Cost-Effective Protein Production in CHO Cells Following Polyethylenimine-Mediated Gene Delivery Showcased by the Production and Crystallization of Antibody Fabs
Abstract
1. Introduction
2. Materials and Methods
2.1. CHO Cells Cultivation
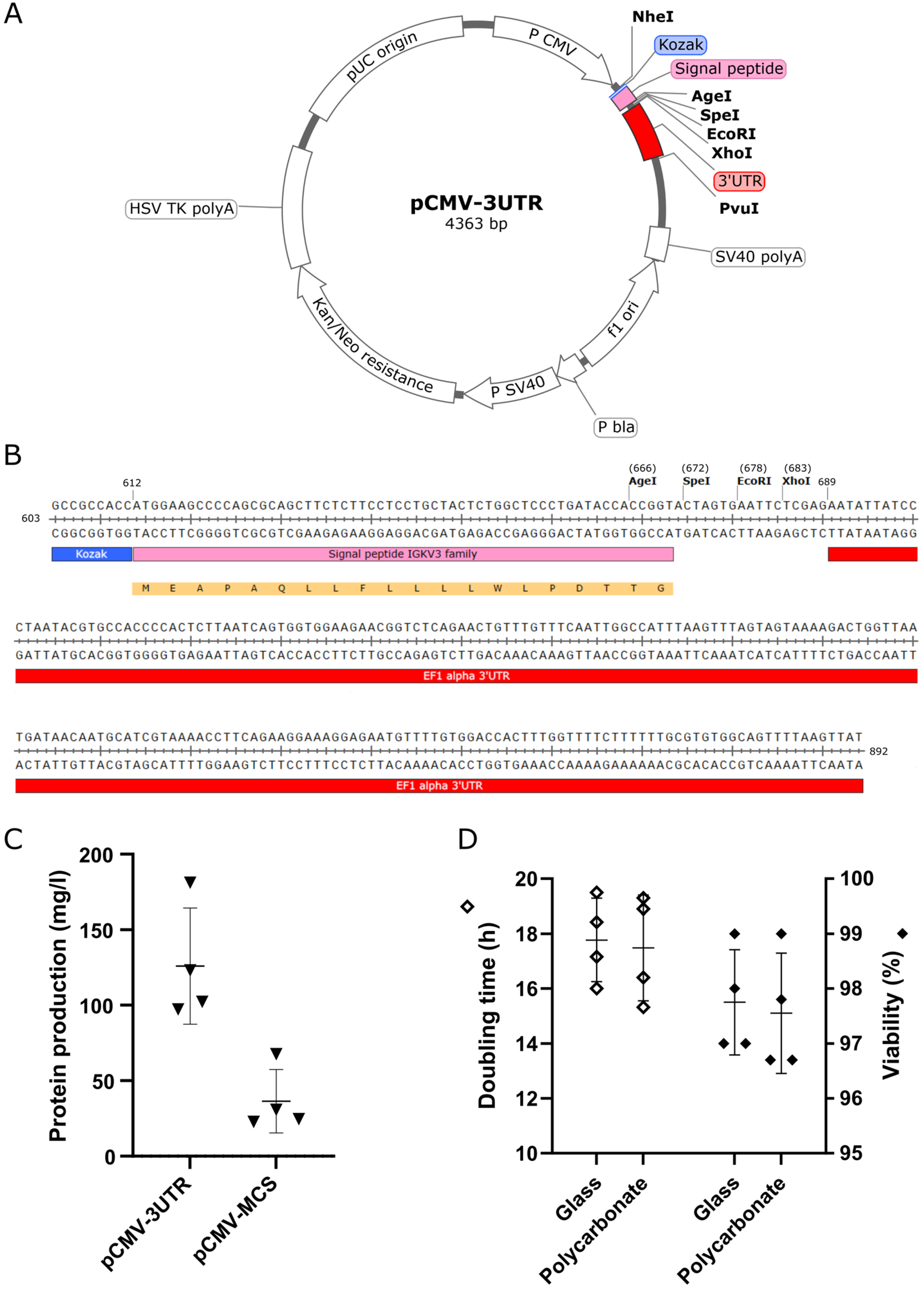
2.2. Preparation of Expression Vectors
2.3. CHO Cells Transfections
2.4. Purification of Recombinant Proteins
2.5. Dynamic Light Scattering
2.6. Crystallization of the Recombinant Antibodies and Their Complexes with Tau 297–391
2.7. Diffraction Experiment and Data Processing
3. Results
3.1. Optimization of the Platform for Transient Gene Expression
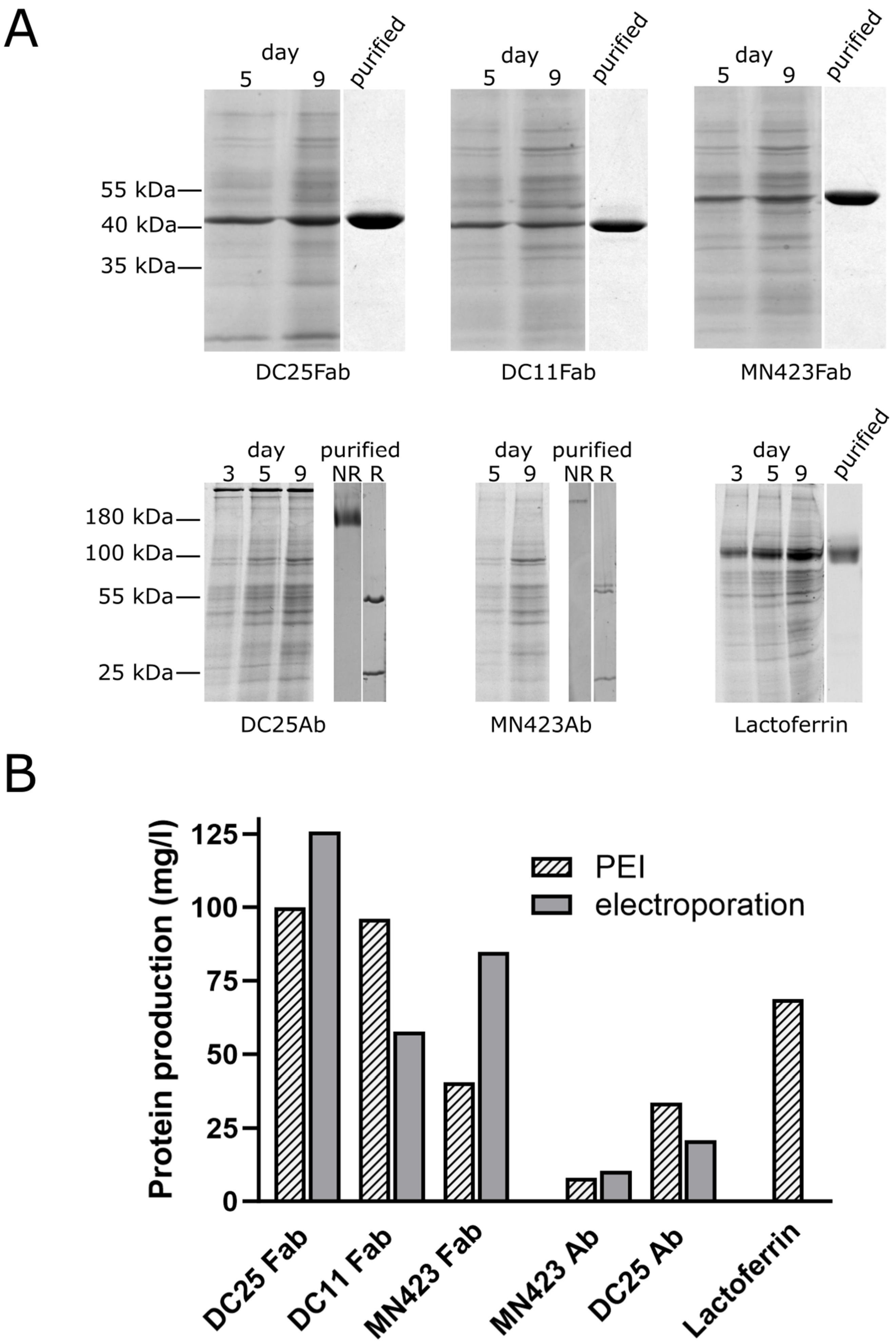
3.2. Production of Recombinant Proteins
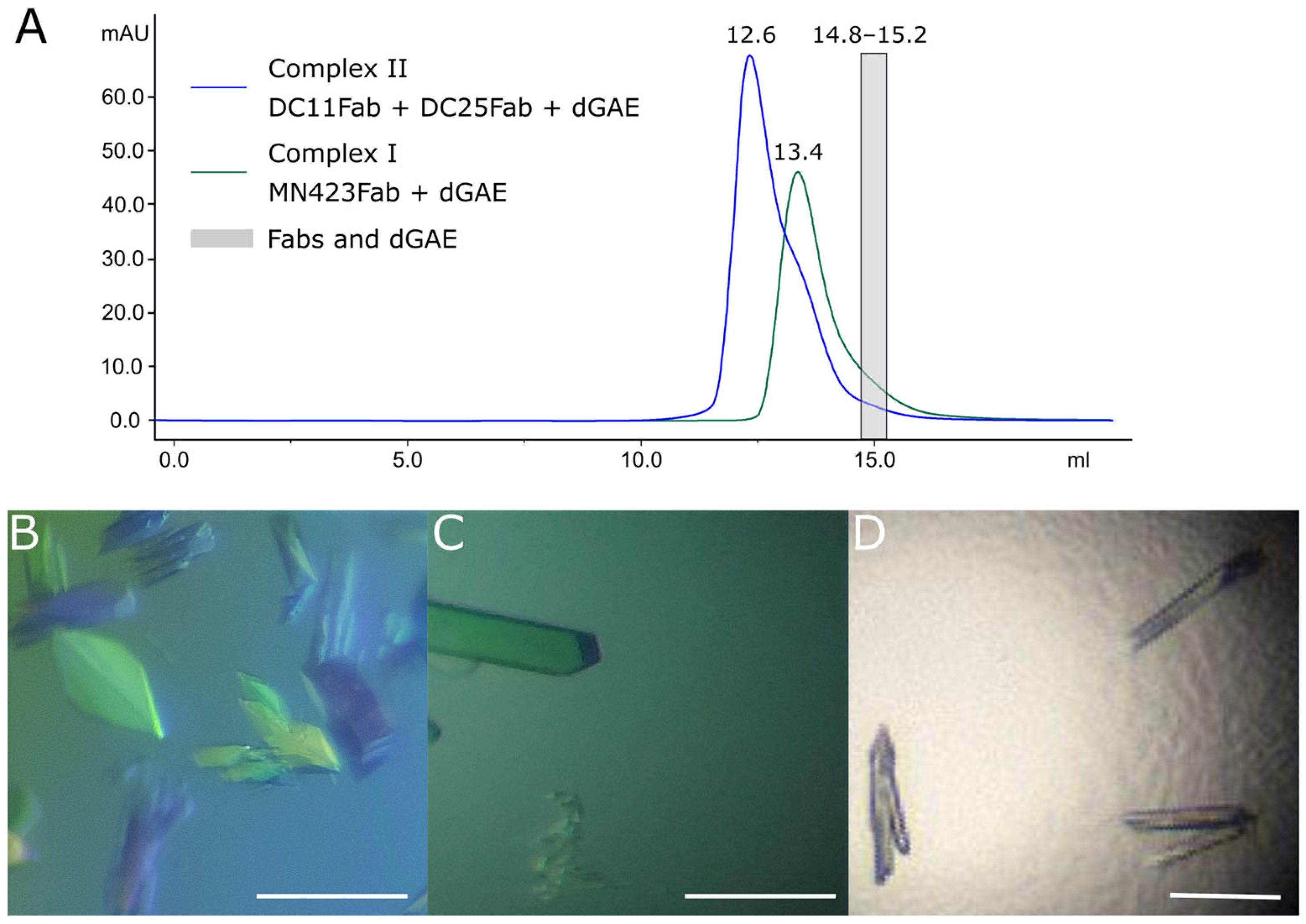
3.3. Crystallography of Alzheimer’s Disease Tau Protein Using Recombinant Antibodies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayyar, B.V.; Arora, S.; O’Kennedy, R. Coming-of-Age of Antibodies in Cancer Therapeutics. Trends Pharmacol. Sci. 2016, 37, 1009–1028. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.; Avila, J.; Schöll, M.; Kovacs, G.G.; Kövari, E.; Skrabana, R.; Evans, L.D.; Kontsekova, E.; Malawska, B.; Silva, R.; et al. A Walk through Tau Therapeutic Strategies. Acta Neuropathol. Commun. 2019, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Kim, W.-U. Targeted Immunotherapy for Autoimmune Disease. Immune Netw. 2022, 22, e9. [Google Scholar] [CrossRef]
- Lutgens, E.; Atzler, D.; Döring, Y.; Duchene, J.; Steffens, S.; Weber, C. Immunotherapy for Cardiovascular Disease. Eur. Heart J. 2019, 40, 3937–3946. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, K.R.; Chou, R.C. Therapeutic Monoclonal Antibodies and Derivatives: Historical Perspectives and Future Directions. Biotechnol. Adv. 2016, 34, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.G.; White, R.N.; Barnard, G.C. Development of a High Cell Density Transient CHO Platform Yielding MAb Titers Greater than 2 g/L in Only 7 Days. Biotechnol. Prog. 2020, 36, e3047. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Ma, W.; Meade, C.L.; Tam, A.S.; Llewellyn, E.; Cornell, R.; Cote, K.; Scarcelli, J.J.; Marshall, J.K.; Tzvetkova, B.; et al. Transient CHO Expression Platform for Robust Antibody Production and Its Enhanced N-Glycan Sialylation on Therapeutic Glycoproteins. Biotechnol. Prog. 2019, 35, e2724. [Google Scholar] [CrossRef]
- Jain, N.K.; Barkowski-Clark, S.; Altman, R.; Johnson, K.; Sun, F.; Zmuda, J.; Liu, C.Y.; Kita, A.; Schulz, R.; Neill, A.; et al. A High Density CHO-S Transient Transfection System: Comparison of ExpiCHO and Expi293. Protein Expr. Purif. 2017, 134, 38–46. [Google Scholar] [CrossRef]
- Chen, R. Bacterial Expression Systems for Recombinant Protein Production: E. Coli and Beyond. Biotechnol. Adv. 2012, 30, 1102–1107. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Steger, K.; Brady, J.; Wang, W.; Duskin, M.; Donato, K.; Peshwa, M. CHO-S Antibody Titers >1 Gram/Liter Using Flow Electroporation-Mediated Transient Gene Expression Followed by Rapid Migration to High-Yield Stable Cell Lines. J. Biomol. Screen. 2015, 20, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Lombardi, R.; Nevoltris, D.; Luthra, A.; Schofield, P.; Zimmermann, C.; Christ, D. Transient Expression of Human Antibodies in Mammalian Cells. Nat. Publ. Group 2017, 13, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Forcato, D.O.; Fili, A.E.; Alustiza, F.E.; Lázaro Martínez, J.M.; Bongiovanni Abel, S.; Olmos Nicotra, M.F.; Alessio, A.P.; Rodríguez, N.; Barbero, C.; Bosch, P. Transfection of Bovine Fetal Fibroblast with Polyethylenimine (PEI) Nanoparticles: Effect of Particle Size and Presence of Fetal Bovine Serum on Transgene Delivery and Cytotoxicity. Cytotechnology 2017, 69, 655–665. [Google Scholar] [CrossRef]
- Sou, S.N.; Polizzi, K.M.; Kontoravdi, C. Evaluation of Transfection Methods for Transient Gene Expression in Chinese Hamster Ovary Cells. Adv. Biosci. Biotechnol. 2013, 04, 1013–1019. [Google Scholar] [CrossRef][Green Version]
- Dekevic, G.; Tasto, L.; Czermak, P.; Salzig, D. Statistical Experimental Designs to Optimize the Transient Transfection of HEK 293T Cells and Determine a Transfer Criterion from Adherent Cells to Larger-Scale Cell Suspension Cultures. J. Biotechnol. 2022, 346, 23–34. [Google Scholar] [CrossRef]
- Stuible, M.; Burlacu, A.; Perret, S.; Brochu, D.; Paul-Roc, B.; Baardsnes, J.; Loignon, M.; Grazzini, E.; Durocher, Y. Optimization of a High-Cell-Density Polyethylenimine Transfection Method for Rapid Protein Production in CHO-EBNA1 Cells. J. Biotechnol. 2018, 281, 39–47. [Google Scholar] [CrossRef]
- Combe, M.; Sokolenko, S. Quantifying the Impact of Cell Culture Media on CHO Cell Growth and Protein Production. Biotechnol. Adv. 2021, 50, 107761. [Google Scholar] [CrossRef]
- Rekena, A.; Livkisa, D.; Loca, D. Factors Affecting Chinese Hamster Ovary Cell Proliferation and Viability. Vide Tehnol. Resur. Environ. Technol. Resour. 2019, 1, 145–248. [Google Scholar] [CrossRef]
- Donaldson, J.; Kleinjan, D.J.; Rosser, S. Synthetic Biology Approaches for Dynamic CHO Cell Engineering. Curr. Opin. Biotechnol. 2022, 78, 102806. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, Y.; Hougland, M.D.; Schmitt, M.G.; Barnard, G.C. Transcriptional and Post-Transcriptional Targeting for Enhanced Transient Gene Expression in CHO Cells. Biotechnol. Lett. 2015, 37, 2379–2386. [Google Scholar] [CrossRef]
- Rajendra, Y.; Hougland, M.D.; Alam, R.; Morehead, T.A.; Barnard, G.C. A High Cell Density Transient Transfection System for Therapeutic Protein Expression Based on a CHO GS-Knockout Cell Line: Process Development and Product Quality Assessment. Biotechnol. Bioeng. 2015, 112, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.T.; Sehlin, D.; Lannfelt, L.; Syvänen, S.; Hultqvist, G. Efficient and Inexpensive Transient Expression of Multispecific Multivalent Antibodies in Expi293 Cells. Biol. Proced. Online 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Kontsekova, E.; Zilka, N.; Kovacech, B.; Skrabana, R.; Novak, M. Identification of Structural Determinants on Tau Protein Essential for Its Pathological Function: Novel Therapeutic Target for Tau Immunotherapy in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2014, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Novak, M.; Kabat, J.; Wischik, C.M. Molecular Characterization of the Minimal Protease Resistant Tau Unit of the Alzheimer’s Disease Paired Helical Filament. EMBO J. 1993, 12, 365–370. [Google Scholar] [CrossRef]
- Jakes, R.; Novak, M.; Davison, M.; Wischik, C.M. Identification of 3- and 4-Repeat Tau Isoforms within the PHF in Alzheimer’s Disease. EMBO J. 1991, 10, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Wischik, C.M.; Novak, M.; Edwards, P.C.; Tichelaar, W.; Klug, A.; Crowther, R.A. Structural Characterization of the Core of the Paired Helical Filament of Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1988, 85, 4884–4888. [Google Scholar] [CrossRef]
- Wischik, C.M.; Novak, M.; Thøgersen, H.C.; Edwards, P.C.; Runswick, M.J.; Jakes, R.; Walker, J.E.; Milstein, C.; Roth, M.; Klug, A. Isolation of a Fragment of Tau Derived from the Core of the Paired Helical Filament of Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1988, 85, 4506–4510. [Google Scholar] [CrossRef]
- Csokova, N.; Skrabana, R.; Liebig, H.D.; Mederlyova, A.; Kontsek, P.; Novak, M. Rapid Purification of Truncated Tau Proteins: Model Approach to Purification of Functionally Active Fragments of Disordered Proteins, Implication for Neurodegenerative Diseases. Protein Expr. Purif. 2004, 35, 366–372. [Google Scholar] [CrossRef]
- Krajciova, G.; Skrabana, R.; Filipcik, P.; Novak, M. Preserving Free Thiols of Intrinsically Disordered Tau Protein without the Use of a Reducing Agent. Anal. Biochem. 2008, 383, 343–345. [Google Scholar] [CrossRef]
- Skrabana, R.; Cehlar, O.; Novak, M. Non-Robotic High-Throughput Setup for Manual Assembly of Nanolitre Vapour-Diffusion Protein Crystallization Screens. J. Appl. Crystallogr. 2012, 45, 1061–1065. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP4 Suite and Current Developments. Acta Crystallogr. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Romanova, N.; Noll, T. Engineered and Natural Promoters and Chromatin-Modifying Elements for Recombinant Protein Expression in CHO Cells. Biotechnol. J. 2018, 13, 1700232. [Google Scholar] [CrossRef] [PubMed]
- Matoulkova, E.; Michalova, E.; Vojtesek, B.; Hrstka, R. The Role of the 3′ Untranslated Region in Post-Transcriptional Regulation of Protein Expression in Mammalian Cells. RNA Biol. 2012, 9, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Moore, M. From Birth to Death: The Complex Lives of Eukaryotic MRNAs. Science 2005, 309, 1514–1518. [Google Scholar] [CrossRef]
- Kovacech, B.; Skrabana, R.; Novak, M. Transition of Tau Protein from Disordered to Misordered in Alzheimer’s Disease. Neurodegener. Dis. 2010, 7, 24–27. [Google Scholar] [CrossRef]
- Vechterova, L.; Kontsekova, E.; Zilka, N.; Ferencik, M.; Ravid, R.; Novak, M. DCII: A Novel Monoclonal Antibody Revealing Alzheimer’s Disease-Specific Tau Epitope. NeuroReport 2003, 14, 87–91. [Google Scholar] [CrossRef]
- Skrabana, R.; Kontsek, P.; Mederlyova, A.; Iqbal, K.; Novak, M. Folding of Alzheimer’s Core PHF Subunit Revealed by Monoclonal Antibody 423. FEBS Lett. 2004, 568, 178–182. [Google Scholar] [CrossRef]
- Ohradanova-Repic, A.; Praženicová, R.; Gebetsberger, L.; Moskalets, T.; Skrabana, R.; Cehlar, O.; Tajti, G.; Stockinger, H.; Leksa, V. Time to Kill and Time to Heal: The Multifaceted Role of Lactoferrin and Lactoferricin in Host Defense. Pharmaceutics 2023, 15, 1056. [Google Scholar] [CrossRef]
- Kaneyoshi, K.; Kuroda, K.; Uchiyama, K.; Onitsuka, M.; Yamano-Adachi, N.; Koga, Y.; Omasa, T. Secretion Analysis of Intracellular “Difficult-to-Express” Immunoglobulin G (IgG) in Chinese Hamster Ovary (CHO) Cells. Cytotechnology 2019, 71, 305–316. [Google Scholar] [CrossRef]
- Mathias, S.; Wippermann, A.; Raab, N.; Zeh, N.; Handrick, R.; Gorr, I.; Schulz, P.; Fischer, S.; Gamer, M.; Otte, K. Unraveling What Makes a Monoclonal Antibody Difficult-to-Express: From Intracellular Accumulation to Incomplete Folding and Degradation via ERAD. Biotechnol. Bioeng. 2020, 117, 5–16. [Google Scholar] [CrossRef]
- Cehlar, O.; Skrabana, R.; Kovac, A.; Kovacech, B.; Novak, M. Crystallization and Preliminary X-Ray Diffraction Analysis of Tau Protein Microtubule-Binding Motifs in Complex with Tau5 and DC25 Antibody Fab Fragments. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2012, 68, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Skrabana, R.; Dvorsky, R.; Sevcik, J.; Novak, M. Monoclonal Antibody MN423 as a Stable Mold Facilitates Structure Determination of Disordered Tau Protein. J. Struct. Biol. 2010, 171, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sevcik, J.; Skrabana, R.; Kontsekova, E.; Novak, M. Structure Solution of Misfolded Conformations Adopted by Intrinsically Disordered Alzheimers Tau Protein. Protein Pept. Lett. 2009, 16, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Tossolini, I.; Gugliotta, A.; López Díaz, F.; Kratje, R.; Prieto, C. Screening of CHO-K1 Endogenous Promoters for Expressing Recombinant Proteins in Mammalian Cell Cultures. Plasmid 2022, 119–120, 102620. [Google Scholar] [CrossRef] [PubMed]
- Kober, L.; Zehe, C.; Bode, J. Optimized Signal Peptides for the Development of High Expressing CHO Cell Lines. Biotechnol. Bioeng. 2013, 110, 1164–1173. [Google Scholar] [CrossRef]
- Haryadi, R.; Ho, S.; Kok, Y.J.; Pu, H.X.; Zheng, L.; Pereira, N.A.; Li, B.; Bi, X.; Goh, L.-T.; Yang, Y.; et al. Optimization of Heavy Chain and Light Chain Signal Peptides for High Level Expression of Therapeutic Antibodies in CHO Cells. PLoS ONE 2015, 10, e0116878. [Google Scholar] [CrossRef]
- Khansarizadeh, M.; Mokhtarzadeh, A.; Rashedinia, M.; Taghdisi, S.M.; Lari, P.; Abnous, K.H.; Ramezani, M. Identification of Possible Cytotoxicity Mechanism of Polyethylenimine by Proteomics Analysis. Hum. Exp. Toxicol. 2016, 35, 377–387. [Google Scholar] [CrossRef]
- Sevcik, J.; Skrabana, R.; Dvorsky, R.; Csokova, N.; Iqbal, K.; Novak, M. X-Ray Structure of the PHF Core C-Terminus: Insight into the Folding of the Intrinsically Disordered Protein Tau in Alzheimer’s Disease. FEBS Lett. 2007, 581, 5872–5878. [Google Scholar] [CrossRef]
- Li, W.; Fan, Z.; Lin, Y.; Wang, T.-Y. Serum-Free Medium for Recombinant Protein Expression in Chinese Hamster Ovary Cells. Front. Bioeng. Biotechnol. 2021, 9, 646363. [Google Scholar] [CrossRef]
| Antibody | Radius [nm] | PD [%] | MW [kDa] |
|---|---|---|---|
| DC11Fab | 2.952 ± 0.012 | 2.3 ± 0.7 | 42 |
| MN423Fab | 3.051 ± 0.009 | 4.7 ± 1.7 | 46 |
| DC25Fab | 2.961 ± 0.012 | 1.9 ± 0.3 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meskova, K.; Martonova, K.; Hrasnova, P.; Sinska, K.; Skrabanova, M.; Fialova, L.; Njemoga, S.; Cehlar, O.; Parmar, O.; Kolenko, P.; et al. Cost-Effective Protein Production in CHO Cells Following Polyethylenimine-Mediated Gene Delivery Showcased by the Production and Crystallization of Antibody Fabs. Antibodies 2023, 12, 51. https://doi.org/10.3390/antib12030051
Meskova K, Martonova K, Hrasnova P, Sinska K, Skrabanova M, Fialova L, Njemoga S, Cehlar O, Parmar O, Kolenko P, et al. Cost-Effective Protein Production in CHO Cells Following Polyethylenimine-Mediated Gene Delivery Showcased by the Production and Crystallization of Antibody Fabs. Antibodies. 2023; 12(3):51. https://doi.org/10.3390/antib12030051
Chicago/Turabian StyleMeskova, Klaudia, Katarina Martonova, Patricia Hrasnova, Kristina Sinska, Michaela Skrabanova, Lubica Fialova, Stefana Njemoga, Ondrej Cehlar, Olga Parmar, Petr Kolenko, and et al. 2023. "Cost-Effective Protein Production in CHO Cells Following Polyethylenimine-Mediated Gene Delivery Showcased by the Production and Crystallization of Antibody Fabs" Antibodies 12, no. 3: 51. https://doi.org/10.3390/antib12030051
APA StyleMeskova, K., Martonova, K., Hrasnova, P., Sinska, K., Skrabanova, M., Fialova, L., Njemoga, S., Cehlar, O., Parmar, O., Kolenko, P., Pevala, V., & Skrabana, R. (2023). Cost-Effective Protein Production in CHO Cells Following Polyethylenimine-Mediated Gene Delivery Showcased by the Production and Crystallization of Antibody Fabs. Antibodies, 12(3), 51. https://doi.org/10.3390/antib12030051





