T330M Substitution in the Sodium-Dependent Phosphate Transporter NaPi2b Abolishes the Efficacy of Monoclonal Antibodies Against MX35 Epitope
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Genomic DNA Preparation for Sequencing
2.2. Sequencing
2.3. Cell Lines and Bacterial Strains
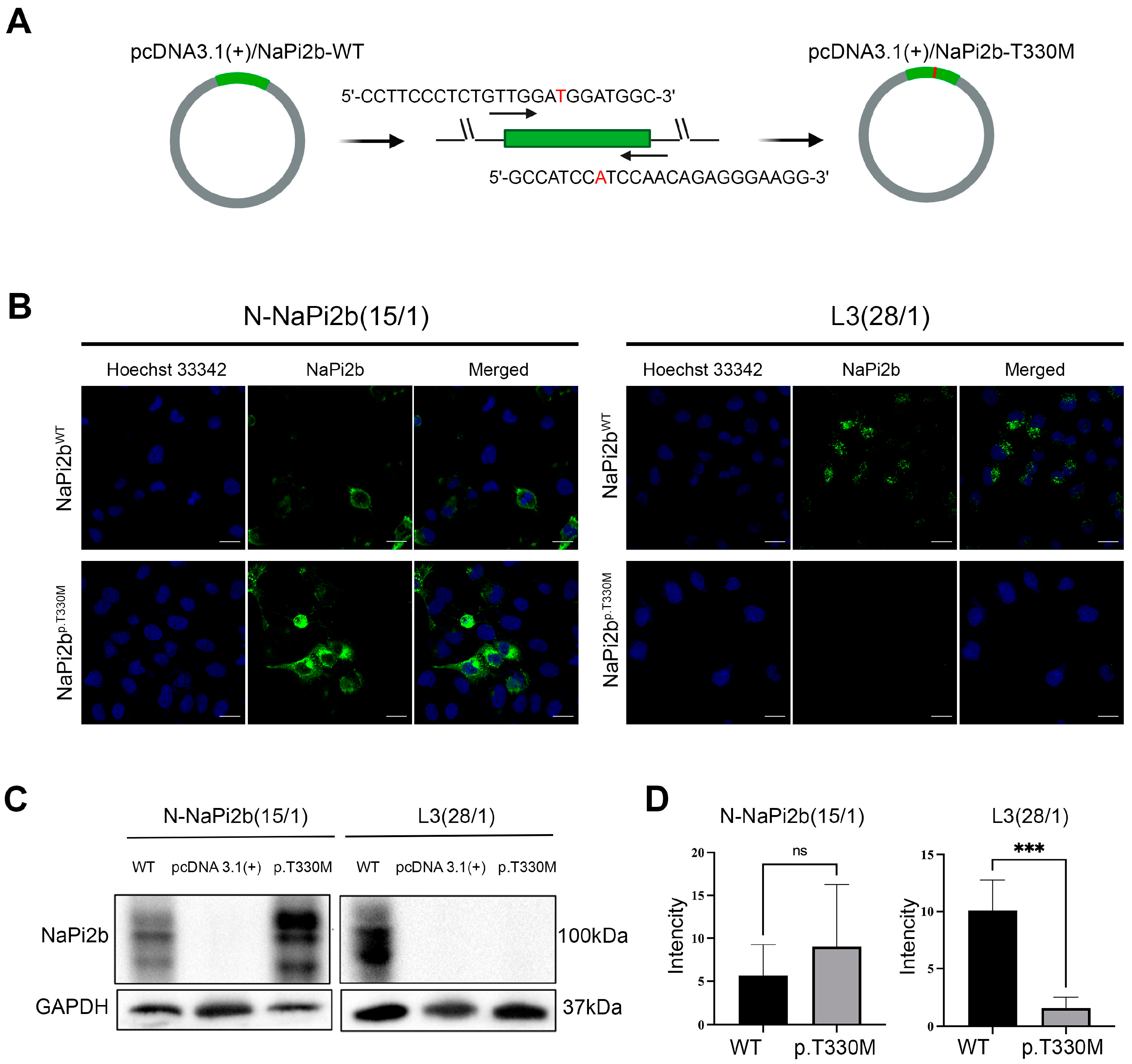
2.4. Site-Directed Mutagenesis
2.5. Transfection
2.6. Western Blot Analysis
2.7. Laser Confocal Microscopy
2.8. Bioinformatic Analysis of Public Databases
3. Results
3.1. Identification of Variations in the MX35 Epitope of the Sodium-Dependent Phosphate Transporter NaPi2b
3.2. Identification of Germline Variants in the MX35 Epitope of the Sodium-Dependent Phosphate Transporter NaPi2b
3.3. The Impact of the Amino Acid Substitution P.T330M on the Recognition of the MX35 Epitope by Monoclonal Antibodies in Ovarian Carcinoma Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henke, O.; Qader, A.Q.; Malle, G.L.; Kuiate, J.R.; Hennig, L.; Demeke, T.; Stroetmann, C.; Henke, A.A.; Alaric, T.T.; Rushanyan, M.; et al. International Cooperation to Fight Cancer’s Late-Stage Presentation in Low- and Middle-Income Countries. Clin. Exp. Metastasis 2023, 40, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kaehler, M.; Cascorbi, I. Germline Variants in Cancer Therapy. Cancer Drug Resist. 2019, 2, 18–30. [Google Scholar] [CrossRef]
- Waarts, M.R.; Stonestrom, A.J.; Park, Y.C.; Levine, R.L. Targeting Mutations in Cancer. J. Clin. Investig. 2022, 132, e154943. [Google Scholar] [CrossRef] [PubMed]
- Stoppa-Lyonnet, D. The Biological Effects and Clinical Implications of BRCA Mutations: Where Do We Go from Here? Eur. J. Hum. Genet. 2016, 24, S3–S9. [Google Scholar] [CrossRef]
- Yin, B.W.T.; Kiyamova, R.; Chua, R.; Caballero, O.L.; Gout, I.; Gryshkova, V.; Bhaskaran, N.; Souchelnytskyi, S.; Hellman, U.; Filonenko, V.; et al. Monoclonal Antibody MX35 Detects the Membrane Transporter NaPi2b (SLC34A2) in Human Carcinomas. Cancer Immun. 2007, 8, 3. [Google Scholar]
- Cheng, X.; Li, P.; Jiang, R.; Meng, E.; Wu, H. ADC: A Deadly Killer of Platinum Resistant Ovarian Cancer. J. Ovarian Res. 2024, 17, 196. [Google Scholar] [CrossRef]
- Kiyamova, R.G.; Vlasenkova, R.A.; Bulatova, L.F. Sodium-Dependent Phosphate Transporter NaPi2b as a Candidate for Targeted Therapy: Features of Structure, Function, and Expression. Adv. Mol. Oncol. 2024, 11, 74–84. [Google Scholar] [CrossRef]
- Nishimura, M.; Naito, S. Tissue-Specific MRNA Expression Profiles of Human Solute Carrier Transporter Superfamilies. Drug Metab. Pharmacokinet. 2008, 23, 22. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A.; Hernando, N.; Forster, I.C.; Biber, J. The SLC34 Family of Sodium-Dependent Phosphate Transporters. Pflügers Arch.-Eur. J. Physiol. 2013, 466, 139–153. [Google Scholar] [CrossRef]
- Banerjee, S.; Drapkin, R.; Richardson, D.L.; Birrer, M. Targeting NaPi2b in Ovarian Cancer. Cancer Treat. Rev. 2023, 112, 102489. [Google Scholar] [CrossRef]
- Vlasenkova, R.; Nurgalieva, A.; Akberova, N.; Bogdanov, M.; Kiyamova, R. Characterization of SLC34A2 as a Potential Prognostic Marker of Oncological Diseases. Biomolecules 2021, 11, 1878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ye, S.; Zhang, M.; Wu, J.; Yan, H.; Li, X.; He, J. High Expression of SLC34A2 Is a Favorable Prognostic Marker in Lung Adenocarcinoma Patients. Tumor Biol. 2017, 39, 1010428317720212. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, D.H.; Kim, J.Y.; Jeoung, N.H.; Lee, I.K.; Bong, J.G.; Jung, E.D. Microarray Analysis of Papillary Thyroid Cancers in Korean. Korean J. Intern. Med. 2010, 25, 399–407. [Google Scholar] [CrossRef]
- Kiyamova, R.; Shyian, M.; Lyzogubov, V.V.; Usenko, V.S.; Gout, T.; Filonenko, V. Immunohistochemical Analysis of NaPi2b Protein (MX35 Antigen) Expression and Subcellular Localization in Human Normal and Cancer Tissues. Exp. Oncol. 2011, 33, 157–161. [Google Scholar]
- Liu, L.; Yang, Y.; Zhou, X.; Yan, X.; Wu, Z. Solute Carrier Family 34 Member 2 Overexpression Contributes to Tumor Growth and Poor Patient Survival in Colorectal Cancer. Biomed. Pharmacother. 2018, 99, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.E.; Yin-Goen, Q.; Alexis, D.; Sirintrapun, J.S.; Harrison, W.; Isett, R.B.; Rossi, M.R.; Moreno, C.S.; Young, A.N.; Osunkoya, A.O. Gene Expression Profiling of Clear Cell Papillary Renal Cell Carcinoma: Comparison with Clear Cell Renal Cell Carcinoma and Papillary Renal Cell Carcinoma. Mod. Pathol. 2014, 27, 222–230. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Lu, H. Knockdown of SLC34A2 Inhibits Hepatocellular Carcinoma Cell Proliferation and Invasion. Oncol. Res. 2016, 24, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Chen, C.; Gao, Y.; Zheng, Z.-S.; Xu, Y.; Yun, M.; Weng, H.-W.; Xie, D.; Ye, S.; Zhang, J.-X. Overexpression of SLC34A2 Is an Independent Prognostic Indicator in Bladder Cancer and Its Depletion Suppresses Tumor Growth via Decreasing C-Myc Expression and Transcriptional Activity. Cell Death Dis. 2017, 8, e2581. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, X.; Zhang, L.; Yang, F.; Qin, L.; Zhang, D.; Qin, Y. SLC34A2 Regulates MiR-25-Gsk3β Signaling Pathway to Affect Tumor Progression in Gastric Cancer Stem Cell-like Cells. Mol. Carcinog. 2018, 57, 440–450. [Google Scholar] [CrossRef]
- Moore, K.N.; Birrer, M.J.; Marsters, J.; Wang, Y.; Choi, Y.; Royer-Joo, S.; Lemahieu, V.; Armstrong, K.; Cordova, J.; Samineni, D.; et al. Phase 1b Study of Anti-NaPi2b Antibody-Drug Conjugate Lifastuzumab Vedotin (DNIB0600A) in Patients with Platinum-Sensitive Recurrent Ovarian Cancer. Gynecol. Oncol. 2020, 158, 631–639. [Google Scholar] [CrossRef]
- Fessler, S.; Dirksen, A.; Collins, S.D.; Xu, L.; Lee, W.; Wang, J.; Eydelloth, R.; Ter-Ovanesyen, E.; Zurita, J.; Ditty, E.; et al. Abstract 2894: XMT-1592, a Site-Specific Dolasynthen-Based NaPi2b-Targeted Antibody-Drug Conjugate for the Treatment of Ovarian Cancer and Lung Adenocarcinoma. Cancer Res. 2020, 80, 2894. [Google Scholar] [CrossRef]
- Bodyak, N.D.; Mosher, R.; Yurkovetskiy, A.V.; Yin, M.; Bu, C.; Conlon, P.R.; Demady, D.R.; DeVit, M.J.; Gumerov, D.R.; Gurijala, V.R.; et al. The Dolaflexin-Based Antibody–Drug Conjugate XMT-1536 Targets the Solid Tumor Lineage Antigen SLC34A2/NaPi2b. Mol. Cancer Ther. 2021, 20, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.E.; Brant, M.G.; Lasalle, M.; Das, S.; Duan, R.; Wong, J.; Ding, T.; Wu, K.J.; Siddappa, D.; Fang, C.; et al. Design and Evaluation of ZD06519, a Novel Camptothecin Payload for Antibody Drug Conjugates. Mol. Cancer Ther. 2024, 23, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Horsley, E.; Jabeen, A.; Veillard, N.; Havenith, K.; Janghra, N.; Alves, P.; Oblette, C.; Kirby, I.; Hogg, P.W.; Zammarchi, F.; et al. Abstract 5085: Preclinical Development of NaPi2b-PL2202, a Novel Camptothecin-Based Antibody-Drug Conjugate Targeting Solid Tumors Expressing NaPi2b. Cancer Res. 2024, 84, 5085. [Google Scholar] [CrossRef]
- Schmitt, S.; Mai, I.; Machui, P.; Herterich, S.; Hauswald, D.; Ochtrop, P.; Cyprys, P.; Kozlowska, I.; Kitowski, A.; Gerlach, M.; et al. Abstract 2622: TUB-040, a Novel Napi2b-Targeting ADC Built with Ethynylphosphonamidate Conjugation Chemistry, Demonstrates High and Long-Lasting Anti-Tumor Efficacy via Topoisomerase-I Inhibition and Excellent Tolerability Predictive of a Wide Therapeutic Window in Humans. Cancer Res. 2024, 84, 2622. [Google Scholar] [CrossRef]
- Xiao, L.; Lian, W.; Liu, Q.; Zong, Q.; Song, S.; Stann, S.; Cai, J.; Xue, T. Abstract 1894: Preclinical Development of YL205, a Novel NaPi2b-Targeting Antibody-Drug Conjugate (ADC) with Novel Topoisomerase I Inhibitor-Based Linker-Payload for Treatment of Solid Tumors. Cancer Res. 2024, 84, 1894. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Z.; Li, Y.; Pan, H.; Qin, X.; Fei, D.; Li, R. Abstract 5907: Pre-Clinical Evaluation of a Novel Antibody Drug Conjugate (ADC) LM-317 Targeting NaPi2b. Cancer Res. 2024, 84, 5907. [Google Scholar] [CrossRef]
- Rojas, A.H.; Wong, J.; Urosev, D.; Lawn, S.; Wu, K.; Konomura, S.; Lasalle, M.; Alonzo, D.A.; Yang, L.; Petersen, M.; et al. Abstract 1533: ZW220, a Novel NaPi2b-Targeting Antibody Drug Conjugate Bearing a Topoisomerase 1 Inhibitor Payload. Cancer Res. 2023, 83, 1533. [Google Scholar] [CrossRef]
- dos Santos, M.L.; Yeda, F.P.; Tsuruta, L.R.; Horta, B.B.; Pimenta, A.A.; Degaki, T.L.; Soares, I.C.; Tuma, M.C.; Okamoto, O.K.; Alves, V.A.F.; et al. Rebmab200, a Humanized Monoclonal Antibody Targeting the Sodium Phosphate Transporter NaPi2b Displays Strong Immune Mediated Cytotoxicity against Cancer: A Novel Reagent for Targeted Antibody Therapy of Cancer. PLoS ONE 2013, 8, e70332. [Google Scholar] [CrossRef]
- Lindegren, S.; Andrade, L.N.S.; Bäck, T.; Machado, C.M.L.; Horta, B.B.; Buchpiguel, C.; Moro, A.M.; Okamoto, O.K.; Jacobsson, L.; Cederkrantz, E.; et al. Binding Affinity, Specificity and Comparative Biodistribution of the Parental Murine Monoclonal Antibody MX35 (Anti-NaPi2b) and Its Humanized Version Rebmab200. PLoS ONE 2015, 10, e0126298. [Google Scholar] [CrossRef]
- Friedman, C.; Anderson, C.; Hays, J.; Lakhani, N.; Buscema, J.; Hamilton, E.; Taylor, S.; Duska, L.; Cloven, N.; Reske, A.; et al. EV257/#1106 Upgrade: Phase 1 Trial of the NaPi2b-Directed Dolaflexin Antibody Drug Conjugate UpRi in Combination with Carboplatin in Patients with Platinum-Sensitive Ovarian Cancer (PSOC). Int. J. Gynecol. Cancer 2024, 34, A215. [Google Scholar] [CrossRef]
- Mattes, M.J.; Look, K.; Furukawa, K.; Pierce, V.K.; Old, L.J.; Lewis, J.L.; Lloyd, K.O. Mouse Monoclonal Antibodies to Human Epithelial Differentiation Antigens Expressed on the Surface of Ovarian Carcinoma Ascites Cells. Cancer Res. 1987, 47, 6741–6750. [Google Scholar] [PubMed]
- Rubin, S.C.; Kostakoglu, L.; Divgi, C.; Federici, M.G.; Finstad, C.L.; Lloyd, K.O.; Larson, S.M.; Hoskins, W.J. Biodistribution and Intraoperative Evaluation of Radiolabeled Monoclonal Antibody MX 35 in Patients with Epithelial Ovarian Cancer. Gynecol. Oncol. 1993, 51, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bulatova, L.F.; Skripova, V.S.; Korotaeva, A.V.; Bogdanov, M.V.; Kiyamova, R.G. Recognition of Mutant Forms of the Sodium-Dependent Phosphate Transporter NaPi2b by monoclonal Antibodies in Ovarian Cancer Cells. Kazan Med. J. 2022, 103, 608–616. [Google Scholar] [CrossRef]
- Korotaeva, A.V.; Bulatova, L.F.; Vlasenkova, R.A.; Kiyamova, R.G. Recognition of Sodium-Dependent Phosphate Transporter Napi2b by Monoclonal Antibodies in Bacterial and Eukaryotic Cells. Biotechnol. STATE ART Perspect. 2022, 1, 128–131. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Guadagnin, G.; Cappello, P.; Novelli, F. Post-Translational Modifications in Tumor-Associated Antigens as a Platform for Novel Immuno-Oncology Therapies. Cancers 2022, 15, 138. [Google Scholar] [CrossRef]
- Dutta, H.; Jain, N. Post-Translational Modifications and Their Implications in Cancer. Front. Oncol. 2023, 13, 1240115. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lubman, D.M. The Role of N-Glycosylation in Cancer. Acta Pharm. Sin. B 2024, 14, 1098–1110. [Google Scholar] [CrossRef]
- Min, J.-Y.; Chun, K.-S.; Kim, D.-H. The Versatile Utility of Cysteine as a Target for Cancer Treatment. Front. Oncol. 2023, 12, 997919. [Google Scholar] [CrossRef]
- Mereiter, S.; Balmaña, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Garrett, T.P.J.; Burgess, A.W.; Gan, H.K.; Luwor, R.B.; Cartwright, G.; Walker, F.; Orchard, S.G.; Clayton, A.H.A.; Nice, E.C.; Rothacker, J.; et al. Antibodies Specifically Targeting a Locally Misfolded Region of Tumor Associated EGFR. Proc. Natl. Acad. Sci. USA 2009, 106, 5082–5087. [Google Scholar] [CrossRef] [PubMed]
- Gryshkova, V.S.; Lituyev, D.S.; Filonenko, V.V.; Kiyamova, R.G. Creation of Cellular Models for the Analysis of Sodium-Dependent Phosphate Transporter NaPi2b, a Potential Marker for Ovarian Cancer. Biopolym. Cell 2009, 25, 95–100. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows–Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Van der Auwera, G.A.; Carneiro, M.O.; Hartl, C.; Poplin, R.; Del Angel, G.; Levy-Moonshine, A.; Jordan, T.; Shakir, K.; Roazen, D.; Thibault, J.; et al. From FastQ Data to High-Confidence Variant Calls: The Genome Analysis Toolkit Best Practices Pipeline. Curr. Protoc. Bioinform. 2013, 43, 11.10.1–11.10.33. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional Annotation of Genetic Variants from High-Throughput Sequencing Data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Kiyamova, R.; Gryshkova, V.; Ovcharenko, G.; Lituyev, D.; Malyuchik, S.; Usenko, V.; Khozhayenko, Y.; Gurtovyy, V.; Yin, B.; Ritter, G.; et al. Development of Monoclonal Antibodies Specific for the Human Sodium-Dependent Phosphate Co-Transporter NaPi2b. Hybridoma 2008, 27, 277–284. [Google Scholar] [CrossRef]
- Gryshkova, V.; Lituiev, D.; Savinska, L.; Ovcharenko, G.; Gout, I.; Filonenko, V.; Kiyamova, R. Generation of Monoclonal Antibodies Against Tumor-Associated Antigen MX35/Sodium-Dependent Phosphate Transporter NaPi2b. Hybridoma 2011, 30, 37–42. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Consortium, I.C.G.; Hudson, T.J.; Anderson, W.; Artez, A.; Barker, A.D.; Bell, C.; Bernabé, R.R.; Bhan, M.K.; Calvo, F.; Eerola, I.; et al. International Network of Cancer Genome Projects. Nature 2010, 464, 993–998. [Google Scholar] [CrossRef]
- Sondka, Z.; Dhir, N.B.; Carvalho-Silva, D.; Jupe, S.; Madhumita; McLaren, K.; Starkey, M.; Ward, S.; Wilding, J.; Ahmed, M.; et al. COSMIC: A Curated Database of Somatic Variants and Clinical Data for Cancer. Nucleic Acids Res. 2023, 52, D1210–D1217. [Google Scholar] [CrossRef]
- Martelotto, L.G.; Ng, C.K.; Filippo, M.R.D.; Zhang, Y.; Piscuoglio, S.; Lim, R.S.; Shen, R.; Norton, L.; Reis-Filho, J.S.; Weigelt, B. Benchmarking Mutation Effect Prediction Algorithms Using Functionally Validated Cancer-Related Missense Mutations. Genome Biol. 2014, 15, 484. [Google Scholar] [CrossRef]
- Chua, E.W.; Goh, C.S. Benchmarking in Silico Tools for the Functional Assessment of DNA Variants Using a Set of Strictly Pharmacogenetic Variants. Sains Malays. 2019, 48, 2151–2159. [Google Scholar] [CrossRef]
- Chen, S.; Francioli, L.C.; Goodrich, J.K.; Collins, R.L.; Kanai, M.; Wang, Q.; Alföldi, J.; Watts, N.A.; Vittal, C.; Gauthier, L.D.; et al. A Genomic Mutational Constraint Map Using Variation in 76,156 Human Genomes. Nature 2024, 625, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Barbitoff, Y.A.; Khmelkova, D.N.; Pomerantseva, E.A.; Slepchenkov, A.V.; Zubashenko, N.A.; Mironova, I.V.; Kaimonov, V.S.; Polev, D.E.; Tsay, V.V.; Glotov, A.S.; et al. Expanding the Russian Allele Frequency Reference via Cross-Laboratory Data Integration: Insights from 7452 Exome Samples. Natl. Sci. Rev. 2024, 11, nwae326. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing Structure Coverage for over 214 Million Protein Sequences. Nucleic Acids Res. 2023, 52, D368–D375. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern Web App for 3D Visualization and Analysis of Large Biomolecular Structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Song, C.H.; Jeong, M.; In, H.; Kim, J.H.; Lin, C.-W.; Han, K.H. Trends in the Development of Antibody-Drug Conjugates for Cancer Therapy. Antibodies 2023, 12, 72. [Google Scholar] [CrossRef]
- Hafeez, U.; Parakh, S.; Gan, H.K.; Scott, A.M. Antibody–Drug Conjugates for Cancer Therapy. Molecules 2020, 25, 4764. [Google Scholar] [CrossRef]
- Ozohanics, O.; Ambrus, A. Hydrogen-Deuterium Exchange Mass Spectrometry: A Novel Structural Biology Approach to Structure, Dynamics and Interactions of Proteins and Their Complexes. Life 2020, 10, 286. [Google Scholar] [CrossRef]
- Jönsson, Å.L.M.; Hilberg, O.; Simonsen, U.; Christensen, J.H.; Bendstrup, E. New Insights in the Genetic Variant Spectrum of SLC34A2 in Pulmonary Alveolar Microlithiasis; a Systematic Review. Orphanet J. Rare Dis. 2023, 18, 130. [Google Scholar] [CrossRef]
- Enemark, A.; Jönsson, Å.L.M.; Kronborg-White, S.; Bendstrup, E. Pulmonary Alveolar Microlithiasis—A Review. Yale J. Biol. Med. 2021, 94, 637–644. [Google Scholar] [PubMed]
- Corut, A.; Senyigit, A.; Ugur, S.A.; Altin, S.; Ozcelik, U.; Calisir, H.; Yildirim, Z.; Gocmen, A.; Tolun, A. Mutations in SLC34A2 Cause Pulmonary Alveolar Microlithiasis and Are Possibly Associated with Testicular Microlithiasis. Am. J. Hum. Genet. 2006, 79, 650–656. [Google Scholar] [CrossRef] [PubMed]
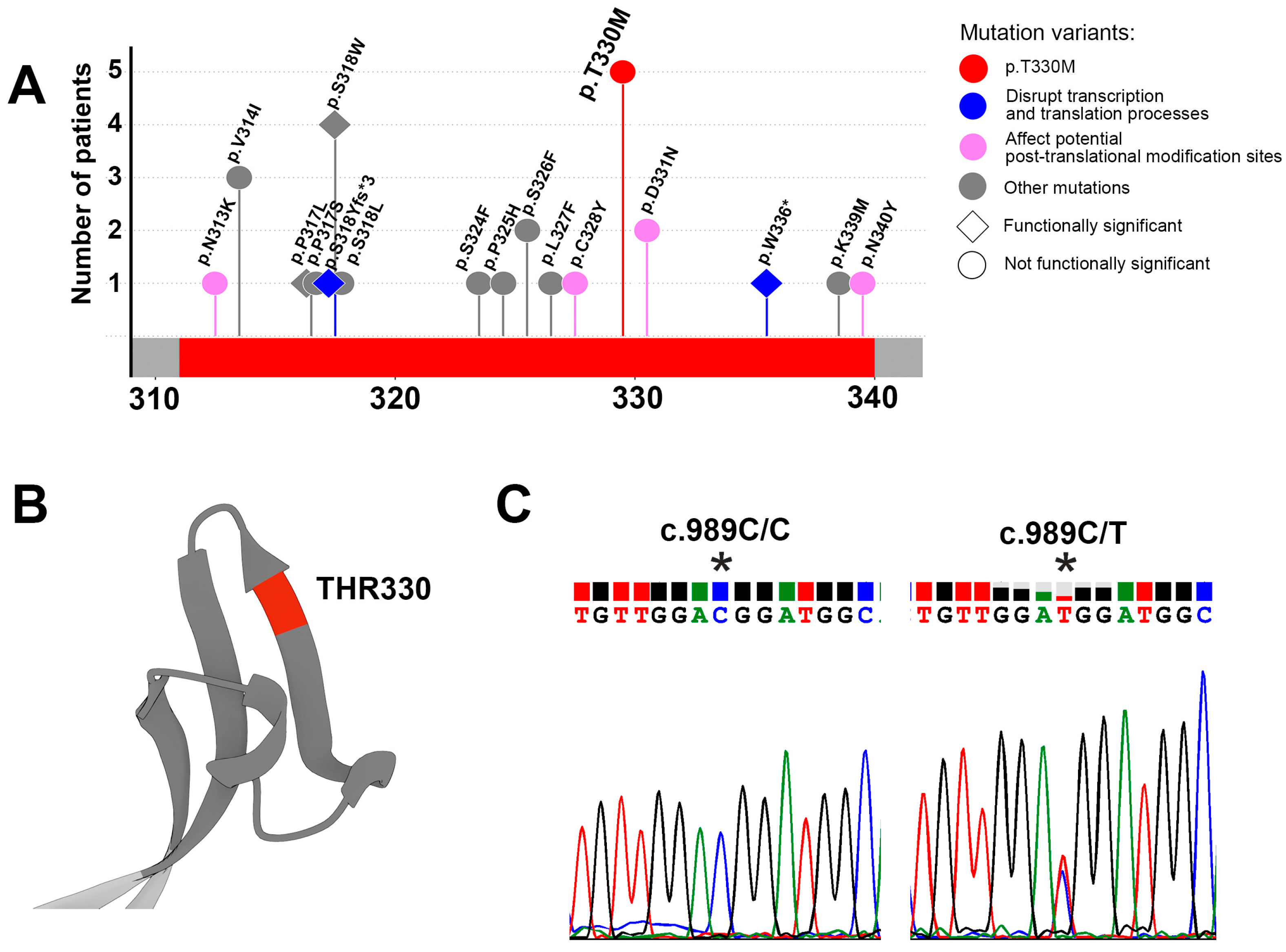
| Diagnosis | Genotype | Total | ||
|---|---|---|---|---|
| Wild Type c.989C/C | Heterozygous c.989C/T | Homozygous c.989T/T | ||
| Breast cancer | 19 | 1 | 0 | 20 |
| Kidney cancer | 14 | 3 | 0 | 17 |
| Ovarian cancer | 26 | 1 | 0 | 27 |
| Healthy (control) | 17 | 0 | 0 | 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulatova, L.F.; Skripova, V.S.; Sagdeeva, A.R.; Vlasenkova, R.A.; Bugaenko, T.A.; Galimova, R.R.; Nesterova, A.I.; Filina, Y.V.; Kiyamova, R.G. T330M Substitution in the Sodium-Dependent Phosphate Transporter NaPi2b Abolishes the Efficacy of Monoclonal Antibodies Against MX35 Epitope. Antibodies 2025, 14, 30. https://doi.org/10.3390/antib14020030
Bulatova LF, Skripova VS, Sagdeeva AR, Vlasenkova RA, Bugaenko TA, Galimova RR, Nesterova AI, Filina YV, Kiyamova RG. T330M Substitution in the Sodium-Dependent Phosphate Transporter NaPi2b Abolishes the Efficacy of Monoclonal Antibodies Against MX35 Epitope. Antibodies. 2025; 14(2):30. https://doi.org/10.3390/antib14020030
Chicago/Turabian StyleBulatova, Leisan F., Vera S. Skripova, Aisylu R. Sagdeeva, Ramilia A. Vlasenkova, Tatiana A. Bugaenko, Rezeda R. Galimova, Alfiya I. Nesterova, Yuliya V. Filina, and Ramziya G. Kiyamova. 2025. "T330M Substitution in the Sodium-Dependent Phosphate Transporter NaPi2b Abolishes the Efficacy of Monoclonal Antibodies Against MX35 Epitope" Antibodies 14, no. 2: 30. https://doi.org/10.3390/antib14020030
APA StyleBulatova, L. F., Skripova, V. S., Sagdeeva, A. R., Vlasenkova, R. A., Bugaenko, T. A., Galimova, R. R., Nesterova, A. I., Filina, Y. V., & Kiyamova, R. G. (2025). T330M Substitution in the Sodium-Dependent Phosphate Transporter NaPi2b Abolishes the Efficacy of Monoclonal Antibodies Against MX35 Epitope. Antibodies, 14(2), 30. https://doi.org/10.3390/antib14020030









