Regulatory T Cell in Kidney Transplant: The Future of Cell Therapy?
Abstract
1. Introduction
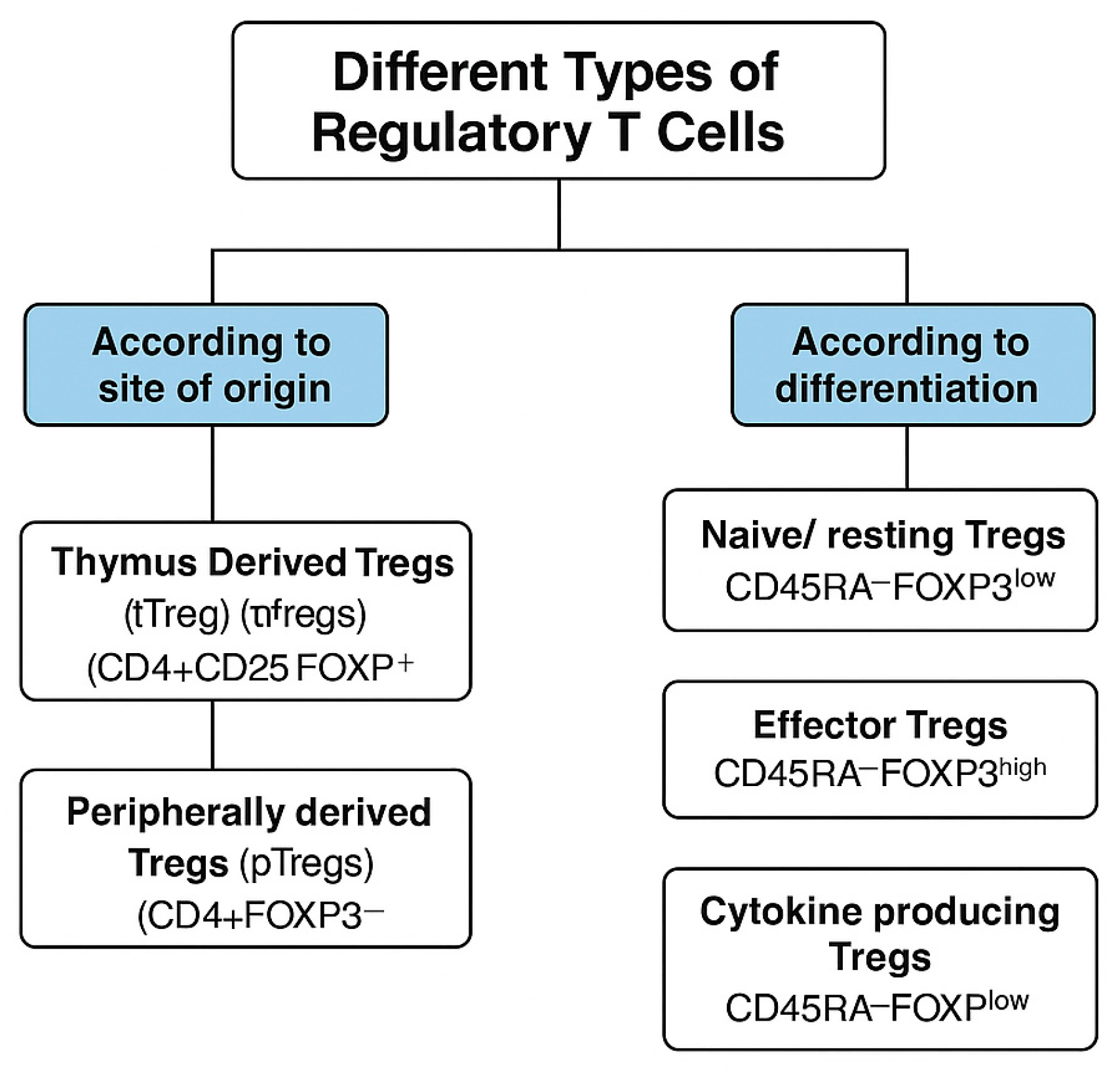
2. Types of Tregs
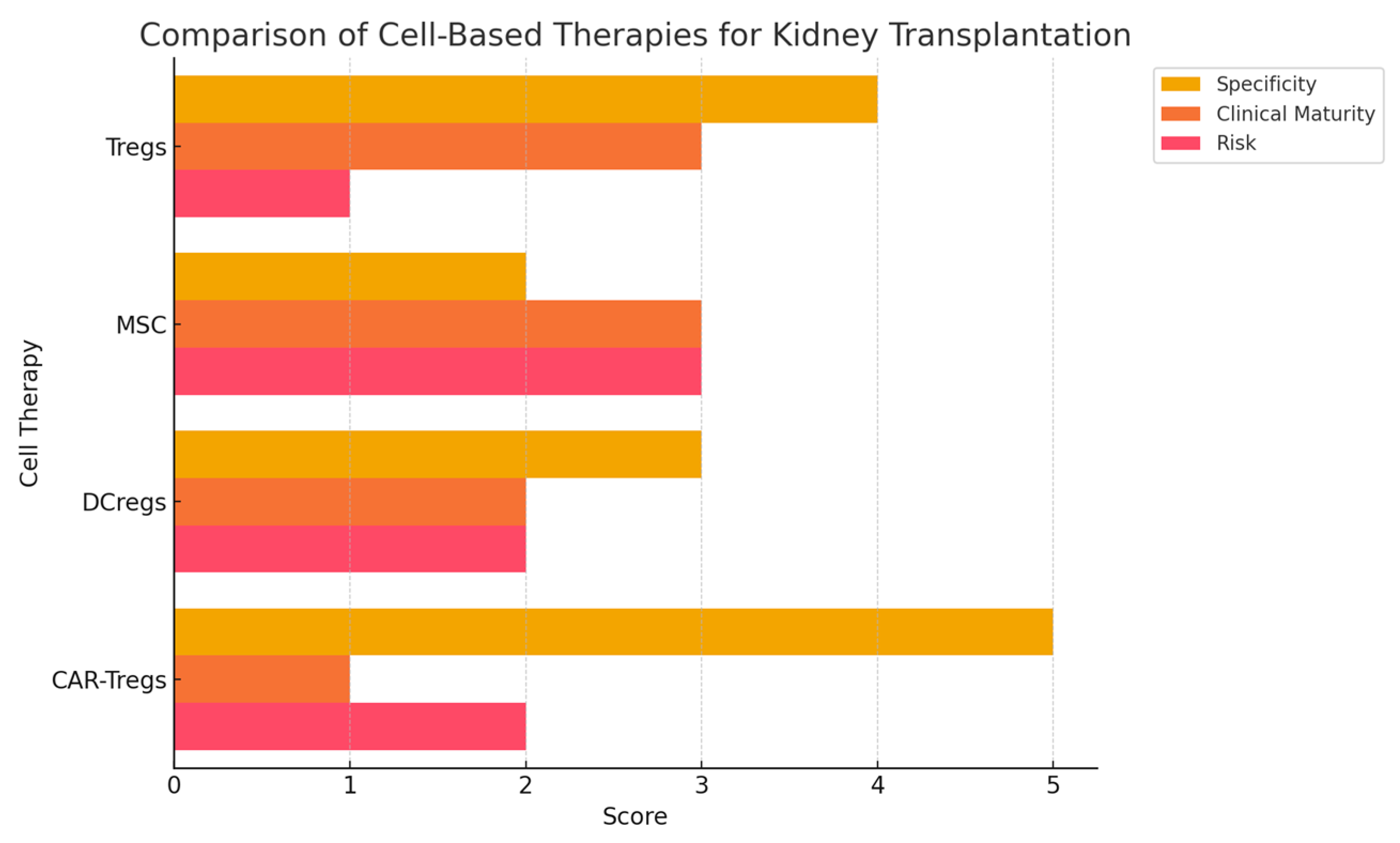
3. Comparison with Other Cell-Based Therapies
- Regulatory Dendritic Cells (DCregs)—These cells induce antigen-specific tolerance by modulating T cell responses. Unlike Tregs, their immunosuppressive effects are indirect and may be less durable.
- Mesenchymal Stromal Cells (MSCs)—MSCs exhibit anti-inflammatory properties and have been tested in renal transplantation, but concerns exist regarding their potential to differentiate into fibrogenic cells.
- Transitional B Cells—These cells suppress immune responses via IL-10 production but are less studied compared to Tregs.
4. Role of Tregs
5. Tregs Immunosuppressive Mechanisms
6. Current Studies
6.1. Early Clinical Trials: Safety and Feasibility of Treg Therapy
6.2. Phase I/II Trials: Immunomodulation and Reduction in Immunosuppressive Burden
6.3. Recent Advances: Antigen-Specific and Genetically Engineered Tregs
6.4. Meta-Analysis and Systematic Reviews: Treg Therapy in Transplantation
6.5. Future Challenges and Research Directions
6.6. Antigen-Specific and Engineered Tregs: The Next Frontier
6.7. Economic and Logistical Considerations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- United States Renal Data System. 2023 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2023.
- Hansen, C.M.; Bachmann, S.; Su, M.; Budde, K.; Choi, M. Calcineurin Inhibitor Associated Nephrotoxicity in Kidney Transplantation—A Transplant Nephrologist’s Perspective. Acta Physiol. 2025, 241, e70047. [Google Scholar] [CrossRef]
- Puri, P.; Bansal, N. Renal Dysfunction After Liver Transplant: Is CNI Nephrotoxicity Overrated. J. Clin. Exp. Hepatol. 2023, 13, 556–558. [Google Scholar] [CrossRef]
- Rubinstein, J.; Toner, K.; Gross, T.; Wistinghausen, B. Diagnosis and management of post-transplant lymphoproliferative disease following solid organ transplantation in children, adolescents, and young adults. Best Pract. Res. Clin. Haematol. 2023, 36, 101446. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, C.L.; Kant, S. Pathophysiology of rejection in kidney transplantation. J. Clin. Med. 2023, 12, 4130. [Google Scholar] [CrossRef] [PubMed]
- Cron, D.C.; Husain, S.A.; King, K.L.; Mohan, S.; Adler, J.T. Increased volume of organ offers and decreased efficiency of kidney placement under circle-based kidney allocation. Am. J. Transplant. 2023, 23, 1209–1220. [Google Scholar] [CrossRef]
- Wang, J.H.; Hart, A. Global perspective on kidney transplantation: United States. Kidney360 2021, 2, 1836–1839. [Google Scholar] [CrossRef]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- UCaldwell, J.S.; Cheng, X.S.; Chertow, G.M.; Goldhaber-Fiebert, J.D. Kidney transplant wait times under waiting list expansion scenarios. JAMA Netw. Open 2025, 8, e251665. [Google Scholar] [CrossRef]
- Cheung, J.; Zahorowska, B.; Suranyi, M.; Wong, J.K.; Diep, J.; Spicer, S.T.; Verma, N.D.; Hodgkinson, S.J.; Hall, B.M. CD4+ CD25+ T regulatory cells in renal transplantation. Front. Immunol. 2022, 13, 1017683. [Google Scholar] [CrossRef]
- Vignali, D.A.; Collison, L.W.; Workman, C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, A.S.; Kedzierska, A.E.; Obojski, A. Identification and classification of distinct surface markers of T regulatory cells. Front. Immunol. 2023, 13, 1055805. [Google Scholar] [CrossRef] [PubMed]
- Arjomandnejad, M.; Kopec, A.L.; Keeler, A.M. CAR-T regulatory (CAR-Treg) cells: Engineering and applications. Biomedicines 2022, 10, 287. [Google Scholar] [CrossRef]
- Santamaria, J.C.; Borelli, A.; Irla, M. Regulatory T cell heterogeneity in the thymus: Impact on their functional activities. Front. Immunol. 2021, 12, 643153. [Google Scholar] [CrossRef] [PubMed]
- Itahashi, K.; Irie, T.; Nishikawa, H. Regulatory T-cell development in the tumor microenvironment. Eur. J. Immunol. 2022, 52, 1216–1227. [Google Scholar] [CrossRef]
- Chen, B.J.; Zhao, J.W.; Zhang, D.H.; Zheng, A.H.; Wu, G.Q. Immunotherapy of cancer by targeting regulatory T cells. Int. Immunopharmacol. 2022, 104, 108469. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, S.; Miyara, M.; Costantino, C.M.; Hafler, D.A. FOXP3+ regulatory T cells in the human immune system. Nat. Rev. Immunol. 2010, 10, 490–500. [Google Scholar] [CrossRef]
- Floess, S.; Freyer, J.; Siewert, C.; Baron, U.; Olek, S.; Polansky, J.; Schlawe, K.; Chang, H.D.; Bopp, T.; Schmitt, E.; et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007, 5, e38. [Google Scholar] [CrossRef]
- McGovern, J.; Holler, A.; Thomas, S.; Stauss, H.J. Forced Fox-P3 expression can improve the safety and antigen-specific function of engineered regulatory T cells. J. Autoimmun. 2022, 132, 102888. [Google Scholar] [CrossRef]
- Bashor, C.J.; Hilton, I.B.; Bandukwala, H.; Smith, D.M.; Veiseh, O. Engineering the next generation of cell-based therapeutics. Nat. Rev. Drug Discov. 2022, 21, 655–675. [Google Scholar] [CrossRef]
- Jiang, Q.; Yang, G.; Liu, Q.; Wang, S.; Cui, D. Function and role of regulatory T cells in rheumatoid arthritis. Front. Immunol. 2021, 12, 626193. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zha, J.; Tang, R.; Chen, G. T-cell immunoglobulin and mucin-domain containing-3 (TIM-3): Solving a key puzzle in autoimmune diseases. Int. Immunopharmacol. 2023, 121, 110418. [Google Scholar] [CrossRef]
- Grover, P.; Goel, P.N.; Greene, M.I. Regulatory T cells: Regulation of identity and function. Front. Immunol. 2021, 12, 750542. [Google Scholar] [CrossRef]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Wilson, M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014, 41, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J. Integrating T cell activation signals to regulate gene expression through cyclosporin-sensitive NFAT. J. Immunol. 2023, 211, 323–324. [Google Scholar] [CrossRef]
- Brunstein, C.G.; Miller, J.S.; Cao, Q.; McKenna, D.H.; Hippen, K.L.; Curtsinger, J.; Defor, T.; Levine, B.L.; June, C.H.; Rubinstein, P.; et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood 2011, 117, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- West, P.K.; McCorkindale, A.N.; Guennewig, B.; Ashhurst, T.M.; Viengkhou, B.; Hayashida, E.; Jung, S.R.; Butovsky, O.; Campbell, I.L.; Hofer, M.J. The cytokines interleukin-6 and interferon-α induce distinct microglia phenotypes. J. Neuroinflamm. 2022, 19, 96. [Google Scholar] [CrossRef]
- Sánchez-Fueyo, A.; Whitehouse, G.; Grageda, N.; Cramp, M.E.; Lim, T.Y.; Romano, M.; Thirkell, S.; Lowe, K.; Fry, L.; Heward, J.; et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am. J. Transplant. 2020, 20, 1125–1136. [Google Scholar] [CrossRef]
- Oberholtzer, N.; Atkinson, C.; Nadig, S.N. Adoptive transfer of regulatory immune cells in organ transplantation. Front. Immunol. 2021, 12, 631365. [Google Scholar] [CrossRef]
- Koyama, I.; Sakaguchi, S.; Todo, S. Clinical efficacy of regulatory T-cell therapy in kidney transplantation: First-in-human phase I study. Transplantation 2020, 104, e107–e114. [Google Scholar]
- Mikami, N.; Sakaguchi, S. Regulatory T cells in autoimmune kidney diseases and transplantation. Nat. Rev. Nephrol. 2023, 19, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.P.; Cepika, A.M.; Agarwal-Hashmi, R.; Saini, G.; Uyeda, M.J.; Louis, D.M.; Cieniewicz, B.; Narula, M.; Amaya Hernandez, L.C.; Harre, N.; et al. Alloantigen-specific type 1 regulatory T cells suppress through CTLA-4 and PD-1 pathways and persist long-term in patients. Sci. Transl. Med. 2021, 13, eabf5264. [Google Scholar] [CrossRef] [PubMed]
- Harden, P.N.; Game, D.S.; Sawitzki, B.; Van der Net, J.B.; Hester, J.; Bushell, A.; Issa, F.; Brook, M.O.; Alzhrani, A.; Schlickeiser, S.; et al. Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients. Am. J. Transplant. 2021, 21, 1603–1611. [Google Scholar] [CrossRef]
- Lu, J.; Li, P.; Du, X.; Liu, Y.; Zhang, B.; Qi, F. Regulatory T cells induce transplant immune tolerance. Transpl. Immunol. 2021, 67, 101411. [Google Scholar] [CrossRef]
- Sawitzki, B.; Harden, P.N.; Reinke, P.; Moreau, A.; Hutchinson, J.A.; Game, D.S.; Tang, Q.; Guinan, E.C.; Battaglia, M.; Burlingham, W.J.; et al. Regulatory cell therapy in kidney transplantation (The ONE Study): A harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet 2020, 395, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zheng, X.; Mathew, J.M.; Gallon, L.; Leventhal, J.R.; Zhang, Z.J. Tackling Chronic Kidney Transplant Rejection: Challenges and Promises. Front. Immunol. 2021, 12, 661643. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Buckner, J.H.; Fitch, M.; Gitelman, S.E.; Gupta, S.; Hellerstein, M.K.; Herold, K.C.; Lares, A.; Lee, M.R.; Li, K.; et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 2015, 7, 315ra189. [Google Scholar] [CrossRef]
- Trzonkowski, P.; Bieniaszewska, M.; Juscinska, J.; Dobyszuk, A.; Krzystyniak, A.; Marek, N.; Myśliwska, J.; Hellmann, A. First-in-man clinical trial of autologous CD4+CD25+CD127− regulatory T cells in patients with graft-versus-host disease and type 1 diabetes. Clin. Immunol. 2009, 133, 22–26. [Google Scholar] [CrossRef]
- Mohseni, Y.R.; Tung, S.L.; Dudreuilh, C.; Lechler, R.I.; Fruhwirth, G.O.; Lombardi, G. The future of regulatory T cell therapy: Promises and challenges of implementing CAR technology. Front. Immunol. 2020, 11, 1608. [Google Scholar] [CrossRef]
- Riet, T.; Chmielewski, M. Regulatory CAR-T cells in autoimmune diseases: Progress and current challenges. Front. Immunol. 2022, 13, 934343. [Google Scholar] [CrossRef]
- Juneja, T.; Kazmi, M.; Mellace, M.; Saidi, R.F. Utilization of Treg Cells in Solid Organ Transplantation. Front. Immunol. 2022, 13, 746889. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Rogers, N.M.; Li, J.; Zhang, G.Y.; Wang, Y.M.; Shaw, K.; O’Connell, P.J.; Alexander, S.I. Antigen Specific Regulatory T Cells in Kidney Transplantation and Other Tolerance Settings. Front. Immunol. 2021, 12, 717594. [Google Scholar] [CrossRef]
- Banas, B.; Krämer, B.K.; Krüger, B.; Kamar, N.; Undre, N. Long-Term Kidney Transplant Outcomes: Role of Prolonged-Release Tacrolimus. Transplant. Proc. 2020, 52, 102–110. [Google Scholar] [CrossRef]
- Mamlouk, O.; Nair, R.; Iyer, S.P.; Edwards, A.; Neelapu, S.S.; Steiner, R.E.; Adkins, S.A.; Hawkins, M.; Saini, N.; Devashish, K.; et al. Safety of CAR T-cell therapy in kidney transplant recipients. Blood J. Am. Soc. Hematol. 2021, 137, 2558–2562. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Lu, Z.; Cheng, C.; Pei, Y.; Chen, L.; Yue, Z.; Lin, A.; Yang, S.; Mo, Y.; Jiang, X. Factors associated with chronic calcineurin inhibitor nephrotoxicity in children with minimal-change disease. Ren. Fail. 2025, 47, 2474743. [Google Scholar] [CrossRef]
- Yu, J.; Wei, X.; Gao, J.; Wang, C.; Wei, W. Role of cyclosporin A in the treatment of kidney disease and nephrotoxicity. Toxicology 2023, 492, 153544. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Epithelial to Mesenchymal Transition in Renal Fibrogenesis: Pathologic Significance, Molecular Mechanism, and Therapeutic Intervention. J. Am. Soc. Nephrol. 2004, 15, 1. [Google Scholar] [CrossRef]
- Dikiy, S.; Rudensky, A.Y. Principles of regulatory T cell function. Immunity 2023, 56, 240–255. [Google Scholar] [CrossRef]
- Shrestha, B.M. Two Decades of Tacrolimus in Renal Transplant: Basic Science and Clinical Evidences. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2017, 15, 1–9. [Google Scholar] [CrossRef][Green Version]
- Hirsch, H.H.; Yakhontova, K.; Lu, M.; Manzetti, J. BK Polyomavirus Replication in Renal Tubular Epithelial Cells Is Inhibited by Sirolimus, but Activated by Tacrolimus Through a Pathway Involving FKBP-12. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2016, 16, 821–832. [Google Scholar] [CrossRef]
- Shen, C.L.; Wu, B.S.; Lien, T.J.; Yang, A.H.; Yang, C.Y. BK Polyomavirus Nephropathy in Kidney Transplantation: Balancing Rejection and Infection. Viruses 2021, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Ambalathingal, G.R.; Francis, R.S.; Smyth, M.J.; Smith, C.; Khanna, R. BK Polyomavirus: Clinical Aspects, Immune Regulation, and Emerging Therapies. Clin. Microbiol. Rev. 2017, 30, 503–528. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Scuderi, C.; Francis, L.; Neller, M.A.; Rehan, S.; Crooks, P.; Ambalathingal, G.R.; Smith, C.; Khanna, R.; John, G.T. T-cell adoptive immunotherapy for BK nephropathy in renal transplantation. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2020, 22, e13399. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 2318–2331. [Google Scholar] [CrossRef]
- Muckenhuber, M.; Wekerle, T. T regulatory cell therapy: The price of specificity. Am. J. Transplant. 2023, 23, 1824–1825. [Google Scholar] [CrossRef]
- Cheng, X.S.; Han, J.; Braggs-Gresham, J.L.; Held, P.J.; Busque, S.; Roberts, J.P.; Tan, J.C.; Scandling, J.D.; Chertow, G.M.; Dor, A. Trends in cost attributable to kidney transplantation evaluation and waiting list management in the United States, 2012–2017. JAMA Netw. Open 2022, 5, e221847. [Google Scholar] [CrossRef]
| Study | Phase | Treg Type | Key Outcome |
|---|---|---|---|
| The ONE Study (2020) [36] | I/II | Polyclonal | Safe, reduced rejection |
| Lai et al. (2018) [37] | I | Ex vivo-expanded | Stable graft function |
| Bluestone et al. (2015) [38] | Pilot | Polyclonal | Well tolerated |
| Koyama et al. (2016) [31] | I/II | Ex vivo-expanded | Tolerance achieved in liver Tx |
| Trzonkowski et al. (2009) [39] | I | Polyclonal | Safe in GVHD; foundational |
| CAR-Treg (ongoing) | I | Genetically engineered | Awaiting results |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matarneh, A.; Patel, M.; Parikh, K.; Karasinski, A.; Kaur, G.; Shah, V.; Ghahramani, N.; Trivedi, N. Regulatory T Cell in Kidney Transplant: The Future of Cell Therapy? Antibodies 2025, 14, 49. https://doi.org/10.3390/antib14020049
Matarneh A, Patel M, Parikh K, Karasinski A, Kaur G, Shah V, Ghahramani N, Trivedi N. Regulatory T Cell in Kidney Transplant: The Future of Cell Therapy? Antibodies. 2025; 14(2):49. https://doi.org/10.3390/antib14020049
Chicago/Turabian StyleMatarneh, Ahmad, Meet Patel, Kinna Parikh, Amanda Karasinski, Gurwant Kaur, Vaqar Shah, Nasrollah Ghahramani, and Naman Trivedi. 2025. "Regulatory T Cell in Kidney Transplant: The Future of Cell Therapy?" Antibodies 14, no. 2: 49. https://doi.org/10.3390/antib14020049
APA StyleMatarneh, A., Patel, M., Parikh, K., Karasinski, A., Kaur, G., Shah, V., Ghahramani, N., & Trivedi, N. (2025). Regulatory T Cell in Kidney Transplant: The Future of Cell Therapy? Antibodies, 14(2), 49. https://doi.org/10.3390/antib14020049






