Anti-MET Antibody Therapies in Non-Small-Cell Lung Cancer: Current Progress and Future Directions
Abstract
1. Introduction
2. Amivantamab
3. Teliso-V
4. Emibetuzumab
5. Other Novel c-MET Therapies
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Graveel, C.R.; Tolbert, D.; Vande Woude, G.F. MET: A critical player in tumorigenesis and therapeutic target. Cold Spring Harb. Perspect. Biol. 2013, 5, a009209. [Google Scholar] [CrossRef]
- Boccaccio, C.; Comoglio, P.M. Invasive growth: A MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer 2006, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Christofori, G. New signals from the invasive front. Nature 2006, 441, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Bottaro, D.P.; Rubin, J.S.; Faletto, D.L.; Chan, A.M.; Kmiecik, T.E.; Vande Woude, G.F.; Aaronson, S.A. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science 1991, 251, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Naldini, L.; Weidner, K.M.; Vigna, E.; Gaudino, G.; Bardelli, A.; Ponzetto, C.; Narsimhan, R.P.; Hartmann, G.; Zarnegar, R.; Michalopoulos, G.K.; et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991, 10, 2867–2878. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef]
- Socinski, M.A.; Pennell, N.A.; Davies, K.D. MET Exon 14 Skipping Mutations in Non-Small-Cell Lung Cancer: An Overview of Biology, Clinical Outcomes, and Testing Considerations. JCO Precis. Oncol. 2021, 5, 653–663. [Google Scholar] [CrossRef]
- Awad, M.M.; Oxnard, G.R.; Jackman, D.M.; Savukoski, D.O.; Hall, D.; Shivdasani, P.; Heng, J.C.; Dahlberg, S.E.; Anne, P.A.J.; Verma, S.; et al. Exon 14 Mutations in Non-Small-Cell Lung Cancer Are Associated With Advanced Age and Stage-Dependent Genomic Amplification and c-Met Overexpression. J. Clin. Oncol. 2016, 34, 721. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14-Mutated or MET-Amplified Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef]
- Yu, H.A.; Suzawa, K.; Jordan, E.; Zehir, A.; Ni, A.; Kim, R.; Kris, M.G.; Hellmann, M.D.; Li, B.T.; Somwar, R.; et al. Concurrent Alterations in EGFR-Mutant Lung Cancers Associated with Resistance to EGFR Kinase Inhibitors and Characterization of MTOR as a Mediator of Resistance. Clin. Cancer Res. 2018, 24, 3108–3118. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.J.; Chan, J.M.; Kubota, D.; Sato, H.; Rizvi, H.; Daneshbod, Y.; Chang, J.C.; Paik, P.K.; Offin, M.; Arcila, M.E.; et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin. Cancer Res. 2020, 26, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, E.; Maeshima, A.; Nakajima, T.; Nakamura, T. Expression of c-met/HGF receptor in human non-small cell lung carcinomas in vitro and in vivo and its prognostic significance. Jpn. J. Cancer Res. 1996, 87, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Guo, M.; Tang, Z.; Toruner, G.A.; Cheng, J.; Medeiros, L.J.; Tang, G. MET Expression Level in Lung Adenocarcinoma Loosely Correlates with MET Copy Number Gain/Amplification and Is a Poor Predictor of Patient Outcome. Cancers 2022, 14, 2433. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Lin, L.; Takahashi, T.; Nomura, M.; Suzuki, M.; Wistuba, I.I.; Fong, K.M.; Lee, H.; Toyooka, S.; Shimizu, N.; et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. Jnci-J. Natl. Cancer I 2005, 97, 339–346. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Yatabe, Y. Mutations of the epidermal growth factor receptor gene and related genes as determinants of epidermal growth factor receptor tyrosine kinase inhibitors sensitivity in lung cancer. Cancer Sci. 2007, 98, 1817–1824. [Google Scholar] [CrossRef]
- Shi, Y.; Au, J.S.K.; Thongprasert, S.; Srinivasan, S.; Tsai, C.M.; Khoa, M.T.; Heeroma, K.; Itoh, Y.; Cornelio, G.; Yang, P.C. A Prospective, Molecular Epidemiology Study of EGFR Mutations in Asian Patients with Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology (PIONEER). J. Thorac. Oncol. 2014, 9, 154–162. [Google Scholar] [CrossRef]
- Stewart, E.L.; Tan, S.Z.; Liu, G.; Tsao, M.S. Known and putative mechanisms of resistance to EGFR targeted therapies in NSCLC patients with EGFR mutations-a review. Transl. Lung Cancer R. 2015, 4, 67–81. [Google Scholar] [CrossRef]
- Huang, L.H.; Fu, L.W. Mechanisms of resistance to EGFR tyrosine kinase inhibitors. Acta Pharm. Sin. B 2015, 5, 390–401. [Google Scholar] [CrossRef]
- Suda, K.; Onozato, R.; Yatabe, Y.; Mitsudomi, T. T790M Mutation A Double Role in Lung Cancer Cell Survival? J. Thorac. Oncol. 2009, 4, 1–4. [Google Scholar] [CrossRef]
- Soria, J.C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Thress, K.S.; Paweletz, C.P.; Felip, E.; Cho, B.C.; Stetson, D.; Dougherty, B.; Lai, Z.; Markovets, A.; Vivancos, A.; Kuang, Y.; et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat. Med. 2015, 21, 560–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tsui, S.T.; Liu, C.; Song, Y.; Liu, D. EGFR C797S mutation mediates resistance to third-generation inhibitors in T790M-positive non-small cell lung cancer. J. Hematol. Oncol. 2016, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.C.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.J.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef]
- Bean, J.; Brennan, C.; Shih, J.Y.; Riely, G.; Viale, A.; Wang, L.; Chitale, D.; Motoi, N.; Szoke, J.; Broderick, S.; et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Mol. Cancer Ther. 2007, 6, 3333s–3334s. [Google Scholar] [CrossRef]
- Drilon, A.; Clark, J.W.; Weiss, J.; Ou, S.H.I.; Camidge, D.R.; Solomon, B.J.; Otterson, G.A.; Villaruz, L.C.; Riely, G.J.; Heist, R.S.; et al. Antitumor activity of crizotinib in lung cancers harboring a exon 14 alteration. Nat. Med. 2020, 26, 47–51. [Google Scholar] [CrossRef]
- FDA. FDA Approves Tepotinib for Metastatic Non-Small Cell Lung Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tepotinib-metastatic-non-small-cell-lung-cancer (accessed on 24 August 2024).
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.Y.; Kim, S.W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Cho, B.C.; Kim, D.W.; Spira, A.I.; Gomez, J.E.; Haura, E.B.; Kim, S.W.; Sanborn, R.E.; Cho, E.K.; Lee, K.H.; Minchom, A.; et al. Amivantamab plus lazertinib in osimertinib-relapsed EGFR-mutant advanced non-small cell lung cancer: A phase 1 trial. Nat. Med. 2023, 29, 2577–2585. [Google Scholar] [CrossRef]
- Krebs, M.; Spira, A.I.; Cho, B.C.; Besse, B.; Goldman, J.W.; Janne, P.A.; Ma, Z.Y.; Mansfield, A.S.; Minchom, A.R.; Ou, S.H.I.; et al. Amivantamab in patients with NSCLC with MET exon 14 skipping mutation: Updated results from the CHRYSALIS study. J. Clin. Oncol. 2022, 40, 9008. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, K.J.; Cho, B.C.; Liu, B.; Paz-Ares, L.; Cheng, S.; Kitazono, S.; Thiagarajan, M.; Goldman, J.W.; Sabari, J.K.; et al. Amivantamab plus Chemotherapy in NSCLC with EGFR Exon 20 Insertions. N. Engl. J. Med. 2023, 389, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, B.C.; Spira, A.I.; Ou, S.H.I.; Waqar, S.N.; Shah, S.; Trani, L.; Chu, P.L.; Thayu, M.; Knoblauch, R.E.; et al. Amivantamab, Lazertinib Plus Platinum-based Chemotherapy in EGFR-mutated Advanced NSCLC: Updated Results from CHRYSALIS-2. J. Thorac. Oncol. 2023, 18, S146–S147. [Google Scholar] [CrossRef]
- Shu, C.A.; Goto, K.; Ohe, Y.; Besse, B.; Lee, S.H.; Wang, Y.S.; Griesinger, F.; Yang, J.C.H.; Felip, E.; Sanborn, R.E.; et al. Amivantamab and lazertinib in patients with EGFR-mutant non-small cell lung (NSCLC) after progression on osimertinib and platinum-based chemotherapy: Updated results from CHRYSALIS-2. J. Clin. Oncol. 2022, 40, 9006. [Google Scholar] [CrossRef]
- Cho, B.C.; Wang, Y.; Li, Y.; Wu, L.; Besse, B.; Marmarelis, M.E.; Goto, K.; Lee, J.S.; Lee, S.H.; Zhang, Y.; et al. Amivantamab in combination with lazertinib in patients with atypical epidermal growth factor receptor (EGFR) mutations excluding exon 20 insertion mutations: Initial results from CHRYSALIS-2. Ann. Oncol. 2022, 33, S1566. [Google Scholar] [CrossRef]
- Leighl, N.B.; Akamatsu, H.; Lim, S.M.; Cheng, Y.; Minchom, A.R.; Marmarelis, M.E.; Sanborn, R.E.; Chih-Hsin Yang, J.; Liu, B.; John, T.; et al. Subcutaneous versus Intravenous Amivantamab, both in Combination with Lazertinib, in Refractory EGFR-mutated NSCLC: Primary Results from the Phase 3 PALOMA-3 Study. J. Clin. Oncol. 2024, 42, JCO2401001. [Google Scholar] [CrossRef]
- Cho, B.C.; Felip, E.; Spira, A.I.; Girard, N.; Lee, J.S.; Lee, S.H.; Ostapenko, Y.V.; Danchaivijitr, P.; Liu, B.; Alip, A.; et al. Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced nonsmall cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Ann. Oncol. 2023, 34, S1306. [Google Scholar] [CrossRef]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.H.; Melosky, B.; Shih, J.Y.; Wang, J.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: Primary results from the phase III MARIPOSA-2 study. Ann. Oncol. 2024, 35, 77–90. [Google Scholar] [CrossRef]
- Strickler, J.H.; Weekes, C.D.; Nemunaitis, J.; Ramanathan, R.K.; Heist, R.S.; Morgensztern, D.; Angevin, E.; Bauer, T.M.; Yue, H.B.; Motwani, M.; et al. First-in-Human Phase I, Dose-Escalation and -Expansion Study of Telisotuzumab Vedotin, an Antibody-Drug Conjugate Targeting c-Met, in Patients With Advanced Solid Tumors. J. Clin. Oncol. 2018, 36, 3298–3306. [Google Scholar] [CrossRef]
- Goldman, J.W.; Horinouchi, H.; Cho, B.C.; Tomasini, P.; Dunbar, M.; Hoffman, D.; Parikh, A.; Blot, V.; Camidge, D.R. Phase 1/1b study of telisotuzumab vedotin (Teliso-V) plus osimertinib (Osi), after failure on prior Osi, in patients with advanced, c-Met overexpressing, EGFR-mutated non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2022, 40, 16. [Google Scholar] [CrossRef]
- Camidge, D.R.; Barlesi, F.; Goldman, J.W.; Morgensztern, D.; Heist, R.; Vokes, E.; Spira, A.; Angevin, E.; Su, W.C.; Hong, D.S.; et al. Phase Ib Study of Telisotuzumab Vedotin in Combination With Erlotinib in Patients With c-Met Protein-Expressing Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2023, 41, 1105. [Google Scholar] [CrossRef]
- Camidge, D.R.; Barlesi, F.; Goldman, J.W.; Morgensztern, D.; Heist, R.; Vokes, E.; Angevin, E.; Hong, D.S.; Rybkin, I.I.; Barve, M.; et al. A Phase 1b Study of Telisotuzumab Vedotin in Combination With Nivolumab in Patients With NSCLC. JTO Clin. Res. Rep. 2022, 3, 100262. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Bar, J.; Horinouchi, H.; Goldman, J.; Moiseenko, F.; Filippova, E.; Cicin, I.; Ciuleanu, T.; Daaboul, N.; Liu, C.; et al. Telisotuzumab Vedotin Monotherapy in Patients With Previously Treated c-Met Protein-Overexpressing Advanced Nonsquamous EGFR-Wildtype Non-Small Cell Lung Cancer in the Phase II LUMINOSITY Trial. J. Clin. Oncol. 2024, 42, JCO2400720. [Google Scholar] [CrossRef] [PubMed]
- Lugini, A.; Goldman, J.W.; Tanizaki, J.; Akamatsu, H.; Xia, S.; Ratajczak, C.; Li, M.; Bolotin, E.; Seraj, J.; Lu, S. A phase III global study of telisotuzumab vedotin versus docetaxel in previously treated patients with c-Met overexpressing, EGFR wildtype, locally advanced/metastatic nonsquamous NSCLC (TeliMET NSCLC-01). Ann. Oncol. 2023, 34, S845. [Google Scholar] [CrossRef]
- Rosen, L.S.; Goldman, J.W.; Algazi, A.P.; Turner, P.K.; Moser, B.; Hu, T.L.; Wang, X.A.; Tuttle, J.; Wacheck, V.; Wooldridge, J.E.; et al. A First-in-Human Phase I Study of a Bivalent MET Antibody, Emibetuzumab (LY2875358), as Monotherapy and in Combination with Erlotinib in Advanced Cancer. Clin. Cancer Res. 2017, 23, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Scagliotti, G.V.; Moro-Sibilot, D.; Kollmeier, J.; Favaretto, A.G.; Cho, E.K.; Grosch, H.; Kimmich, M.; Girard, N.; Tsai, C.M.; Hsia, T.C. A randomized, controlled, open label phase II study of erlotinib (E) with or without the MET antibody emibetuzumab (Emi) as first-line treatment for EGFRmt non-small cell lung cancer (NSCLC) patients who have disease control after an 8-week lead-in treatment with erlotinib. J. Clin. Oncol. 2017, 35, 9019. [Google Scholar] [CrossRef]
- Scagliotti, G.; Moro-Sibilot, D.; Kollmeier, J.; Favaretto, A.; Cho, E.K.; Grosch, H.; Kimmich, M.; Girard, N.; Tsai, C.M.; Hsia, T.C.; et al. A Randomized-Controlled Phase 2 Study of the MET Antibody Emibetuzumab in Combination with Erlotinib as First-Line Treatment for Mutation-Positive NSCLC Patients. J. Thorac. Oncol. 2020, 15, 80–90. [Google Scholar] [CrossRef]
- Camidge, D.R.; Moran, T.; Demedts, I.; Grosch, H.; Mileham, K.; Molina, J.; Juan-Vidal, O.; Bepler, G.; Goldman, J.W.; Park, K.; et al. A Randomized, Open-Label Phase II Study Evaluating Emibetuzumab Plus Erlotinib and Emibetuzumab Monotherapy in MET Immunohistochemistry Positive NSCLC Patients with Acquired Resistance to Erlotinib. Clin. Lung Cancer 2022, 23, 300–310. [Google Scholar] [CrossRef]
- Harding, J.J.; Zhu, A.X.; Bauer, T.M.; Choueiri, T.K.; Drilon, A.; Voss, M.H.; Fuchs, C.S.; Abou-Alfa, G.K.; Wijayawardana, S.R.; Wang, X.A.; et al. A Phase Ib/II Study of Ramucirumab in Combination with Emibetuzumab in Patients with Advanced Cancer. Clin. Cancer Res. 2019, 25, 5202–5211. [Google Scholar] [CrossRef]
- Spigel, D.R.; Ervin, T.J.; Ramlau, R.A.; Daniel, D.B.; Goldschmidt, J.H.; Blumenschein, G.R.; Krzakowski, M.J.; Robinet, G.; Godbert, B.; Barlesi, F.; et al. Randomized Phase II Trial of Onartuzumab in Combination With Erlotinib in Patients With Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2013, 31, 4105. [Google Scholar] [CrossRef]
- Spigel, D.R.; Edelman, M.J.; O’Byrne, K.; Paz-Ares, L.; Mocci, S.; Phan, S.; Shames, D.S.; Smith, D.; Yu, W.; Paton, V.E.; et al. Results From the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J. Clin. Oncol. 2017, 35, 412. [Google Scholar] [CrossRef]
- Camidge, D.R.; Janku, F.; Martinez-Bueno, A.; Catenacci, D.V.T.; Lee, J.; Lee, S.H.; Dowlati, A.; Rohrberg, K.S.; Navarro, A.; Moon, Y.W.; et al. Safety and preliminary clinical activity of the MET antibody mixture, Sym015 in advanced non-small cell lung cancer (NSCLC) patients with MET amplification/exon 14 deletion (MET). J. Clin. Oncol. 2020, 38, 15. [Google Scholar] [CrossRef]
- Drilon, A.E.; Awad, M.M.; Gadgeel, S.M.; Villaruz, L.C.; Sabari, J.K.; Perez, J.; Daly, C.; Patel, S.; Li, S.; Seebach, F.A.; et al. A phase 1/2 study of REGN5093-M114, a METxMET antibody-drug conjugate, in patients with mesenchymal epithelial transition factor (MET)-overexpressing NSCLC. J. Clin. Oncol. 2022, 40, 16. [Google Scholar] [CrossRef]
- FDA. FDA Approves Lazertinib with Amivantamab-Vmjw for Non-Small Lung Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lazertinib-amivantamab-vmjw-non-small-lung-cancer (accessed on 24 August 2024).
- Belzer, A.; Nguyen, M.O.; Talsania, A.; Haldas, J.; Smith, J.; Leventhal, J.S. Spectrum of Dermatologic Adverse Events Associated With Amivantamab Use. JAMA Dermatol. 2023, 159, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Bar, J.; Horinouchi, H.; Goldman, J.W.; Moiseenko, F.V.; Filippova, E.; Cicin, I.; Bradbury, P.A.; Daaboul, N.; Tomasini, P.; et al. Telisotuzumab vedotin (Teliso-V) monotherapy in patients (pts) with previously treated c-Met-overexpressing (OE) advanced non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2022, 40, 9016. [Google Scholar] [CrossRef]
- Kishi, K.; Sakai, H.; Seto, T.; Kozuki, T.; Nishio, M.; Imamura, F.; Nokihara, H.; Satouchi, M.; Nakagawa, S.; Tahata, T.; et al. First-line onartuzumab plus erlotinib treatment for patients with MET-positive and EGFR mutation-positive non-small-cell lung cancer. Cancer Treat. Res. Commun. 2019, 18, 100113. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.R.; Govindan, R.; Zvirbule, Z.; Braiteh, F.; Rittmeyer, A.; Belda-Iniesta, C.; Isla, D.; Cosgriff, T.; Boyer, M.; Ueda, M.; et al. Efficacy and Safety Results From a Phase II, Placebo-Controlled Study of Onartuzumab Plus First-Line Platinum-Doublet Chemotherapy for Advanced Squamous Cell Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2017, 18, 43–49. [Google Scholar] [CrossRef]
- Wakelee, H.; Zvirbule, Z.; De Braud, F.; Kingsley, C.D.; Mekhail, T.; Lowe, T.; Schutte, W.; Lena, H.; Lawler, W.; Braiteh, F.; et al. Efficacy and Safety of Onartuzumab in Combination With First-Line Bevacizumab- or Pemetrexed-Based Chemotherapy Regimens in Advanced Non-Squamous Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2017, 18, 50–59. [Google Scholar] [CrossRef]
- Oh, S.Y.; Lee, Y.W.; Lee, E.J.; Kim, J.H.; Park, Y.; Heo, S.G.; Yu, M.R.; Hong, M.H.; DaSilva, J.; Daly, C.; et al. Preclinical Study of a Biparatopic METxMET Antibody-Drug Conjugate, REGN5093-M114, Overcomes MET-driven Acquired Resistance to EGFR TKIs in EGFR-mutant NSCLC. Clin. Cancer Res. 2023, 29, 221–232. [Google Scholar] [CrossRef]
- Cho, B.; Ahn, M.-J.; Kim, T.; Kim, C.; Shim, B.; Han, J.-Y.; Drilon, A.; Lena, H.; Gomez, J.; Awad, M.; et al. Early safety, tolerability, and efficacy of REGN5093 in patients (pts) with MET-altered advanced non-small cell lung cancer (aNSCLC) from a first in human (FIH) study. Ann. Oncol. 2022, 33, S1085. [Google Scholar] [CrossRef]
- McGrath, L.; Zheng, Y.; Christ, S.; Sachs, C.C.; Khelifa, S.; Windmüller, C.; Sweet, S.; Kim, Y.J.; Sutton, D.; Sulikowski, M.; et al. Evaluation of the relationship between target expression and in vivo anti-tumor efficacy of AZD9592. Cancer Res. 2023, 83, 5737. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. First in Human Study of AZD9592 in Solid Tumors (EGRET). Available online: https://clinicaltrials.gov/study/NCT05647122 (accessed on 24 August 2024).
- ClinicalTrials.gov. A Study of RC108-ADC in Subjects With Advanced Malignant Solid Tumors. Available online: https://clinicaltrials.gov/study/NCT04617314 (accessed on 25 August 2024).
- Jorgensen, J.T.; Mollerup, J. Companion Diagnostics and Predictive Biomarkers for MET-Targeted Therapy in NSCLC. Cancers 2022, 14, 2150. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N.; Hong, L.; Zhang, J.; Heymach, J.; Hong, D.; Le, X. Beyond epidermal growth factor receptor: MET amplification as a general resistance driver to targeted therapy in oncogene-driven non-small-cell lung cancer. ESMO Open 2021, 6, 100319. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Luo, J.; Chang, J.; Rekhtman, N.; Arcila, M.; Drilon, A. MET-dependent solid tumours—Molecular diagnosis and targeted therapy. Nat. Rev. Clin. Oncol. 2020, 17, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.X.; Jie, G.L.; Li, A.N.; Liu, S.Y.; Sun, H.; Zheng, M.M.; Zhou, J.Y.; Zhang, J.T.; Zhang, X.C.; Zhou, Q.; et al. MET amplification identified by next-generation sequencing and its clinical relevance for MET inhibitors. Exp. Hematol. Oncol. 2021, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Hsu, R.; Benjamin, D.J.; Nagasaka, M. The Development and Role of Capmatinib in the Treatment of MET-Dysregulated Non-Small Cell Lung Cancer-A Narrative Review. Cancers 2023, 15, 3561. [Google Scholar] [CrossRef]
- Recondo, G.; Bahcall, M.; Spurr, L.F.; Che, J.; Ricciuti, B.; Leonardi, G.C.; Lo, Y.C.; Li, Y.Y.; Lamberti, G.; Nguyen, T.; et al. Molecular Mechanisms of Acquired Resistance to MET Tyrosine Kinase Inhibitors in Patients with MET Exon 14-Mutant NSCLC. Clin. Cancer Res. 2020, 26, 2615–2625. [Google Scholar] [CrossRef]
- Ou, S.I.; Young, L.; Schrock, A.B.; Johnson, A.; Klempner, S.J.; Zhu, V.W.; Miller, V.A.; Ali, S.M. Emergence of Preexisting MET Y1230C Mutation as a Resistance Mechanism to Crizotinib in NSCLC with MET Exon 14 Skipping. J. Thorac. Oncol. 2017, 12, 137–140. [Google Scholar] [CrossRef]
- Riedel, R.; Fassunke, J.; Tumbrink, H.L.; Scheel, A.H.; Heydt, C.; Hieggelke, L.; Scheffler, M.; Heimsoeth, A.; Nogova, L.; Michels, S.; et al. Resistance to MET inhibition in MET-dependent NSCLC and therapeutic activity after switching from type I to type II MET inhibitors. Eur. J. Cancer 2023, 179, 124–135. [Google Scholar] [CrossRef]
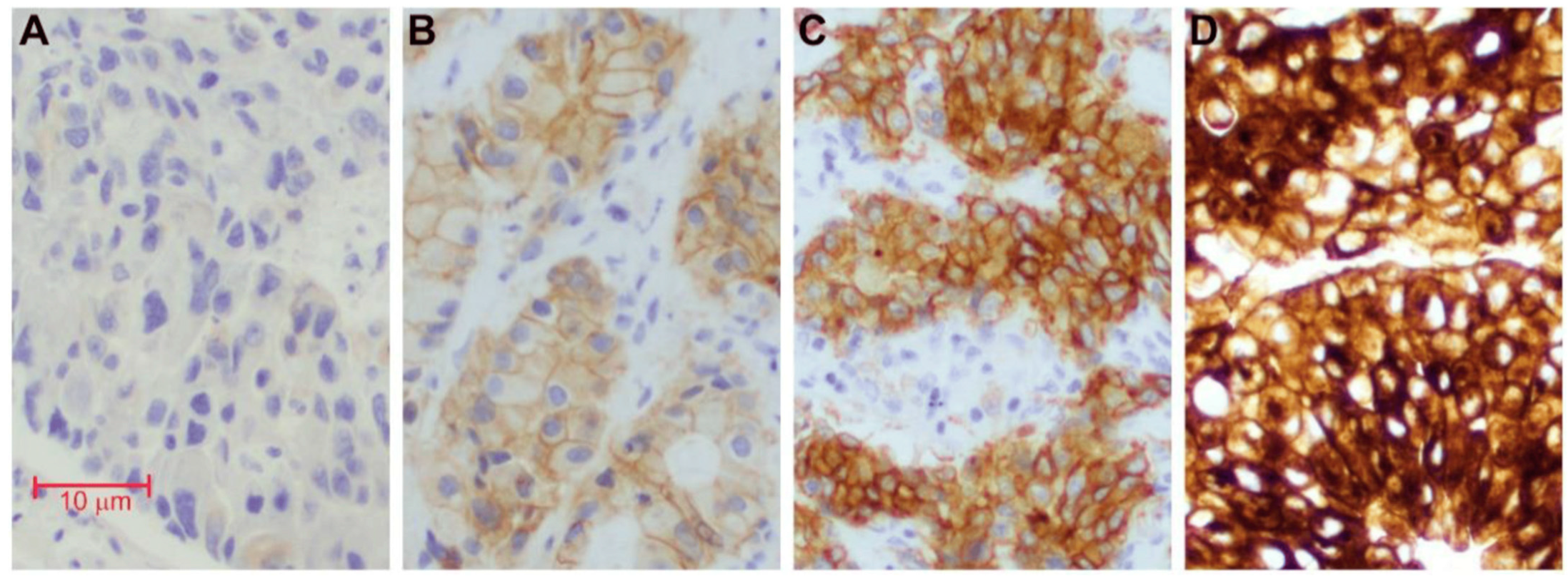
| Drug | Trial | Target Population | Results |
|---|---|---|---|
| Amivantamab | CHRYSALIS (NCT02609776) | EGFR Exon20ins (n = 81) progressed on chemotherapy [29] | ORR, 40% mDOR, 11.1 mo mPFS, 8.31 mo |
| Amivantamab plus lazertinib | EGFRm with progression on osimertinib but chemotherapy-naïve (n = 45) [30] | ORR, 36% mDOR, 9.6 mo mPFS, 4.9 mo | |
| Amivantamab | MET positive with MetEx14 mutation (n = 36) [31] | ORR, 33.3% mPFS, 6.7 months | |
| Amivantamab plus capmatinib | METalmark (NCT05488314) | MetEx14 mutation and MET-amplified (n = 161) | Ongoing |
| Amivantamab plus CT | PAPILLION (NCT04538664) | Ami-CT (n = 153) vs. CT alone (n = 155) as 1L for EGFR Exon20ins mutation [32] | Ami-CT vs. CT ORR, 73% vs. 47% mPFS, 11.4 vs. 6.7 mo |
| Amivantamab plus lazertinib plus CT | CHRYSALIS-2 (NCT04077463) | EGFRm with progression on or after EGFR TKI (n = 20) [33] | ORR, 50% mPFS, 14.0 months |
| Amivantamab plus lazertinib | post-chemo/post-osimertinib EGFRm Exon19del or L858R (n = 162; 50 efficacy evaluable) [34] | ORR, 36% Clinical benefit rate, 58% | |
| Amivantamab plus lazertinib | Atypical non-exon 20 insertion EGFRm (n = 40) in post-afatinib and treatment-naïve [35] | PR in 60% patients ORR, 56.7% | |
| Amivantamab plus lazertinib | PALOMA-3 (NCT05388669) | subcutaneous vs. intravenous administration in post-CT/post-osimertinib EGFRm (n = 418) [36] | ORR, 30% vs. 33% mPFS, 6.1 mo vs. 4.3 mo |
| Amivantamb plus lazertinib | MARIPOSA (NCT04487080) | Amivantamb plus lazertinib (n = 429) versus osimertinib (n = 429) as 1L EGFRm [37] | Ami-laz vs. osi ORR, 86% vs. 85% mDOR, 25.8 vs. 16.8 mo mPFS, 23.7 mo vs. 16.6 mo |
| Amivantamab–lazertinib–chemotherapy | MARIPOSA-2 (NCT04988295) | Ami-laz-CT vs. ami-CT versus CT alone (2:1:2 ratio, n = 657) in EGFRm after progression on osimertinib [38] | Ami-laz-CT vs. ami-CT vs. CT ORR, 63% vs. 64% vs. 36% mPFS, 8.2 vs. 8.3 vs. 4.2 mo |
| Teliso-V | NCT02099058 | Advanced NSCLC with c-MET overexpression (n = 16) [39] | 3/16 partial reponse DOR, 4.8 month mPFS, 5.7 month |
| Teliso-V plus osimertinib | c-MET overexpressing, EGFRm, after failure on previous osimertinib (n = 18) [40] | ORR, 56% | |
| Teliso-V plus erlotinib | c-MET positive EGFRm (n = 26) and EGFR WT (n = 8) [41] | mPFS, 5.9 mo EGFRm: ORR, 32.1% Non-T790M: mPFS, 6.8 mo | |
| Teliso-V plus nivolumab | c-MET positive EGFRm (n = 27) [42] | ORR, 7.4% mPFS, 7.2 month | |
| Teliso-V | Luminosity (NCT03539536) | MET overexpressing (details elaborated on in text) [43] | NSQ EGFR-WT: ORR, 52.2% NSQ EGFRm: ORR, 11.6% SQ EGFR-WT: ORR, 11.1% |
| Teliso-V | TeliMET NSCLC-01 (NCT04928846) | Teliso-V vs. docetaxel in c-MET overexpressing, EGFR-WT, NSQ with progression [44] | Ongoing |
| Teliso-V | NCT05513703 | Previously untreated MET Amp NSQ NSCLC with no targetable mutation | Results not published |
| Emibetuzumab plus erlotinib | NCT01287546 | EM + ER vs. EM in NSCLC (n = 4 vs. 14, respectively) [45] | 1/4 EM partial response 2/14 EM-ER partial response |
| Emibetuzumab plus erlotinib | NCT01897480 | EM + ER (n = 71) vs. ER (n = 70) in MET-positive with erlotinib resistance [46,47] | EM-ER vs. ER mPFS 9.3 vs. 9.5 mo High MET expression: mPFS, 20.7 vs. 5.4 mo |
| Emibetuzumab plus erlotinib | NCT01900652 | EM + ER vs. EM (n = 111, 3:1 ratio) with ER resistance [48] | EM-ER vs. EM ORR, 3.0% vs. 4.3% |
| Emibetuzumab plus ramucirumab | NCT02082210 | Advanced NSCLC (n = 15) [49] | mPFS, 6.6 mo 1/15 partial response 12/15 stable disease |
| Onartuzumab plus erlotininb | NCT00854308 | onartuzumab plus ER vs. ER in ITT (n = 137) and MET-pos (n = 66) [50] | Onartuzumab + ER vs. ER ORR, 5.8% vs. 4.4% mPFS, 2.2 vs. 2.6 mo |
| Onartuzumab plus erlotininb | METLung trial (NCT01456325) | onartuzumab plus ER vs. ER in MET-pos after chemo progression [51] | Onartuzumab + ER vs. ER mOS, 6.8 vs. 9.1 mo |
| Sym015 | NCT02648724 | MetEx14 (n = 12) or MET amp (n = 8) [52] | 5/20 PR mPFS, 5.5 mo MET TKI naïve (n = 10): ORR, 50%, 6.5 mo |
| REGN5093-M114 | NCT04982224 | MET-overexpressing NSCLC with no approved therapies [53] | Ongoing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Hsu, R. Anti-MET Antibody Therapies in Non-Small-Cell Lung Cancer: Current Progress and Future Directions. Antibodies 2024, 13, 88. https://doi.org/10.3390/antib13040088
Wang K, Hsu R. Anti-MET Antibody Therapies in Non-Small-Cell Lung Cancer: Current Progress and Future Directions. Antibodies. 2024; 13(4):88. https://doi.org/10.3390/antib13040088
Chicago/Turabian StyleWang, Kinsley, and Robert Hsu. 2024. "Anti-MET Antibody Therapies in Non-Small-Cell Lung Cancer: Current Progress and Future Directions" Antibodies 13, no. 4: 88. https://doi.org/10.3390/antib13040088
APA StyleWang, K., & Hsu, R. (2024). Anti-MET Antibody Therapies in Non-Small-Cell Lung Cancer: Current Progress and Future Directions. Antibodies, 13(4), 88. https://doi.org/10.3390/antib13040088






