Proteolysis-Targeting Chimera (PROTAC) Delivery into the Brain across the Blood-Brain Barrier
Abstract
1. Introduction
2. Discussion
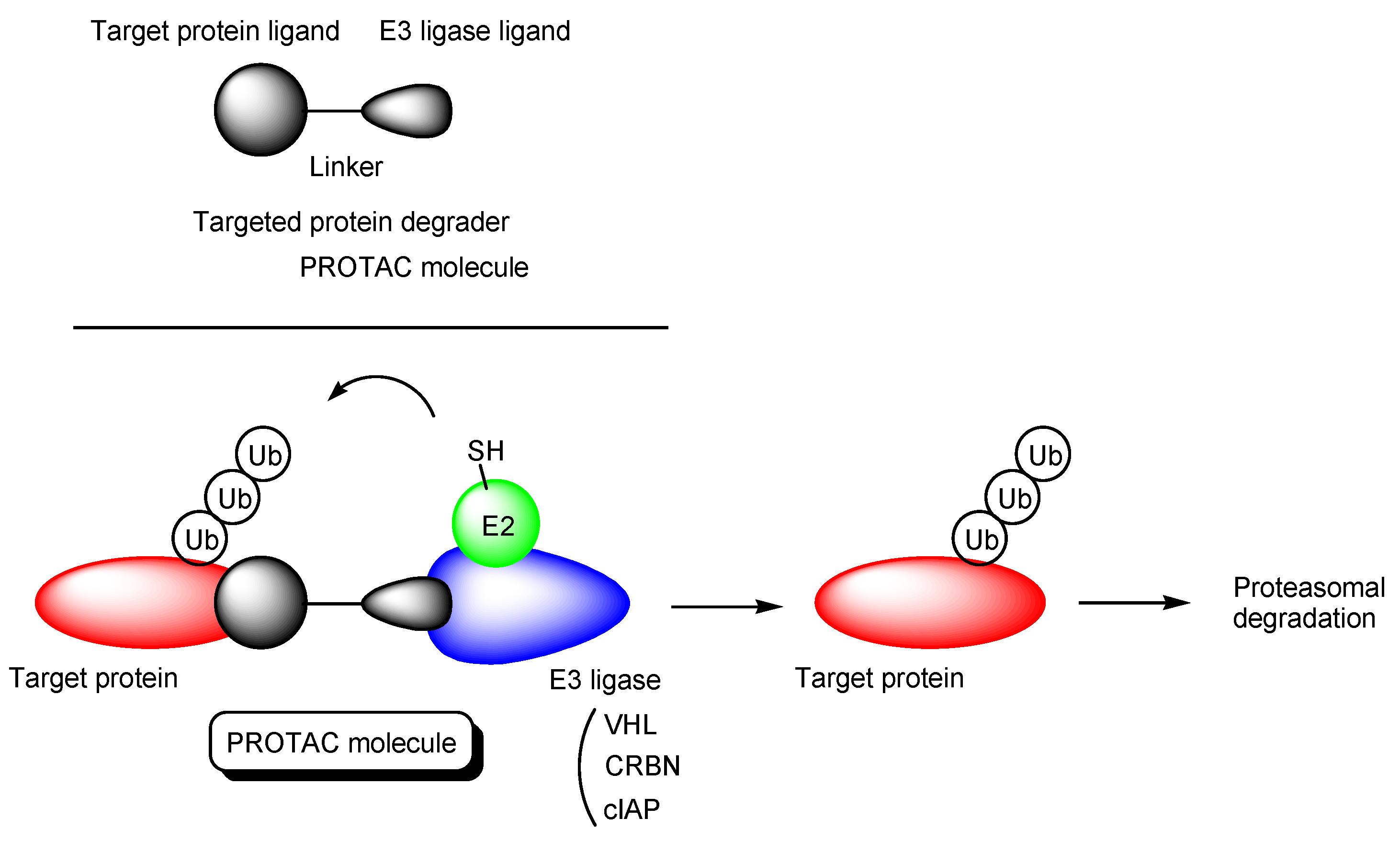
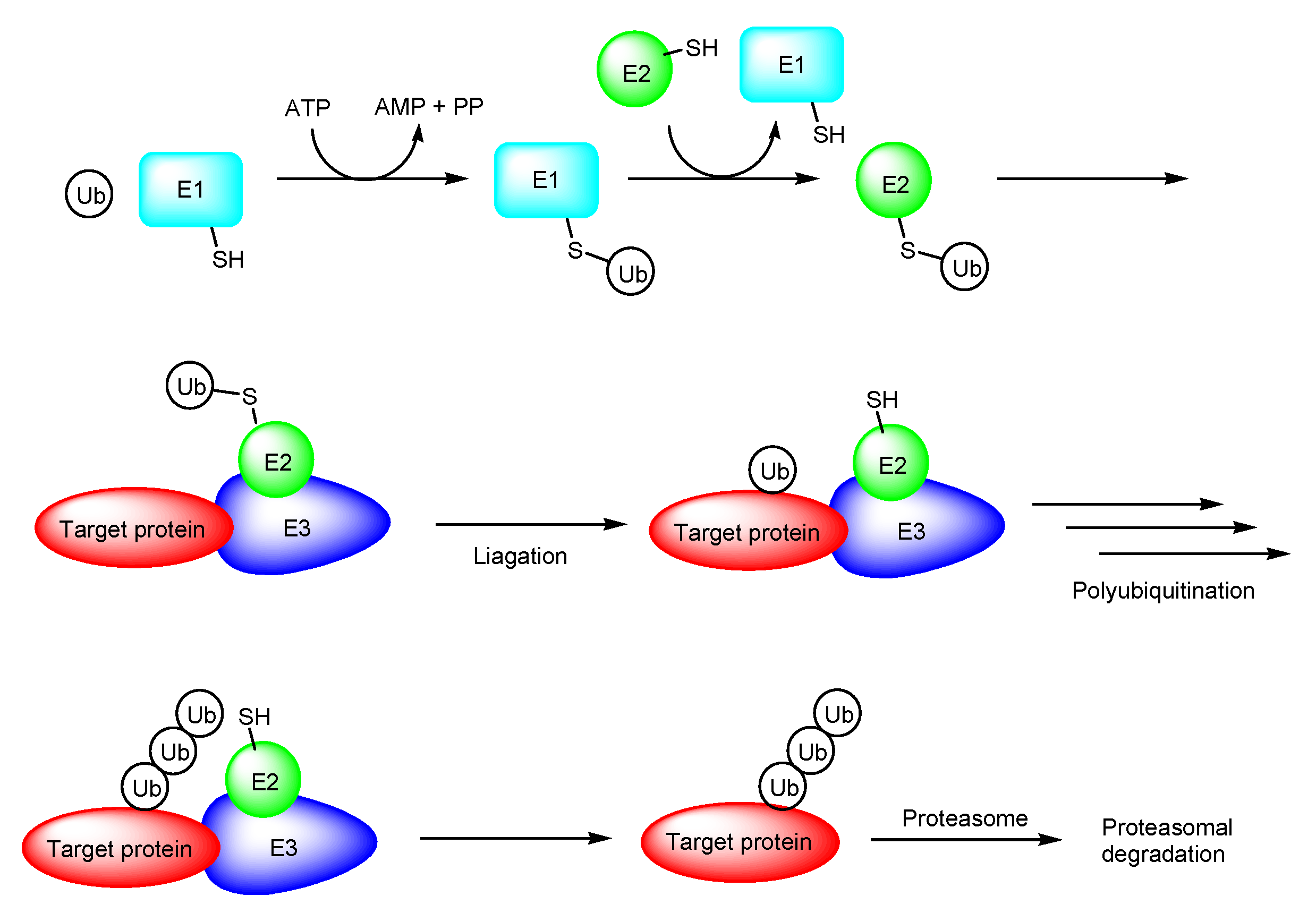
2.1. PROTACs
- (i)
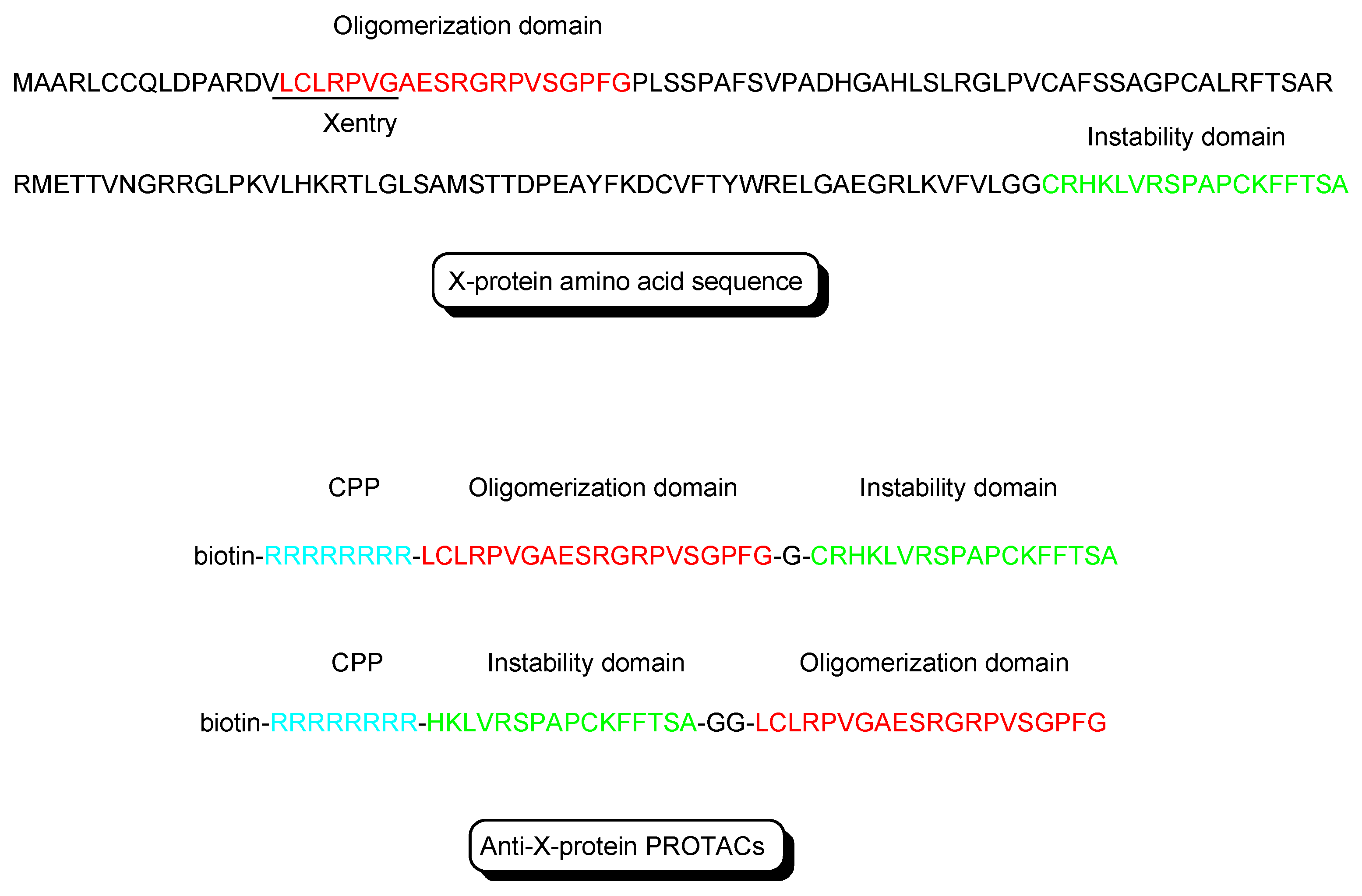
- X-protein, derived from the hepatitis B virus (HBV), induces hepatocellular carcinoma (HCC). Thus, the elimination of X-protein by a PROTAC approach can prevent HCC. X-proteins were oligomerized through the oligomerization domain that could be an X-protein ligand. Anti-X-protein peptide-based PROTACs with (a) the oligomerization domain of the X-protein as an X-protein ligand, (b) degron peptide of the X-protein as an E3 ligase ligand, and (c) R8 as a CPP (Figure 4) entered cells and destroyed the X-protein in HepG2 cells. In general, a degron is a peptide sequence within a protein and is recognized by an E3 ligase. The instability domain as a degron peptide would induce proteasomal degradation of the X-protein [17].
- (ii)
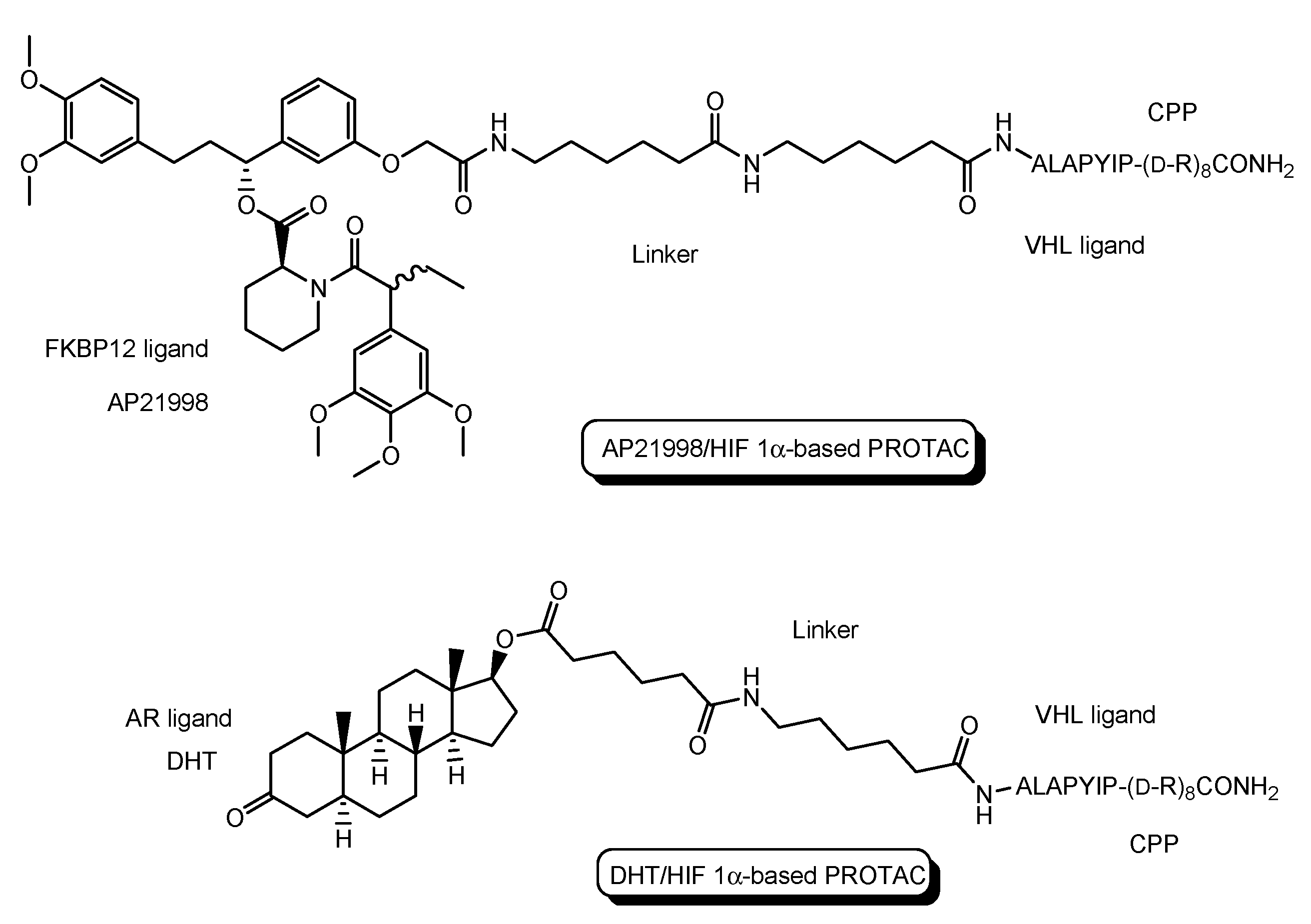
- The first in vivo examples of small molecule-based PROTACs were demonstrated targeting the FK506 binding protein (FKBP12) or androgen receptor (AR). AP21998/hypoxia inducible factor (HIF) 1α-based PROTAC (Figure 5) is composed of (a) AP21998 as an FKBP12 ligand, (b) the ALAPYIP sequence as a VHL ligand, and (c) D-R8 fused to the C-terminus. FKBP12 was fused to enhanced green fluorescent protein (EGFP) by a vector, to monitor loss of intracellular fluorescence by degradation due to PROTACs. After hydroxylation of the central proline in the ALAPYIP sequence of HIF 1α by a proline hydroxylase, the hydroxylated ALAPYIP became recognized by VHL. On the other hand, dihydrotestosterone (DHT)/HIF 1α-based PROTAC (Figure 5) is composed of (a) DHT as an androgen receptor (AR) ligand, (b) the ALAPYIP sequence as a VHL ligand after hydroxylation of the central proline in the ALAPYIP sequence by a proline hydroxylase, and (c) D-R8 fused to the C-terminus. The AR was fused to GFP. In fact, the AP21998/ HIF 1α-based PROTAC degraded EGFP-FKBP12 in a VHL-dependent manner using HeLaEGFP-FKBP cells. The fluorescence of FKBP12-EGFP was lost in the cells. The DHT/HIF1 α-based PROTAC induced the degradation of the AR using HEK293GFP-AR cells [18]. These findings suggested that PROTACs were transported into cells across the membrane by virtue of CPPs. D-R8 is more stable to L-protein-mediated enzymatic degradation than L-R8.
- (iii)
- PROTACs for Alzheimer’s disease (AD) were developed. The tau protein abnormally aggregates in AD patients’ brains. Peptide-based PROTACs with (a) a tau ligand, (b) an E3 ligase ligand, and (c) D-R8 fused to the C-terminus of the E3 ligase ligand were designed. Among them, TH006, with (b) the ALAPYIP sequence as a VHL ligand (Figure 6), demonstrated the highest tau degradation in in vitro assay using tau-overexpressed SH-SY5Y cells, and furthermore, it reduced the toxicity of amyloid β (Aβ), and lowered the tau level in an AD mouse model [19].
- (iv)
- Moreover, peptide-based PROTACs with (a) a tau ligand, (b) Kelch-like ECH-associated protein-1 (Keap1) ligand as an E3 ligase ligand forming the Keap1- Cullin (Cul3) E3 ligase complex, and (c) D-R8 at the C-terminus (Figure 6) showed degradation of intracellular tau in vitro using tau-EGFP over-expressed SH-SY5Y cells [20].
- (v)
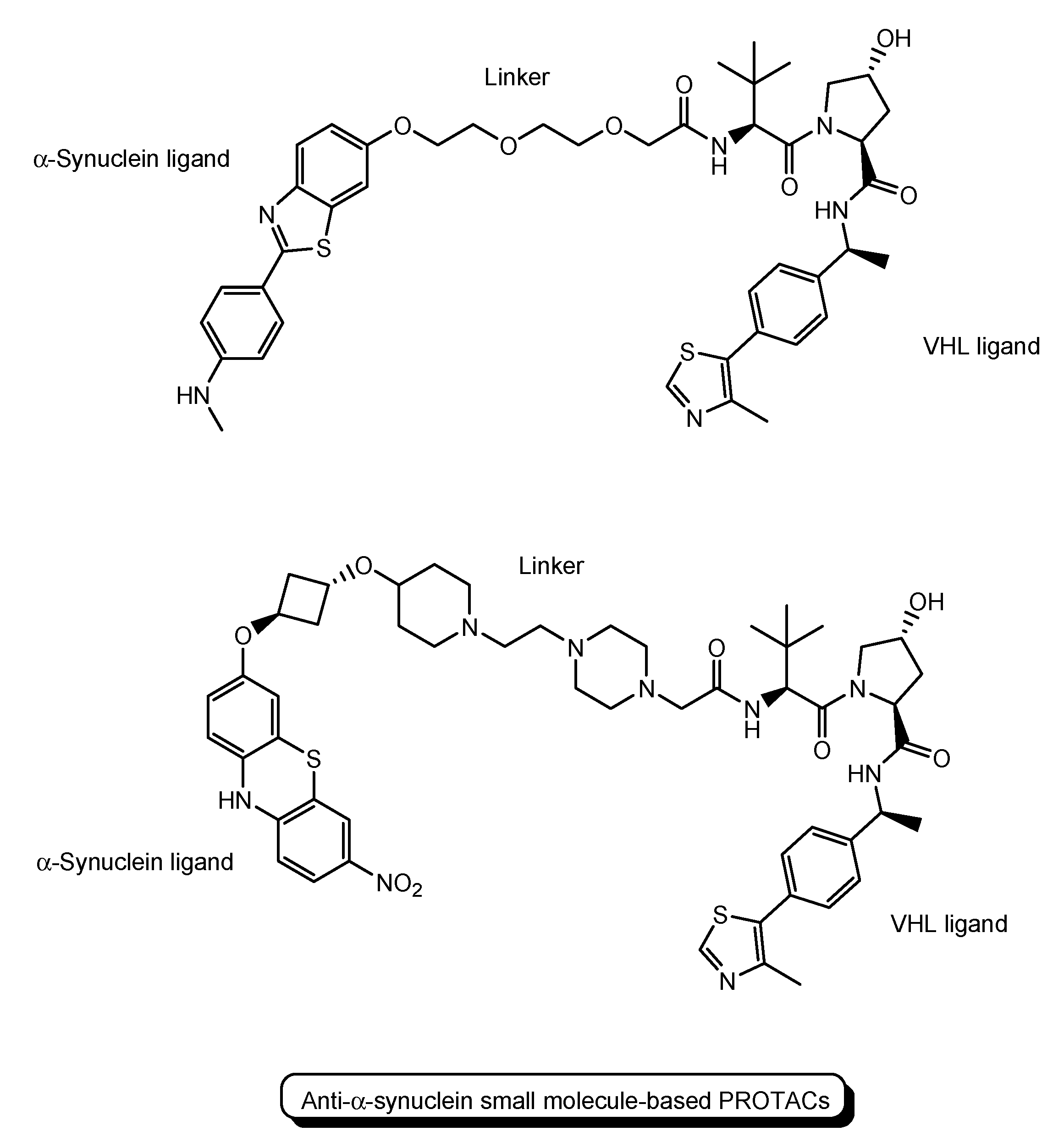
- PROTACs for Parkinson’s disease (PD) were developed. α-Synuclein protein aggregation is a prominent feature in PD patients’ brains. Peptide-based PROTACs with (a) an α-synuclein ligand, (b) an E3 ligase ligand at the carboxyl terminus, and (c) TAT at the N-terminus (Figure 6) suppressed the cellular α-synuclein level in the primary cultured cortical neurons [21]. Although CPPs delivered PROTACs into cells, they lacked cell selectivity. Positively charged TAT was internalized through receptor-mediated endocytosis using ubiquitously expressed negatively charged heparan sulfate proteoglycans (HSPGs) as a receptor on the cell surface. Furthermore, passive diffusion also lacks cell selectivity. VHL and CRBN are ubiquitously expressed in various tissues [22]. To avoid wrong distribution and off-target side effects, cell-selective internalization should be established using well-designed compounds.
2.2. Clinical Trials of PROTACs
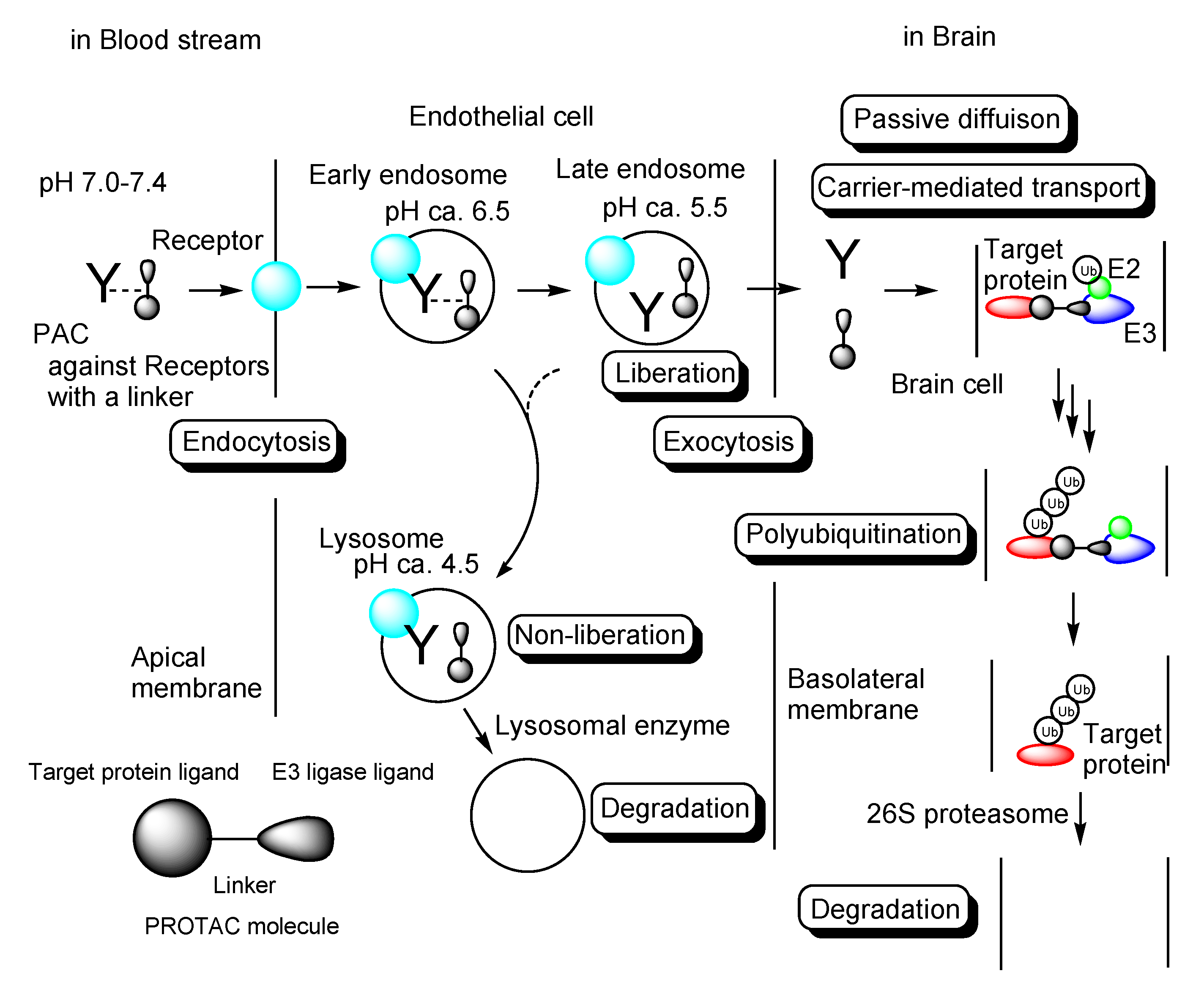
2.3. The Possibilities of PROTACs for CNS Diseases
2.4. The Implements of PROTACs for CNS Diseases
2.5. Promising PROTACs for CNS Diseases
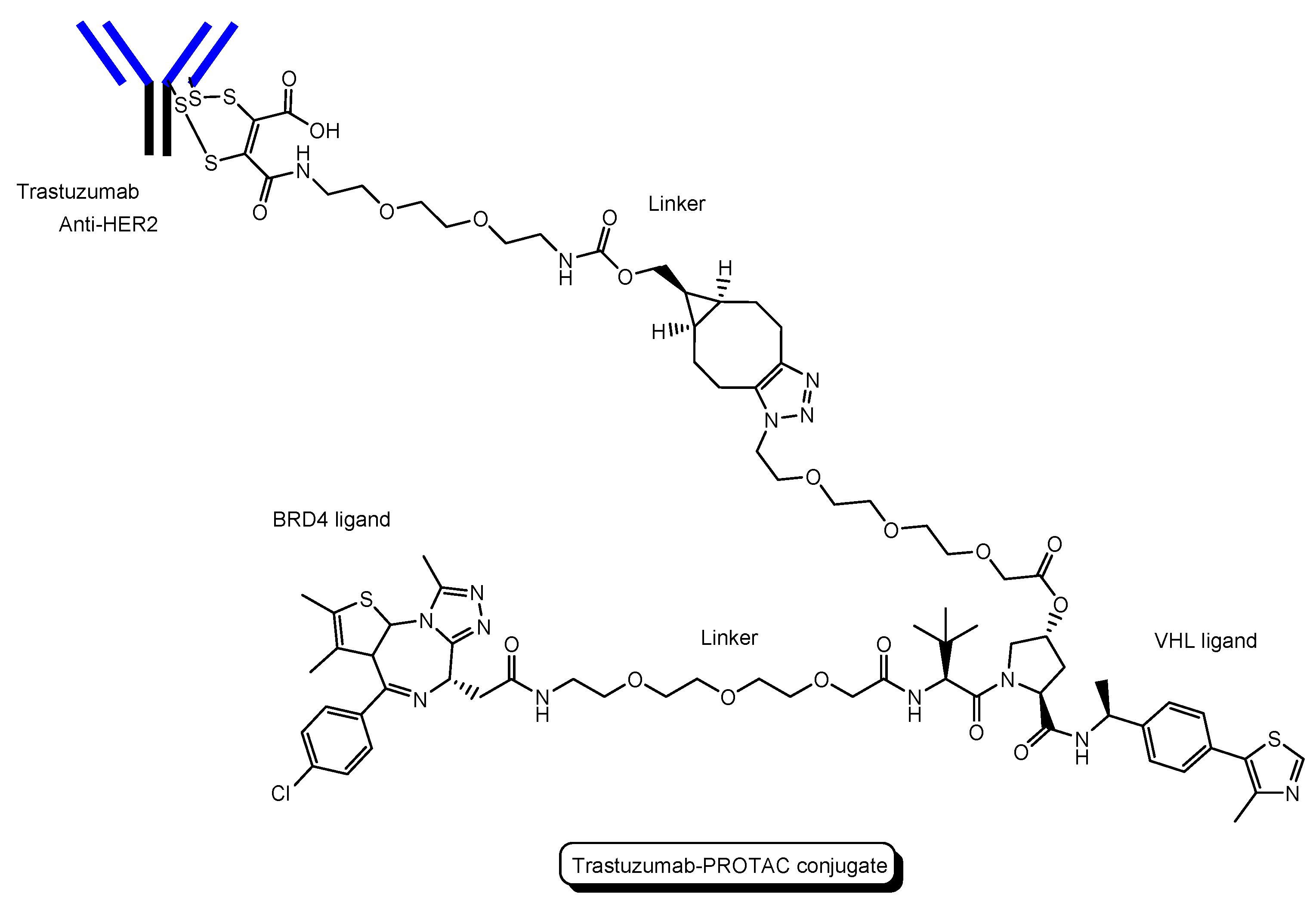
- (i)
- A trastuzumab-PROTAC conjugate with a BRD4 ligand and a VHL ligand (Figure 12) showed BRD4 degradation only in HER2 positive breast cancer cell lines [46]. Trastuzumab (Herceptin) is a humanized, recombinant monoclonal antibody against the extracellular domain of human epidermal growth factor type 2 (HER2). This trastuzumab-PROTAC was internalized into cells through receptor-mediated endocytosis using HER2. It was thought that S-S bonds that connected linkers and a trastuzumab were reductively cleaved in endosomes [47] and that ester bonds that connected a linker and a PROTAC were enzymatically cleaved in endosomes and/or lysosomes. Cleaved PROTACs were implied to have been transported into the cytosol across the membrane of endosomes and/or lysosomes through passive diffusion, because PROTACs showed BRD4 degradation by the ubiquitin-proteasome system.
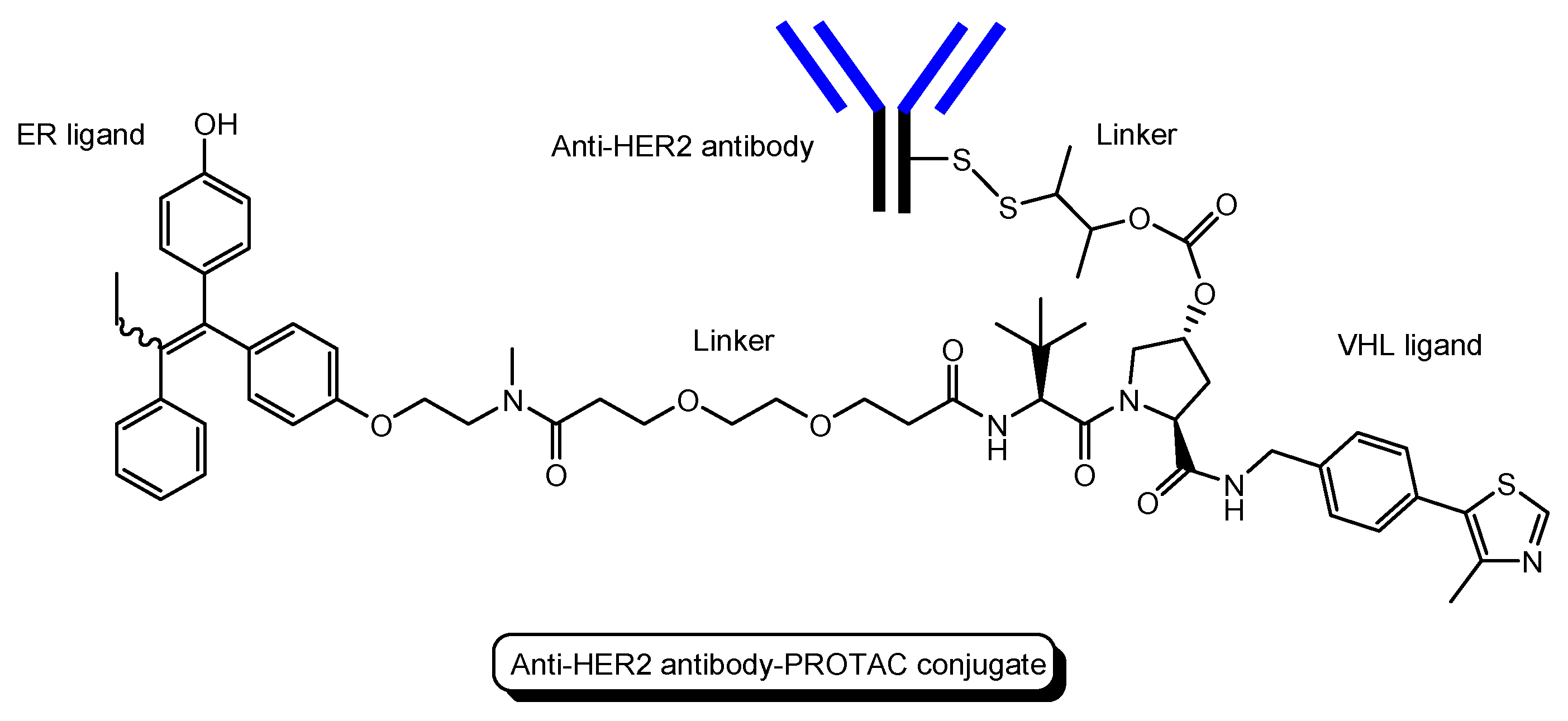
- (ii)
- Several antibody-PROTAC conjugates with an ER ligand and an E3 ligase ligand were designed and evaluated for their ER degradation activity. Among them, an anti-HER2 antibody-PROTAC conjugate with an ER ligand and a VHL ligand (Figure 13) demonstrated the highest ERα degradation (99%) using MCF7-neo/HER2 cells that were stably transfected to overexpress HER2 [48]. Thus, this PAC entered cells through receptor-mediated endocytosis using HER2. It was thought that cleaved PROTACs were transported into the cytosol across the membrane of endosomes and/or lysosomes through passive diffusion.
- (iii)
- Six-transmembrane epithelial antigen of prostate 1 (STEAP1) is a membrane protein overexpressed in cancer cells. Anti-STEAP1 antibody-PROTAC conjugates with a BRD4 ligand and a VHL ligand, particularly STEAP1-5a (drug-to-antibody ratio (DAR) 6.0) (Figure 14), afforded degradation of the BRD4 protein with a DC50 value of 0.67 nM using PC3-S1 prostate cancer cells [49]. The DC50 value is the concentration at which the target is degraded by fifty percent. Modified anti-STEAP1 antibody-PROTAC conjugates with a BRD4 ligand and a VHL ligand, particularly STEAP1-9d (DAR 5.9) (Figure 12), afforded the highest degradation of the BRD4 protein with a DC50 value of 0.025 nM using PC3-S1 prostate cancer cells [50]. Anti-STEAP1 antibody-PROTAC conjugates entered cells through receptor-mediated endocytosis using STEAP1. Enzymatically cleaved PROTACs were transferred to the cytosol from lysosomes probably through passive diffusion. Whereas STEAP1-5a was cut by proteases, STEAP1-9d was cut by phosphatases. Currently, PACs have been investigated mainly for cancer therapy. However, this strategy can be applied to other diseases including CNS diseases.
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, Y.; Ma, D.; Wang, Y. The PROTAC technology in drug development. Cell Biochem. Funct. 2019, 37, 21–30. [Google Scholar] [CrossRef]
- He, M.; Cao, C.; Ni, Z.; Liu, Y.; Song, P.; Hao, S.; He, Y.; Sun, X.; Rao, Y. PROTACs: Great opportunities for academia and industry (an update from 2020 to 2021). Signal Transduct. Target Ther. 2022, 7, 181. [Google Scholar] [CrossRef]
- Segarra, M.; Aburto, M.A.; Acker-Palmer, A. Blood-Brain Barrier Dynamics to Maintain Brain Homeostasis. Trends Neurosci. 2021, 44, 393–405. [Google Scholar] [CrossRef]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef]
- Kimura, Y.; Morita, S.; Matsuo, M.; Ueda, K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer Sci. 2007, 98, 1303–1310. [Google Scholar] [CrossRef]
- Tashima, T. Brain Cancer Chemotherapy through a Delivery System across the Blood-Brain Barrier into the Brain Based on Receptor-Mediated Transcytosis Using Monoclonal Antibody Conjugates. Biomedicines 2022, 10, 1597. [Google Scholar] [CrossRef]
- Tashima, T. Delivery of Intravenously Administered Antibodies Targeting Alzheimer’s Disease-Relevant Tau Species into the Brain Based on Receptor-Mediated Transcytosis. Pharmaceutics 2022, 14, 411. [Google Scholar] [CrossRef]
- Tashima, T. Intriguing possibilities and beneficial aspects of transporter-conscious drug design. Bioorg. Med. Chem. 2015, 23, 4119–4131. [Google Scholar] [CrossRef]
- Tashima, T. Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood–Brain Barrier Using Receptor-Mediated Transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Kleiger, G.; Mayor, T. Perilous journey: A tour of the ubiquitin-proteasome system. Trends Cell Biol. 2014, 24, 352–359. [Google Scholar] [CrossRef]
- Pohl, C.; Dikic, I. Cellular quality control by the ubiquitin-proteasome system and autophagy. Science 2019, 366, 818–822. [Google Scholar] [CrossRef]
- Burslem, G.M.; Crews, C.M. Small-Molecule Modulation of Protein Homeostasis. Chem. Rev. 2017, 117, 11269–11301. [Google Scholar] [CrossRef]
- Ohoka, N. Development of Protein Knockdown Technology as Emerging Drug Discovery Strategy. Yakugaku Zasshi 2018, 138, 1135–1143. [Google Scholar] [CrossRef]
- Troup, R.I.; Fallan, C.; Baud, M.G.J. Current strategies for the design of PROTAC linkers: A critical review. Explor. Target Antitumor Ther. 2020, 1, 273–312. [Google Scholar] [CrossRef]
- Poongavanam, V.; Atilaw, Y.; Siegel, S.; Giese, A.; Lehmann, L.; Meibom, D.; Erdelyi, M.; Kihlberg, J. Linker-dependent folding rationalizes PROTAC cell permeability. J. Med. Chem. 2022, 65, 13029–13040. [Google Scholar] [CrossRef]
- Poongavanam, V.; Kölling, F.; Giese, A.; Göller, A.H.; Lehmann, L.; Meibom, D.; Kihlberg, J. Predictive Modeling of PROTAC Cell Permeability with Machine Learning. ACS Omega 2022, 8, 5901–5916. [Google Scholar] [CrossRef]
- Montrose, K.; Krissansen, G.W. Design of a PROTAC that antagonizes and destroys the cancer-forming X-protein of the hepatitis B virus. Biochem. Biophys. Res. Commun. 2014, 453, 735–740. [Google Scholar] [CrossRef]
- Schneekloth, J.S., Jr.; Fonseca, F.N.; Koldobskiy, M.; Mandal, A.; Deshaies, R.; Sakamoto, K.; Crews, C.M. Chemical genetic control of protein levels: Selective in vivo targeted degradation. J. Am. Chem. Soc. 2004, 12, 3748–3754. [Google Scholar] [CrossRef]
- Chu, T.-T.; Gao, N.; Li, Q.Q.; Chen, P.-G.; Yang, X.-F.; Chen, Y.-X.; Zhao, Y.-F.; Li, Y.-M. Specific knockdown of endogenous tau protein by peptide-directed ubiquitin-proteasome degradation. Cell Chem. Biol. 2016, 23, 453–461. [Google Scholar] [CrossRef]
- Lu, M.; Liu, T.; Jiao, Q.; Ji, J.; Tao, M.; Liu, Y.; You, Q.; Jiang, Z. Discovery of a Keap1-dependent peptide PROTAC to knockdown tau by ubiquitination-proteasome degradation pathway. Eur. J. Med. Chem. 2018, 146, 251–259. [Google Scholar] [CrossRef]
- Qu, J.; Ren, X.; Xue, F.; He, Y.; Zhang, R.; Zheng, Y.; Huang, H.; Wang, W.; Zhang, J. Specific Knockdown of α-Synuclein by Peptide Directed Proteasome Degradation Rescued Its Associated Neurotoxicity. Cell Chem. Biol. 2020, 27, 751–762. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Jung, Y.M.; Park, J.H.; Yoo, H.S.; Park, J. Discovery of E3 Ligase Ligands for Target Protein Degradation. Molecules 2022, 27, 6515. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric Molecules That Target Proteins to the Skp1-Cullin-F Box Complex for Ubiquitination and Degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef]
- Smith, A.R.; Pucheault, M.; Tae, H.T.; Crews, C.M. Targeted Intracellular Protein Degradation Induced by a Small Molecule: En Route to Chemical Proteomics. Bioorg. Med. Chem. Lett. 2008, 18, 5904–5908. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Pedrucci, F.; Pappalardo, C.; Marzaro, G.; Ferri, N.; Ferlin, A.; De Toni, L. Proteolysis Targeting Chimeric Molecules: Tuning Molecular Strategies for a Clinically Sound Listening. Int. J. Mol. Sci. 2022, 23, 6630. [Google Scholar] [CrossRef]
- Chambers-Richards, T.; Chireh, B.; D’Arcy, C. Unmet health care needs: Factors predicting satisfaction with health care services among community-dwelling Canadians living with neurological conditions. BMC Health Serv. Res. 2022, 22, 1256. [Google Scholar] [CrossRef]
- Mamun, A.A.; Uddin, S.; Kabir, T.; Khanum, S.; Sarwar, S.; Mathew, B.; Rauf, A.; Ahmed, M.; Md Ashraf, G.M. Exploring the Promise of Targeting Ubiquitin-Proteasome System to Combat Alzheimer’s Disease. Neurotox. Res. 2020, 38, 8–17. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, J.H.; Rubinsztein, D.C. Tau degradation: The ubiquitin–proteasome system versus the autophagy-lysosome system. Prog. Neurobiol. 2013, 105, 49–59. [Google Scholar] [CrossRef]
- Silva, M.C.; Ferguson, F.M.; Cai, Q.; Donovan, K.A.; Nandi, G.; Patnaik, D.; Zhang, T.; Huang, H.T.; Lucente, D.E.; Dickerson, B.C.; et al. Targeted degradation of aberrant tau in frontotemporal dementia patient-derived neuronal cell models. eLife 2019, 8, e45457. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kurimchak, A.M.; Herrera-Montávez, C.; Montserrat-Sangrà, S.; Araiza-Olivera, D.; Hu, J.; Neumann-Domer, R.; Kuruvilla, M.; Bellacosa, A.; Testa, J.R.; Jin, J.; et al. The drug efflux pump MDR1 promotes intrinsic and acquired resistance to PROTACs in cancer cells. Sci. Signal. 2022, 15, eabn2707. [Google Scholar] [CrossRef] [PubMed]
- Vagrys, D.; Davidson, J.; Chen, I.; Hubbard, R.E.; Davis, B. Exploring IDP–Ligand Interactions: Tau K18 as a Test Case. Int. J. Mol. Sci. 2020, 21, 5257. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.; Jiang, T.; Li, S.; Ye, J.; Zhen, G.; Wang, X.; Liu, Y.; Deng, M.; Ke, D.; et al. A Novel Small-molecule PROTAC Selectively Promotes Tau Clearance to Improve Cognitive Functions in Alzheimer-like Models. Theranostics 2021, 11, 5279–5295. [Google Scholar] [CrossRef]
- Liang, M.; Gu, L.; Zhang, H.; Min, J.; Wang, Z.; Ma, Z.; Zhang, C.; Zeng, S.; Pan, Y.; Yan, D.; et al. Design, Synthesis, and Bioactivity of Novel Bifunctional Small Molecules for Alzheimer’s disease. ACS Omega 2022, 7, 26308–26315. [Google Scholar] [CrossRef]
- Kargbo, R.B. PROTAC Compounds Targeting α-Synuclein Protein for Treating Neurogenerative Disorders: Alzheimer’s and Parkinson’s Diseases. ACS Med. Chem. Lett. 2020, 11, 1086–1087. [Google Scholar] [CrossRef]
- Crew, A.P.; Dong, H.; Berlin, M.; Sparks, S.M. Proteolysis Targeting Chimeric (PROTAC) Compound with E3 Ubiquitin Ligase Binding Activity and Targeting Alpha-Synuclein Protein for Treating Neurovegetative Diseases. WO 2020/041331 A1, 27 February 2020. [Google Scholar]
- Bonfanti, S.; Lionetti, M.C.; Fumagalli, M.R.; Chirasani, V.R.; Tiana, G.; Dokholyan, N.V.; Zapperi, S.; La Porta, C.A.M. Molecular mechanisms of heterogeneous oligomerization of huntingtin proteins. Sci. Rep. 2019, 9, 7615. [Google Scholar] [CrossRef]
- Irfan, Z.; Khanam, S.; Karmakar, V.; Firdous, S.M.; El Khier, B.S.I.A.; Khan, I.; Rehman, M.U.; Khan, A. Pathogenesis of Huntington’s Disease: An Emphasis on Molecular Pathways and Prevention by Natural Remedies. Brain Sci. 2022, 12, 1389. [Google Scholar] [CrossRef]
- Tomoshige, S.; Nomura, S.; Ohgane, K.; Hashimoto, Y.; Ishikawa, M. Discovery of Small Molecules that Induce the Degradation of Huntingtin. Angew. Chem. Int. Ed. 2017, 56, 11530–11533. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G.; et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef]
- Kida, S.; Kinoshita, M.; Tanaka, S.; Okumura, M.; Koshimura, Y.; Morimoto, H. Non-clinical evaluation of a blood-brain barrier-penetrating enzyme for the treatment of mucopolysaccharidosis type I. Mol. Genet. Metab. 2019, 126, S83–S84. [Google Scholar] [CrossRef]
- Okuyama, T.; Eto, Y.; Sakai, N.; Minami, K.; Yamamoto, T.; Sonoda, H.; Yamaoka, M.; Tachibana, K.; Hirato, T.; Sato, Y. Iduronate-2-Sulfatase with Anti-human Transferrin Receptor Antibody for Neuropathic Mucopolysaccharidosis II: A Phase 1/2 Trial. Mol. Ther. 2019, 27, 456–464. [Google Scholar] [CrossRef]
- Dragovich, P.S. Degrader-antibody conjugates. Chem. Soc. Rev. 2022, 51, 3886–3897. [Google Scholar] [CrossRef]
- Hong, K.B.; An, H. Degrader-Antibody Conjugates: Emerging New Modality. J. Med. Chem. 2023, 66, 140–148. [Google Scholar] [CrossRef]
- Maneiro, M.; Forte, N.; Shchepinova, M.M.; Kounde, C.S.; Chudasama, V.; Baker, J.R.; Tate, E.W. Antibody-PROTAC Conjugates Enable HER2-Dependent Targeted Protein Degradation of BRD4. ACS Chem. Biol. 2020, 15, 1306–1312. [Google Scholar] [CrossRef]
- Lin, A.Y.; Dinner, N.S. Moxetumomab pasudotox for hairy cell leukemia: Preclinical development to FDA approval. Blood Adv. 2019, 3, 2905–2910. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Adhikari, P.; Blake, R.A.; Blaquiere, N.; Chen, J.; Cheng, Y.X.; den Besten, W.; Han, J.; Hartman, S.J.; He, J.; et al. Antibody-mediated delivery of chimeric protein degraders which target estrogen receptor alpha (ERα). Bioorg. Med. Chem. Lett. 2020, 30, 126907. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Pillow, T.H.; Blake, R.A.; Sadowsky, J.D.; Adaligil, E.; Adhikari, P.; Bhakta, S.; Blaquiere, N.; Chen, J.; Cruz-Chuh, J.D.; et al. Antibody-Mediated Delivery of Chimeric BRD4 Degraders. Part 1: Exploration of Antibody Linker, Payload Loading, and Payload Molecular Properties. J. Med. Chem. 2021, 64, 2534–2575. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Pillow, T.H.; Blake, R.A.; Sadowsky, J.D.; Adaligil, E.; Adhikari, P.; Chen, J.; Corr, N.; Cruz-Chuh, J.D.; Del Rosario, G.D.; et al. Antibody-Mediated Delivery of Chimeric BRD4 Degraders. Part 2: Improvement of In Vitro Antiproliferation Activity and In Vivo Antitumor Efficacy. J. Med. Chem. 2021, 64, 2576–2607. [Google Scholar] [CrossRef]
- Mankarious, S.; Lee, M.; Fischer, S.; Pyun, K.H.; Ochs, H.D.; Oxelius, V.A.; Wedgwood, J.W. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 1988, 112, 634–640. [Google Scholar]
- Juan, A.; Noblejas-López, M.D.M.; Arenas-Moreira, M.; Alonso-Moreno, C.; Ocaña, A. Options to Improve the Action of PROTACs in Cancer: Development of Controlled Delivery Nanoparticles. Front. Cell Dev. Biol. 2022, 9, 2021. [Google Scholar] [CrossRef]
- Cimas, F.J.; Niza, E.; Juan, A.; Noblejas-López, M.d.M.; Bravo, I.; Lara-Sanchez, A.; Alonso-Moreno, C.; Ocaña, A. Controlled Delivery of BET-PROTACs: In Vitro Evaluation of MZ1-Loaded Polymeric Antibody Conjugated Nanoparticles in Breast Cancer. Pharmaceutics 2020, 12, 986. [Google Scholar] [CrossRef]
- Liu, A.P.; Aguet, F.; Danuser, G.; Schmid, S.L. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J. Cell Biol. 2010, 191, 1381–1393. [Google Scholar] [CrossRef]
- Cureton, D.K.; Harbison, C.E.; Cocucci, E.; Parrish, C.R.; Kirchhausen, T. Limited Transferrin Receptor Clustering Allows Rapid Diffusion of Canine Parvovirus into Clathrin Endocytic Structures. J. Virol. 2012, 86, 5330–5340. [Google Scholar] [CrossRef]
- Laughlin, C.D.; D’Aquili, E.G. Biogenetic Structuralism; Columbia University Press: New York, NY, USA, 1974. [Google Scholar]
- Leavy, S.A. Biogenetic Structuralism. Yale J. Biol. Med. 1976, 49, 420–421. [Google Scholar]




| # | Drug | Route | E3 Ligand | Target Protein Ligand | Disease | Sponsor | Phase | Study Start Date | Study Completion Date | ClinicalTrials.gov Identifier (accessed on 15 January 2023) | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
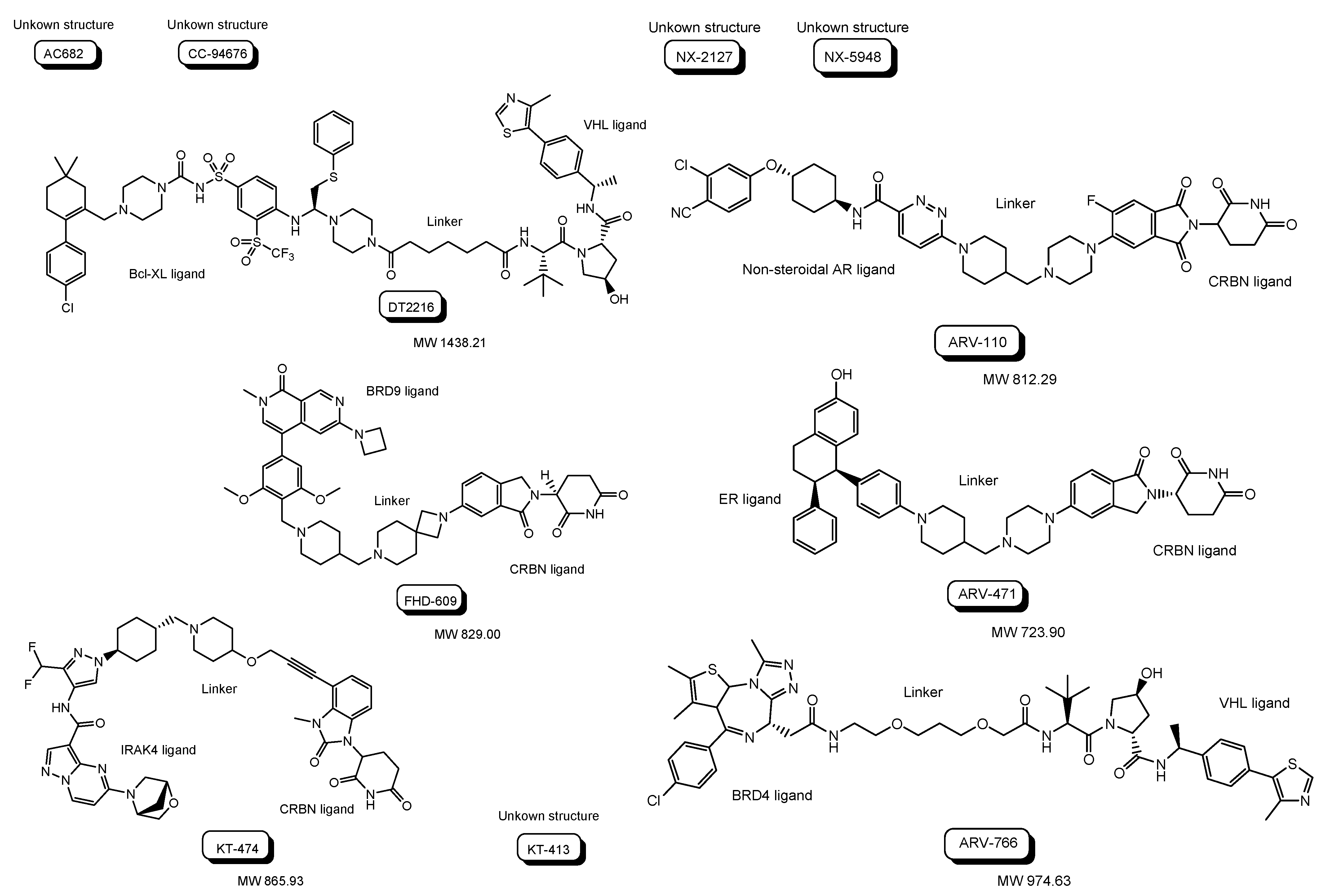
| (i) | AC682 | Oral | CRBN | ER | Cancer | Accutar Biotechnology, Inc. | Phase 1 | 12 November 2021 | September 2023 | NCT05080842 | Recruiting |
| (ii) | CC-94676 | Oral | CRBN | Non-steroidal AR | Cancer | Celgene | Phase 1 | 22 June 2020 | 27 February 2025 | NCT04428788 | Recruiting |
| (iii) | DT2216 | Intravenous | VHL | Bcl-XL | Cancer | Dialectic Therapeutics, Inc. | Phase 1 | 25 August 2021 | 15 April 2023 | NCT04886622 | Recruiting |
| (iv) | FHD-609 | Intravenous | CRBN | BRD9 | Cancer | Foghorn Therapeutics, Inc. | Phase 1 | 17 August 2021 | 31 May 2025 | NCT04965753 | Recruiting |
| (v) | KT-474 | Oral | CRBN | IRAK4 | Cancer | Kymera Therapeutics, Inc. | Phase 1 | 23 February 2021 | 20 October 2022 | NCT04772885 | Completed |
| (vi) | KT-413 | Intravenous | CRBN | IRAK4 | Cancer | Kymera Therapeutics, Inc. | Phase 1 | 13 June 2022 | May 2025 | NCT05233033 | Recruiting |
| (vii) | NX-2127 | Oral | CRBN | BTK | Cancer | Nurix Therapeutics, Inc. | Phase 1 | 5 May 2021 | November 2023 | NCT04830137 | Recruiting |
| (viii) | NX-5948 | Oral | CRBN | BTK | Cancer | Nurix Therapeutics, Inc. | Phase 1 | 13 April 2022 | May 2024 | NCT05131022 | Recruiting |
| (ix) | ARV-110 | Oral | CRBN | Non-steroidal AR | Cancer | Arvinas Androgen Receptor, Inc. | Phase 1 Phase 2 | 1 March 2019 | 31 October 2023 | NCT03888612 | Recruiting |
| (x) | ARV-471 | Oral | CRBN | ER ligand | Cancer | Arvinas Estrogen Receptor, Inc. | Phase 1 Phase 2 | 5 August 2019 | June 2023 | NCT04072952 | Recruiting |
| (xi) | ARV-766 | Oral | VHL | BRD4 | Cancer | Arvinas Androgen Receptor, Inc. | Phase 1 Phase 2 | 2 September 2021 | 27 June 2025 | NCT05067140 | Recruiting |
| # | Administrated drug | Formulation | Receptor for endocytosis | Disease | Vector | Target Protein Ligand | E3 Ligand | Status | References |
|---|---|---|---|---|---|---|---|---|---|
| (1) | Anti-X-protein peptide-based PROTACs | PROTACs with the oligomerization domain, degron peptide, and R8 | Heparan sulfate proteoglycans | Cancer | R8 and X-entry as CPPs | Oligomerization domain of the X-protein as an X-protein ligand | Degron peptide of the X-protein as an E3 ligand | In vitro basic research | [17] |
| (2) | AP21998/ hypoxia inducible factor (HIF) 1α-based PROTAC | PROTAC with AP21998, ALAPYIP sequence, and D-R8 | Heparan sulfate proteoglycans | - | D-R8 as a CPP | AP21998 as an FK506 binding protein (FKBP12) ligand, | ALAPYIP sequence as a VHL ligand | In vitro Basic research | [18] |
| (3) | Dihydrotestosterone (DHT)/HIF 1α-based PROTAC | PROTAC with DHT, ALAPYIP sequence, and D-R8 | Heparan sulfate proteoglycans | Cancer | D-R as a CPP | DHT as an androgen receptor (AR) ligand | ALAPYIP sequence as a VHL ligand | In vitro Basic research | [18] |
| (4) | TH006 | Peptide-based PROTAC with a tau ligand, ALAPYIP sequence, and D-R8 | Heparan sulfate proteoglycans | Alzheimer’s disease | D-R as a CPP | Tau ligand | ALAPYIP sequence as a VHL ligand | In vitro Basic research | [19] |
| (5) | Peptide-based PROTAC | Peptide-based PROTAC with a tau ligand, Kelch-like ECH-associated protein-1 (Keap1) ligand, and D-R8 | Heparan sulfate proteoglycans | Alzheimer’s disease | D-R as a CPP | Tau ligand | Keap1 ligand as an E3 ligase ligand | In vitro Basic research | [20] |
| (6) | Peptide-based PROTAC | Peptide-based PROTACs with an α-synuclein ligand, the E3 ligase ligand, and TAT | Heparan sulfate proteoglycans | Parkinson’s disease | TAT as a CPP | α-Synuclein ligand | E3 ligase ligand | In vitro Basic research | [21] |
| (7) | Protac-1 as the first PROTAC | Protac with ovalicin and the IκBα phosphopeptide | - | - | -- | Ovalicin to bind methionine aminopeptidase-2 | IκBα phosphopeptide to bind β-TRCP contained in the Skp1-Cullin-F box E3 ligase complex | In vitro Basic research | [23] |
| (8) | The first small molecule-based PROTAC | PROTAC with a non-steroidal AR ligand and nutlin | - | Cancer | - | Non-steroidal AR ligand | Nutlin as an E3 ligase ligand | In vitro Basic research | [24] |
| (9) | ARV-110 as the first PROTAC entering the clinical trial test | PTOTAC with a non-steroidal AR ligand and a CRBN ligand | - | Cancer | - | Non-steroidal AR ligand | CRBN ligand | phase 1 phase 2 | (Table 1) [25,26] |
| (10) | Eight PROTACs under clinical trials | Small molecule-based PROTAC with a target protein ligand and an E3 ligase ligand | - | Canceer | - | Target protein ligand | E3 ligase ligand | phase 1 | (Table 1) [25,26] |
| (11) | Three PROTACs under clinical trials including ARV-110 | Small molecule-based PROTAC with a target protein ligand and an E3 ligase ligand | - | Cancer | - | Target protein ligand | E3 ligase ligand | phase 1 phase 2 | (Table 1) [25,26] |
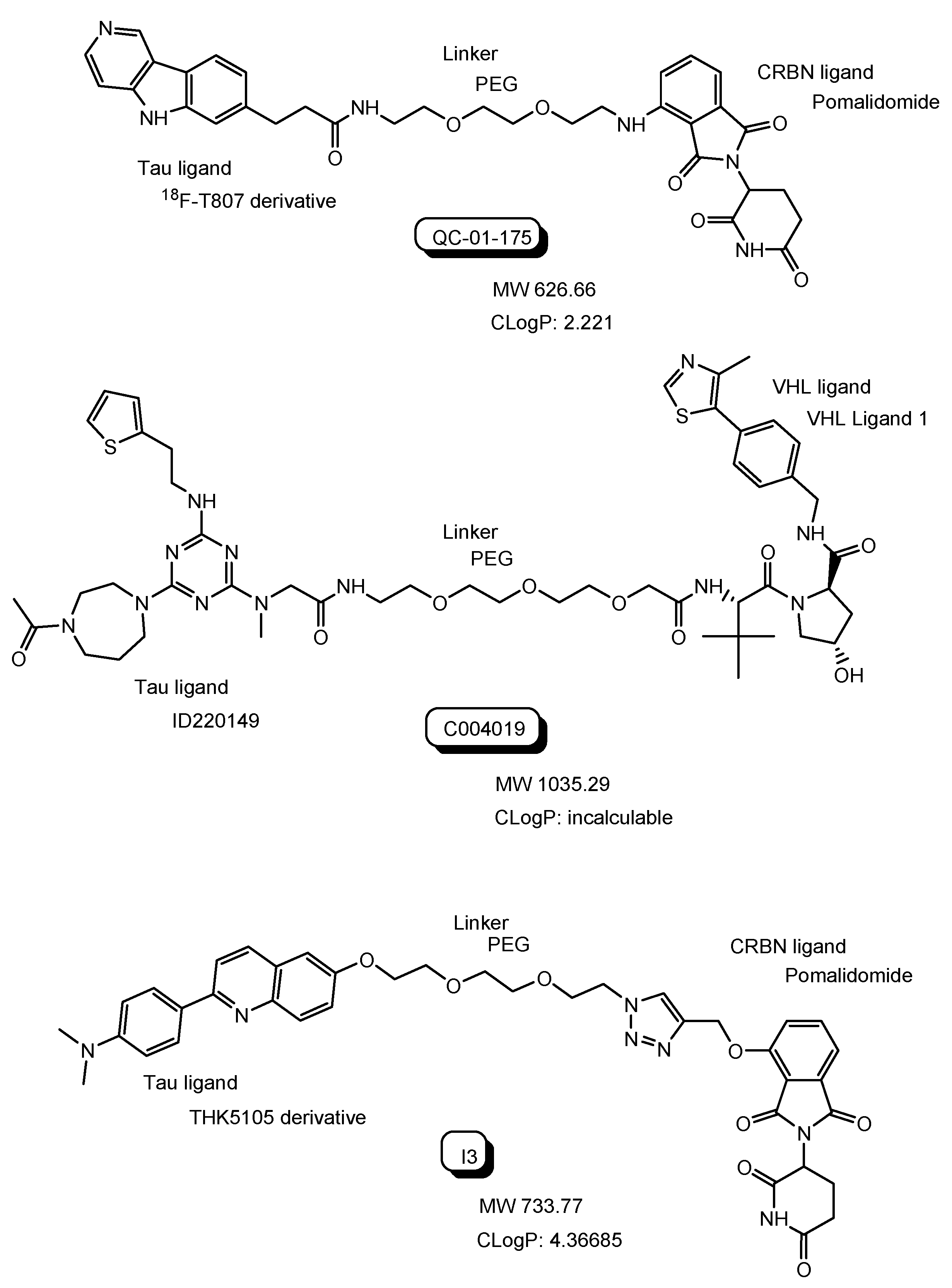
| (12) | QC-01-175 | PROTAC with 18F-T807 derivative and a CRBN ligand | - | Alzheimer’s disease | - | 18F-T807 derivative as a tau ligand | CRBN ligand | In vitro Basic research | [30] |
| (13) | C004019 | PROTAC with ID220149 and a VHL ligand | - | Alzheimer’s disease | - | ID220149 as a tau ligand | VHL ligand | In vivo Basic research | [34] |
| (14) | I3 | PROTACs with THK5105 derivative and a CRBN ligand | - | Alzheimer’s disease | - | THK5105 derivative as a tau ligand | CRBN ligand | In vitro Basic research | [35] |
| (15) | Small molecule-based PROTACs | PROTACs with an α-synuclein ligand and a VHL ligand | - | Parkinson’s disease | - | α-Synuclein ligand | VHL ligand | In vitro Basic research | [36,37] |
| (16) | Small molecule-based PROTACs | PROTACs with a huntingtin ligand and a cIAP ligand | - | Huntington’s disease | - | Huntingtin ligand | cIAP ligand | In vitro Basic research | [40] |
| (17) | Idursulfase beta | Anti-transferrin receptor antibody fused to iduronate-2-sulfatase | Transferrin receptor | Hunter syndrome | Anti-transferrin receptor antibody | - | - | Launched in 2021 | [43] |
| (18) | Trastuzumab-PROTAC conjugate | PROTAC-antibody conjugate with a BRD4 ligand and a VHL ligand | HER2 | Cancer | Anti-HER2 antibody | BRD4 ligand | VHL ligand | In vitro Basic research | [46] |
| (19) | Anti-HER2 antibody-PROTAC conjugate | PROTAC-antibody conjugate with an ER ligand and a VHL ligand | HER2 | Cancer | Anti-HER2 antibody | ER ligand | VHL ligand | In vitro Basic research | [48] |
| (20) | STEAP1-5a | PROTAC-antibody conjugate with a BRD4 ligand and a VHL ligand | STEAP1 | Cancer | Anti-STEAP1 antibody | BRD4 ligand | VHL ligand | In vitro Basic research | [49] |
| (21) | STEAP1-9d | PROTAC-antibody conjugate with a BRD4 ligand and a VHL ligand | STEAP1 | Cancer | Anti-STEAP1 antibody | BRD4 ligand | VHL ligand | In vitro Basic research | [50] |
| (22) | NanoPROTACs covered with trastuzumab | PROTAC-encapsulated nanoparticles covered with antibodies | HER2 | Cancer | Anti-HER2 antibody | BRD4 ligand | VHL ligand | In vitro Basic research | [53] |
| (23) | PROTAC-antibody conjugate | PROTAC-antibody conjugate with a tau ligand and an E3 ligase ligand | Transferrin receptor | Alzheimer’s disease | Anti-transferrin receptor antibody | Tau ligand | E3 ligase ligand | Under analysis in Tashima lab | - |
| (24) | NanoPROTACs covered with antibodies | PROTAC-encapsulated nanoparticles covered with antibodies | Transferrin receptor | Alzheimer’s disease | Anti-transferrin receptor antibody | Tau ligand | E3 ligase ligand | Under analysis in Tashima lab | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tashima, T. Proteolysis-Targeting Chimera (PROTAC) Delivery into the Brain across the Blood-Brain Barrier. Antibodies 2023, 12, 43. https://doi.org/10.3390/antib12030043
Tashima T. Proteolysis-Targeting Chimera (PROTAC) Delivery into the Brain across the Blood-Brain Barrier. Antibodies. 2023; 12(3):43. https://doi.org/10.3390/antib12030043
Chicago/Turabian StyleTashima, Toshihiko. 2023. "Proteolysis-Targeting Chimera (PROTAC) Delivery into the Brain across the Blood-Brain Barrier" Antibodies 12, no. 3: 43. https://doi.org/10.3390/antib12030043
APA StyleTashima, T. (2023). Proteolysis-Targeting Chimera (PROTAC) Delivery into the Brain across the Blood-Brain Barrier. Antibodies, 12(3), 43. https://doi.org/10.3390/antib12030043






