A Bead-Based Nonradioactive Immunoassay for Autoantibody Testing in a Mouse Model of Myasthenia Gravis
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Model of MG
2.2. Detection of Pathogenic and Anti-AChR Autoantibodies
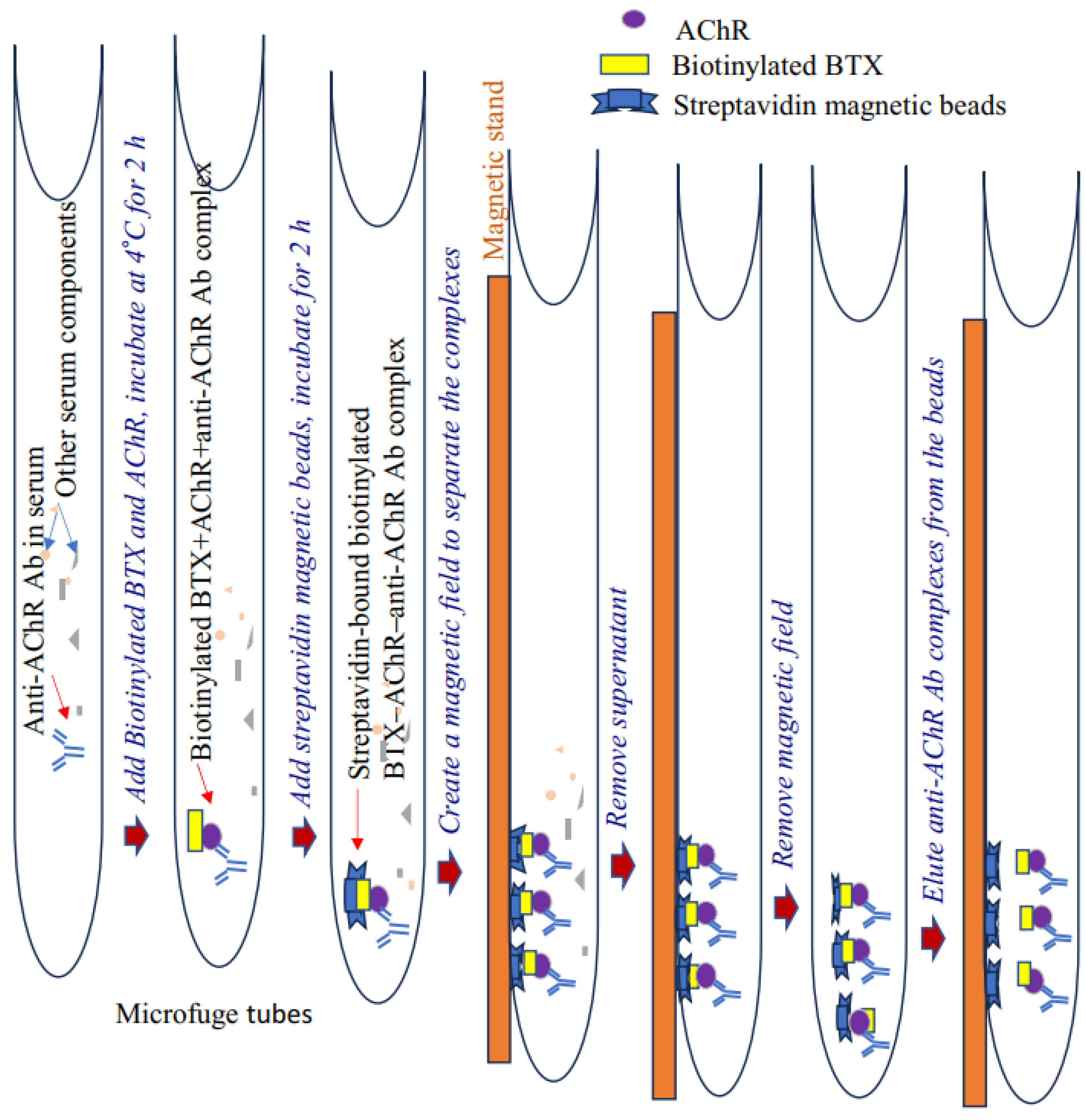
2.3. Assay Steps (Time Required: Approximately 2 to 4 h)
2.3.1. Immunoprecipitation
Materials
- PBS;
- PBS–Triton X-100 buffer (0.01% Triton X-100 in 1× PBS = 40 μL of Triton X-100 in 400 mL of 1× PBS);
- Affinity-purified Torpedo/mouse AChR—in-house purified (11);
- Alpha-Bungarotoxin Conjugates (#B1196; Invitrogen, MA, USA);
- Phenylmethylsulfonyl fluoride (Sigma, MO, USA), 100 mM in EtOH stock solution;
- Protease inhibitor cocktail (Sigma);
- Magnetic streptavidin beads (#5917; Cell Signaling Technology, MA, USA);
- Magnetic stand/rack (Invitrogen/StemCell Technologies, MA, USA).
Procedure
- Add 1 mL of 0.01% PBS–Triton X-100 buffer to prelabeled 1.5 mL microfuge tubes placed on ice. Triton X-100 (detergent) is necessary in order to separate magnetic beads on the tube surface efficiently.
- Add 2 μL of purified Torpedo or mouse AChR (concentration: 1 μg/mL), 2 μL of biotinylated BTX, and 3 μL of serum (from CFA- or CFA/AChR-immunized mice) to each tube. In addition, add 10 μL of phenylmethylsulfonyl fluoride (100 mM) and 10 μL of protease inhibitor cocktail. Incubate the tube on a rocker in a cold room for 2 h for immunoprecipitation.
- Prerinse the streptavidin magnetic beads with 300 µL of 0.01% PBS–Triton X-100 buffer. Preclearing is unnecessary for some beads (e.g., Dynabeads [Pierce]). Gently pulse-vortex the beads and add 3 μL of bead suspension into each tube. Wrap the tube lid with parafilm (to prevent leakage) and incubate for 2 h to overnight at 4 °C on a rotator.
- Briefly centrifuge the sample at a low speed (500 g) to move any solution from the lid into the microfuge tube. Place each tube on a magnetic stand/rack (Invitrogen/ThermoScientific) and incubate for 5 min to separate the beads from the extract.
- Carefully and gently aspirate (manually or with a vacuum aspirator) to remove the supernatant without disturbing the pellet in the microfuge tube.
- Add 1 mL of 0.01% PBS–Triton X-100 (wash solution) to each tube. Remove it from the magnetic stand, tap to resuspend, and return to the stand. Incubate for 5 min and then discard the supernatant, leaving the beads in the tube.
- Repeat step 6.
- Add 15 μL of PBS to each tube. Resuspend the beads in the buffer by tapping the tube.
2.3.2. Immunoblotting (Any Standard Method; Time Required: Approximately 6–8 h)
Materials
- Nondenaturing 4× Laemmli sample buffer (#1610747; Biorad, CA, USA);
- Mini-PROTEAN TGX Precast Gels (4–20%; #4561095; Biorad);
- Rainbow marker (Invitrogen, MA, USA);
- Tris-buffered saline (TBS50 mM Tris–HCl and 150 mM NaCl; pH: 7.5);
- Polyvinylidene fluoride membrane (Biorad);
- Tris–glycine transfer buffer (Biorad);
- Methanol (Sigma);
- Bovine serum albumin (Sigma);
- Secondary antibodies: sheep antimouse IgG–horseradish peroxidase antibody (HRP; #ab6808; abcam, MA, USA) or goat antimouse IgG2b (#M32407; Caltag Laboratories, Burlingame, CA, USA);
- Enhanced chemiluminescence (#32109; Pierce Biotechnology, MA, USA) reagent;
- Amersham Imager 680 (Amersham, NJ, USA).
Procedure
- Add 4 μL of 4× nonreducing sample loading buffer to each sample tube and tap to mix.
- Incubate each sample in a heating block (at 95 °C) for 5 min. Magnetically separate the beads and collect the supernatant containing the AChR-antibody-containing immune complex (optional). The supernatant can be stored at −80 °C for up to a month for immunoblot analysis.
- Load the samples together with beads (at room temperature) onto a 4–20% precast gel. In addition, load a rainbow marker (7–10 μL) into a gel well.
- Run the gel in TBS running buffer at 125 V for 1 h.
- To avoid a nonspecific background on the membrane, carefully slice off the bead-containing wells from the gel using a razor blade. Gently remove any remaining beads on the gel with water from a squirt bottle.
- Follow any standard immunoblot transfer protocol to transfer protein from the gel to a polyvinylidene fluoride (PVDF) membrane.
- After transfer, wash the wet PVDF membrane with distilled water 6 to 7 times to remove the methanol used in the transfer buffer. Label the front/back side of the membrane.
- Rinse the membrane in TBS once and replace TBS with approximately 25 mL of blocking buffer (1% bovine serum albumin in 0.5% TBS-Tween 20). Incubate the membrane for 30 min on a rocker.
- Wash the membrane and incubate with secondary antibodies (sheep antimouse IgG-HRP; dilution: 1:5000 [1 μL of secondary antibody in 5 mL of blocking buffer]) on a rocker for 1 h at room temperature.
- 10.
- Wash the blot thrice for 5 min with 0.5% TBS-Tween 20 buffer (approximately 15 mL).
- 11.
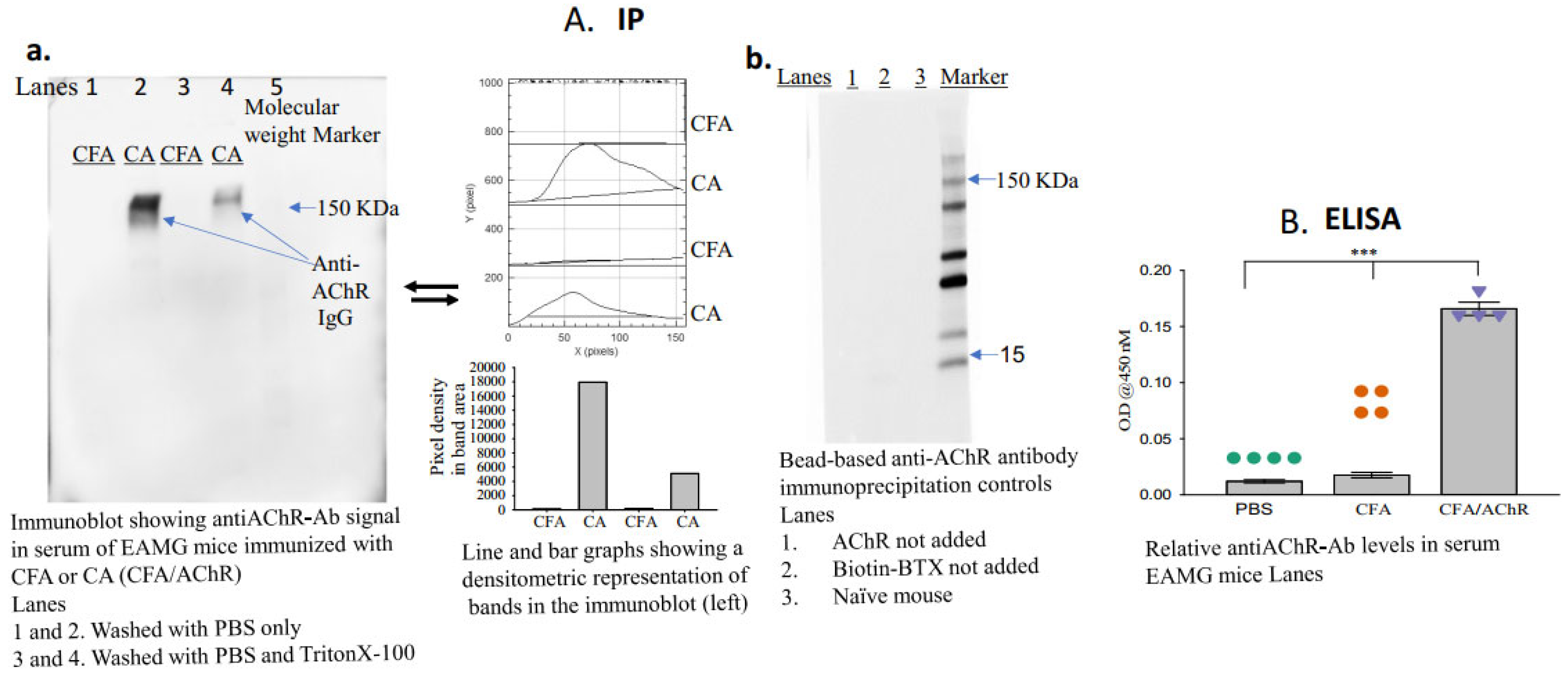
- Apply the enhanced chemiluminescence reagent, image the blot (using a Gel Imager, Amersham imager 680, GE Healthcare Life Sciences, Little Chalfont, Buckinghamshire, UK), and measure the density of each band (ImageJ.net 1.53F, National Institutes of Health, Bethesda, MD, USA) representing the titer of anti-AChR autoantibodies in each sample (Figure 2A(a,b)).
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilhus, N.E.; Tzartos, S.; Evoli, A.; Palace, J.; Burns, T.M.; Verschuuren, J.J.G.M. Myasthenia Gravis. Nat. Rev. Dis. Primers 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Koneczny, I.; Herbst, R. Myasthenia Gravis: Pathogenic Effects of Autoantibodies on Neuromuscular Architecture. Cells 2019, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Howard, F.M., Jr.; Lennon, V.A.; Finley, J.; Matsumoto, J.; Elveback, L.R. Clinical Correlations of Antibodies that Bind, Block, or Modulate Human Acetylcholine Receptors in Myasthenia Gravis. Ann. N. Y. Acad. Sci. 1987, 505, 526–538. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Gold, C.; Hagenacker, T.; Melzer, N.; Ruck, T. Understanding the Burden of Refractory Myasthenia Gravis. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419832242. [Google Scholar] [CrossRef] [PubMed]
- Christadoss, P.; Poussin, M.; Deng, C. Animal Models of Myasthenia Gravis. Clin. Immunol. 2000, 94, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.L.; Barrantes, F.J. Autoimmune Attack of the Neuromuscular Junction in Myasthenia Gravis: Nicotinic Acetylcholine Receptors and Other Targets. ACS Chem. Neurosci. 2019, 10, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Michail, M.; Zouvelou, V.; Belimezi, M.; Haroniti, A.; Zouridakis, M.; Zisimopoulou, P. Analysis of nAChR Autoantibodies Against Extracellular Epitopes in MG Patients. Front. Neurol. 2022, 13, 858998. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Yang, H. Serological Diagnosis of Myasthenia Gravis and Its Clinical Significance. Ann. Transl. Med. 2023, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Assini, A.; Gandoglia, I.; Damato, V.; Rikani, K.; Evoli, A.; Del Sette, M. Myasthenia Gravis Associated with Anti-MuSK Antibodies Developed after SARS-CoV-2 Infection. Eur. J. Neurol. 2021, 28, 3537–3539. [Google Scholar] [CrossRef] [PubMed]
- Bokoliya, S.; Patil, S.; Nagappa, M.; Taly, A. A Simple, Rapid and Non-Radiolabeled Immune Assay to Detect Anti-AChR Antibodies in Myasthenia Gravis. Lab. Med. 2019, 50, 229–235. [Google Scholar] [CrossRef]
- Wu, B.; Goluszko, E.; Huda, R.; Tüzün, E.; Christadoss, P. Experimental Autoimmune Myasthenia Gravis in the Mouse. Curr. Protoc. Immunol. 2011, 100, 15–18. [Google Scholar] [CrossRef]
- Ibtehaj, N.; Bahauddin, A.; Ivannikov, M.; Rytting, E.; Jamaluddin, M.; Liang, Y.; Sun, J.; Haller, S.L.; Wu, X.; Huda, R. B Cell-Specific mAb-siRNA Conjugates Improve Experimental Myasthenia. J. Autoimmun. 2023, 135, 102983. [Google Scholar] [CrossRef] [PubMed]
- Damato, V.; Spagni, G.; Monte, G.; Woodhall, M.; Jacobson, L.; Falso, S.; Smith, T.; Iorio, R.; Waters, P.; Irani, S.R.; et al. Clinical Value of Cell-Based Assays in the Characterisation of Seronegative Myasthenia Gravis. J. Neurol. Neurosurg. Psychiatry 2022, 93, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, C.; Chang, T.; Zhang, X.; Yang, H.; Gao, F.; Feng, J.; Liu, H.; Chen, S.; Wang, L.; et al. A Multicentre, Prospective, Double-Blind Study Comparing the Accuracy of Autoantibody Diagnostic Assays in Myasthenia Gravis: The SCREAM Study. Lancet Reg. Health West. Pac. 2023, 38, 100846. [Google Scholar] [CrossRef] [PubMed]
- Gambino, C.M.; Agnello, L.; Ciaccio, A.M.; Scazzone, C.; Vidali, M.; Di Stefano, V.; Milano, S.; Brighina, F.; Candore, G.; Lo Sasso, B.; et al. Detection of Antibodies against the Acetylcholine Receptor in Patients with Myasthenia Gravis: A Comparison of Two Enzyme Immunoassays and a Fixed Cell-Based Assay. J. Clin. Med. 2023, 12, 4781. [Google Scholar] [CrossRef] [PubMed]
- Young, H.S.; Herbette, L.G.; Skita, V. Alpha-bungarotoxin binding to acetylcholine receptor membranes studied by low angle X-ray diffraction. Biophys. J. 2003, 85, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Ibtehaj, N.; Huda, R. High-dose BAFF receptor specific mAb-siRNA conjugate generates Fas-expressing B cells in lymph nodes and high-affinity serum autoantibody in a myasthenia mouse model. Clin. Immunol. 2017, 176, 122–130. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahauddin, A.; Curtis, K.; Guptarak, J.; Huda, R. A Bead-Based Nonradioactive Immunoassay for Autoantibody Testing in a Mouse Model of Myasthenia Gravis. Antibodies 2024, 13, 53. https://doi.org/10.3390/antib13030053
Bahauddin A, Curtis K, Guptarak J, Huda R. A Bead-Based Nonradioactive Immunoassay for Autoantibody Testing in a Mouse Model of Myasthenia Gravis. Antibodies. 2024; 13(3):53. https://doi.org/10.3390/antib13030053
Chicago/Turabian StyleBahauddin, Afrin, Kyra Curtis, Jutatip Guptarak, and Ruksana Huda. 2024. "A Bead-Based Nonradioactive Immunoassay for Autoantibody Testing in a Mouse Model of Myasthenia Gravis" Antibodies 13, no. 3: 53. https://doi.org/10.3390/antib13030053
APA StyleBahauddin, A., Curtis, K., Guptarak, J., & Huda, R. (2024). A Bead-Based Nonradioactive Immunoassay for Autoantibody Testing in a Mouse Model of Myasthenia Gravis. Antibodies, 13(3), 53. https://doi.org/10.3390/antib13030053




