EMab-300 Detects Mouse Epidermal Growth Factor Receptor-Expressing Cancer Cell Lines in Flow Cytometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plasmids
2.2. Antibodies
2.3. Cell Lines
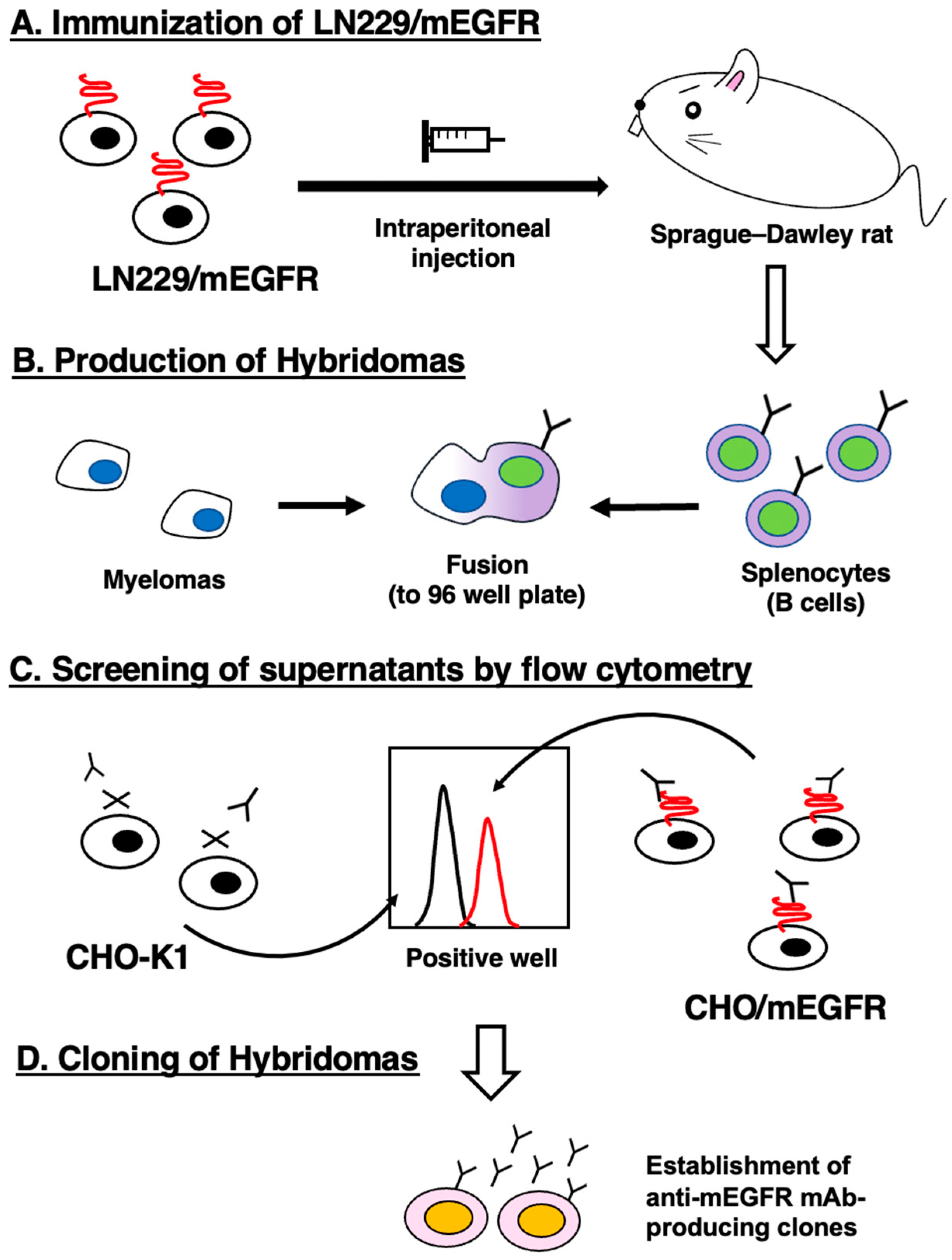
2.4. Development of Hybridomas
2.5. Purification of EMab-300
2.6. Flow Cytometric Analysis
2.7. Determination of KD using Flow Cytometry
3. Results
3.1. Development of Anti-mEGFR mAbs Using the CBIS Method
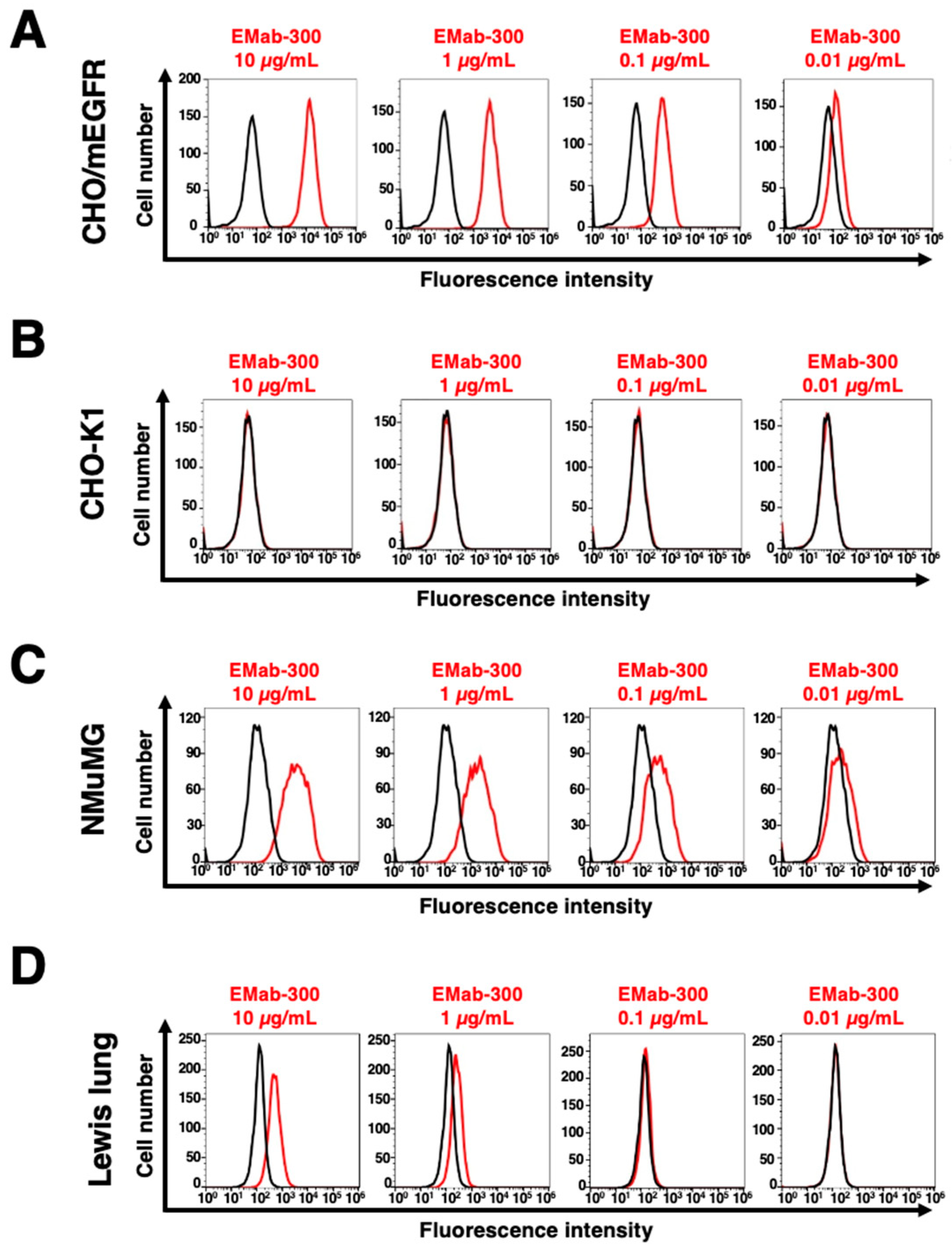
3.2. Flow Cytometric Analysis Using EMab-300
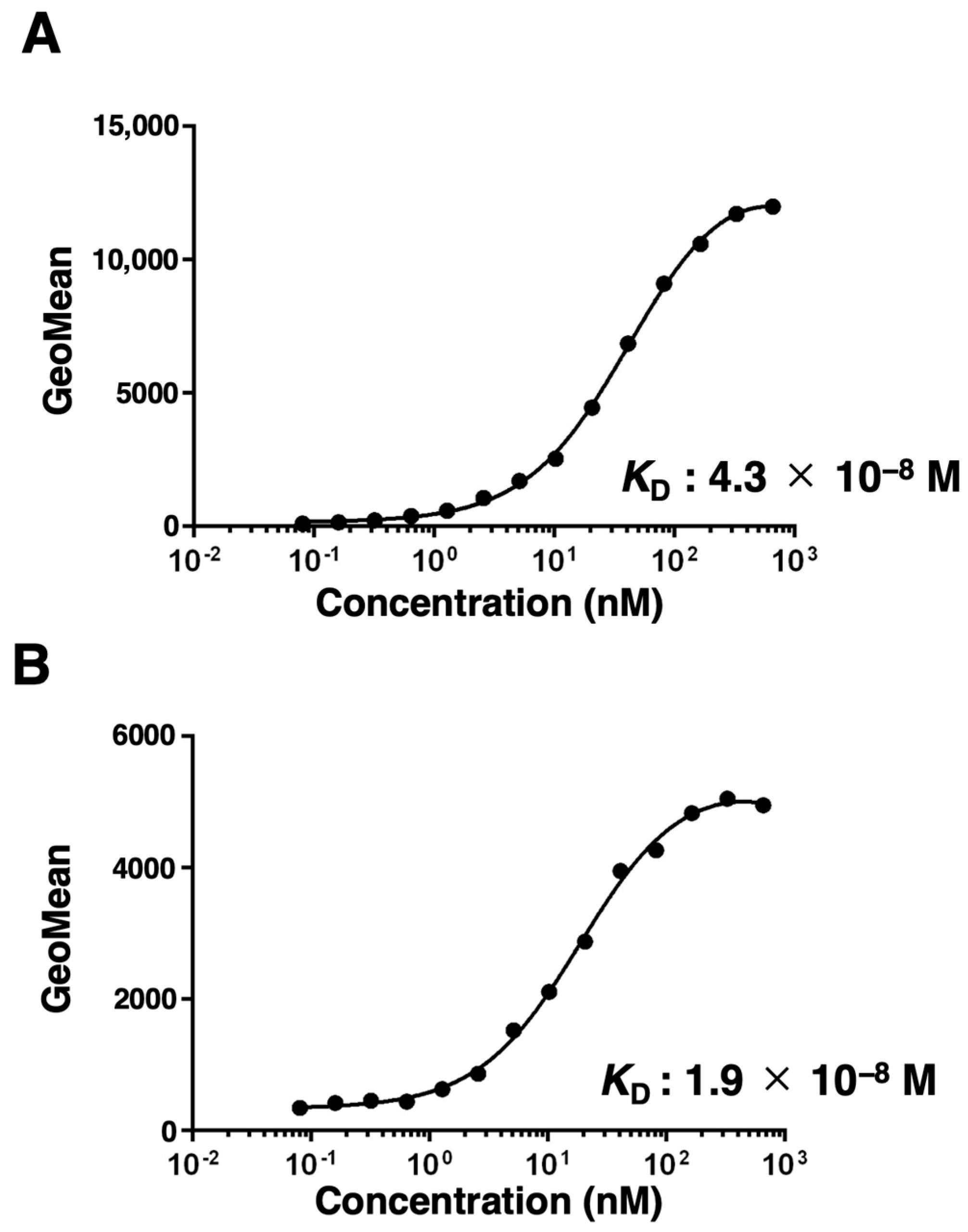
3.3. Kinetic Analysis of EMab-300 Using Flow Cytometry
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zandi, R.; Larsen, A.B.; Andersen, P.; Stockhausen, M.T.; Poulsen, H.S. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell. Signal. 2007, 19, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.J.; Sequist, L.V.; Lin, J.J. Third-generation EGFR and ALK inhibitors: Mechanisms of resistance and management. Nat. Rev. Clin. Oncol. 2022, 19, 499–514. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tsao, L.C.; Force, J.; Hartman, Z.C. Mechanisms of Therapeutic Antitumor Monoclonal Antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Zubair, T.; Bandyopadhyay, D. Small Molecule EGFR Inhibitors as Anti-Cancer Agents: Discovery, Mechanisms of Action, and Opportunities. Int. J. Mol. Sci. 2023, 24, 2651. [Google Scholar] [CrossRef]
- Li, S.; Schmitz, K.R.; Jeffrey, P.D.; Wiltzius, J.J.; Kussie, P.; Ferguson, K.M. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 2005, 7, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Jonker, D.J.; O’Callaghan, C.J.; Karapetis, C.S.; Zalcberg, J.R.; Tu, D.; Au, H.J.; Berry, S.R.; Krahn, M.; Price, T.; Simes, R.J.; et al. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 2007, 357, 2040–2048. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Ohishi, T.; Kaneko, M.K.; Yoshida, Y.; Takashima, A.; Kato, Y.; Kawada, M. Current Targeted Therapy for Metastatic Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 1702. [Google Scholar] [CrossRef] [PubMed]
- Bardelli, A.; Jänne, P.A. The road to resistance: EGFR mutation and cetuximab. Nat. Med. 2012, 18, 199–200. [Google Scholar] [CrossRef]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Day, C.P.; Merlino, G.; Van Dyke, T. Preclinical mouse cancer models: A maze of opportunities and challenges. Cell 2015, 163, 39–53. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Trivedi, S.; Concha-Benavente, F.; Gibson, S.P.; Reeder, C.; Ferrone, S.; Ferris, R.L. CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 707–716. [Google Scholar] [CrossRef]
- Kubach, J.; Hubo, M.; Amendt, C.; Stroh, C.; Jonuleit, H. IgG1 anti-epidermal growth factor receptor antibodies induce CD8-dependent antitumor activity. Int. J. Cancer 2015, 136, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Itai, S.; Fujii, Y.; Kaneko, M.K.; Yamada, S.; Nakamura, T.; Yanaka, M.; Saidoh, N.; Chang, Y.W.; Handa, S.; Takahashi, M.; et al. H(2)Mab-77 is a Sensitive and Specific Anti-HER2 Monoclonal Antibody Against Breast Cancer. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 143–148. [Google Scholar] [CrossRef]
- Asano, T.; Ohishi, T.; Takei, J.; Nakamura, T.; Nanamiya, R.; Hosono, H.; Tanaka, T.; Sano, M.; Harada, H.; Kawada, M.; et al. Anti-HER3 monoclonal antibody exerts antitumor activity in a mouse model of colorectal adenocarcinoma. Oncol. Rep. 2021, 46, 173. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Suzuki, H.; Asano, T.; Tanaka, T.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-EpCAM Monoclonal Antibody for Various Applications. Antibodies 2022, 11, 41. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Ohishi, T.; Takei, J.; Sano, M.; Nakamura, T.; Hosono, H.; Yanaka, M.; Asano, T.; Sayama, Y.; Harada, H.; et al. Anti-EpCAM monoclonal antibody exerts antitumor activity against oral squamous cell carcinomas. Oncol. Rep. 2020, 44, 2517–2526. [Google Scholar] [CrossRef] [PubMed]
- Sayama, Y.; Kaneko, M.K.; Takei, J.; Hosono, H.; Sano, M.; Asano, T.; Kato, Y. Establishment of a novel anti-TROP2 monoclonal antibody TrMab-29 for immunohistochemical analysis. Biochem. Biophys. Rep. 2021, 25, 100902. [Google Scholar] [CrossRef]
- Tanaka, T.; Ohishi, T.; Asano, T.; Takei, J.; Nanamiya, R.; Hosono, H.; Sano, M.; Harada, H.; Kawada, M.; Kaneko, M.K.; et al. An anti-TROP2 monoclonal antibody TrMab-6 exerts antitumor activity in breast cancer mouse xenograft models. Oncol. Rep. 2021, 46, 132. [Google Scholar] [CrossRef]
- Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Chang, Y.W.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Monoclonal Antibody L(1)Mab-13 Detected Human PD-L1 in Lung Cancers. Monoclon. Antib. Immunodiagn. Immunother. 2018, 37, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Saidoh, N.; Chang, Y.W.; Handa, S.; Harada, H.; Kagawa, Y.; Ichii, O.; et al. PMab-52: Specific and Sensitive Monoclonal Antibody Against Cat Podoplanin for Immunohistochemistry. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 224–230. [Google Scholar] [CrossRef]
- Furusawa, Y.; Kaneko, M.K.; Nakamura, T.; Itai, S.; Fukui, M.; Harada, H.; Yamada, S.; Kato, Y. Establishment of a Monoclonal Antibody PMab-231 for Tiger Podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2019, 38, 89–95. [Google Scholar] [CrossRef]
- Furusawa, Y.; Takei, J.; Sayama, Y.; Yamada, S.; Kaneko, M.K.; Kato, Y. Development of an anti-bear podoplanin monoclonal antibody PMab-247 for immunohistochemical analysis. Biochem. Biophys. Rep. 2019, 18, 100644. [Google Scholar] [CrossRef]
- Furusawa, Y.; Yamada, S.; Itai, S.; Nakamura, T.; Takei, J.; Sano, M.; Harada, H.; Fukui, M.; Kaneko, M.K.; Kato, Y. Establishment of a monoclonal antibody PMab-233 for immunohistochemical analysis against Tasmanian devil podoplanin. Biochem. Biophys. Rep. 2019, 18, 100631. [Google Scholar] [CrossRef] [PubMed]
- Goto, N.; Suzuki, H.; Tanaka, T.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Monoclonal Antibody PMab-292 Against Ferret Podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2022; in press. [Google Scholar]
- Furusawa, Y.; Yamada, S.; Itai, S.; Sano, M.; Nakamura, T.; Yanaka, M.; Handa, S.; Mizuno, T.; Maeda, K.; Fukui, M.; et al. Establishment of Monoclonal Antibody PMab-202 Against Horse Podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2018, 37, 233–237. [Google Scholar] [CrossRef]
- Kato, Y.; Yamada, S.; Furusawa, Y.; Itai, S.; Nakamura, T.; Yanaka, M.; Sano, M.; Harada, H.; Fukui, M.; Kaneko, M.K. PMab-213: A monoclonal antibody for immunohistochemical analysis against pig podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2019, 38, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Yamada, S.; Nakamura, T.; Sano, M.; Sayama, Y.; Itai, S.; Takei, J.; Harada, H.; Fukui, M.; Kaneko, M.K.; et al. PMab-235: A monoclonal antibody for immunohistochemical analysis against goat podoplanin. Heliyon 2019, 5, e02063. [Google Scholar] [CrossRef]
- Kato, Y.; Furusawa, Y.; Yamada, S.; Itai, S.; Takei, J.; Sano, M.; Kaneko, M.K. Establishment of a monoclonal antibody PMab-225 against alpaca podoplanin for immunohistochemical analyses. Biochem. Biophys. Rep. 2019, 18, 100633. [Google Scholar] [CrossRef]
- Kato, Y.; Furusawa, Y.; Itai, S.; Takei, J.; Nakamura, T.; Sano, M.; Harada, H.; Yamada, S.; Kaneko, M.K. Establishment of an Anticetacean Podoplanin Monoclonal Antibody PMab-237 for Immunohistochemical Analysis. Monoclon. Antib. Immunodiagn. Immunother. 2019, 38, 108–113. [Google Scholar] [CrossRef]
- Kato, Y.; Furusawa, Y.; Sano, M.; Takei, J.; Nakamura, T.; Yanaka, M.; Okamoto, S.; Handa, S.; Komatsu, Y.; Asano, T.; et al. Development of an Anti-Sheep Podoplanin Monoclonal Antibody PMab-256 for Immunohistochemical Analysis of Lymphatic Endothelial Cells. Monoclon. Antib. Immunodiagn. Immunother. 2020, 39, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Asano, T.; Sano, M.; Takei, J.; Hosono, H.; Nanamiya, R.; Nakamura, T.; Yanaka, M.; Harada, H.; Fukui, M.; et al. Development of Monoclonal Antibody PMab-269 Against California Sea Lion Podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 124–133. [Google Scholar] [CrossRef]
- Yamada, S.; Kaneko, M.K.; Sayama, Y.; Asano, T.; Sano, M.; Yanaka, M.; Nakamura, T.; Okamoto, S.; Handa, S.; Komatsu, Y.; et al. Development of Novel Mouse Monoclonal Antibodies Against Human CD19. Monoclon. Antib. Immunodiagn. Immunother. 2020, 39, 45–50. [Google Scholar] [CrossRef]
- Furusawa, Y.; Kaneko, M.K.; Kato, Y. Establishment of C(20)Mab-11, a novel anti-CD20 monoclonal antibody, for the detection of B cells. Oncol. Lett. 2020, 20, 1961–1967. [Google Scholar] [CrossRef]
- Furusawa, Y.; Kaneko, M.K.; Kato, Y. Establishment of an Anti-CD20 Monoclonal Antibody (C(20)Mab-60) for Immunohistochemical Analyses. Monoclon. Antib. Immunodiagn. Immunother. 2020, 39, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Tawara, M.; Suzuki, H.; Goto, N.; Tanaka, T.; Kaneko, M.K.; Kato, Y. A Novel Anti-CD44 Variant 9 Monoclonal Antibody C44Mab-1 was Developed for Immunohistochemical Analyses Against Colorectal Cancers. Curr. Issues Mol. Biol. 2023, 45, 3658–3673. [Google Scholar] [CrossRef]
- Kudo, Y.; Suzuki, H.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 variant 5 Monoclonal Antibody C44Mab-3 for Multiple Applications against Pancreatic Carcinomas. Antibodies 2023, 12, 31. [Google Scholar] [CrossRef]
- Ejima, R.; Suzuki, H.; Tanaka, T.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-CD44 Variant 6 Monoclonal Antibody C(44)Mab-9 for Multiple Applications against Colorectal Carcinomas. Int. J. Mol. Sci. 2023, 24, 4007. [Google Scholar] [CrossRef]
- Yamada, S.; Itai, S.; Nakamura, T.; Yanaka, M.; Kaneko, M.K.; Kato, Y. Detection of high CD44 expression in oral cancers using the novel monoclonal antibody, C(44)Mab-5. Biochem. Biophys. Rep. 2018, 14, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Itai, S.; Fujii, Y.; Nakamura, T.; Chang, Y.W.; Yanaka, M.; Saidoh, N.; Handa, S.; Suzuki, H.; Harada, H.; Yamada, S.; et al. Establishment of CMab-43, a Sensitive and Specific Anti-CD133 Monoclonal Antibody, for Immunohistochemistry. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 231–235. [Google Scholar] [CrossRef]
- Asano, T.; Nanamiya, R.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of Antihuman Killer Cell Lectin-Like Receptor Subfamily G Member 1 Monoclonal Antibodies for Flow Cytometry. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 76–80. [Google Scholar] [CrossRef]
- Nanamiya, R.; Takei, J.; Asano, T.; Tanaka, T.; Sano, M.; Nakamura, T.; Yanaka, M.; Hosono, H.; Kaneko, M.K.; Kato, Y. Development of Anti-Human CC Chemokine Receptor 9 Monoclonal Antibodies for Flow Cytometry. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Takei, J.; Asano, T.; Nanamiya, R.; Nakamura, T.; Yanaka, M.; Hosono, H.; Tanaka, T.; Sano, M.; Kaneko, M.K.; Harada, H.; et al. Development of Anti-human T Cell Immunoreceptor with Ig and ITIM Domains (TIGIT) Monoclonal Antibodies for Flow Cytometry. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Tanaka, T.; Sano, M.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 3 Monoclonal Antibodies for Flow Cytometry. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 107–112. [Google Scholar] [CrossRef]
- Tanaka, T.; Nanamiya, R.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Sano, M.; Asano, T.; Kaneko, M.K.; Kato, Y. Development of Anti-Mouse CC Chemokine Receptor 8 Monoclonal Antibodies for Flow Cytometry. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 65–70. [Google Scholar] [CrossRef]
- Tamura, R.; Oi, R.; Akashi, S.; Kaneko, M.K.; Kato, Y.; Nogi, T. Application of the NZ-1 Fab as a crystallization chaperone for PA tag-inserted target proteins. Protein Sci. 2019, 28, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Matsunaga, Y.; Arimori, T.; Kitago, Y.; Ogasawara, S.; Kaneko, M.K.; Kato, Y.; Takagi, J. Tailored placement of a turn-forming PA tag into the structured domain of a protein to probe its conformational state. J. Cell Sci. 2016, 129, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, M.; Neyazaki, M.; Nogi, T.; Kato, Y.; Takagi, J. PA tag: A versatile protein tagging system using a super high affinity antibody against a dodecapeptide derived from human podoplanin. Protein Expr. Purif. 2014, 95, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kaneko, M.K.; Kato, Y. MAP Tag: A Novel Tagging System for Protein Purification and Detection. Monoclon. Antib. Immunodiagn. Immunother. 2016, 35, 293–299. [Google Scholar] [CrossRef]
- Wakasa, A.; Kaneko, M.K.; Kato, Y.; Takagi, J.; Arimori, T. Site-specific epitope insertion into recombinant proteins using the MAP tag system. J. Biochem. 2020, 168, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kaneko, M.K.; Kuno, A.; Uchiyama, N.; Amano, K.; Chiba, Y.; Hasegawa, Y.; Hirabayashi, J.; Narimatsu, H.; Mishima, K.; et al. Inhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet-aggregation-stimulating domain. Biochem. Biophys. Res. Commun. 2006, 349, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Chalise, L.; Kato, A.; Ohno, M.; Maeda, S.; Yamamichi, A.; Kuramitsu, S.; Shiina, S.; Takahashi, H.; Ozone, S.; Yamaguchi, J.; et al. Efficacy of cancer-specific anti-podoplanin CAR-T cells and oncolytic herpes virus G47Delta combination therapy against glioblastoma. Mol. Ther. Oncolytics 2022, 26, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Waseda, M.; Ishii, T.; Kaneko, M.K.; Kato, Y.; Kaneko, S. Improved anti-solid tumor response by humanized anti-podoplanin chimeric antigen receptor transduced human cytotoxic T cells in an animal model. Genes Cells 2022, 27, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Tamura-Sakaguchi, R.; Aruga, R.; Hirose, M.; Ekimoto, T.; Miyake, T.; Hizukuri, Y.; Oi, R.; Kaneko, M.K.; Kato, Y.; Akiyama, Y.; et al. Moving toward generalizable NZ-1 labeling for 3D structure determination with optimized epitope-tag insertion. Acta. Crystallogr. D Struct. Biol. 2021, 77, 645–662. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Ohishi, T.; Nakamura, T.; Inoue, H.; Takei, J.; Sano, M.; Asano, T.; Sayama, Y.; Hosono, H.; Suzuki, H.; et al. Development of Core-Fucose-Deficient Humanized and Chimeric Anti-Human Podoplanin Antibodies. Monoclon. Antib. Immunodiagn. Immunother. 2020, 39, 167–174. [Google Scholar] [CrossRef]
- Abe, S.; Kaneko, M.K.; Tsuchihashi, Y.; Izumi, T.; Ogasawara, S.; Okada, N.; Sato, C.; Tobiume, M.; Otsuka, K.; Miyamoto, L.; et al. Antitumor effect of novel anti-podoplanin antibody NZ-12 against malignant pleural mesothelioma in an orthotopic xenograft model. Cancer Sci. 2016, 107, 1198–1205. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Abe, S.; Ogasawara, S.; Fujii, Y.; Yamada, S.; Murata, T.; Uchida, H.; Tahara, H.; Nishioka, Y.; Kato, Y. Chimeric Anti-Human Podoplanin Antibody NZ-12 of Lambda Light Chain Exerts Higher Antibody-Dependent Cellular Cytotoxicity and Complement-Dependent Cytotoxicity Compared with NZ-8 of Kappa Light Chain. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 25–29. [Google Scholar] [CrossRef]
- Ito, A.; Ohta, M.; Kato, Y.; Inada, S.; Kato, T.; Nakata, S.; Yatabe, Y.; Goto, M.; Kaneda, N.; Kurita, K.; et al. A Real-Time Near-Infrared Fluorescence Imaging Method for the Detection of Oral Cancers in Mice Using an Indocyanine Green-Labeled Podoplanin Antibody. Technol. Cancer Res. Treat. 2018, 17, 1533033818767936. [Google Scholar] [CrossRef]
- Shiina, S.; Ohno, M.; Ohka, F.; Kuramitsu, S.; Yamamichi, A.; Kato, A.; Motomura, K.; Tanahashi, K.; Yamamoto, T.; Watanabe, R.; et al. CAR T Cells Targeting Podoplanin Reduce Orthotopic Glioblastomas in Mouse Brains. Cancer Immunol. Res. 2016, 4, 259–268. [Google Scholar] [CrossRef]
- Kuwata, T.; Yoneda, K.; Mori, M.; Kanayama, M.; Kuroda, K.; Kaneko, M.K.; Kato, Y.; Tanaka, F. Detection of Circulating Tumor Cells (CTCs) in Malignant Pleural Mesothelioma (MPM) with the “Universal” CTC-Chip and An Anti-Podoplanin Antibody NZ-1.2. Cells 2020, 9, 888. [Google Scholar] [CrossRef] [PubMed]
- Nishinaga, Y.; Sato, K.; Yasui, H.; Taki, S.; Takahashi, K.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; Nakamura, S.; et al. Targeted Phototherapy for Malignant Pleural Mesothelioma: Near-Infrared Photoimmunotherapy Targeting Podoplanin. Cells 2020, 9, 1019. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kaneko, M.K.; Kunita, A.; Ito, H.; Kameyama, A.; Ogasawara, S.; Matsuura, N.; Hasegawa, Y.; Suzuki-Inoue, K.; Inoue, O.; et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008, 99, 54–61. [Google Scholar] [CrossRef]
- Kato, Y.; Vaidyanathan, G.; Kaneko, M.K.; Mishima, K.; Srivastava, N.; Chandramohan, V.; Pegram, C.; Keir, S.T.; Kuan, C.T.; Bigner, D.D.; et al. Evaluation of anti-podoplanin rat monoclonal antibody NZ-1 for targeting malignant gliomas. Nucl. Med. Biol. 2010, 37, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Rapraeger, A. Altered structure of the hybrid cell surface proteoglycan of mammary epithelial cells in response to transforming growth factor-beta. J. Cell Biol. 1988, 107, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schaller, N.; Cardner, M.; Diepenbruck, M.; Saxena, M.; Tiede, S.; Lüönd, F.; Ivanek, R.; Beerenwinkel, N.; Christofori, G. A Hierarchical Regulatory Landscape during the Multiple Stages of EMT. Dev. Cell 2019, 48, 539–553.e6. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Okita, Y.; Kimura, M.; Xie, R.; Chen, C.; Shen, L.T.; Kojima, Y.; Suzuki, H.; Muratani, M.; Saitoh, M.; Semba, K.; et al. The transcription factor MAFK induces EMT and malignant progression of triple-negative breast cancer cells through its target GPNMB. Sci. Signal. 2017, 10, 9397. [Google Scholar] [CrossRef]
- Kellar, A.; Egan, C.; Morris, D. Preclinical Murine Models for Lung Cancer: Clinical Trial Applications. Biomed. Res. Int. 2015, 2015, 621324. [Google Scholar] [CrossRef]
- Xin, M.; Lin, D.; Yan, N.; Li, H.; Li, J.; Huang, Z. Oxaliplatin facilitates tumor-infiltration of T cells and natural-killer cells for enhanced tumor immunotherapy in lung cancer model. Anticancer Drugs 2022, 33, 117–123. [Google Scholar] [CrossRef]
- Canon, J.; Rex, K.; Saiki, A.Y.; Mohr, C.; Cooke, K.; Bagal, D.; Gaida, K.; Holt, T.; Knutson, C.G.; Koppada, N.; et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature 2019, 575, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Suzuki, H.; Ohishi, T.; Asano, T.; Tanaka, T.; Yanaka, M.; Nakamura, T.; Yoshikawa, T.; Kawada, M.; Kaneko, M.K.; et al. Antitumor activities of a defucosylated anti-EpCAM monoclonal antibody in colorectal carcinoma xenograft models. Int. J. Mol. Med. 2023, 51, 5221. [Google Scholar] [CrossRef] [PubMed]
- Nanamiya, R.; Takei, J.; Ohishi, T.; Asano, T.; Tanaka, T.; Sano, M.; Nakamura, T.; Yanaka, M.; Handa, S.; Tateyama, N.; et al. Defucosylated Anti-Epidermal Growth Factor Receptor Monoclonal Antibody (134-mG(2a)-f) Exerts Antitumor Activities in Mouse Xenograft Models of Canine Osteosarcoma. Monoclon. Antib. Immunodiagn. Immunother. 2022, 41, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H.; Suzuki, H.; Ohishi, T.; Kawada, M.; Kaneko, M.K.; Kato, Y. A Defucosylated Mouse Anti-CD10 Monoclonal Antibody (31-mG(2a)-f) Exerts Antitumor Activity in a Mouse Xenograft Model of CD10-Overexpressed Tumors. Monoclon. Antib. Immunodiagn. Immunother. 2022, 41, 59–66. [Google Scholar] [CrossRef]
- Kawabata, H.; Ohishi, T.; Suzuki, H.; Asano, T.; Kawada, M.; Suzuki, H.; Kaneko, M.K.; Kato, Y. A Defucosylated Mouse Anti-CD10 Monoclonal Antibody (31-mG(2a)-f) Exerts Antitumor Activity in a Mouse Xenograft Model of Renal Cell Cancers. Monoclon. Antib. Immunodiagn. Immunother. 2022, 41, 320–327. [Google Scholar] [CrossRef]
- Asano, T.; Tanaka, T.; Suzuki, H.; Li, G.; Ohishi, T.; Kawada, M.; Yoshikawa, T.; Kaneko, M.K.; Kato, Y. A Defucosylated Anti-EpCAM Monoclonal Antibody (EpMab-37-mG(2a)-f) Exerts Antitumor Activity in Xenograft Model. Antibodies 2022, 11, 74. [Google Scholar] [CrossRef]
- Tateyama, N.; Nanamiya, R.; Ohishi, T.; Takei, J.; Nakamura, T.; Yanaka, M.; Hosono, H.; Saito, M.; Asano, T.; Tanaka, T.; et al. Defucosylated Anti-Epidermal Growth Factor Receptor Monoclonal Antibody 134-mG(2a)-f Exerts Antitumor Activities in Mouse Xenograft Models of Dog Epidermal Growth Factor Receptor-Overexpressed Cells. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 177–183. [Google Scholar] [CrossRef]
- Takei, J.; Ohishi, T.; Kaneko, M.K.; Harada, H.; Kawada, M.; Kato, Y. A defucosylated anti-PD-L1 monoclonal antibody 13-mG(2a)-f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Biochem. Biophys. Rep. 2020, 24, 100801. [Google Scholar] [CrossRef]
- Takei, J.; Kaneko, M.K.; Ohishi, T.; Hosono, H.; Nakamura, T.; Yanaka, M.; Sano, M.; Asano, T.; Sayama, Y.; Kawada, M.; et al. A defucosylated antiCD44 monoclonal antibody 5mG2af exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Oncol. Rep. 2020, 44, 1949–1960. [Google Scholar] [CrossRef]
- Asano, T.; Kaneko, M.K.; Takei, J.; Tateyama, N.; Kato, Y. Epitope Mapping of the Anti-CD44 Monoclonal Antibody (C44Mab-46) Using the REMAP Method. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 156–161. [Google Scholar] [CrossRef]
- Sano, M.; Kaneko, M.K.; Aasano, T.; Kato, Y. Epitope Mapping of an Antihuman EGFR Monoclonal Antibody (EMab-134) Using the REMAP Method. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Epitope Mapping System: RIEDL Insertion for Epitope Mapping Method. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Nanamiya, R.; Sano, M.; Asano, T.; Yanaka, M.; Nakamura, T.; Saito, M.; Tanaka, T.; Hosono, H.; Tateyama, N.; Kaneko, M.K.; et al. Epitope Mapping of an Anti-Human Epidermal Growth Factor Receptor Monoclonal Antibody (EMab-51) Using the RIEDL Insertion for Epitope Mapping Method. Monoclon. Antib. Immunodiagn. Immunother. 2021, 40, 149–155. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, N.; Suzuki, H.; Tanaka, T.; Ishikawa, K.; Ouchida, T.; Kaneko, M.K.; Kato, Y. EMab-300 Detects Mouse Epidermal Growth Factor Receptor-Expressing Cancer Cell Lines in Flow Cytometry. Antibodies 2023, 12, 42. https://doi.org/10.3390/antib12030042
Goto N, Suzuki H, Tanaka T, Ishikawa K, Ouchida T, Kaneko MK, Kato Y. EMab-300 Detects Mouse Epidermal Growth Factor Receptor-Expressing Cancer Cell Lines in Flow Cytometry. Antibodies. 2023; 12(3):42. https://doi.org/10.3390/antib12030042
Chicago/Turabian StyleGoto, Nohara, Hiroyuki Suzuki, Tomohiro Tanaka, Kenichiro Ishikawa, Tsunenori Ouchida, Mika K. Kaneko, and Yukinari Kato. 2023. "EMab-300 Detects Mouse Epidermal Growth Factor Receptor-Expressing Cancer Cell Lines in Flow Cytometry" Antibodies 12, no. 3: 42. https://doi.org/10.3390/antib12030042
APA StyleGoto, N., Suzuki, H., Tanaka, T., Ishikawa, K., Ouchida, T., Kaneko, M. K., & Kato, Y. (2023). EMab-300 Detects Mouse Epidermal Growth Factor Receptor-Expressing Cancer Cell Lines in Flow Cytometry. Antibodies, 12(3), 42. https://doi.org/10.3390/antib12030042






