Real-Life Use of Component-Specific IgE in IgE-Mediated Cow’s Milk Protein Allergy in a Spanish Paediatric Allergy Centre
Abstract
:1. Introduction
2. Methods
2.1. Study Population
- Positive skin prick test for cow’s milk (≥3 mm);
- Specific IgE levels for cow’s milk and or milk components of >0.35 k U/L.
2.2. Intervention
2.3. Outcome Measurements
2.4. Clinical Characterisation of Subjects
2.5. Data Analysis
3. Results
3.1. Study Population
3.2. Primary Outcome
3.2.1. Completion of Treatment
3.2.2. Skin Prick Test and Milk-Component-Specific IgE Levels
3.3. Secondary Outcomes
Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CMPA | Cow’s milk protein allergy |
| OFC | Oral food challenge |
| SPT | Skin prick test |
| CI | Confidence interval |
| AAI | Adrenaline autoinjector |
| EAACI | European Academy of Allergy and Clinical Immunology |
| OIT | Oral immunotherapy |
References
- Wood, R.A.; Sicherer, S.H.; Vickery, B.P.; Jones, S.M.; Liu, A.H.; Fleischer, D.M.; Henning, A.K.; Mayer, L.; Burks, A.W.; Grishin, A.; et al. The natural history of milk allergy in an observational cohort. J. Allergy Clin. Immunol. 2013, 131, 805–812.e4. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, A.A.; Sprikkelman, A.B.; Grimshaw, K.E.; Roberts, G.; Grabenhenrich, L.; Rosenfeld, L.; Siegert, S.; Dubakiene, R.; Rudzeviciene, O.; Reche, M.; et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children—EuroPrevall birth cohort. Allergy 2015, 70, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.; Vespasiani, G.T.; Ricci, G.; Miniaci, A.; di Palmo, E.; Pession, A. Cow’s milk protein allergy as a model of food allergies. Nutrients 2021, 13, 1525. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27, 1–250. [Google Scholar] [CrossRef] [PubMed]
- Dramburg, S.; Hilger, C.; Santos, A.F.; Vecillas, L.d.L.; Aalberse, R.C.; Acevedo, N.; Aglas, L.; Altmann, F.; Arruda, K.L.; Asero, R.; et al. EAACI Molecular Allergology User’s Guide 2.0. Pediatr. Allergy Immunol. 2023, 34, e13854. [Google Scholar] [CrossRef] [PubMed]
- Cerecedo, I.; Zamora, J.; Shreffler, W.G.; Lin, J.; Bardina, L.; Dieguez, M.C.; Wang, J.; Muriel, A.; de la Hoz, B.; Sampson, H.A. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray-based immunoassay. J. Allergy Clin. Immunol. 2008, 122, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ara, M.C.; Boyano-Martínez, M.T.; Díaz-Pena, J.M.; Martín-Muñoz, M.F.; Martin-Esteban, M. Cow’s milk-specific immunoglobulin E levels as predictors of clinical reactivity in the follow-up of the cow’s milk allergy infants. Clin. Exp. Allergy 2004, 34, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Cronin, C.; Ramesh, Y.; De Pieri, C.; Velasco, R.; Trujillo, J. ‘Early Introduction’ of Cow’s Milk for Children with IgE-Mediated Cow’s Milk Protein Allergy: A Review of Current and Emerging Approaches for CMPA Management. Nutrients 2023, 15, 1397. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, B.; Lopes de Oliveira, L.C.; Grabenhenrich, L.; Schulz, G.; Niggemann, B.; Wahn, U.; Beyer, K. Individual cow’s milk allergens as prognostic markers for tolerance development? Clin. Exp. Allergy 2012, 42, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Chatchatee, P.; Järvinen, K.M.; Bardina, L.; Beyer, K.; Sampson, H.A. Identification of IgE- and IgG-binding epitopes on αs1-casein: Differences in patients with persistent and transient cow’s milk allergy. J. Allergy Clin. Immunol. 2001, 107, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Chatchatee, P.; Järvinen, K.M.; Bardina, L.; Vila, L.; Beyer, K.; Sampson, H.A. Identification of IgE and IgG binding epitopes on β- and κ-casein in cow’s milk allergic patients. Clin. Exp. Allergy 2001, 31, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Pajno, G.B.; Fernandez-Rivas, M.; Arasi, S.; Roberts, G.; Akdis, C.A.; Alvaro-Lozano, M.; Beyer, K.; Bindslev-Jensen, C.; Burks, W.; Ebisawa, M.; et al. EAACI Guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy Eur. J. Allergy Clin. Immunol. 2018, 73, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.B.; Luyt, D. Home-based cow’s milk reintroduction using a milk ladder in children less than 3 years old with IgE-mediated cow’s milk allergy. Clin. Exp. Allergy 2019, 49, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Cronin, C.; McGinley, A.M.; Flores, L.; McKiernan, A.; Velasco, R.; Hourihane, J.O.; Trujillo, J. Primary care as a setting for introducing milk using the milk ladder in children with IgE-mediated cow’s milk protein allergy. Clincal Transl. Allergy 2023, 13, e12286. [Google Scholar] [CrossRef] [PubMed]
- Dumpler, J.; Huppertz, T.; Kulozik, U. Invited review: Heat stability of milk and concentrated milk: Past, present, and future research objectives. J. Dairy Sci. 2020, 103, 10986–11007. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Yokooji, T.; Taogoshi, T. Common food allergens and their IgE-binding epitopes. Allergol. Int. 2015, 64, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Campbell, D.E.; Motosue, M.S.; Campbell, R.L. Global Trends in Anaphylaxis Epidemiology and Clinical Implications. J. Allergy Clin. Immunol. Pract. 2020, 8, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, J.; Cronin, C. Benefit of educational intervention on Autoinjector Technique for caregivers and paediatric patients with food allergies: A literature review. Allergol. Immunopathol. 2022, 50, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Calvani, M.; Cardinale, F.; Martelli, A.; Muraro, A.; Pucci, N.; Savino, F.; Zappalà, D.; Panetta, V.; The Italian Society of Pediatric Allergy and Immunology (SIAIP) Anaphylaxis’ Study Group. Risk factors for severe pediatric food anaphylaxis in Italy. Pediatr. Allergy Immunol. 2011, 22, 813–819. [Google Scholar] [CrossRef] [PubMed]

| Patients (n = 200) | |
|---|---|
| Sex—male | 126 (63%) |
| Age in months at diagnosis—median (IQR) | 5 (4–7) |
| Prematurity | 4 (2%) |
| Feeding before diagnosis | |
| Breast fed | 181 (90.5%) |
| Bottle fed | 10 (5%) |
| Mixed | 9 (4.5%) |
| Unknown | 0 (0%) |
| Duration of breastfeeding (if breastfed) in months | 5 (4–6) |
| Atopic dermatitis | 122 (61%) |
| Asthma/viral-induced wheeze | 95 (47.5%) |
| Allergic rhinitis | 39 (19.2%) |
| Any food allergy | 79 (39.5%) |
| Egg | 63 (31.5%) |
| Peanut | 13 (6.5%) |
| Other nuts | 13 (6.5%) |
| Other food | 26 (13%) |
| Any family history of atopic conditions | 132 (66%) |
| Food allergy | 36 (18%) |
| Atopic dermatitis | 32 (16%) |
| Asthma | 52 (26%) |
| Allergic rhinitis | 82 (41%) |
| Carry autoinjectors | 95 (47.5%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Success n = 122 (%) | p-Value | OR (95% CI) | p-Value | |
| Sex | 0.390 | |||
| Male | 74 (58.7) | |||
| Female | 48 (64.9) | |||
| Prematurity | 0.106 | |||
| Yes | 4 (100) | |||
| No | 118 (60.2) | |||
| Atopic dermatitis | 0.107 | |||
| Yes | 69 (56.6) | |||
| No | 53 (67.9) | |||
| Asthma/viral-induced wheeze | <0.001 * OR 0.248 (95% CI: 0.135–0.453) | OR 0.583 (0.259–1.315) | p = 0.194 | |
| Yes | 42 (44.2) | |||
| No | 80 (76.2) | |||
| Allergic rhinitis | <0.001 * OR 0.205 (95% CI: 0.097–0.439) | OR 0.612 (0.218–1.722) | p = 0.353 | |
| Yes | 12 (30.8) | |||
| No | 110 (68.3) | |||
| Egg allergy | 0.003 * OR 0.404 (95% CI: 0.219–0.744) | OR 0.856 (0.354–2.069) | p = 0.730 | |
| Yes | 29 (46) | |||
| No | 93 (67.9) | |||
| Peanut allergy | 0.004 * OR 0.171 (95% CI: 0.046–0.644) | OR 0.492 (0.1–2.427) | p = 0.384 | |
| Yes | 3 (23.1) | |||
| No | 119 (63.6) | |||
| Anaphylaxis prior to diagnosis | 0.732 | |||
| Yes | 5 (55.6) | |||
| No | 117 (61.3) | |||
| Carry autoinjector | <0.001 * OR 0.063 (95% CI: 0.031–0.129) | OR 0.148 (0.06–0.367) | p < 0.001 ¥ | |
| Yes | 16 (22.5) | |||
| No | 106 (82.2) | |||
| Allergic reaction to milk during treatment (accidental exposure) | <0.001 * OR 0.224 (95% CI: 0.12–0.418) | OR 0.396 (0.178–0.881) | p = 0.023 ¥ | |
| Yes | 48 (45.3) | |||
| No | 74 (78.7) | |||
| Anaphylaxis during treatment (accidental exposure) | <0.001 * OR 0.024 (95% CI: 0.006–0.104) | OR 0.066 (0.013–0.331) | p < 0.001 ¥ | |
| Yes | 2 (5.9) | |||
| No | 120 (72.3) | |||
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Success (Mean (95% CI)) | Fail (Mean (95% CI)) | p-Value | OR (95% CI) | p-Value | |
| Skin prick tests (SPTs) at diagnosis | |||||
| SPT—whole milk (mm) | 2.43 (2.01–2.84) | 3.63 (2.90–4.36) | 0.002 * | 1.036 (0.848–1.265) | 0.732 |
| SPT—alpha-lactalbumin (mm) | 3.17 (2.566–3.78) | 4.33 (3.44–5.21) | 0.028 * | 1.017 (0.891–1.16) | 0.804 |
| SPT—beta-lactoglobulin (mm) | 3.33 (2.82–3.85) | 4.3 (3.39–5.21) | 0.05 * | 1.047 (0.905–1.211) | 0.537 |
| SPT—casein (mm) | 2.16 (1.75–2.58) | 3.33 (2.64–4.01) | 0.002 * | 0.984 (0.789–1.227) | 0.884 |
| Specific IgE levels at diagnosis | |||||
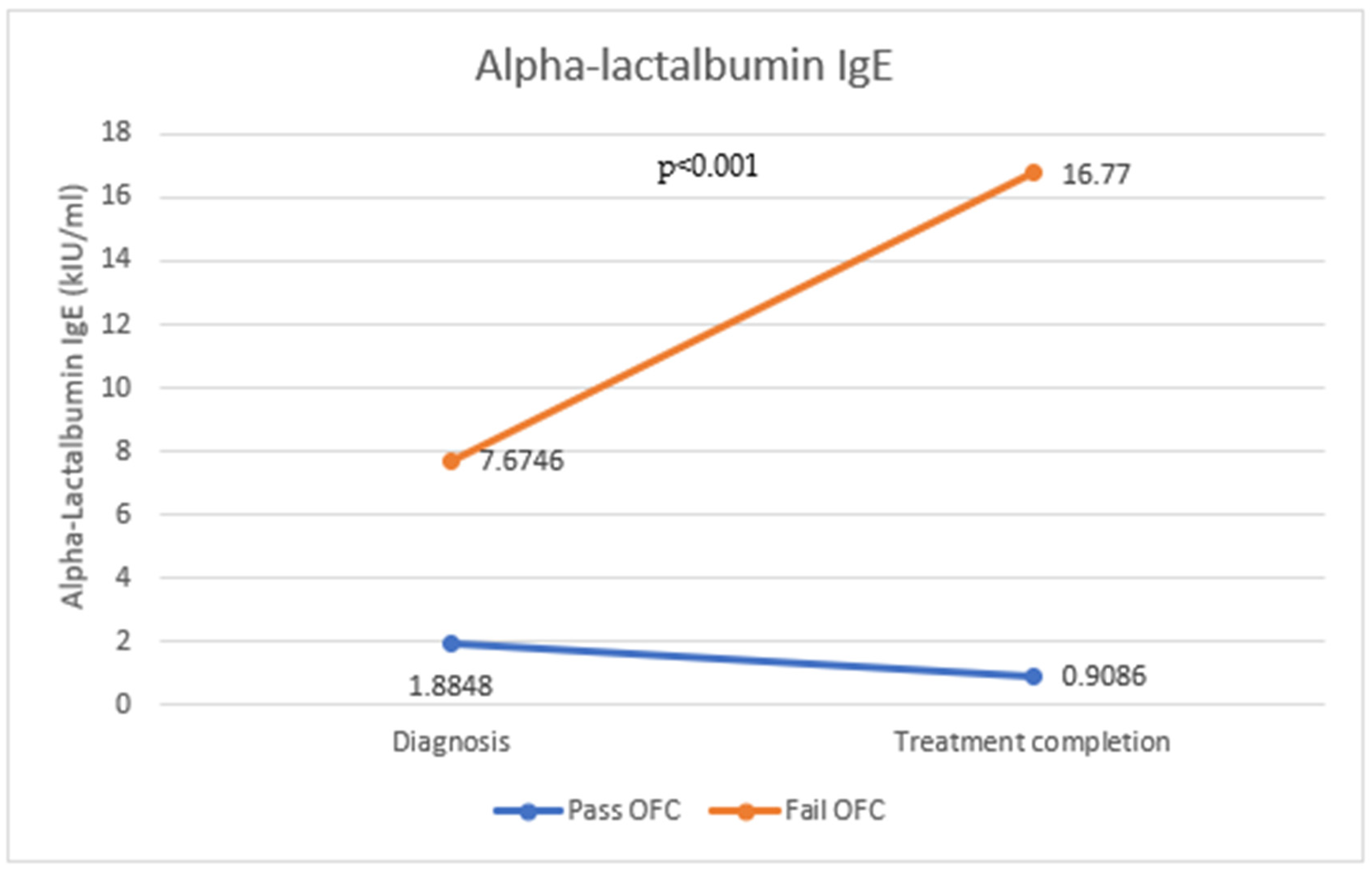
| Alpha-lactalbumin-specific IgE (kIU/mL) | 1.844 (1.05–2.62) | 7.59 (4.65–10.53) | <0.001 * | 1.08 (0.98–1.189) | 0.12 |
| Beta-lactoglobulin-specific IgE (kIU/mL) | 2.29 (1.52–3.06) | 5.31 (3.16–7.46) | 0.003 * | 1.014 (0.937–1.096) | 0.734 |
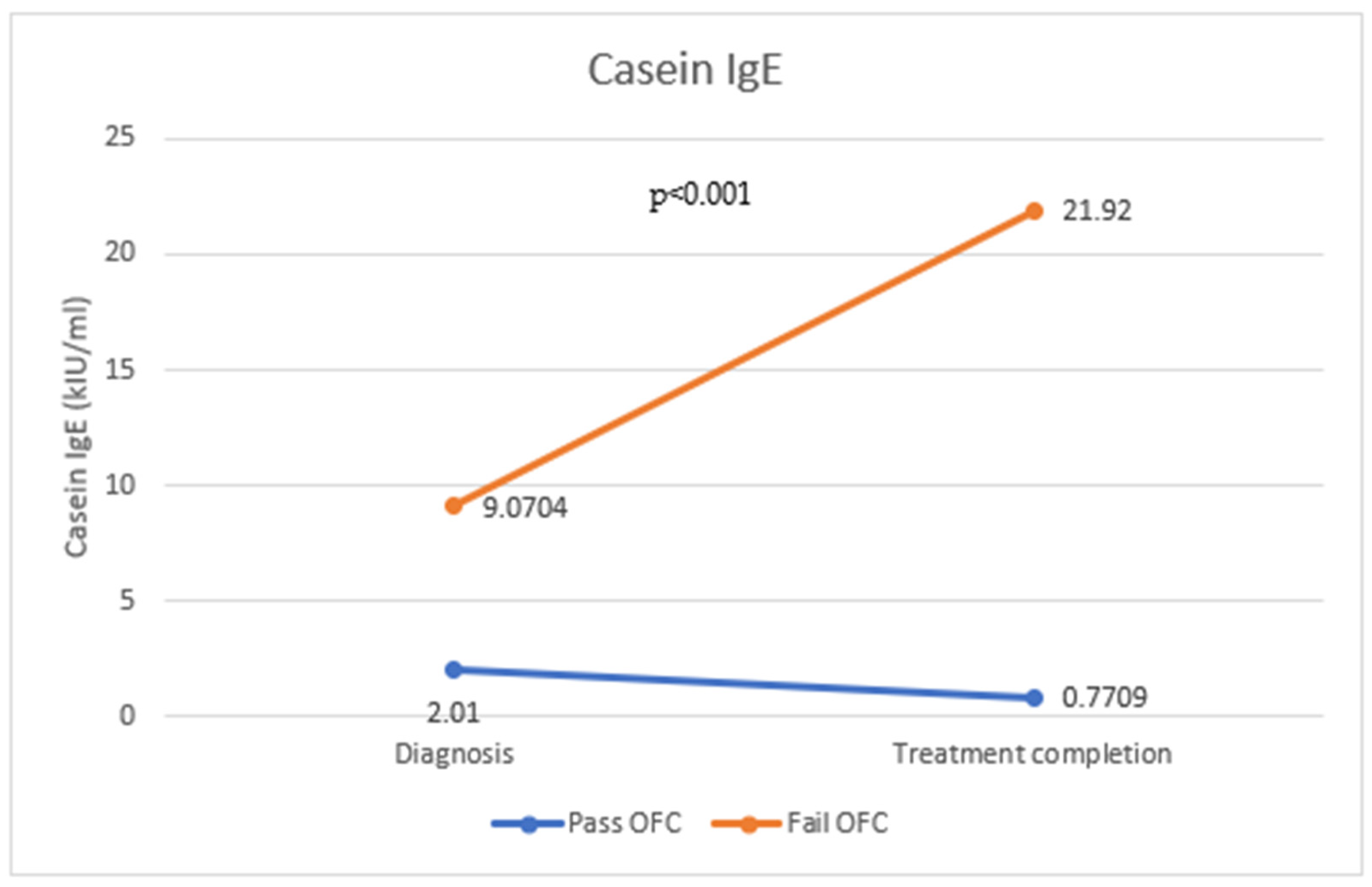
| Casein-specific IgE (kIU/mL) | 1.93 (1.28–2.59) | 9.45 (6.43–12.47) | <0.001 * | 1.129 (1.019–1.251) | 0.02 ¥ |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Anaphylaxis (Mean (95% CI)) | No Anaphylaxis (Mean (95% CI)) | p-Value | OR (95% CI) | p-Value | |
| Skin prick tests (SPTs) at diagnosis | |||||
| SPT—whole milk (mm) | 3.84 (2.64–5.04) | 2.7 (2.31–3.1) | 0.029 * | 0.977 (0.782–1.220) | 0.836 |
| SPT—alpha-lactalbumin (mm) | 4.21 (2.83–5.6) | 3.5 (2.9–4.05) | 0.306 | ||
| SPT—beta-lactoglobulin (mm) | 4.12 (2.85–5.39) | 3.63 (3.11–4.14) | 0.449 | ||
| SPT—casein (mm) | 3.5 (2.45–4.54) | 2.44 (2.05–2.83) | 0.037 * | 0.938 (0.738–1.194) | 0.605 |
| Specific IgE levels at diagnosis | |||||
| Alpha-lactalbumin-specific IgE (kIU/mL) | 6.14 (1.86–10.42) | 3.66 (2.36–4.96) | 0.155 | ||
| Beta-lactoglobulin-specific IgE (kIU/mL) | 4.5 (1.58–7.43) | 3.26 (2.24–4.28) | 0.344 | ||
| Casein-specific IgE (kIU/mL) | 9.17 (5.17–13.16) | 3.99 (2.62–5.36) | 0.004 * | 0.98 (0.938–1.024) | 0.361 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cronin, C.; Muñoz Archidona, C.; Fernández Prudencio, B.; Gallagher, A.; Velasco Zuniga, R.; Trujillo Wurttele, J. Real-Life Use of Component-Specific IgE in IgE-Mediated Cow’s Milk Protein Allergy in a Spanish Paediatric Allergy Centre. Antibodies 2023, 12, 76. https://doi.org/10.3390/antib12040076
Cronin C, Muñoz Archidona C, Fernández Prudencio B, Gallagher A, Velasco Zuniga R, Trujillo Wurttele J. Real-Life Use of Component-Specific IgE in IgE-Mediated Cow’s Milk Protein Allergy in a Spanish Paediatric Allergy Centre. Antibodies. 2023; 12(4):76. https://doi.org/10.3390/antib12040076
Chicago/Turabian StyleCronin, Caoimhe, Cristina Muñoz Archidona, Beatriz Fernández Prudencio, Aoife Gallagher, Roberto Velasco Zuniga, and Juan Trujillo Wurttele. 2023. "Real-Life Use of Component-Specific IgE in IgE-Mediated Cow’s Milk Protein Allergy in a Spanish Paediatric Allergy Centre" Antibodies 12, no. 4: 76. https://doi.org/10.3390/antib12040076
APA StyleCronin, C., Muñoz Archidona, C., Fernández Prudencio, B., Gallagher, A., Velasco Zuniga, R., & Trujillo Wurttele, J. (2023). Real-Life Use of Component-Specific IgE in IgE-Mediated Cow’s Milk Protein Allergy in a Spanish Paediatric Allergy Centre. Antibodies, 12(4), 76. https://doi.org/10.3390/antib12040076







