Abstract
Seronegative rheumatoid arthritis (SNRA) is characterized by the absence of both rheumatoid factor (RF) and antibodies against the cyclic citrullinated protein (ACPA) in serum. However, the differences between the two forms of RA are more complex and have not yet been definitively characterized. Several lines of evidences support the idea that there are specific elements of the two forms, including genetic background, epidemiology, pathogenesis, severity of progression over time, and response to therapy. Clinical features that may differentiate SNRA from SPRA are also suggested by data obtained from classical radiology and newer imaging techniques. Although new evidence seems to provide additional help in differentiating the two forms of RA, their distinguishing features remain largely elusive. It should also be emphasized that the distinctive features of RA forms, if not properly recognized, can lead to the underdiagnosis of SNRA, potentially missing the period called the “window of opportunity” that is critical for early diagnosis, timely treatment, and better prognosis. This review aims to summarize the data provided in the scientific literature with the goal of helping clinicians diagnose SNRA as accurately as possible, with emphasis on the most recent findings available.
1. Introduction
Rheumatoid arthritis (RA) is an inflammatory disease that primarily affects synovial joints through an autoimmune mechanism. If not treated properly, the disease can lead to bone erosion, joint deformities, and disability. Arthritis can also cause serious extra-articular disorders, including interstitial lung disease, vasculitis, and lymphoma [1,2]. According to the latest 2010 ACR/EULAR criteria [3], the diagnosis is based on a scoring system calculated using symptom duration, the number and type of joints affected, altered acute-phase reactants, and the presence of autoantibodies, such as rheumatoid factor (RF) and/or anti-citrullinated protein antibodies (ACPAs), in serum [4]. Because the presence of RF and ACPA in serum is not necessary for the diagnosis of RA, a substantial number of patients presenting with the typical clinical features of RA in the absence of these autoantibodies can be diagnosed as having RA. The form of RA without RF and/or ACPA is termed seronegative RA (SNRA) [5,6]. Numerous observations have reported that the clinical presentation, course severity, and response to therapy appear to be significantly different between SNRA and seropositive RA (SPRA) [7,8,9]. In recent years, the focus on seronegative forms of RA has increased due to clinicians’ sensitivity to the different clinical presentations of RA, as well as the advent of increasingly sophisticated means of both molecular and imaging investigations. In addition, the availability of therapeutic means that can act on different effector functions of immunity has indirectly clarified further distinctive aspects between SNRA and SPRA. The purpose of this review is to summarize the distinct elements that have emerged over time regarding the epidemiological, pathogenetic, and clinical features that help to distinguish SNRA from SPRA.
2. Epidemiology
Available epidemiological data have traditionally reported a lower prevalence of SNRA than SPRA, ranging from 20 to 30 percent of total cases of RA [10,11]. However, the incidence of SNRA has been reported to be increased in recent decades [12,13]. Many hypotheses have been advanced to explain this finding. One possible cause is the increasing age of the general population. In fact, late-onset RA occurring in elderly patients is commonly seronegative, suggesting that the dysregulation of inflammation, typical in people of old age, may underlie SNRA [14,15,16]. Another cause is an overall reduction in smoking habits, with cigarette smoking being a strong risk factor for protein citrullination [17]. It is believed that the process of citrullination, by changing the self-nature of joint antigens to non-self-antigens, induces an autoimmune process that leads to the generation of ACPA and causes the humoral and cellular immune systems to attack the altered joint antigens, resulting in synovitis with tissue damage. Therefore, it is likely that the reduction in smoking increased the incidence ratio between SNRA and the seropositive forms through this immunological mechanism [18]. Other factors reported to explain the increased incidence/prevalence of SNRA are changes in the microbiome, possibly from chronic triggers by gut flora via microbial DNA and pepdidoglycans [19], and some environmental factors, including increased occupational exposure to crystalline free silica [20]. Of course, further studies are needed to clarify whether the upward trend in the incidence/prevalence of SNRA compared with that of SNPA is a finding that can proceed over time and whether there are additional genetic and/or environmental factors causing it, which have yet to be elucidated.
3. Pathogenesis
The first distinction between SNRA and SPRA is their different genetic backgrounds. Although RA is a polygenic disease, some genetic risk factors have been identified for SNRA. Among them, the HLA-B*08/DRB1*03 haplotype is one of the genetic markers most frequently associated with SNRA, while the classical HLA-DRB1*04 and *10 alleles have been shown to be risk factors exclusively for SPRA [21,22]. Non-HLA genes also play a relevant role in determining susceptibility to RA, including mutations in genes coding for Janus kinase (JAK)/signal transducer and activator of transcription (STAT) proteins, which are risk factors for SPRA but not for SNRA [23,24]. A genome-wide association study revealed an association with single-nucleotide polymorphism of non-HLA genes ANKRD55 [25] and CLYBL [26] in SNRA but not in SPRA. It is possible to speculate that mutations in non-HLA genes represent changes in the innate-type immune response through the modulation of the synthesis of cytokines and other soluble factors. Indeed, innate immunity seems to be more prominent in SNRA than in SPRA, while antigen/autoantigen presentation to T lymphocytes by HLA molecules represents a key element of adaptive immunity that is more typical of SPRA pathogenesis.
The study of the expression of miRNA is a new exciting field of research aiming to identify biomarkers for differentiating SNRA from SPRA. In this regard, it was recently reported that the miRNAs has-miR-362-5p and has-miR-708-3p were upregulated in SNRA but not in SPRA. Other miRNAs were found to be downregulated differently in the two forms of RA, including the mRNAs expressed exclusively in SPRA and others common to both forms [27,28]. Table 1 summarizes the different genetic backgrounds and miRNA expressions between SNRA and SPRA.

Table 1.
Susceptibility to SNRA and SPRA is favored by the presence of HLA and non-HLA genetic factors and miRNA expression.
Some studies were conducted to determine the differences between SPRA and SNRA at the cellular level. The synovial histological score for CD4+ T cells, CD68+ cells in the lining layer, and sublining CD3+ and vessel CD31+ positive cells was less abundant in undifferentiated seronegative arthritis than in differentiated SPRA [28]. It has also been reported that synovium-infiltrating monocytes and macrophages predominate in SNRA [21]. In an attempt to identify biomarkers that can differentiate SNRA from psoriatic arthritis (PsA) because they share some clinical features, a study was conducted that analyzed the synovial histopathology of the two diseases. It was reported that plasma cells predominate in the synovium of SNRA, while mast cells predominate in PsA [31].
An immunohistochemical analysis of the synovium also revealed a higher percentage of tissue-resident dendritic cells and a reduced expression of the PD-1 inhibitory receptor on T cells in SNRA compared with its seropositive counterpart [32]. Therefore, the finding that the immune checkpoint inhibitor PD-1 can induce SNRA in the course of cancer therapy is of particular interest [33]. This observation is discussed in more detail in a later section of this review for its potential therapeutic implications. Table 2 shows the inflammatory cells detected in the synovial membrane during SNRA and SPRA.

Table 2.
Immunological features of SNRA and SPRA.
Interestingly, SNRA occurrence has been reported during asthma therapy with anti-IL4/IL-13 biologics with the activation of the IL-23-IL-17 axis, suggesting a protective role of T helper-2 (TH-2) cells in the disease [34]. This evidence further supports the idea that SNRA is a form of RA that diverges substantially from SPRA and suggests a similarity of SNRA with SpA, which depends primarily on IL-17 [5,35,36]. Several observations point out that SNRA has a more variable outcome, generally associated with a better prognosis than SPRA [37,38]. It is interesting to note the reported association between SNRA with NLRP3 inflammasome activation. In this regard, studies have demonstrated a role for interleukin-beta (IL1β), a key component of the inflammasome, in the pathogenesis of SNRA [39]. The pathogenetic relationship between SNRA and IL-1β may explain the favorable response to the interleukin-1 receptor antagonist (IL-1ra), as observed in some patients with SNRA, and the minor response to JAK inhibitor (JAKi) therapy of SNRA compared with SPRA, as reported in some studies [40,41,42]. This can be related to the fact that IL-1 does not depend on the JAK/STAT transduction pathway. As is well known, the activation of the NLRP3 inflammasome by monosodium urate crystals with the release of IL-1β plays a major role in the initiation of gout flare [43]. Interestingly, elevated uric acid levels and crystal deposition are occasionally observed in SNRA but not in SPRA, indirectly suggesting an at least partially autoinflammatory nature of SNRA [43]. Although it is not easy to give an explanation for these observations, they suggest a possible pathogenetic link between SNRA and crystal deposition arthritis. Similarly, an autoinflammatory nature has also been proposed for spondyloarthritis (SpA) [44]. To elucidate the possible autoinflammatory component of SNRA, further studies using methods to study the inflammasome and the genetic substrate of this form of RA are needed. In addition, it should be noted that the study of synovial histology is providing very promising results due to the precise characterization of the cells that infiltrate this tissue. Using the methods of histochemistry and flow cytometry, many research groups are trying to identify new biomarkers that can differentiate SNRA from SPRA. Although synovial biopsy is an invasive procedure, it cannot be ruled out that, in the near future, the results obtained may allow for the development of serologic tests that allow for differential diagnosis through simpler diagnostic tests. Pathogenetic characterization, of course, not only has a scientific or diagnostic purpose but also appears essential for the setting of targeted therapies and the possible realization of the so-called personalized therapy tailored to the individual.
4. Diagnosis
As discussed earlier, the diagnosis of RA based on 2010 ACR/EULAR criteria is likely when the patient’s signs and symptoms reach a score of at least 6 [3]. However, this classification criterion may not be optimal for the diagnosis of SNRA. In fact, because RF and ACPA contribute significantly to this score, a seronegative disease must have a higher clinical and inflammatory severity than SPRA to compensate for the lack of serologic markers [45,46]. Although studies are underway to identify as-yet unknown autoantibodies, such as anti-modified protein antibodies (AMPAs), that could help in the diagnosis of SNRA, these efforts have so far been unsuccessful, and we currently have no biomarkers for this condition [47,48]. In a cohort study, it was shown that the diagnosis of SNRA was significantly delayed compared with that of SPRA, even when the previous 1987 RA diagnostic criteria were met [49]. Inadequate classification criteria could lead to a delay in the initiation of therapy in SNRA, resulting in the inability to take advantage of the so-called “window of opportunity” [50], which is critical for achieving early remission of the disease [51].
Therefore, it is likely that, based on the 2010 ACR/EULAR criteria, SNRA is underdiagnosed, suggesting that its incidence/prevalence is actually higher than that reported in the literature. The results of registry studies indicating a low incidence of SNRA may therefore be attributed, at least in part, to a missed diagnosis due to the new classification [52,53]. Thus, the clinical differences between SNRA and SPRA have been carefully analyzed in several studies to help physicians make a correct diagnosis, in addition to the official classification criteria. In initial studies, detectable differences with standard radiography were reported to distinguish possible differences between the two conditions at the hand level [54,55]. Notably, the van der Heijde-modified total Sharp score was significantly lower in patients with SNRA for both erosions and joint space narrowing than in those with SPRA. Interestingly, the erosion subscore in SNRA was higher in the carpal and proximal interphalangeal compartments and in the distal ulna, while the joints of the feet were virtually spared, with a greater involvement of the large and carpal joints in SNRA than in SPRA [56,57]. However, in SPRA, joint damage was significantly more evident in metacarpophalangeal joints II, III, and V, with bone demineralization [58]. In general, the involvement of the metacarpophalangeal joint appears to be a hallmark of SPRA, whereas it is more rarely found in SNRA [59].
To clarify the differences between SNRA and SPRA and improve the accuracy of diagnosis, other criteria have been proposed based on imaging techniques other than standard radiography. Musculoskeletal ultrasound (MSK-US) has emerged in recent decades as a useful and inexpensive tool for diagnosing RA. Several studies have pointed out that this method is very effective in detecting the presence of synovitis compared with clinical examination [60,61]. The usefulness of this tool was emphasized with the inclusion of this method as a means of determining the presence of synovitis in the 2010 ACR/EULAR diagnostic criteria for RA [3,62]. While for the diagnosis of SPRA, some studies have not found significant advantages of MSK-US over physical examination [63], this imaging tool has proven very useful in helping physicians diagnose SNRA. A low degree of synovitis and frequent tenosynovitis involvement, which are hallmarks of SNRA, can be detected with a high accuracy via MSK-US [64,65], as recently summarized in a systematic review of the literature [66]. In a recent study that exploited MSK-US for the evaluation of tendon involvement in SNRA, inflammation of the synovial sheaths of the extensor tendons of the carpus, particularly the sixth compartment, and of the finger flexors, particularly the third finger, was found to be a hallmark of the disease. The usefulness of microvascular flow imaging was also demonstrated in the same study [67]. The results obtained by MSK-US, indicating that the presence of tenosynovitis is more frequent in SNRA than in SPRA and that the extra synovial compartment is often involved in SNRA, were also confirmed by MRI investigation [68,69]. Therefore, there is broad agreement that MSK-US provides significant help in differentiating between SNRA and SPRA [70]. The most important clinical features that differentiate SNRA from SPRA are summarized in Table 3.

Table 3.
Typical clinical involvement of SNRA and SPRA.
However, clinical similarities with SpA pose a risk of misdiagnosis, which could lead to an overestimation of the prevalence of SNRA [72]. Given the substantial differences between SNRA and SNRA, several studies have even questioned the actual existence of SNRA. SNRA presentation may show similarities with different types of SpAs, including PsA, axial SpA (axSpA), and undifferentiated SpA. In particular, PsA, as discussed earlier, has the most similar clinical features to SNRA [73]. With the refinement of methods and instruments, some typical features of SpA, such as enthesitis, can be detected with sufficient accuracy, allowing for a proper differential diagnosis [74,75]. At a more advanced stage, however, axial manifestations may appear, which may cause SNRA to be reclassified as axSpA [76].
It cannot be excluded that, in clinical practice, some rheumatic diseases diagnosed as SNRA are SpA with predominantly peripheral expression [71,77]. In some real-world studies, it has been reported that the initial diagnosis of SNRA has often been changed over time to that of SpA [77,78]. In a study with a 23-year follow-up, more than 60 percent of patients initially classified as having SNRA turned out to have SpA over time [79]. This aspect is of paramount importance in the clinical setting. Indeed, RA and SpA share some initial treatments, such as TNFi. However, after the eventual primary or secondary failure of these treatments, the drugs used in the two diseases are very different. In RA, anti-interleukin-6-receptor drugs or drugs that interfere with adaptive immunity are used, although the latter, as we discussed earlier, are not very effective in SNRA. The treatment of SpA, however, makes use of drugs that interfere with the IL-23/IL17 axis, which are ineffective in the two different forms of RA. A correct diagnosis is therefore crucial for the prescriptive appropriateness of the rheumatic patient and a satisfactory therapeutic outcome.
Differences in the comorbidities most frequently associated with SNRAs or SPRAs were investigated in a large study of data obtained from Swedish registries. Specifically, SNRA showed an increase in atrial fibrillation, type II diabetes, psychiatric disorders, neoplasms, and peripheral neuropathy compared with its seropositive counterpart [80]. In another study, cardiovascular risk was shown to be significantly higher in SPRA than in SNRA [81]. Finally, fibromyalgia was significantly associated with SNRA but not SPRA [82], although not all studies have confirmed this finding [83].
5. Severity of the Disease
An unresolved issue concerns the long-term prognosis of SNRA compared with SPRA, both in terms of joint damage and extra-articular manifestations [77]. Many prospective studies have underlined a better prognosis for SNRA than SPRA, even when presenting with a higher disease activity [84,85,86]. Moreover, the two forms of RA appear to be different diseases in some respects, as SNRA has distinct genetic characteristics and appears to develop under peculiar environmental conditions [87,88,89]. SNRA is generally considered a benign form of RA, and this has been attributed to the absence of autoantibodies in serum. In fact, several studies have shown that the erosive evolution of RA is associated with the presence of ACPA, regardless of disease activity or other clinical manifestations [90,91]. However, not all studies agree on the benign nature of SNRA [14]. It has been objected that studies demonstrating the greater severity of SNRA could be due to a bias arising from the current 2010 ACR/EULAR classification criteria. In fact, since these criteria assign a significant score to the presence of RF and/or ACPA, their absence in serum must be compensated for by increased joint involvement and inflammatory status to reach the score necessary for a diagnosis of probable RA [45,92], as discussed above. Prospective registry studies will be able to clarify whether SNRA actually represents a form of RA with a benign course or, conversely, whether its pathogenicity is currently underestimated.
6. The Response to Therapy of SNRA Is Different from That of SPRA
According to the ACR/EULAR recommendations, the initial therapy of RA should be based on the use of conventional synthetic antirheumatic drugs (csDMARDs) [93]. The first-line csDMARD suggested by most experts, regardless of serologic status, is methotrexate (MTX). However, several studies have shown that MTX is less effective in SNRA than in SPRA [94,95]. It has also been shown that MTX is not as effective in improving the retention rate of biologic drugs such as TNF inhibitors (TNFis) in SNRA compared with SPRA [96,97]. Since the enhanced action of biologics from combination therapy with MTX is attributed to the ability of MTX to inhibit the production of anti-drug antibodies (ADAs) by B lymphocytes [98], this evidence supports the hypothesis that adaptive immunity is less involved in SNRA than in SPRA. In support of this hypothesis is the observed low efficacy of drugs that target B or T cells, such as anti-CD20 or anti-CD80/CD86 biologics, respectively, in patients with SNRA compared with seropositive patients [99,100,101,102].
However, several lines of evidence suggest that SNRA is, in general, more responsive to therapy than SPRA, with a more favorable long-term radiographic outcome [79,84,85,103]. It is also noteworthy that SNRA, unlike SNRA, generally responds positively to switching to a second anti-TNFalpha after failure of the first and often does not require switching to other cytokine-targeted therapies [104]. Studies on the efficacy of Janus kinase inhibitors (JAKis) and their retention rates, which can inhibit the action of multiple pro-inflammatory cytokines in patients with SNRA, are not yet conclusive, and further studies are needed to clarify these important issues [41,105,106]. The results of several clinical trials and real-world data on response to therapy in SNRA and SPRA are summarized in Table 4. However, randomized clinical trials specific to SNRA have not yet been conducted. Therefore, this represents an important unmet need for the optimization of different clinical forms of RA.

Table 4.
Response to therapy of SNRA and SPRA with conventional and advanced drugs.
As introduced in an earlier section of this review, interesting evidence regarding possible alternative therapies to SNRAs comes from the observation that inflammatory rheumatic diseases, termed immune-related rheumatologic adverse events (Rh-irAEs), can develop during cancer therapy with immune checkpoint inhibitors (ICIs) [107]. In particular, the occurrence of RA is particularly frequent and is seronegative in most cases [108]. In fact, it has been reported that RA appearing during ICI therapy actually resembles SNRA both clinically and serologically. In addition to the absence of RF and ACPA, morning stiffness and the absence of erosive joint damage were found to be present in the vast majority of patients with ICI-induced RA [109,110]. These findings indicate that the dysregulation of PD-1-mediated signaling could be at least partly responsible for the pathogenesis of SNRA via an antibody-independent mechanism, suggesting the use of PD-1 agonists as a potential new therapeutic option for RA. Encouraging results of a phase II trial on the use of the humanized monoclonal antibody peresolimab were recently published. However, although patients with SNRA and SPRA were included in the study, an analysis of the response of the two subgroups was not performed [111]. Taken together, all these results underscore that the response to SNRA therapy is a very complex issue. It should also be considered that the research and development of new drugs in immuno-rheumatology are particularly active. Therefore, it cannot be ruled out that new drugs that are more effective than the current ones may become available in the next few years and that their use may indirectly clarify the pathogenetic and clinical differences between SNRA and SPRA. Figure 1 summarizes the distinguishing features between SNRA and SNRA that have emerged so far.
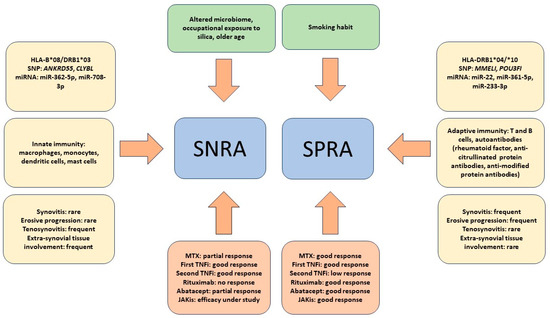
Figure 1.
The figure summarizes the main factors described so far as being associated with seronegative rheumatoid arthritis (SNRA) or seropositive rheumatoid arthritis (SPRA). Epidemiological and environmental factors are depicted in the upper green quadrants. The yellow quadrants on either side of the figure summarize HLA and non-HLA genetic factors, microRNA (miR) expression, cells of immunity most involved in pathogenesis, and clinical features. The red quadrants at the bottom of the figure summarize the response to therapy with both conventional drugs, such as methotrexate (MTX), and biologic drugs, such as TNF inhibitors (TNFis), anti-CD20 (rituximab), and anti-CD80/86 (abatacept), as well as to Janus kinase inhibitors (JAKis).
7. Conclusions
SNRA represents an inflammatory rheumatic condition that is difficult to classify uniquely. Distinctive genetic features and the greater involvement of innate versus adaptive immunity represent characteristic features of this form of RA. This translates not only into distinctive immunopathologic features, such as less synovial involvement and a more frequent occurrence of tendinitis, but also into features of different prognoses and responses to therapy as compared with SPRA. Prospective and controlled studies are therefore needed to delineate an increasingly accurate picture of this form of RA, which could also allow for a better differentiation from SpA with which SNRA seems to share numerous pathogenetic and clinical features.
Author Contributions
M.P. and M.I.S. were involved in the conception and design of the revision, participated in the discussion and writing of the final draft, and critically reviewed it for intellectual content. They also prepared the first draft of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Figus, F.A.; Piga, M.; Azzolin, I.; McConnell, R.; Iagnocco, A. Rheumatoid arthritis: Extra-articular manifestations and comorbidities. Autoimmun. Rev. 2021, 20, 102776. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Kay, J.; Upchurch, K.S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford) 2012, 51 (Suppl. S6), vi5–vi9. [Google Scholar] [CrossRef]
- Pratt, A.G.; Isaacs, J.D. Seronegative rheumatoid arthritis: Pathogenetic and therapeutic aspects. Best Pract Res. Clin. Rheumatol. 2014, 28, 651–659. [Google Scholar] [CrossRef]
- Lenti, M.V.; Rossi, C.M.; Melazzini, F.; Gastaldi, M.; Bugatti, S.; Rotondi, M.; Bianchi, P.I.; Gentile, A.; Chiovato, L.; Montecucco, C.; et al. Seronegative autoimmune diseases: A challenging diagnosis. Autoimmun. Rev. 2022, 21, 103143. [Google Scholar] [CrossRef]
- Sokolova, M.V.; Schett, G.; Steffen, U. Autoantibodies in Rheumatoid Arthritis: Historical Background and Novel Findings. Clin. Rev. Allergy Immunol. 2022, 63, 138–151. [Google Scholar] [CrossRef]
- Kolarz, B.; Podgorska, D.; Podgorski, R. Insights of rheumatoid arthritis biomarkers. Biomarkers 2021, 26, 185–195. [Google Scholar] [CrossRef]
- Derksen, V.; Huizinga, T.W.J.; van der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 437–446. [Google Scholar] [CrossRef]
- Curtis, J.R.; Jain, A.; Askling, J.; Bridges, S.L., Jr.; Carmona, L.; Dixon, W.; Finckh, A.; Hyrich, K.; Greenberg, J.D.; Kremer, J.; et al. A comparison of patient characteristics and outcomes in selected European and U.S. rheumatoid arthritis registries. Semin. Arthritis Rheum. 2010, 40, 2–14 e11. [Google Scholar] [CrossRef]
- Courvoisier, D.S.; Chatzidionysiou, K.; Mongin, D.; Lauper, K.; Mariette, X.; Morel, J.; Gottenberg, J.E.; Bergstra, S.A.; Suarez, M.P.; Codreanu, C.; et al. The impact of seropositivity on the effectiveness of biologic anti-rheumatic agents: Results from a collaboration of 16 registries. Rheumatology 2021, 60, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Davis, J.; Matteson, E.L.; Crowson, C.S. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann. Rheum. Dis. 2020, 79, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Matthijssen, X.M.E.; Huizinga, T.W.J.; van der Helm-van Mil, A.H.M. Increasing incidence of autoantibody-negative RA is replicated and is partly explained by an aging population. Ann. Rheum. Dis. 2022, 81, e69. [Google Scholar] [CrossRef]
- Matthijssen, X.M.E.; Niemantsverdriet, E.; Huizinga, T.W.J.; van der Helm-van Mil, A.H.M. Enhanced treatment strategies and distinct disease outcomes among autoantibody-positive and -negative rheumatoid arthritis patients over 25 years: A longitudinal cohort study in the Netherlands. PLoS Med. 2020, 17, e1003296. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, S.; Takeuchi, T.; Kaneko, Y. Effects of Aging on Rheumatoid Factor and Anticyclic Citrullinated Peptide Antibody Positivity in Patients With Rheumatoid Arthritis. J. Rheumatol. 2023, 50, 330–334. [Google Scholar] [CrossRef]
- Turkcapar, N.; Demir, O.; Atli, T.; Kopuk, M.; Turgay, M.; Kinikli, G.; Duman, M. Late onset rheumatoid arthritis: Clinical and laboratory comparisons with younger onset patients. Arch. Gerontol. Geriatr. 2006, 42, 225–231. [Google Scholar] [CrossRef]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Regueiro, C.; Rodriguez-Rodriguez, L.; Lopez-Mejias, R.; Nuno, L.; Triguero-Martinez, A.; Perez-Pampin, E.; Corrales, A.; Villalba, A.; Lopez-Golan, Y.; Abasolo, L.; et al. A predominant involvement of the triple seropositive patients and others with rheumatoid factor in the association of smoking with rheumatoid arthritis. Sci. Rep. 2020, 10, 3355. [Google Scholar] [CrossRef]
- Bergot, A.S.; Giri, R.; Thomas, R. The microbiome and rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101497. [Google Scholar] [CrossRef]
- Morotti, A.; Sollaku, I.; Franceschini, F.; Cavazzana, I.; Fredi, M.; Sala, E.; De Palma, G. Systematic Review and Meta-analysis on the Association of Occupational Exposure to Free Crystalline Silica and Rheumatoid Arthritis. Clin. Allergy Immunol. 2022, 62, 333–345. [Google Scholar] [CrossRef]
- De Stefano, L.; D’Onofrio, B.; Manzo, A.; Montecucco, C.; Bugatti, S. The Genetic, Environmental, and Immunopathological Complexity of Autoantibody-Negative Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 12386. [Google Scholar] [CrossRef] [PubMed]
- Padyukov, L. Genetics of rheumatoid arthritis. Semin. Immunopathol. 2022, 44, 47–62. [Google Scholar] [CrossRef]
- Saevarsdottir, S.; Stefansdottir, L.; Sulem, P.; Thorleifsson, G.; Ferkingstad, E.; Rutsdottir, G.; Glintborg, B.; Westerlind, H.; Grondal, G.; Loft, I.C.; et al. Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the seropositive subset. Ann. Rheum. Dis. 2022, 81, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Li, X.; Lin, A.; Larsson, S.C. Interleukins and rheumatoid arthritis: Bi-directional Mendelian randomization investigation. Semin. Arthritis Rheum. 2022, 53, 151958. [Google Scholar] [CrossRef] [PubMed]
- Eyre, S.; Bowes, J.; Diogo, D.; Lee, A.; Barton, A.; Martin, P.; Zhernakova, A.; Stahl, E.; Viatte, S.; McAllister, K.; et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat. Genet. 2012, 44, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Bossini-Castillo, L.; de Kovel, C.; Kallberg, H.; van’t Slot, R.; Italiaander, A.; Coenen, M.; Tak, P.P.; Posthumus, M.D.; Wijmenga, C.; Huizinga, T.; et al. A genome-wide association study of rheumatoid arthritis without antibodies against citrullinated peptides. Ann. Rheum. Dis. 2015, 74, e15. [Google Scholar] [CrossRef]
- He, X.H.; Xiao, Y.T.; Chen, W.Y.; Wang, M.J.; Wu, X.D.; Mei, L.Y.; Gao, K.X.; Huang, Q.C.; Huang, R.Y.; Chen, X.M. In silico analysis of serum miRNA profiles in seronegative and seropositive rheumatoid arthritis patients by small RNA sequencing. PeerJ 2023, 11, e15690. [Google Scholar] [CrossRef]
- Alivernini, S.; Tolusso, B.; Petricca, L.; Bui, L.; Di Mario, C.; Gigante, M.R.; Di Sante, G.; Benvenuto, R.; Fedele, A.L.; Federico, F.; et al. Synovial Predictors of Differentiation to Definite Arthritis in Patients With Seronegative Undifferentiated Peripheral Inflammatory Arthritis: microRNA Signature, Histological, and Ultrasound Features. Front. Med. 2018, 5, 186. [Google Scholar] [CrossRef]
- Ding, B.; Padyukov, L.; Lundstrom, E.; Seielstad, M.; Plenge, R.M.; Oksenberg, J.R.; Gregersen, P.K.; Alfredsson, L.; Klareskog, L. Different patterns of associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in the extended major histocompatibility complex region. Arthritis Rheum. 2009, 60, 30–38. [Google Scholar] [CrossRef]
- Chang, C.; Xu, L.; Zhang, R.; Jin, Y.; Jiang, P.; Wei, K.; Xu, L.; Shi, Y.; Zhao, J.; Xiong, M.; et al. MicroRNA-Mediated Epigenetic Regulation of Rheumatoid Arthritis Susceptibility and Pathogenesis. Front. Immunol. 2022, 13, 838884. [Google Scholar] [CrossRef]
- Alivernini, S.; Bruno, D.; Tolusso, B.; Bui, L.; Petricca, L.; Gigante, M.R.; Birra, D.; Fedele, A.L.; Peluso, G.; Federico, F.; et al. Differential synovial tissue biomarkers among psoriatic arthritis and rheumatoid factor/anti-citrulline antibody-negative rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 116. [Google Scholar] [CrossRef]
- Argyriou, A.; Wadsworth, M.H., 2nd; Lendvai, A.; Christensen, S.M.; Hensvold, A.H.; Gerstner, C.; van Vollenhoven, A.; Kravarik, K.; Winkler, A.; Malmstrom, V.; et al. Single cell sequencing identifies clonally expanded synovial CD4(+) T(PH) cells expressing GPR56 in rheumatoid arthritis. Nat. Commun. 2022, 13, 4046. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, F.; Bogdanos, D.; Dimitroulas, T.; Sakkas, L.; Daoussis, D. Immune checkpoint inhibitor-induced musculoskeletal manifestations. Rheumatol. Int. 2021, 41, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Bridgewood, C.; Wittmann, M.; Macleod, T.; Watad, A.; Newton, D.; Bhan, K.; Amital, H.; Damiani, G.; Giryes, S.; Bragazzi, N.L.; et al. T Helper 2 IL-4/IL-13 Dual Blockade with Dupilumab Is Linked to Some Emergent T Helper 17—Type Diseases, Including Seronegative Arthritis and Enthesitis/Enthesopathy, but Not to Humoral Autoimmune Diseases. J. Investig. Dermatol. 2022, 142, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Yarwood, A.; Huizinga, T.W.; Worthington, J. The genetics of rheumatoid arthritis: Risk and protection in different stages of the evolution of RA. Rheumatology 2016, 55, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Bardin, T.; Legrand, L.; Naveau, B.; Marcelli-Barge, A.; Debeyre, N.; Lathrop, G.M.; Poirier, J.C.; Schmid, M.; Ryckewaert, A.; Dryll, A. HLA antigens and seronegative rheumatoid arthritis. Ann. Rheum. Dis. 1985, 44, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Kaarela, K.; Kautiainen, H. Continuous progression of radiological destruction in seropositive rheumatoid arthritis. J. Rheumatol. 1997, 24, 1285–1287. [Google Scholar]
- Choi, S.; Lee, K.H. Clinical management of seronegative and seropositive rheumatoid arthritis: A comparative study. PLoS ONE 2018, 13, e0195550. [Google Scholar] [CrossRef]
- Martin-Sanchez, F.; Diamond, C.; Zeitler, M.; Gomez, A.I.; Baroja-Mazo, A.; Bagnall, J.; Spiller, D.; White, M.; Daniels, M.J.; Mortellaro, A.; et al. Inflammasome-dependent IL-1beta release depends upon membrane permeabilisation. Cell Death Differ. 2016, 23, 1219–1231. [Google Scholar] [CrossRef]
- Bird, P.; Littlejohn, G.; Butcher, B.; Smith, T.; O’Sullivan, C.; Witcombe, D.; Griffiths, H. Real-world evaluation of effectiveness, persistence, and usage patterns of monotherapy and combination therapy tofacitinib in treatment of rheumatoid arthritis in Australia. Clin. Rheumatol. 2022, 41, 53–62. [Google Scholar] [CrossRef]
- Sugawara, M.; Fujieda, Y.; Noguchi, A.; Tanimura, S.; Shimizu, Y.; Nakagawa, I.; Yoshimura, M.; Abe, N.; Kono, M.; Kato, M.; et al. Prediction of the intolerance or non-responder to Janus kinase inhibitors in patients with rheumatoid arthritis: A preliminary retrospective study with integrative cluster analysis. Clin. Exp. Rheumatol. 2022, 40, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, E.; Kim, J.W.; Suh, C.H.; Kim, H.A. Efficacy and drug retention of tofacitinib in rheumatoid arthritis: From the nationwide Korean College of Rheumatology Biologics registry. Clin. Exp. Rheumatol. 2023, 41, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Dalbeth, N.; Gosling, A.L.; Gaffo, A.; Abhishek, A. Gout. Lancet 2021, 397, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Mauro, D.; Thomas, R.; Guggino, G.; Lories, R.; Brown, M.A.; Ciccia, F. Ankylosing spondylitis: An autoimmune or autoinflammatory disease? Nat. Rev. Rheumatol. 2021, 17, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, L.B.; Lillegraven, S.; Lie, E.; Aga, A.B.; Olsen, I.C.; Hammer, H.B.; Uhlig, T.; Jonsson, M.K.; van der Heijde, D.; Kvien, T.K.; et al. Patients with seronegative RA have more inflammatory activity compared with patients with seropositive RA in an inception cohort of DMARD-naive patients classified according to the 2010 ACR/EULAR criteria. Ann. Rheum. Dis. 2017, 76, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Boeters, D.M.; Gaujoux-Viala, C.; Constantin, A.; van der Helm-van Mil, A.H.M. The 2010 ACR/EULAR criteria are not sufficiently accurate in the early identification of autoantibody-negative rheumatoid arthritis: Results from the Leiden-EAC and ESPOIR cohorts. Semin. Arthritis Rheum. 2017, 47, 170–174. [Google Scholar] [CrossRef]
- Trouw, L.A.; Mahler, M. Closing the serological gap: Promising novel biomarkers for the early diagnosis of rheumatoid arthritis. Autoimmun. Rev. 2012, 12, 318–322. [Google Scholar] [CrossRef]
- Kwon, E.J.; Ju, J.H. Impact of Posttranslational Modification in Pathogenesis of Rheumatoid Arthritis: Focusing on Citrullination, Carbamylation, and Acetylation. Int. J. Mol. Sci. 2021, 22, 10576. [Google Scholar] [CrossRef]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Burgers, L.E.; Raza, K.; van der Helm-van Mil, A.H. Window of opportunity in rheumatoid arthritis—Definitions and supporting evidence: From old to new perspectives. RMD Open 2019, 5, e000870. [Google Scholar] [CrossRef]
- Coffey, C.M.; Crowson, C.S.; Myasoedova, E.; Matteson, E.L.; Davis, J.M., 3rd. Evidence of Diagnostic and Treatment Delay in Seronegative Rheumatoid Arthritis: Missing the Window of Opportunity. Mayo Clin. Proc. 2019, 94, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Muilu, P.; Rantalaiho, V.; Kautiainen, H.; Virta, L.J.; Eriksson, J.G.; Puolakka, K. Increasing incidence and shifting profile of idiopathic inflammatory rheumatic diseases in adults during this millennium. Clin. Rheumatol. 2019, 38, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T.; Aletaha, D.; Silman, A.J.; Naden, R.L.; Felson, D.T.; Aggarwal, R.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; et al. The 2010 American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: Phase 2 methodological report. Arthritis Rheum. 2010, 62, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Burns, T.M.; Calin, A. The hand radiograph as a diagnostic discriminant between seropositive and seronegative ‘rheumatoid arthritis’: A controlled study. Ann. Rheum. Dis. 1983, 42, 605–612. [Google Scholar] [CrossRef] [PubMed]
- el-Khoury, G.Y.; Larson, R.K.; Kathol, M.H.; Berbaum, K.S.; Furst, D.E. Seronegative and seropositive rheumatoid arthritis: Radiographic differences. Radiology 1988, 168, 517–520. [Google Scholar] [CrossRef]
- Panayi, G.S.; Celinska, E.; Emery, P.; Griffin, J.; Welsh, K.I.; Grahame, R.; Gibson, T. Seronegative and seropositive rheumatoid arthritis: Similar diseases. Br. J. Rheumatol. 1987, 26, 172–180. [Google Scholar] [CrossRef]
- Carbonell-Bobadilla, N.; Soto-Fajardo, C.; Amezcua-Guerra, L.M.; Batres-Marroquin, A.B.; Vargas, T.; Hernandez-Diazcouder, A.; Jimenez-Rojas, V.; Medina-Garcia, A.C.; Pineda, C.; Silveira, L.H. Patients with seronegative rheumatoid arthritis have a different phenotype than seropositive patients: A clinical and ultrasound study. Front. Med. 2022, 9, 978351. [Google Scholar] [CrossRef]
- Gadeholt, O.; Hausotter, K.; Eberle, H.; Klink, T.; Pfeil, A. Differing X-ray patterns in seronegative and seropositive rheumatoid arthritis. Clin. Rheumatol. 2019, 38, 2403–2410. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, M.; Zhang, Y.; Xie, Y.; Cao, J.; Pan, Y. Seronegative rheumatic arthritis has milder inflammation and bone erosion in an ultrasound study of disease-modifying anti-rheumatic drugs (DMARDs)-naive Chinese cohort. Ann. Transl. Med. 2022, 10, 661. [Google Scholar] [CrossRef]
- Naredo, E.; Bonilla, G.; Gamero, F.; Uson, J.; Carmona, L.; Laffon, A. Assessment of inflammatory activity in rheumatoid arthritis: A comparative study of clinical evaluation with grey scale and power Doppler ultrasonography. Ann. Rheum. Dis. 2005, 64, 375–381. [Google Scholar] [CrossRef]
- Brown, A.K.; Conaghan, P.G.; Karim, Z.; Quinn, M.A.; Ikeda, K.; Peterfy, C.G.; Hensor, E.; Wakefield, R.J.; O’Connor, P.J.; Emery, P. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum. 2008, 58, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Colebatch, A.N.; Edwards, C.J.; Ostergaard, M.; van der Heijde, D.; Balint, P.V.; D’Agostino, M.A.; Forslind, K.; Grassi, W.; Haavardsholm, E.A.; Haugeberg, G.; et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 804–814. [Google Scholar] [CrossRef] [PubMed]
- van de Stadt, L.A.; Bos, W.H.; Meursinge Reynders, M.; Wieringa, H.; Turkstra, F.; van der Laken, C.J.; van Schaardenburg, D. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: A prospective cohort study. Arthritis Res. Ther. 2010, 12, R98. [Google Scholar] [CrossRef] [PubMed]
- Minowa, K.; Ogasawara, M.; Murayama, G.; Gorai, M.; Yamada, Y.; Nemoto, T.; Matsuki, Y.; Sugisaki, N.; Ando, S.; Kon, T.; et al. Predictive grade of ultrasound synovitis for diagnosing rheumatoid arthritis in clinical practice and the possible difference between patients with and without seropositivity. Mod. Rheumatol. 2016, 26, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Freeston, J.E.; Wakefield, R.J.; Conaghan, P.G.; Hensor, E.M.; Stewart, S.P.; Emery, P. A diagnostic algorithm for persistence of very early inflammatory arthritis: The utility of power Doppler ultrasound when added to conventional assessment tools. Ann. Rheum. Dis. 2010, 69, 417–419. [Google Scholar] [CrossRef]
- Lage-Hansen, P.R.; Lindegaard, H.; Chrysidis, S.; Terslev, L. The role of ultrasound in diagnosing rheumatoid arthritis, what do we know? An updated review. Rheumatol. Int. 2017, 37, 179–187. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Wang, M.; Qi, Q.; Song, Q.; Sun, B.; Li, C.; Dong, Y. Evaluation of tenosynovitis in patients with seronegative rheumatoid arthritis using microvascular flow imaging. Med. Eng. Phys. 2022, 110, 103839. [Google Scholar] [CrossRef]
- den Hollander, N.K.; Verstappen, M.; Sidhu, N.; van Mulligen, E.; Reijnierse, M.; van der Helm-van Mil, A.H.M. Hand and foot MRI in contemporary undifferentiated arthritis: In which patients is MRI valuable to detect rheumatoid arthritis early? A large prospective study. Rheumatology 2022, 61, 3963–3973. [Google Scholar] [CrossRef]
- Sahbudin, I.; Singh, R.; De Pablo, P.; Rankin, E.; Rhodes, B.; Justice, E.; Derrett-Smith, E.; Amft, N.; Narayan, N.; McGrath, C.; et al. The value of ultrasound-defined tenosynovitis and synovitis in the prediction of persistent arthritis. Rheumatology 2023, 62, 1057–1068. [Google Scholar] [CrossRef]
- Mandl, P.; Navarro-Compan, V.; Terslev, L.; Aegerter, P.; van der Heijde, D.; D’Agostino, M.A.; Baraliakos, X.; Pedersen, S.J.; Jurik, A.G.; Naredo, E.; et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 2015, 74, 1327–1339. [Google Scholar] [CrossRef]
- Gadeholt, O. Rheumatoid arthritis is not a single disease. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S104), 20–21. [Google Scholar] [PubMed]
- Mease, P.J.; Bhutani, M.K.; Hass, S.; Yi, E.; Hur, P.; Kim, N. Comparison of Clinical Manifestations in Rheumatoid Arthritis vs. Spondyloarthritis: A Systematic Literature Review. Rheumatol. Ther. 2022, 9, 331–378. [Google Scholar] [CrossRef] [PubMed]
- Felbo, S.K.; Terslev, L.; Ostergaard, M. Imaging in peripheral and axial psoriatic arthritis: Contributions to diagnosis, follow-up, prognosis and knowledge of pathogenesis. Clin. Exp. Rheumatol. 2018, 36 (Suppl. S114), 24–34. [Google Scholar]
- Taniguchi, Y.; Kumon, Y.; Takata, T.; Sano, S.; Ohnishi, T.; Nogami, M.; Ogawa, Y.; Terada, Y. Imaging assessment of enthesitis in spondyloarthritis. Ann. Nucl. Med. 2013, 27, 105–111. [Google Scholar] [CrossRef]
- Fournie, B.; Margarit-Coll, N.; Champetier de Ribes, T.L.; Zabraniecki, L.; Jouan, A.; Vincent, V.; Chiavassa, H.; Sans, N.; Railhac, J.J. Extrasynovial ultrasound abnormalities in the psoriatic finger. Prospective comparative power-doppler study versus rheumatoid arthritis. Joint. Bone Spine 2006, 73, 527–531. [Google Scholar] [CrossRef]
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–84. [Google Scholar] [CrossRef]
- Paalanen, K.; Rannio, K.; Rannio, T.; Asikainen, J.; Hannonen, P.; Sokka, T. Does early seronegative arthritis develop into rheumatoid arthritis? A 10-year observational study. Clin. Exp. Rheumatol. 2019, 37, 37–43. [Google Scholar]
- Paalanen, K.; Puolakka, K.; Nikiphorou, E.; Hannonen, P.; Sokka, T. Is seronegative rheumatoid arthritis true rheumatoid arthritis? A nationwide cohort study. Rheumatology 2021, 60, 2391–2395. [Google Scholar] [CrossRef]
- Jantti, J.K.; Kaarela, K.; Lehtinen, K.E. Seronegative oligoarthritis: A 23-year follow-up study. Clin. Rheumatol. 2002, 21, 353–356. [Google Scholar] [CrossRef]
- Tidblad, L.; Westerlind, H.; Delcoigne, B.; Askling, J.; Saevarsdottir, S. Comorbidities at diagnosis of rheumatoid arthritis: A population-based case-control study. Rheumatology 2021, 60, 3760–3769. [Google Scholar] [CrossRef]
- van Boheemen, L.; van Beers-Tas, M.H.; Kroese, J.M.; van de Stadt, L.A.; van Schaardenburg, D.; Nurmohamed, M.T. Cardiovascular risk in persons at risk of developing rheumatoid arthritis. PLoS ONE 2020, 15, e0237072. [Google Scholar] [CrossRef]
- Doss, J.; Mo, H.; Carroll, R.J.; Crofford, L.J.; Denny, J.C. Phenome-Wide Association Study of Rheumatoid Arthritis Subgroups Identifies Association Between Seronegative Disease and Fibromyalgia. Arthritis Rheumatol. 2017, 69, 291–300. [Google Scholar] [CrossRef]
- Wolfe, F.; Walitt, B. No Association of Fibromyalgia and Seronegative Rheumatoid Arthritis-The Need for Uniform Application of Fibromyalgia Criteria in Research Studies: Comment on the Article by Doss et al. Arthritis Rheumatol. 2017, 69, 679–680. [Google Scholar] [CrossRef] [PubMed]
- van der Helm-van Mil, A.H.; Verpoort, K.N.; Breedveld, F.C.; Toes, R.E.; Huizinga, T.W. Antibodies to citrullinated proteins and differences in clinical progression of rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R949–R958. [Google Scholar] [CrossRef] [PubMed]
- Asikainen, J.; Nikiphorou, E.; Kaarela, K.; Lindqvist, E.; Hakkinen, A.; Kautiainen, H.; Hannonen, P.; Rannio, T.; Sokka, T. Is long-term radiographic joint damage different between men and women? Prospective longitudinal data analysis of four early RA cohorts with greater than 15 years follow-up. Clin. Exp. Rheumatol. 2016, 34, 641–645. [Google Scholar] [PubMed]
- Goekoop-Ruiterman, Y.P.; de Vries-Bouwstra, J.K.; Allaart, C.F.; van Zeben, D.; Kerstens, P.J.; Hazes, J.M.; Zwinderman, A.H.; Ronday, H.K.; Han, K.H.; Westedt, M.L.; et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis Rheum. 2005, 52, 3381–3390. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Alfredsson, L.; Rantapaa-Dahlqvist, S.; Berglin, E.; Stolt, P.; Padyukov, L. What precedes development of rheumatoid arthritis? Ann. Rheum. Dis. 2004, 63 (Suppl. S2), ii28–ii31. [Google Scholar] [CrossRef]
- Huizinga, T.W.; Amos, C.I.; van der Helm-van Mil, A.H.; Chen, W.; van Gaalen, F.A.; Jawaheer, D.; Schreuder, G.M.; Wener, M.; Breedveld, F.C.; Ahmad, N.; et al. Refining the complex rheumatoid arthritis phenotype based on specificity of the HLA-DRB1 shared epitope for antibodies to citrullinated proteins. Arthritis Rheum. 2005, 52, 3433–3438. [Google Scholar] [CrossRef]
- Lundstrom, E.; Kallberg, H.; Alfredsson, L.; Klareskog, L.; Padyukov, L. Gene-environment interaction between the DRB1 shared epitope and smoking in the risk of anti-citrullinated protein antibody-positive rheumatoid arthritis: All alleles are important. Arthritis Rheum. 2009, 60, 1597–1603. [Google Scholar] [CrossRef]
- Bugatti, S.; Manzo, A.; Montecucco, C.; Caporali, R. The Clinical Value of Autoantibodies in Rheumatoid Arthritis. Front. Med. 2018, 5, 339. [Google Scholar] [CrossRef]
- Willemze, A.; Trouw, L.A.; Toes, R.E.; Huizinga, T.W. The influence of ACPA status and characteristics on the course of RA. Nat. Rev. Rheumatol. 2012, 8, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Barra, L.; Pope, J.E.; Orav, J.E.; Boire, G.; Haraoui, B.; Hitchon, C.; Keystone, E.C.; Thorne, J.C.; Tin, D.; Bykerk, V.P.; et al. Prognosis of seronegative patients in a large prospective cohort of patients with early inflammatory arthritis. J. Rheumatol. 2014, 41, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewe, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Wevers-de Boer, K.; Visser, K.; Heimans, L.; Ronday, H.K.; Molenaar, E.; Groenendael, J.H.; Peeters, A.J.; Westedt, M.L.; Collee, G.; de Sonnaville, P.B.; et al. Remission induction therapy with methotrexate and prednisone in patients with early rheumatoid and undifferentiated arthritis (the IMPROVED study). Ann. Rheum. Dis. 2012, 71, 1472–1477. [Google Scholar] [CrossRef]
- Nordberg, L.B.; Lillegraven, S.; Aga, A.B.; Sexton, J.; Olsen, I.C.; Lie, E.; Berner Hammer, H.; Uhlig, T.; van der Heijde, D.; Kvien, T.K.; et al. Comparing the disease course of patients with seronegative and seropositive rheumatoid arthritis fulfilling the 2010 ACR/EULAR classification criteria in a treat-to-target setting: 2-year data from the ARCTIC trial. RMD Open 2018, 4, e000752. [Google Scholar] [CrossRef]
- Greenwood, M.; Shipa, M.; Yeoh, S.A.; Roussou, E.; Mukerjee, D.; Ehrenstein, M.R. Methotrexate reduces withdrawal rates of TNF inhibitors due to ineffectiveness in rheumatoid arthritis but only in patients who are seropositive. Ann. Rheum. Dis. 2020, 79, 1516–1517. [Google Scholar] [CrossRef]
- Hernandez-Breijo, B.; Brenis, C.M.; Plasencia-Rodriguez, C.; Martinez-Feito, A.; Novella-Navarro, M.; Pascual-Salcedo, D.; Balsa, A. Methotrexate Reduces the Probability of Discontinuation of TNF Inhibitors in Seropositive Patients With Rheumatoid Arthritis. A Real-World Data Analysis. Front. Med. 2021, 8, 692557. [Google Scholar] [CrossRef]
- Fernandez, C.A. Pharmacological strategies for mitigating anti-TNF biologic immunogenicity in rheumatoid arthritis patients. Curr. Opin. Pharmacol. 2023, 68, 102320. [Google Scholar] [CrossRef]
- Isaacs, J.D.; Cohen, S.B.; Emery, P.; Tak, P.P.; Wang, J.; Lei, G.; Williams, S.; Lal, P.; Read, S.J. Effect of baseline rheumatoid factor and anticitrullinated peptide antibody serotype on rituximab clinical response: A meta-analysis. Ann. Rheum. Dis. 2013, 72, 329–336. [Google Scholar] [CrossRef]
- Sokolove, J.; Schiff, M.; Fleischmann, R.; Weinblatt, M.E.; Connolly, S.E.; Johnsen, A.; Zhu, J.; Maldonado, M.A.; Patel, S.; Robinson, W.H. Impact of baseline anti-cyclic citrullinated peptide-2 antibody concentration on efficacy outcomes following treatment with subcutaneous abatacept or adalimumab: 2-year results from the AMPLE trial. Ann. Rheum. Dis. 2016, 75, 709–714. [Google Scholar] [CrossRef]
- Norris-Grey, C.; Cambridge, G.; Moore, S.; Reddy, V.; Leandro, M. Long-term persistence of rituximab in patients with rheumatoid arthritis: An evaluation of the UCL cohort from 1998 to 2020. Rheumatology 2022, 61, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Alten, R.; Mariette, X.; Flipo, R.M.; Caporali, R.; Buch, M.H.; Patel, Y.; Marsal, S.; Sanmarti, R.; Nurmohamed, M.T.; Griffiths, H.; et al. Retention of subcutaneous abatacept for the treatment of rheumatoid arthritis: Real-world results from the ASCORE study: An international 2-year observational study. Clin. Rheumatol. 2022, 41, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, H.; van Aken, J.; Lard, L.R.; Visser, K.; Ronday, H.K.; Hulsmans, H.M.; Speyer, I.; Westedt, M.L.; Peeters, A.J.; Allaart, C.F.; et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: A double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007, 56, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Shipa, M.R.A.; Di Cicco, M.; Balogh, E.; Nitu, N.A.; Mainuddin, M.D.; Bhadauria, N.; Mukerjee, D.; Roussou, E. Drug-survival profiling of second-line biologic therapy in rheumatoid arthritis: Choice of another tumour necrosis factor inhibitor or a biologic of different mode of action? Mod. Rheumatol. 2023, 33, 700–707. [Google Scholar] [CrossRef]
- Bird, P.; Hall, S.; Nash, P.; Connell, C.A.; Kwok, K.; Witcombe, D.; Thirunavukkarasu, K. Treatment outcomes in patients with seropositive versus seronegative rheumatoid arthritis in Phase III randomised clinical trials of tofacitinib. RMD Open 2019, 5, e000742. [Google Scholar] [CrossRef]
- Paroli, M.; Becciolini, A.; Bravi, E.; Andracco, R.; Nucera, V.; Parisi, S.; Ometto, F.; Lumetti, F.; Farina, A.; Del Medico, P.; et al. Long-Term Retention Rate of Tofacitinib in Rheumatoid Arthritis: An Italian Multicenter Retrospective Cohort Study. Medicina 2023, 59, 1480. [Google Scholar] [CrossRef]
- Braaten, T.J.; Brahmer, J.R.; Forde, P.M.; Le, D.; Lipson, E.J.; Naidoo, J.; Schollenberger, M.; Zheng, L.; Bingham, C.O.; Shah, A.A.; et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann. Rheum. Dis. 2020, 79, 332–338. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Chang, M.H.; Nigrovic, P.A. Antibody-dependent and -independent mechanisms of inflammatory arthritis. JCI Insight 2019, 4, 6483516. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Delfino, D.V.; Fierabracci, A. Recent insights into the role of the PD-1/PD-L1 pathway in immunological tolerance and autoimmunity. Autoimmun. Rev. 2013, 12, 1091–1100. [Google Scholar] [CrossRef]
- Tuttle, J.; Drescher, E.; Simon-Campos, J.A.; Emery, P.; Greenwald, M.; Kivitz, A.; Rha, H.; Yachi, P.; Kiley, C.; Nirula, A. A Phase 2 Trial of Peresolimab for Adults with Rheumatoid Arthritis. N. Engl. J. Med. 2023, 388, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).