Antibodies against Platelet Glycoproteins in Clinically Suspected VITT Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
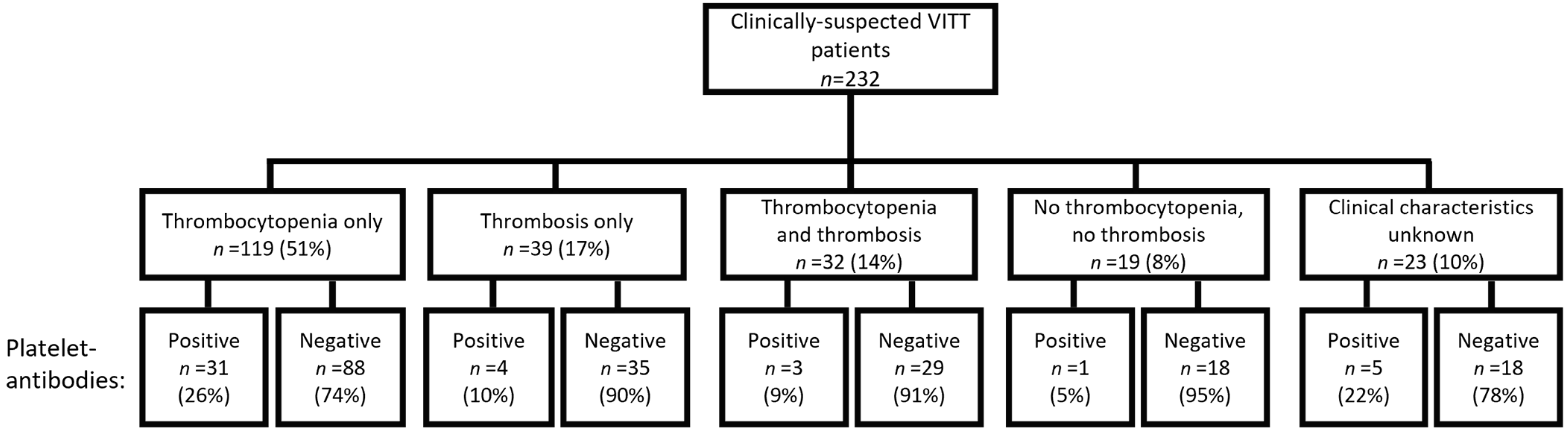
3.1. Patient Characteristics
3.2. Platelet-Antibodies in HIT-like VITT Patients
3.3. Clinical Characteristics in Clinically-Suspected VITT Patients with Platelet-Antibodies
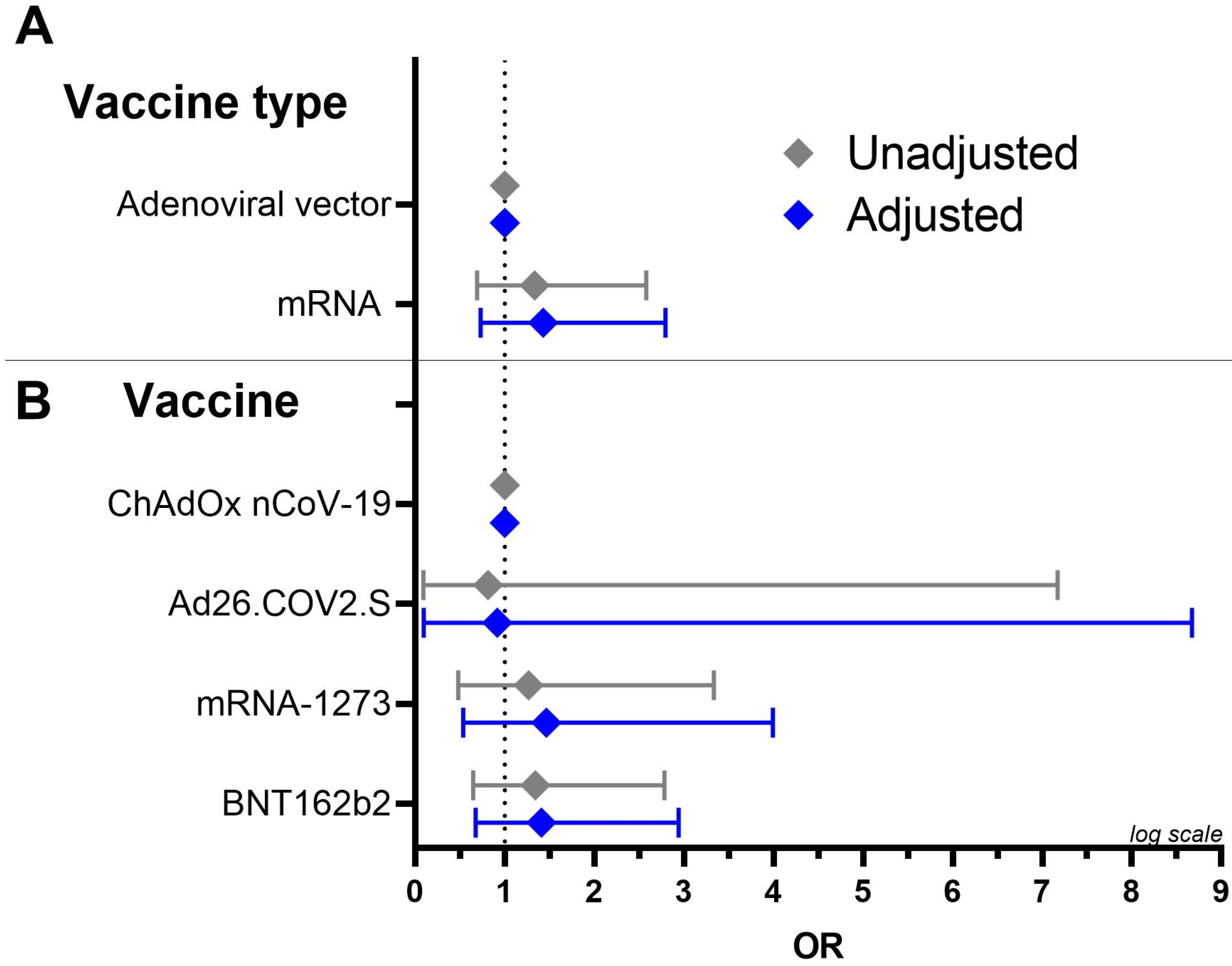
3.4. Presence of Platelet-Antibodies in Relation to Vaccines
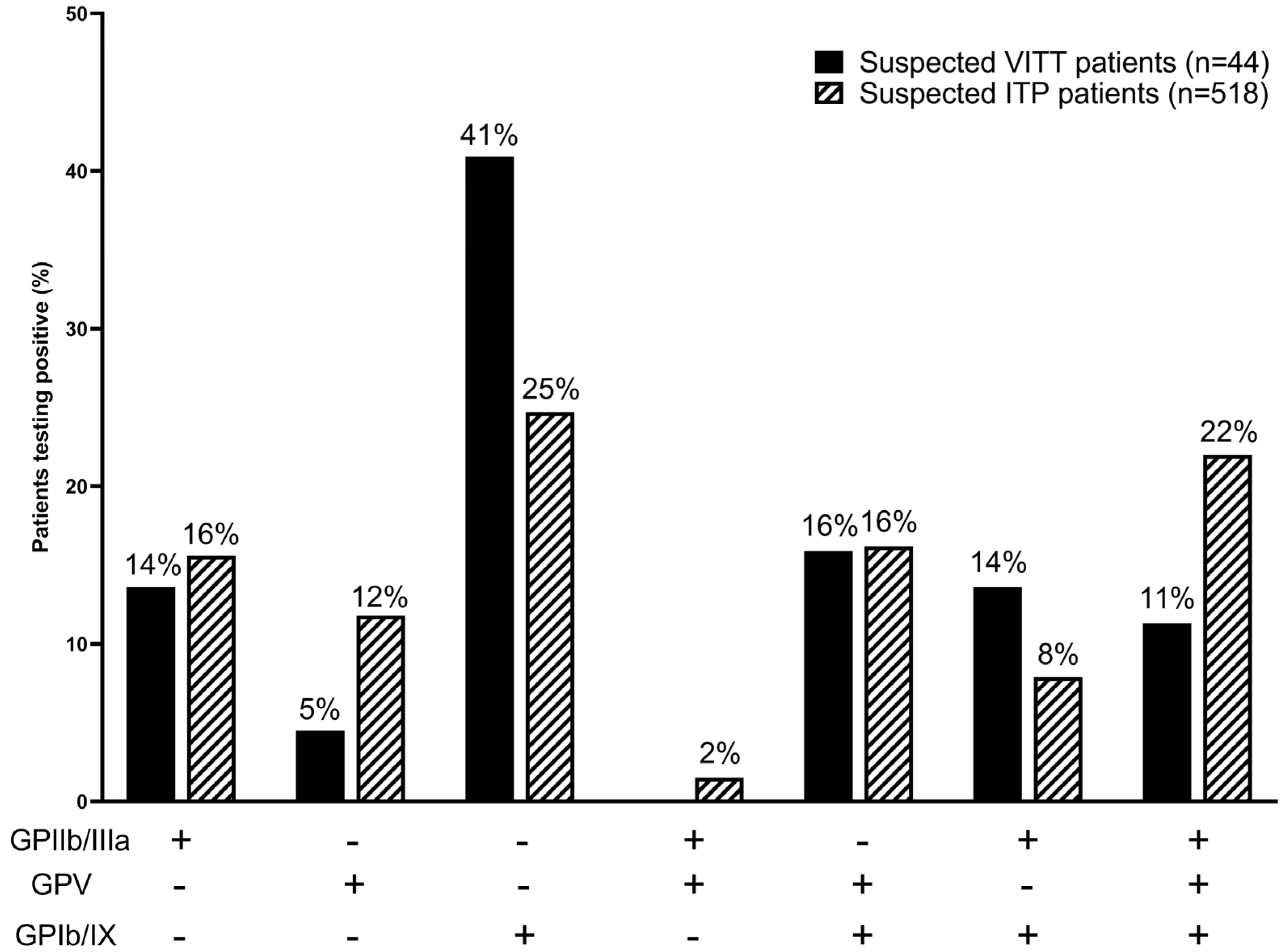
3.5. Platelet-Antibody Profiles
3.6. TPO Levels of Clinically-Suspected VITT Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef]
- Arepally, G.M. Clinical platelet disorders heparin-induced thrombocytopenia. Blood 2017, 129, 2864–2872. [Google Scholar] [CrossRef]
- Arepally, G.M.; Cines, D.B. Pathogenesis of heparin-induced thrombocytopenia. Transl. Res. 2020, 225, 131–140. [Google Scholar] [CrossRef]
- Nicolai, L.; Leunig, A.; Pekayvaz, K.; Esefeld, M.; Anjum, A.; Rath, J.; Riedlinger, E.; Ehreiser, V.; Mader, M.; Eivers, L.; et al. Thrombocytopenia and splenic platelet-directed immune responses after IV ChAdOx1 nCov-19 administration. Blood 2022, 140, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Kiefel, V. Platelet antibodies in immune thrombocytopenia and related conditions. J. Lab. Med. 2020, 44, 273–284. [Google Scholar] [CrossRef]
- Stéphan, F.; Cheffi, M.A.; Kaplan, C.; Maillet, J.M.; Novara, A.; Fagon, J.Y.; Bonnet, F. Autoantibodies against platelet glycoproteins in critically ill patients with thrombocytopenia. Am. J. Med. 2000, 108, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Porcelijn, L.; Schmidt, D.E.; Oldert, G.; Hofstede-van Egmond, S.; Kapur, R.; Zwaginga, J.J.; de Haas, M. Evolution and Utility of Antiplatelet Autoantibody Testing in Patients with Immune Thrombocytopenia. Transfus. Med. Rev. 2020, 34, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Porcelijn, L.; Schmidt, D.E.; Ellen van der Schoot, C.; Vidarsson, G.; de Haas, M.; Kapur, R. Anti-glycoprotein Ibα autoantibodies do not impair circulating thrombopoietin levels in immune thrombocytopenia patients. Haematologica 2020, 105, e172. [Google Scholar] [CrossRef]
- Petito, E.; Colonna, E.; Falcinelli, E.; Mezzasoma, A.M.; Cesari, E.; Giglio, E.; Fiordi, T.; Almerigogna, F.; Villa, A.; Gresele, P. Anti-severe acute respiratory syndrome coronavirus-2 adenoviral-vector vaccines trigger subclinical antiplatelet autoimmunity and increase of soluble platelet activation markers. Br. J. Haematol. 2022, 198, 257–266. [Google Scholar] [CrossRef]
- Kiefel, V.; Santoso, S.; Weisheit, M.; Mueller-Eckhardt, C. Monoclonal antibody--specific immobilization of platelet antigens (MAIPA): A new tool for the identification of platelet-reactive antibodies. Blood 1987, 70, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Foxcroft, Z.; Campbell, K.; Merieux, Y.; Urbaniak, S.; Brierley, M.; Rigal, D.; Ouwehand, W.H.; Metcalfe, P. Report on the 13th international society of blood transfusion platelet immunology workshop. Vox Sang. 2007, 93, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Folman, C.C.; Von Dem Borne, A.E.G.K.; Rensink, I.H.J.A.M.; Gerritsen, W.; Van Der Schoot, C.E.; De Haas, M.; Aarden, L. Sensitive measurement of thrombopoietin by a monoclonal antibody based sandwich enzyme-linked immunosorbent assay. Thromb. Haemost. 1997, 78, 1262–1267. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.T.; Brighton, C.; Black, S. Updated Proposed Brighton Collaboaration Process for Developing a Standard Case Definition for Study of New Clinical Syndrome X, as Applied to Thrombosis with Thrombocytopenia Syndrome (TTS). 2021. Available online: https://brightoncollaboration.org/wp-content/uploads/2023/08/TTS-Interim-Case-Definition-v10.16.3-May-23-2021.pdf (accessed on 23 January 2024).
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Bissola, A.L.; Daka, M.; Arnold, D.M.; Smith, J.W.; Moore, J.C.; Clare, R.; Ivetic, N.; Kelton, J.G.; Nazy, I. The clinical and laboratory diagnosis of vaccine-induced immune thrombotic thrombocytopenia. Blood Adv. 2022, 6, 4228–4235. [Google Scholar] [CrossRef]
- Nazy, I.; Sachs, U.J.; Arnold, D.M.; McKenzie, S.E.; Choi, P.; Althaus, K.; Ahlen, M.T.; Sharma, R.; Grace, R.F.; Bakchoul, T. Recommendations for the clinical and laboratory diagnosis of VITT against COVID-19: Communication from the ISTH SSC Subcommittee on Platelet Immunology. J. Thromb. Haemost. 2021, 19, 1585–1588. [Google Scholar] [CrossRef]
- Emmons, R.V.B.; Reid, D.M.; Cohen, R.L.; Meng, G.; Young, N.S.; Dunbar, C.E.; Shulman, N.R. Human Thrombopoietin Levels Are High When Thrombocytopenia Is Due to Megakaryocytic Deficiency and Low When Due to Increased Platelet Destruction. Blood 1996, 87, 4068–4071. [Google Scholar] [CrossRef]
- Kuter, D.J.; Phil, D.; Gernsheimer, T.B. Thrombopoietin and Platelet Production in Chronic Immune Thrombocytopenia. Hematol./Oncol. Clin. 2009, 23, 1193–1211. [Google Scholar] [CrossRef]
- Rogers, P.; Walker, I.; Yeung, J.; Khan, A.; Gangi, A.; Mobashwera, B.; Ayto, R.; Shah, A.; Hermans, J.; Murchison, A.; et al. Thrombus Distribution in Vaccine-induced Immune Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. Radiology 2022, 305, 590–596. [Google Scholar] [CrossRef]
- Saudagar, V.; Patil, S.; Goh, S.; Pothiawala, S. Vigilance regarding immune thrombocytopenic purpura after COVID-19 vaccine. Ir. J. Med. Sci. 2022, 191, 919. [Google Scholar] [CrossRef]
- Shah, S.R.A.; Dolkar, S.; Mathew, J.; Vishnu, P. COVID-19 vaccination associated severe immune thrombocytopenia. Exp. Hematol. Oncol. 2021, 10, 42. [Google Scholar] [CrossRef]
- Liao, P.W.; Teng, C.L.J.; Chou, C.W. Immune Thrombocytopenia Induced by the Chimpanzee Adenovirus-Vectored Vaccine against SARS-CoV-2 Infection. Vaccines 2021, 9, 1486. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Nazy, I.; Smith, J.W.; Kelton, J.G.; Arnold, D.M. Platelet autoantibodies in the bone marrow of patients with immune thrombocytopenia. Blood Adv. 2020, 4, 2962. [Google Scholar] [CrossRef]
- Kaushansky, K. The molecular mechanisms that control thrombopoiesis. J. Clin. Investig. 2005, 115, 3339. [Google Scholar] [CrossRef]
- Provan, D.; Arnold, D.M.; Bussel, J.B.; Chong, B.H.; Cooper, N.; Gernsheimer, T.; Ghanima, W.; Godeau, B.; González-López, T.J.; Grainger, J.; et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019, 3, 3780–3817. [Google Scholar] [CrossRef]
- Kuter, D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br. J. Haematol. 2021, 195, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Beltrami-Moreira, M.; Al-Samkari, H.; Cuker, A.; DiRaimo, J.; Gernsheimer, T.; Kruse, A.; Kessler, C.; Kruse, C.; Leavitt, A.D.; et al. SARS-CoV-2 vaccination and ITP in patients with de novo or preexisting ITP. Blood 2022, 139, 1564–1574. [Google Scholar] [CrossRef]
- Kapur, R.; Kustiawan, I.; Vestrheim, A.; Koeleman, C.A.M.; Visser, R.; Einarsdottir, H.K.; Porcelijn, L.; Jackson, D.; Kumpel, B.; Deelder, A.M.; et al. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood 2014, 123, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Moulis, G.; Crickx, E.; Thomas, L.; Massy, N.; Mahévas, M.; Valnet-Rabier, M.B.; Atzenhoffer, M.; Michel, M.; Godeau, B.; Bagheri, H.; et al. De novo and relapsed immune thrombocytopenia after COVID-19 vaccines: Results of French safety monitoring. Blood 2022, 139, 2561–2565. [Google Scholar] [CrossRef]
- Mingot-Castellano, M.E.; Butta, N.; Canaro, M.; Del Castillo Solano, M.D.C.G.; Sánchez-González, B.; Jiménez-Bárcenas, R.; Pascual-Izquierdo, C.; Caballero-Navarro, G.; Ureña, L.E.; González-López, T. COVID-19 Vaccines and Autoimmune Hematologic Disorders. Vaccines 2022, 10, 961. [Google Scholar] [CrossRef]
- Lee, E.J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 96, 534. [Google Scholar] [CrossRef] [PubMed]
- The ITP Support Association—Vaccinations and ITP. Available online: https://www.itpsupport.org.uk/index.php/en/vaccinations-and-itp (accessed on 26 April 2023).
- Welsh, K.J.; Baumblatt, J.; Chege, W.; Goud, R.; Nair, N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2021, 39, 3329. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Banerjee, M. Immune Thrombocytopenia Secondary to COVID-19: A Systematic Review. SN Compr. Clin. Med. 2020, 2, 2048. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.G.; Alanazi, N.; Yousef, A.; Alanazi, N.; Alotaibi, B.; Aljurf, M.; El Fakih, R. COVID-19 associated with immune thrombocytopenia: A systematic review and meta-analysis. Expert Rev. Hematol. 2022, 15, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Schipperus, M.R.; Nelson, V.S.; Amini, S.N. Immuuntrombocytopenie (ITP): Hoofdpunten uit de richtlijn van 2020 met aanbevelingen voor diagnostiek en behandeling. Ned. Tijdschr. Hematol. 2021, 18, 20-8. [Google Scholar]
- Koilpillai, S.; Dominguez, B.; Khan, A.; Carlan, S. Severe case of refractory immune thrombocytopenic purpura requiring splenectomy after the COVID-19 vaccine. BMJ Case Rep. 2022, 15, e250153. [Google Scholar] [CrossRef]
- Confirmed Cases|Coronavirus Dashboard|Government.nl. Cited. Available online: https://coronadashboard.government.nl/landelijk/positief-geteste-mensen (accessed on 8 February 2023).
| Clinically Suspected Patients (n = 232) | Positive for Platelet Antibodies (n = 44) | Negative for Platelet Antibodies (n = 188) | |
|---|---|---|---|
| Demographics | |||
| Median age (IQR) | 62 (53–68) | 62 (54–69) | 60 (53–69) |
| Female sex (no.(%)) | 111 (48%) | 24 (55%) | 87 (46%) |
| Male sex (no.(%)) | 121 (53%) | 20 (45%) | 101 (54%) |
| Vaccination | |||
| Vaccine type (no.(%)) | |||
| Adenoviral vector vaccines | 119 (51%) | 20 (45%) | 99 (53%) |
| ChAdOx1 nCoV-19 | 112 (48%) | 19 (43%) | 93 (50%) |
| Ad26.COV2.S | 7 (3%) | 1 (2%) | 6 (3%) |
| mRNA vaccines | 113 (49%) | 24 (55%) | 89 (47%) |
| mRNA-1273 | 34 (15%) | 7 (16%) | 27 (14%) |
| BTN162b2 | 79 (34%) | 17 (39%) | 62 (33%) |
| Days between admission and vaccination | |||
| Mean (IQR) | 21 (8–28) | 24 (9–29) | 21 (8–28) |
| Number of vaccination (no.(%)) | |||
| First dose | 37 (16%) | 10 (23%) | 31 (17%) |
| Second dose | 68 (29%) | 15 (34%) | 55 (29%) |
| Third dose | 2 (1%) | - | 2 (1%) |
| No information on dose | 125 (54%) | 19 (43%) | 100 (53%) |
| Clinical characteristics (no.(%)) | |||
| Thrombocytopenia (<100 × 109/L) | 151 (65%) | 34 (77%) | 117 (62%) |
| Median platelet count (IQR) | 51 (18–99) | 35 (8–63) | 55 (21–108) |
| No thrombocytopenia | 55 (24%) | 4 (9%) | 51 (27%) |
| No data on platelet count | 26 (11%) | 6 (14%) | 20 (11%) |
| Thrombosis | 71 (31%) | 7 (16%) | 64 (34%) |
| No thrombosis | 129 (56%) | 31 (71%) | 98 (52%) |
| No data on thrombosis available | 32 (14%) | 6 (14%) | 26 (14%) |
| Thrombocytopenia and thrombosis | 32 (14%) | 3 (7%) | 29 (15%) |
| Thrombocytopenia only | 119 (51%) | 31 (71%) | 88 (47%) |
| Thrombosis only | 39 (17%) | 4 (9%) | 35 (19%) |
| Neither thrombocytopenia nor thrombosis | 19 (8%) | 1 (2%) | 18 (10%) |
| No data on both thrombocytopenia and thrombosis | 23 (10%) | 5 (11%) | 18 (10%) |
| Laboratory tests | |||
| Anti-PF4 ELISA negative (OD < 1.0) | 212 (91%) | 42 (96%) | 170 (90%) |
| PIPAA negative | 206 (97%) | 38 (90%) | 168 (99%) |
| PIPAA positive | 6 (3%) | 4 (10%) | 2 (1%) |
| Anti-PF4 ELISA weak-positive (1.0 ≤ OD < 2.0) | 7 (3%) | 1 (2%) | 6 (3%) |
| PIPAA negative | 6 (86%) | 1 (100%) | 5 (83%) |
| PIPAA positive | 1 (14%) | - | 1 (17%) |
| Anti-PF4 ELISA positive (OD ≥ 2.0) | 13 (6%) | 1 (2%) | 12 (6%) |
| PIPAA negative | 3 (23%) | 1 (100%) | 2 (17%) |
| PIPAA positive | 10 (77%) | - | 10 (83%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meier, R.T.; Porcelijn, L.; Hofstede-van Egmond, S.; Caram-Deelder, C.; Coutinho, J.M.; Henskens, Y.M.C.; Kruip, M.J.H.A.; Stroobants, A.K.; Zwaginga, J.J.; van der Schoot, C.E.; et al. Antibodies against Platelet Glycoproteins in Clinically Suspected VITT Patients. Antibodies 2024, 13, 35. https://doi.org/10.3390/antib13020035
Meier RT, Porcelijn L, Hofstede-van Egmond S, Caram-Deelder C, Coutinho JM, Henskens YMC, Kruip MJHA, Stroobants AK, Zwaginga JJ, van der Schoot CE, et al. Antibodies against Platelet Glycoproteins in Clinically Suspected VITT Patients. Antibodies. 2024; 13(2):35. https://doi.org/10.3390/antib13020035
Chicago/Turabian StyleMeier, Romy T., Leendert Porcelijn, Suzanne Hofstede-van Egmond, Camila Caram-Deelder, Jonathan M. Coutinho, Yvonne M. C. Henskens, Marieke J. H. A. Kruip, An K. Stroobants, Jaap J. Zwaginga, C. Ellen van der Schoot, and et al. 2024. "Antibodies against Platelet Glycoproteins in Clinically Suspected VITT Patients" Antibodies 13, no. 2: 35. https://doi.org/10.3390/antib13020035
APA StyleMeier, R. T., Porcelijn, L., Hofstede-van Egmond, S., Caram-Deelder, C., Coutinho, J. M., Henskens, Y. M. C., Kruip, M. J. H. A., Stroobants, A. K., Zwaginga, J. J., van der Schoot, C. E., de Haas, M., & Kapur, R. (2024). Antibodies against Platelet Glycoproteins in Clinically Suspected VITT Patients. Antibodies, 13(2), 35. https://doi.org/10.3390/antib13020035






