA (yes), n (%) | 36 (50.7%) | 47 (50.0%) | 0.929 |
| Sustained 12-month LDA (yes), n (%) | 22 (31.0%) | 35 (37.2%) | 0.403 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apostolova, Z.; Shivacheva, T.; Georgiev, T. Survival Outcomes and Prognostic Factors in Rheumatoid Arthritis Patients Receiving Biologic or Targeted Synthetic Therapy: Real-World Data. Antibodies 2025, 14, 54. https://doi.org/10.3390/antib14030054
Apostolova Z, Shivacheva T, Georgiev T. Survival Outcomes and Prognostic Factors in Rheumatoid Arthritis Patients Receiving Biologic or Targeted Synthetic Therapy: Real-World Data. Antibodies. 2025; 14(3):54. https://doi.org/10.3390/antib14030054
Chicago/Turabian StyleApostolova, Zhaklin, Tanya Shivacheva, and Tsvetoslav Georgiev. 2025. "Survival Outcomes and Prognostic Factors in Rheumatoid Arthritis Patients Receiving Biologic or Targeted Synthetic Therapy: Real-World Data" Antibodies 14, no. 3: 54. https://doi.org/10.3390/antib14030054
APA StyleApostolova, Z., Shivacheva, T., & Georgiev, T. (2025). Survival Outcomes and Prognostic Factors in Rheumatoid Arthritis Patients Receiving Biologic or Targeted Synthetic Therapy: Real-World Data. Antibodies, 14(3), 54. https://doi.org/10.3390/antib14030054





