A Novel Method for Preparing Uniform Micro-Sized Dry Powder Formulations, Including Aggregation-Controlled VHH
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of the VHH-Loaded Powders
2.3. Physicochemical Evaluation
2.3.1. High-Performance Liquid Chromatography (HPLC) Analysis
2.3.2. Bio-Layer Interferometry
2.3.3. Scanning Electron Microscopy (SEM)
2.3.4. Laser Diffraction
2.3.5. Andersen Cascade Impactor
3. Results and Discussion
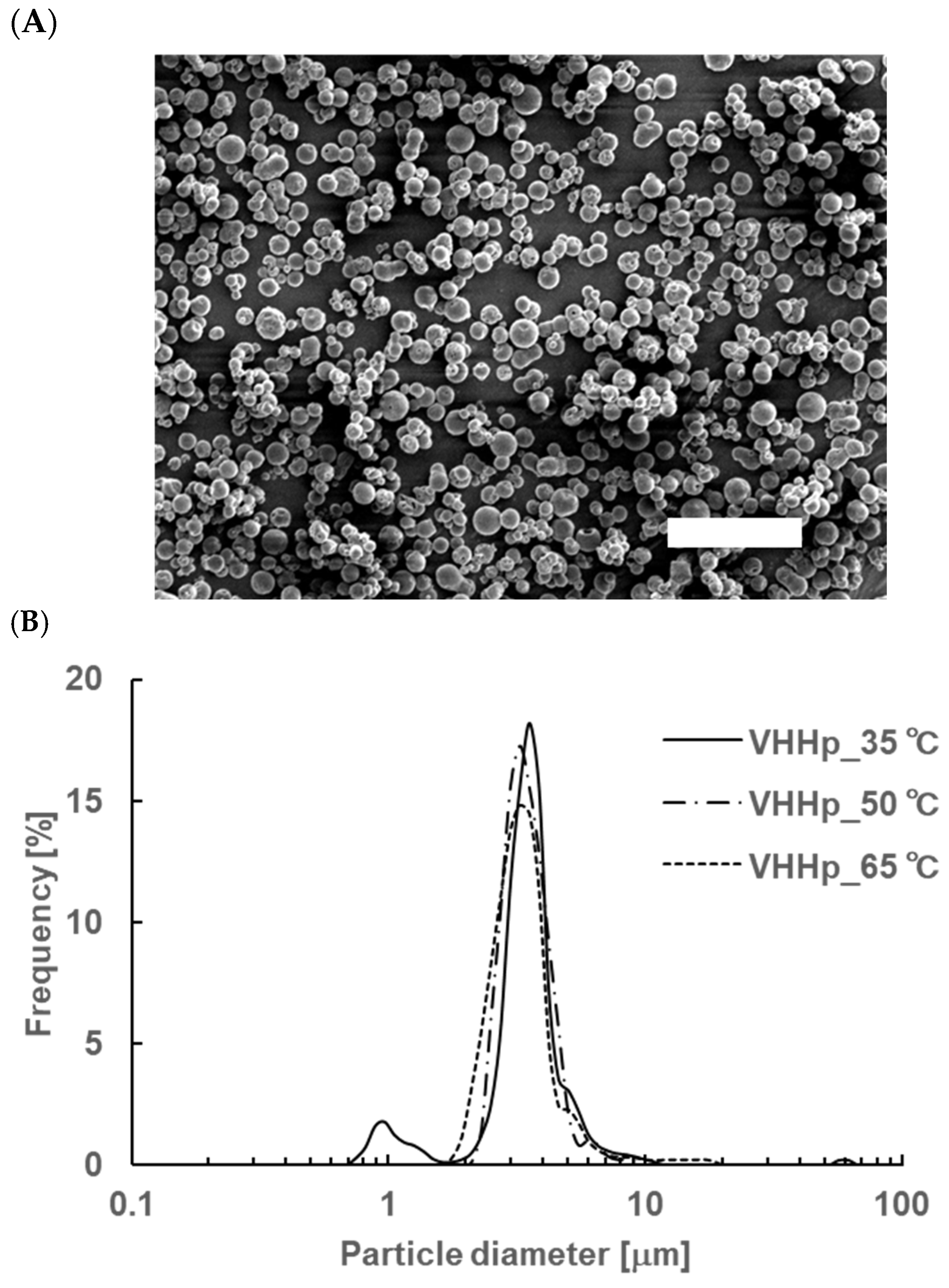
3.1. Appearance and Particle Size Distribution of VHHps
3.2. VHH Properties in VHHps
3.3. Inhalation Properties of VHHps
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Van Der Linden, R.; De Geus, B.; Stok, W.; Bos, W.; Van Wassenaar, D.; Verrips, T.; Frenken, L. Induction of Immune Responses and Molecular Cloning of the Heavy Chain Antibody Repertoire of Lama Glama. J. Immunol. Methods 2000, 240, 185–195. [Google Scholar] [CrossRef]
- Rothbauer, U.; Zolghadr, K.; Tillib, S.; Nowak, D.; Schermelleh, L.; Gahl, A.; Backmann, N.; Conrath, K.; Muyldermans, S.; Cardoso, M.C.; et al. Targeting and Tracing Antigens in Live Cells with Fluorescent Nanobodies. Nat. Methods 2006, 3, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Maass, D.R.; Sepulveda, J.; Pernthaner, A.; Shoemaker, C.B. Alpaca (Lama Pacos) as a Convenient Source of Recombinant Camelid Heavy Chain Antibodies (VHHs). J. Immunol. Methods 2007, 324, 13–25. [Google Scholar] [CrossRef] [PubMed]
- De Simone, E.A.; Saccodossi, N.; Ferrari, A.; Leoni, J. Development of ELISAs for the Measurement of IgM and IgG Subclasses in Sera from Llamas (Lama Glama) and Assessment of the Humoral Immune Response against Different Antigens. Vet. Immunol. Immunopathol. 2008, 126, 64–73. [Google Scholar] [CrossRef]
- Blanc, M.R.; Anouassi, A.; Abed, M.A.; Tsikis, G.; Canepa, S.; Labas, V.; Belghazi, M.; Bruneau, G. A One-step Exclusion-binding Procedure for the Purification of Functional Heavy-chain and Mammalian-type Γ-globulins from Camelid Sera. Biotechnol. Appl. Biochem. 2009, 54, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kunz, P.; Zinner, K.; Mücke, N.; Bartoschik, T.; Muyldermans, S.; Hoheisel, J.D. The Structural Basis of Nanobody Unfolding Reversibility and Thermoresistance. Sci. Rep. 2018, 8, 7934. [Google Scholar] [CrossRef]
- Davies, J.; Riechmann, L. Antibody VH Domains as Small Recognition Units. Nat. Biotechnol. 1995, 13, 475–479. [Google Scholar] [CrossRef]
- Gray, A.; Bradbury, A.R.M.; Knappik, A.; Plückthun, A.; Borrebaeck, C.A.K.; Dübel, S. Animal-Free Alternatives and the Antibody Iceberg. Nat. Biotechnol. 2020, 38, 1234–1239. [Google Scholar] [CrossRef]
- Suzuki, T.; Mochizuki, Y.; Kimura, S.; Akazawa-Ogawa, Y.; Hagihara, Y.; Nemoto, N. Anti-Survivin Single-Domain Antibodies Derived from an Artificial Library Including Three Synthetic Random Regions by in Vitro Selection Using CDNA Display. Biochem. Biophys. Res. Commun. 2018, 503, 2054–2060. [Google Scholar] [CrossRef] [PubMed]
- Moutel, S.; Bery, N.; Bernard, V.; Keller, L.; Lemesre, E.; De Marco, A.; Ligat, L.; Rain, J.C.; Favre, G.; Olichon, A.; et al. NaLi-H1: A Universal Synthetic Library of Humanized Nanobodies Providing Highly Functional Antibodies and Intrabodies. eLife 2016, 5, e16228. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Kumachi, S.; Matsunaga, Y.; Sato, M.; Wakabayashi-Nakao, K.; Masaki, H.; Yonehara, R.; Motohashi, M.; Nemoto, N.; Tsuchiya, M. Construction of a Humanized Artificial VHH Library Reproducing Structural Features of Camelid VHHs for Therapeutics. Antibodies 2022, 11, 10. [Google Scholar] [CrossRef]
- Jabbal, S.; Poli, G.; Lipworth, B. Does Size Really Matter?: Relationship of Particle Size to Lung Deposition and Exhaled Fraction. J. Allergy Clin. Immunol. 2017, 139, 2013–2014.e1. [Google Scholar] [CrossRef] [PubMed]
- Heyder, J. Particle Transport onto Human Airway Surfaces. Eur. J. Respir. Dis. 1982, 119, 29–50. [Google Scholar]
- Norikane, Y.; Nakamura, H.; Ohgaki, M.; Sekicuchi, Y. Liquid Droplet Ejecting Method, Liquid Droplet Ejection Apparatus, Inkjet Recording Apparatus, Production Method of Fine Particles, Fine Particle Production Apparatus, and Toner. 2011. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=WO2011115303 (accessed on 26 November 2024).
- Suzuki, H.; Moritani, T.; Morinaga, T.; Seto, Y.; Sato, H.; Onoue, S. Amorphous Solid Dispersion of Cyclosporine A Prepared with Fine Droplet Drying Process: Physicochemical and Pharmacokinetic Characterization. Int. J. Pharm. 2017, 519, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Tabata, A.; Moritani, T.; Morinaga, T.; Mizumoto, T.; Seto, Y.; Onoue, S. Design and Characterizations of Inhalable Poly(Lactic-Co-Glycolic Acid) Microspheres Prepared by the Fine Droplet Drying Process for a Sustained Effect of Salmon Calcitonin. Molecules 2020, 25, 1311. [Google Scholar] [CrossRef]
- Vázquez-Rey, M.; Lang, D.A. Aggregates in Monoclonal Antibody Manufacturing Processes. Biotechnol. Bioeng. 2011, 108, 1494–1508. [Google Scholar] [CrossRef]
- Chiou, D.; Langrish, T.A.G.; Braham, R. The Effect of Temperature on the Crystallinity of Lactose Powders Produced by Spray Drying. J. Food Eng. 2008, 86, 288–293. [Google Scholar] [CrossRef]
- Maas, S.G.; Schaldach, G.; Littringer, E.M.; Mescher, A.; Griesser, U.J.; Braun, D.E.; Walzel, P.E.; Urbanetz, N.A. The Impact of Spray Drying Outlet Temperature on the Particle Morphology of Mannitol. Powder Technol. 2011, 213, 27–35. [Google Scholar] [CrossRef]
- Kaerger, J.S.; Edge, S.; Price, R. Influence of Particle Size and Shape on Flowability and Compactibility of Binary Mixtures of Paracetamol and Microcrystalline Cellulose. Eur. J. Pharm. Sci. 2004, 22, 173–179. [Google Scholar] [CrossRef]
- Wang, Z.; Solomos, M.; Axnanda, S.; Chen, C.; Figus, M.; Schenck, L.; Sun, C.C. Varied Bulk Powder Properties of Micro-Sized API within Size Specifications as a Result of Particle Engineering Methods. Pharmaceutics 2022, 14, 1901. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Villen, F.; Gallego, I.; Sainz-Ramos, M.; Ordoyo-Pascual, J.; Ruiz-Alonso, S.; Saenz-del-Burgo, L.; O’Mahony, C.; Pedraz, J.L. Stability of Monoclonal Antibodies as Solid Formulation for Auto-Injectors: A Pilot Study. Pharmaceutics 2023, 15, 2049. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.P.; Hicks, D.; Shi, K.; Moeller, N.H.; Hoppe, B.; Lake, E.W.; Baehr, C.; Pravetoni, M.; Aihara, H.; LeBeau, A.M. Identification and biophysical characterization of a novel domain-swapped camelid antibody specific for fentanyl. J. Biol. Chem. 2024, 300, 107502. [Google Scholar] [CrossRef]
- Koenig, P.-A.; Das, H.; Liu, H.; Kümmerer, B.M.; Gohr, F.N.; Jenster, L.-M.; Schiffelers, L.D.J.; Tesfamariam, Y.M.; Uchima, M.; Wuerth, J.D.; et al. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science 2021, 371, eabe6230. [Google Scholar] [CrossRef]
- Akita, T.; Kimura, R.; Akaguma, S.; Nagai, M.; Nakao, Y.; Tsugane, M.; Suzuki, H.; Oka, J.; Yamashita, C. Usefulness of Cell-Penetrating Peptides and Penetration Accelerating Sequence for Nose-to-Brain Delivery of Glucagon-like Peptide-2. J. Control. Release 2021, 335, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Guérin, M.; Lepeltier, E. Nanomedicines via the Pulmonary Route: A Promising Strategy to Reach the Target? Drug Deliv. Transl. Res. 2024, 14, 2276–2297. [Google Scholar] [CrossRef]
- Glover, W.; Chan, H.K.; Eberl, S.; Daviskas, E.; Verschuer, J. Effect of Particle Size of Dry Powder Mannitol on the Lung Deposition in Healthy Volunteers. Int. J. Pharm. 2008, 349, 314–322. [Google Scholar] [CrossRef]

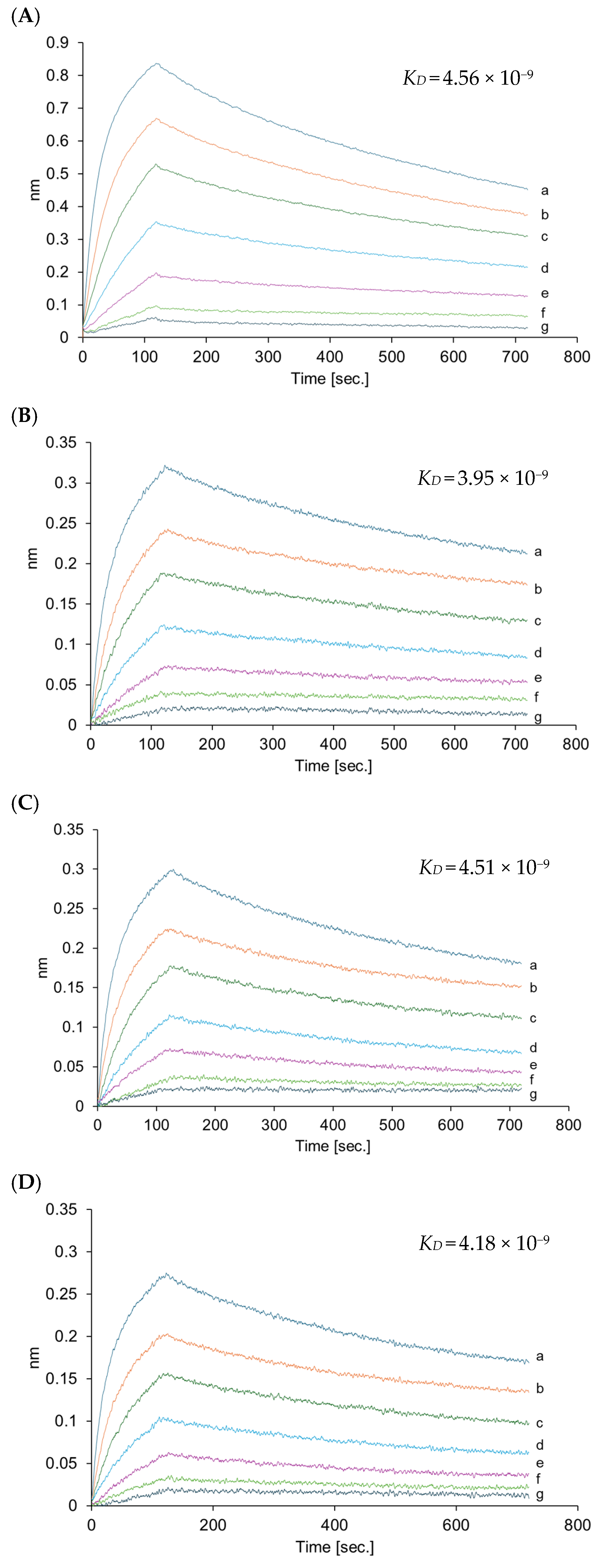
| Native VHH | VHHp (35 °C) | VHHp (50 °C) | VHHp (65 °C) | |
|---|---|---|---|---|
| Aggregation (%) | 0.00 | 1.77 | 3.09 | 9.06 |
| KD (M) | 4.56 × 10−9 | 3.95 × 10−9 | 4.51 × 10−9 | 4.18 × 10−9 |
| kon (M/s) | 2.08 × 105 | 1.57 × 105 | 1.74 × 105 | 1.86 × 105 |
| koff (s−1) | 9.48 × 10−4 | 6.18 × 10−4 | 7.87 × 10−4 | 7.75 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moritani, T.; Masaki, H.; Yonehara, R.; Suzuki, T.; Arai, H.; Tsuchiya, M.; Nemoto, N. A Novel Method for Preparing Uniform Micro-Sized Dry Powder Formulations, Including Aggregation-Controlled VHH. Antibodies 2025, 14, 29. https://doi.org/10.3390/antib14020029
Moritani T, Masaki H, Yonehara R, Suzuki T, Arai H, Tsuchiya M, Nemoto N. A Novel Method for Preparing Uniform Micro-Sized Dry Powder Formulations, Including Aggregation-Controlled VHH. Antibodies. 2025; 14(2):29. https://doi.org/10.3390/antib14020029
Chicago/Turabian StyleMoritani, Tatsuru, Hidekazu Masaki, Ryo Yonehara, Takeru Suzuki, Hidenao Arai, Masayuki Tsuchiya, and Naoto Nemoto. 2025. "A Novel Method for Preparing Uniform Micro-Sized Dry Powder Formulations, Including Aggregation-Controlled VHH" Antibodies 14, no. 2: 29. https://doi.org/10.3390/antib14020029
APA StyleMoritani, T., Masaki, H., Yonehara, R., Suzuki, T., Arai, H., Tsuchiya, M., & Nemoto, N. (2025). A Novel Method for Preparing Uniform Micro-Sized Dry Powder Formulations, Including Aggregation-Controlled VHH. Antibodies, 14(2), 29. https://doi.org/10.3390/antib14020029






