Bioprocess Development and Characterization of a 13C-Labeled Hybrid Bispecific Antibody Produced in Escherichia coli
Abstract
1. Introduction
2. Methods
2.1. Bacterial Strain and Plasmids
2.2. Fermentations
2.3. Analytical Measurements for Monitoring Fermentation
2.4. Downstream Processing
2.5. Mass Spectrometry Analysis and Determination of % 13C-Incorporation
3. Results
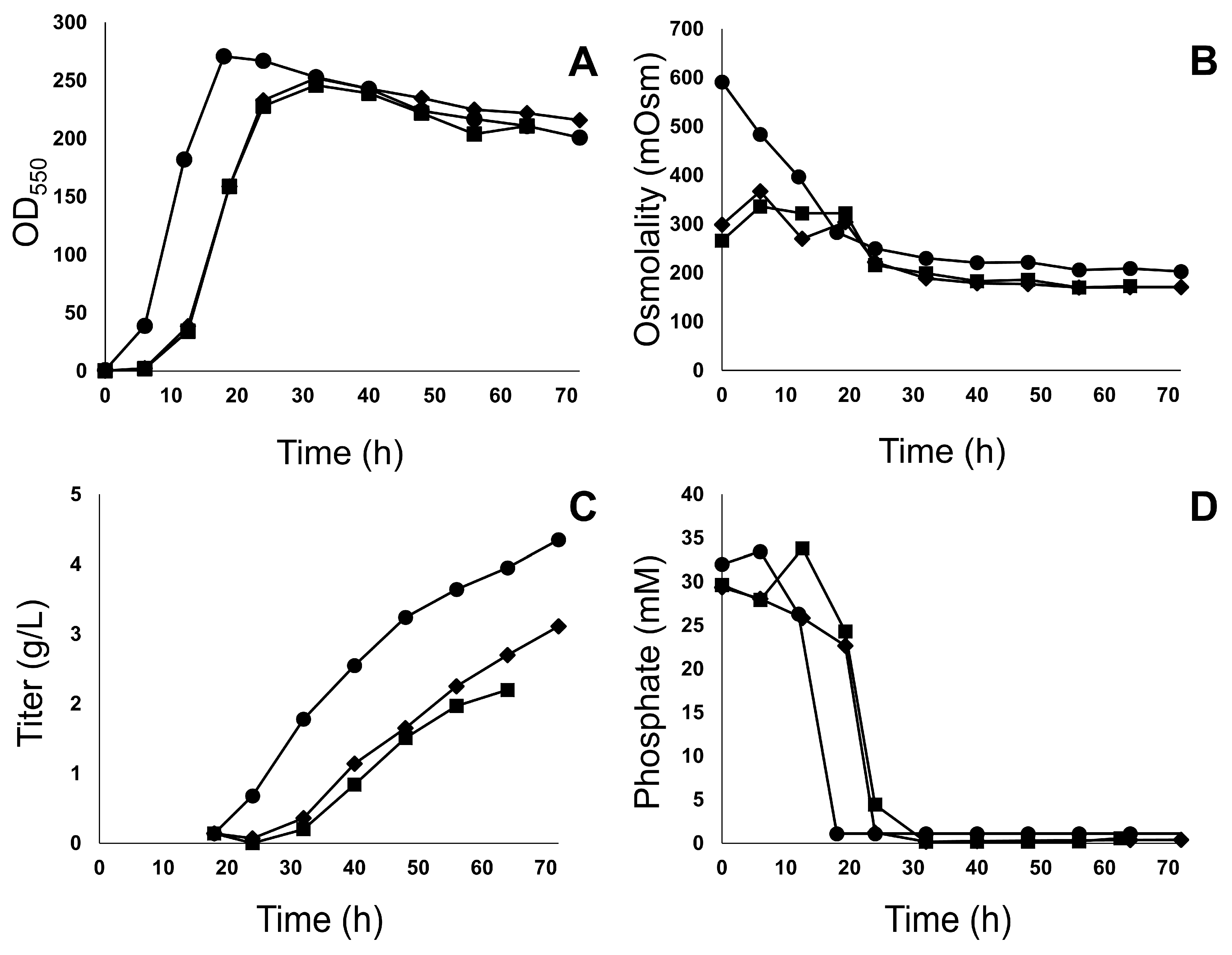
3.1. Fermentation
3.2. Mass Spectrometry Analysis and % 13C-Incorporation
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.Z.; Li, H.J.; Wu, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Kaplon, H.; Chenoweth, A.; Crescioli, S.; Reichert, J.M. Antibodies to watch in 2022. MAbs 2022, 14, 2014296. [Google Scholar] [CrossRef]
- Carter, P.J.; Rajpal, A. Designing antibodies as therapeutics. Cell 2022, 185, 2789–2805. [Google Scholar] [CrossRef]
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef]
- Sevy, A.M.; Meiler, J. Antibodies: Computer-Aided Prediction of Structure and Design of Function. Microbiol. Spectr. 2014, 2, 173–190. [Google Scholar] [CrossRef]
- Garidel, P.; Hegyi, M.; Bassarab, S.; Weichel, M. A rapid, sensitive and economical assessment of monoclonal antibody conformational stability by intrinsic tryptophan fluorescence spectroscopy. Biotechnol. J. 2008, 3, 1201–1211. [Google Scholar] [CrossRef]
- Thiagarajan, G.; Semple, A.; James, J.K.; Cheung, J.K.; Shameem, M. A comparison of biophysical characterization techniques in predicting monoclonal antibody stability. MAbs 2016, 8, 1088–1097. [Google Scholar] [CrossRef]
- Arbogast, L.W.; Brinson, R.G.; Marino, J.P. Mapping monoclonal antibody structure by 2D 13C NMR at natural abundance. Anal. Chem. 2015, 87, 3556–3561. [Google Scholar] [CrossRef]
- Harris, L.J.; Skaletsky, E.; McPherson, A. Crystallographic structure of an intact IgG1 monoclonal antibody. J. Mol. Biol. 1998, 275, 861–872. [Google Scholar] [CrossRef]
- Sears, A.E.; Albiez, S.; Gulati, S.; Wang, B.; Kiser, P.; Kovacik, L.; Engel, A.; Stahlberg, H.; Palczewski, K. Single particle cryo-EM of the complex between interphotoreceptor retinoid-binding protein and a monoclonal antibody. FASEB J. 2020, 34, 13918–13934. [Google Scholar] [CrossRef]
- Robotham, A.C.; Kelly, J.F. Chapter 1—LC-MS characterization of antibody-based therapeutics: Recent highlights and future prospects. In Approaches to the Purification, Analysis and Characterization of Antibody-Based Therapeutics; Matte, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. [Google Scholar]
- Kim, I.Y.; Suh, S.H.; Lee, I.K.; Wolfe, R.R. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp. Mol. Med. 2016, 48, e203. [Google Scholar] [CrossRef]
- Barbet, J.; Bardies, M.; Bourgeois, M.; Chatal, J.F.; Cherel, M.; Davodeau, F.; Faivre-Chauvet, A.; Gestin, J.F.; Kraeber-Bodere, F. Radiolabeled antibodies for cancer imaging and therapy. Methods Mol. Biol. 2012, 907, 681–697. [Google Scholar] [PubMed]
- Hiroaki, H. Recent applications of isotopic labeling for protein NMR in drug discovery. Expert Opin. Drug Discov. 2013, 8, 523–536. [Google Scholar] [CrossRef]
- Pan, S.; Aebersold, R.; Chen, R.; Rush, J.; Goodlett, D.R.; McIntosh, M.W.; Zhang, J.; Brentnall, T.A. Mass spectrometry based targeted protein quantification: Methods and applications. J. Proteome Res. 2009, 8, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.T.; Brinson, R.G.; Hoopes, J.T.; McClung, C.; Ke, N.; Kashi, L.; Berkmen, M.; Kelman, Z. Platform development for expression and purification of stable isotope labeled monoclonal antibodies in Escherichia coli. MAbs 2018, 10, 992–1002. [Google Scholar] [PubMed]
- Martinovic, S.; Veenstra, T.D.; Anderson, G.A.; Pasa-Tolic, L.; Smith, R.D. Selective incorporation of isotopically labeled amino acids for identification of intact proteins on a proteome-wide level. J. Mass. Spectrom. 2002, 37, 99–107. [Google Scholar] [CrossRef]
- Ridgway, J.B.; Presta, L.G.; Carter, P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 1996, 9, 617–621. [Google Scholar] [CrossRef]
- Bachmann, B.J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 1972, 36, 525–557. [Google Scholar] [CrossRef]
- Sutcliffe, J.G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb. Symp. Quant. Biol. 1979, 43 Pt 1, 77–90. [Google Scholar] [CrossRef]
- Simmons, L.C.; Reilly, D.; Klimowski, L.; Raju, T.S.; Meng, G.; Sims, P.; Hong, K.; Shields, R.L.; Damico, L.A.; Rancatore, P.; et al. Expression of full-length immunoglobulins in Escherichia coli: Rapid and efficient production of aglycosylated antibodies. J. Immunol. Methods 2002, 263, 133–147. [Google Scholar] [CrossRef]
- Veeravalli, K.; Schindler, T.; Dong, E.; Yamada, M.; Hamilton, R.; Laird, M.W. Strain engineering to reduce acetate accumulation during microaerobic growth conditions in Escherichia coli. Biotechnol. Prog. 2018, 34, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Taussky, H.H.; Shorr, E. A microcolorimetric method for the determination of inorganic phosphorus. J. Biol. Chem. 1953, 202, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Giese, G.; Persson, J. Improved assembly of bispecific antibodies from knob and hole half-antibodies. Biotechnol. Prog. 2015, 31, 1315–1322. [Google Scholar] [CrossRef]
- Macchi, F.D.; Yang, F.; Li, C.; Wang, C.; Dang, A.N.; Marhoul, J.C.; Zhang, H.M.; Tully, T.; Liu, H.; Yu, X.C.; et al. Absolute Quantitation of Intact Recombinant Antibody Product Variants Using Mass Spectrometry. Anal. Chem. 2015, 87, 10475–10482. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Izadi, S.; Callahan, M.; Deperalta, G.; Wecksler, A.T. Antibody-receptor interactions mediate antibody-dependent cellular cytotoxicity. J. Biol. Chem. 2021, 297, 100826. [Google Scholar] [CrossRef]
- Klein, C.; Schaefer, W.; Regula, J.T. The use of CrossMAb technology for the generation of bi- and multispecific antibodies. MAbs 2016, 8, 1010–1020. [Google Scholar] [CrossRef] [PubMed]

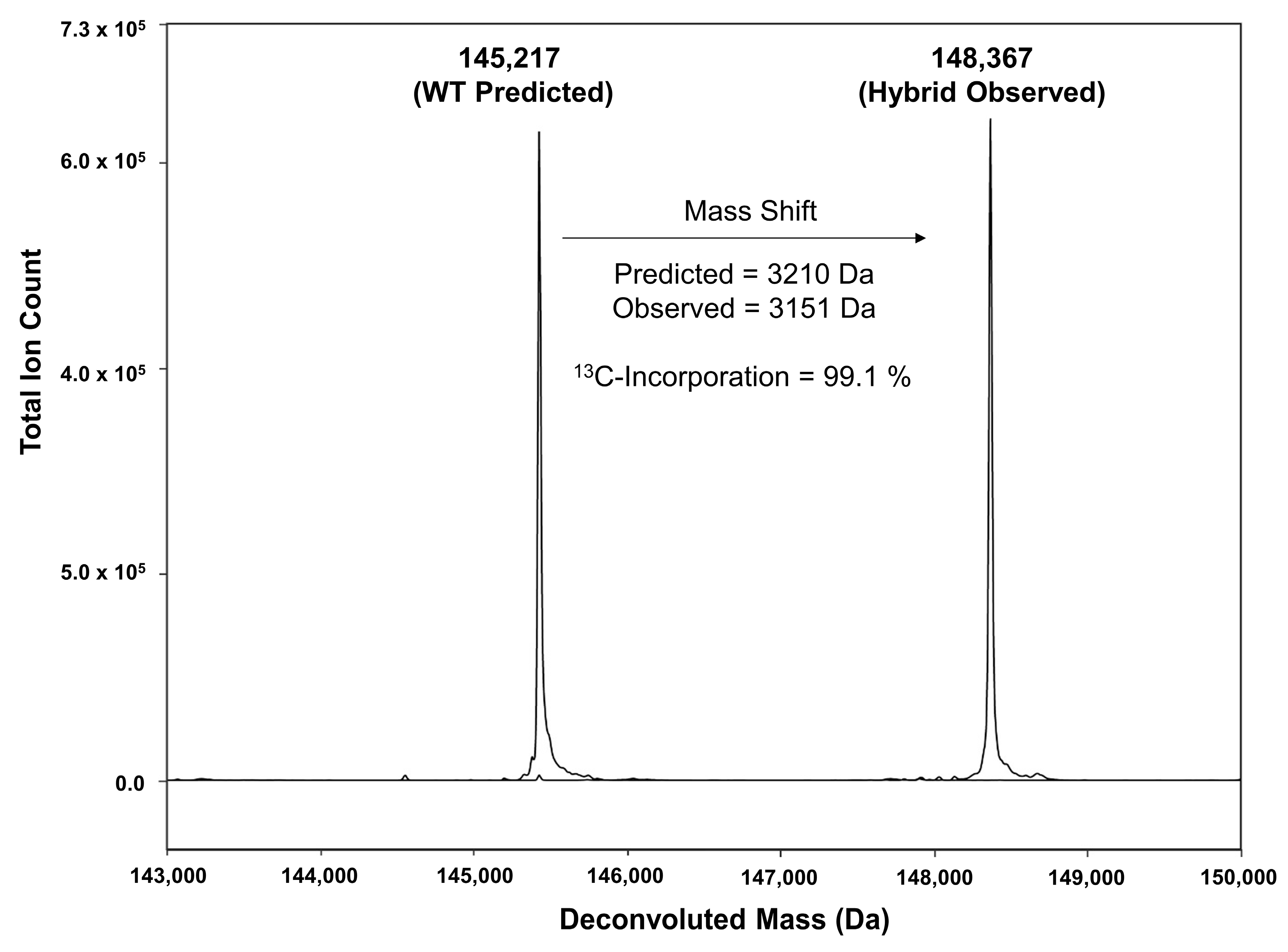
| WT Mass Predicted (Da) | Number of Carbons | Expected Mass Shift (Da) | 13C-labeling Mass Predicted (Da) | Mass Observed (Da) | 13C-Incorporation Calculated (%) | |
|---|---|---|---|---|---|---|
| hBsAb | 145,217 | 3210 | 3178 | 148,395 | 148,367 | 99.1 |
| Knob-hAb | 72,711 | --- | --- | --- | 72,712 | --- |
| Hole-hAb (Labeled) | 72,506 | 3210 | 3178 | 75,684 | 75,665 | 99.4 |
| Knob-HC | 48,912 | --- | --- | 48,912 | --- | |
| Hole-HC (labeled) | 48,707 | 2169 | 2147 | 50,854 | 50,835 | 99.1 |
| Knob-LC | 23,815 | --- | --- | --- | 23,815 | --- |
| Hole-LC (labeled) | 23,815 | 1041 | 1031 | 24,846 | 24,837 | 99.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wecksler, A.T.; Lundin, V.; Williams, A.J.; Veeravalli, K.; Reilly, D.E.; Grieco, S.-H. Bioprocess Development and Characterization of a 13C-Labeled Hybrid Bispecific Antibody Produced in Escherichia coli. Antibodies 2023, 12, 16. https://doi.org/10.3390/antib12010016
Wecksler AT, Lundin V, Williams AJ, Veeravalli K, Reilly DE, Grieco S-H. Bioprocess Development and Characterization of a 13C-Labeled Hybrid Bispecific Antibody Produced in Escherichia coli. Antibodies. 2023; 12(1):16. https://doi.org/10.3390/antib12010016
Chicago/Turabian StyleWecksler, Aaron T., Victor Lundin, Ambrose J. Williams, Karthik Veeravalli, Dorothea E. Reilly, and Sung-Hye Grieco. 2023. "Bioprocess Development and Characterization of a 13C-Labeled Hybrid Bispecific Antibody Produced in Escherichia coli" Antibodies 12, no. 1: 16. https://doi.org/10.3390/antib12010016
APA StyleWecksler, A. T., Lundin, V., Williams, A. J., Veeravalli, K., Reilly, D. E., & Grieco, S.-H. (2023). Bioprocess Development and Characterization of a 13C-Labeled Hybrid Bispecific Antibody Produced in Escherichia coli. Antibodies, 12(1), 16. https://doi.org/10.3390/antib12010016






