Abstract
Bentonite-based organic amendments may have the potential to enhance soil microbial properties. The experiment was carried out from 2014 to 2017 comprising four treatments: NPK fertilizer (nitrogen, phosphorus and potassium mineral fertilizer as a control), NPK + cattle manure, NPK + bentonite, and NPK + combination of manure with bentonite (MB) to verify this hypothesis. The effect of treatments on seven different soil microbial properties was measured: dehydrogenase activity (DHA), bacterial phospholipid fatty acid content, fungal phospholipid fatty acid content, microbial biomass carbon (Cmic), 16S rDNA, 18S rDNA, and ammonia-oxidizing bacteria in soil. The results showed that solely bentonite treatment increases the bacterial and fungal biomass, which was further confirmed by the increased 16S rDNA and 18s rDNA gene copy numbers. The only significantly decreased values upon treatment with solely bentonite were recorded for DHA and Cmic. The ammonia-oxidizing bacteria population increased with the sole application of bentonite and reached its maximum value when bentonite was applied with manure. The MB treatment showed the highest value for all seven measured properties. In summary, the application of bentonite solely might increase or decrease the soil activity, but its addition, along with manure, always promotes an abundance of soil microorganisms and their activity. The co-application of bentonite with manure altered the soil microbial properties in a 3-year field experiment in favor of increased microbial biomass, which is beneficial for agriculture and environment and reveals the potential for the restoration of polluted lands.
1. Introduction
Soil erosion is a consequence of unsustainable land management; it leads to increase in land degradation and a decrease in soil fertility, and it directly affects agricultural productivity [1]. Inorganic fertilizers are frequently used to increase soil fertility and crop yield. However, they are not a permanent solution to tackle soil infertility. The long-term application of mineral fertilizers into soils with a low percentage of organic compounds accelerates the mineralization of soil organic matter (SOM) and disrupts the natural metabolic processes of soil organisms. This leads to a reduction in the SOM content, nutritional imbalance, and soil acidification [2]. Therefore, natural amendments, such as bentonite and its combination with manure, could be useful tools to maintain or increase the SOM in a sustainable way.
Bentonite immobilizes toxic xenobiotic compounds (herbicides and antibiotics) present in the soil [3,4] and is widely used for the reclamation of polluted land [5,6]. Bentonite clay composite has also been evaluated as a tool for the remediation of heavy metal-contaminated soil [7]. Bentonite amendment successfully accelerates salt removal, reduces salt content, and improves the physical and chemical properties of coastal land. Bentonite addition is a main soil amelioration technique that is successfully used in the remediation and restoration of highly saline coastal land. Bentonite can improve the ability of a soil to retain nutrients and is used as a chemical and physical soil conditioner due to the high cationic exchange capacity that helps in the restoration of bare land and supports plant growth [8].
SOM plays an essential role in long-term soil conservation and restoration by sustaining its fertility and, hence, in sustainable agricultural production, due to the improvement of the physical, chemical, and biological properties of soils [9]. Many previous studies have reported that the application of organic material to the soil has increased the soil organic carbon storage [10].
Manure addition to the soil increases the SOM; soil with a higher proportion of organic matter has a higher cation exchange capacity (CEC) owing to the negative charge on organic matter. A high CEC helps in nutrient retention and nutrient availability in the soil [11]. Bentonite can vary its bulk density as a function of the soil moisture and temperature, thereby generating a reaction space for a simultaneously applied organic fertilizer [5,12]. Therefore, the use of bentonite together with manure may be an effective and sustainable way to achieve improved soil quality.
This study aims to investigate the impact of solely applied bentonite and its combination with manure on different soil microbial properties. The following soil microbial properties were selected as indicators of soil health and quality in the individual experiment variants: the microbial biomass carbon (Cmic), dehydrogenase activity (DHA), and soil microbial community abundance. The hypothesis is that the use of bentonite and manure together should result in a mutual modification of their properties; hence, the combination of both could give positive effects better than the simple sum of the individual effects of each one. The hypotheses were tested within the field experiments, which were performed from 2014 to 2017.
2. Materials and Methods
2.1. Experimental Field Site and Experiment Design
The experimental field was located at the locality of Rapotín in the Czech Republic (Figure 1), situated at an altitude of about 345 m a.s.l. This area comes under the temperate continental climate zone, with a mean annual temperature of 7 °C and a mean annual precipitation of about 705 mm. The seasonal distribution of rainfall at the Rapotín locality is around 400–450 mm during the vegetation season and 250–300 mm within the winter period.
Figure 1.
Localization of the field experiment within the Czech Republic.
Field-scale experiments were conducted during the cropping season from 2014 to 2017. The design of an experiments consisted of an application of four soil treatments, which were: (1) only NPK (mineral fertilizer) as a control, (2) NPK + cattle manure (50 t/ha), (3) NPK + bentonite (6 t/ha), and (4) NPK + combination of manure (50 t/ha) with bentonite (6 t/ha) (MB). Manure was added at the start of the experiment, whereas the bentonite and mineral fertilizer (NPK) were applied each year. The doses of nutrient (N, P, K) applied to each crop were: 220 kg/ha N, 35 kg/ha P, and 225 kg/ha K in the year 2014/2015 under the maize species LAVENA (FAO 250) for the silage; 160 kg/ha N, 30 kg/ha P, and 80 kg/ha K in the year 2016 under the spring barley species KWS IRINA; 170 kg/ha N, 30 kg/ha P, and 80 kg/ha K in the year 2016/2017 under the winter wheat species FAKIR. The dosage of cattle manure of 50 t/ha was added as recommended [13]. The experimental area was divided as follows: three small-scale-plots (10 × 10 m) per each of four variants of the soil amendment (12 small-scale plots overall). The amendments (bentonite, manure, and the combination of both) were manually spread on the small-scale plot and incorporated by deep plowing to a depth of 0–25 cm.
2.2. Soil and Amendments Characteristics
2.2.1. Soil Properties
The soil at the experimental area was the loamy sand Luvisol. For the more detailed introduction of this experimental soil, the analyses of the physical and chemical properties were realized at the beginning of the experiment (see in Table 1).

Table 1.
Soil physical and chemical properties.
2.2.2. Bentonite Properties
The applied bentonite (Ekobent B) was brought from the Czech producer KERAMOST, s.c.: humidity 5%; pH (KCl) value 9.0; grain 0–2 mm; content of montmorillonite 70%; SiO2 535 g/kg; Al2O3 24 g/kg; Na2O 2.5 g/kg; K2O 7.5 g/kg; Li2O 1 g/kg; P2O5 1 g/kg.
2.2.3. Manure Production and Properties
The manure was from deep bedding with straw litter with the manure removal frequency from the barn every 8 weeks. After removal and before the manure application on the field, it was stored on the covered dunghill with tarpaulin for 2 months. The chemical properties of the manure (the content in dry matter) were total organic carbon 427 g/kg, N-NH4+ 1.39 g/kg; Ntot 23.5 g/kg; P2O5 9.13 g/kg; K2O 23.9 g/kg; CaO 21.7 g/kg; MgO 6.30 g/kg; pH (KCl) value 8.6; and C:N ratio 24.1:1.
2.2.4. Combined Manure and Bentonite Production and Properties
Bentonite + manure production: After removal from the barn and before the manure application on the field, manure was mixed with bentonite and stored on the covered dunghill with tarpaulin for 2 months. The chemical properties of the combined manure with bentonite (the content in dry matter): total organic carbon 429 g/kg, N-NH4+ 2.69 g/kg; Ntot 29.1 g/kg; P2O5 10.5 g/kg; K2O 24.8 g/kg; CaO 26.1 g/kg; MgO 7.74 g/kg; pH value (KCl) 9.3; and C:N ratio 20.1:1.2.3.
2.3. Sample Collection
The soil samples were collected immediately after the third cropping season in October 2017. Three spatially independent mixed soil subsamples from each experimental variant were collected in the following way: a portion of topsoil from the depth 0–15 cm was taken by a soil drill at five spots of an experimental field and mixed in a plastic sampling bag. Thus, a total of 12 samples (3 per each of 4 variants) were collected. The samples were transported to the laboratory and they were homogenized there by sieving them through a 2 mm mesh under sterile conditions. The samples for the analysis of the enzyme assays were stored at 0–4 °C until they were used (within one week). The samples for the qPCR and PLFA (phospholipid fatty acid) analysis were freeze-dried. The dry mass and maximum water holding capacity (WHC max) were estimated in each sample prior to the measurement of the Cmic and dehydrogenase activity (DHA). The water holding capacity of the samples was adjusted to 50–60% of the WHC max, and they were re-activated by incubation in the dark at 22 °C for 4–5 days in closed bottles of volume 10-fold higher than the sample volume.
2.4. Microbial Biomass Carbon
The fumigation extraction method was used to determine the soil Cmic. This method is based on the lysis of the microbial cell upon treatment with chloroform for 24hr. The sample sets were duplicated and only one set was subjected to fumigation, followed by the extraction of K2SO4 and the comparison of the fumigated and non-fumigated samples [14].
2.5. Dehydrogenase Activities
Dehydrogenase acivity DHA was measured using triphenyltetrazolium chloride (TTC). The methodology was modified according to [15] based on [16]. A soil sample (3 g) was mixed with magnesium oxide (MgO) and sealed with the standard solution (triphenyl tetrazolium chloride + distilled water). The samples were incubated at 37 °C for 24 h. Triphenylformazan (TPF) was extracted from the samples using methyl alcohol, resulting in a change in the color of the solution. The intensity of the color was measured using a spectrophotometer (DR 3900, Hach Lang, Duesseldorf, Germany) at a wavelength of 485 nm. The DHA was calculated according to the calibration curve and expressed in µg TPF/g/h.
2.6. DNA Extraction and Real-Time qPCR
The DNeasy PowerSoil Kit (Qiagen, Hilden, Germany) was used to extract DNA from 0.5 g of the lyophilized soil. The soil DNA extracts were subjected to real-time PCR for quantification of partial bacterial (16S rDNA) and fungal (18S rDNA) rDNA. Each sample was spiked with the DNA of the plasmid vector derived from pUC18 serving as an internal standard for the valuation of the yield efficiency and contamination with PCR inhibitors. Picodrop was used to quantify the isolated DNA. CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories) was applied to perform SYBR-green assays. The primers used were 1132R (5′ GGGTTGCGCTCGTTGC 3′) and 1108F (5′ ATGGYTGTCGTCAGCTCGTG 3′) for bacteria [17], and FR1 (5′ AICCATTCAATCGGTAIT 3′) and FF390 (5′ CGATAACGAACGAGACCT 3′) for fungi [18]. The combination of the primer was used for the quantification of ammonia-oxidizing bacterial (AOB) 16S rDNA, RT1R (5′ CGTCCTCTCAGACCARCTACTG 3′), CTO189FC (5′ GGAGGAAAGTAGGGGATCG 3′), and CTO189FA/B (5′ GGAGRAAAGCAGGGGATCG 3′) primers at a 2:1:2 ratio [19]. The DNA of pUC18-derivate (internal standard) was quantified by qPCR using SQPR2 (5′ CTCGTATGTTGTGTGGAA 3′) and SQP (5′ GTTTTCCCAGTCACGAC 3′) primers [20].
The SYBR-green type real-time qPCR was conducted on the CFX96™ Real-Time PCR Cycler (Bio-Rad, USA). The same PCR programming (for the determination of respective target genes—i.e., referred to each microbial group) as reported in the original works (Amann et al. 1995, Vainio et Hantula 2000, Hermansson et al. 2001) was followed [17,18,19]. A 5× HOT FIREPol® EvaGreen®qPCR Supermix (Solis Biodyne, Estonia) was used for the PCR reaction assay.
2.7. Quantification of Microbial Biomass
The samples for the phospholipidic fatty acid (PLFA) analysis were extracted from the mixture of chloroform-methanol-phosphate buffer (1:2:0.8) [21]. The phospholipids were separated using solid-phase extraction cartridges (LiChrolut Si 40, Merck). Then, the samples were subjected to mild alkaline methanolysis and extracted to hexane as a final solvent [22]. The free methyl esters of the phospholipid fatty acids were analyzed using gas chromatography-mass spectrometry (Agilent 7890A with a Flame ionization detector FID detector). The GC instrument was equipped with a split/splitless injector, and a CP Sil 88 column was applied for separation (100 m, 0.25 mm i.d., 0.2 µm film thickness). The temperature program started at 80 °C and was held for 1 min in a splitless mode. Thereafter, the splitter was opened, and the oven was heated to 160 °C at a rate of 20 °C/min. The second temperature ramp was up to 225 °C at a rate of 5 °C/min; this temperature was maintained for 12 min.
Methylated fatty acids were identified according to their mass spectra and using a mixture of the chemical standards obtained from Sigma/Matreya LLC. The fungal biomass (FPLFA) was quantified based on the 18:2ω6,9 content, and bacterial biomass (BPLFA) was quantified as a sum of i14:0, i15:0, a15:0, 16:1ω7t, 16:1ω9, 16:1ω7, 10Me-16:0, i17:0, a17:0, cy17:0, 17:0, 10Me-17:0, 10Me-18:0, and cy19:0. The fatty acids found in both bacteria and fungi—15:0, 16:0, and 18:1ω7—were excluded from the analysis. The relative content of the individual PLFA molecules was also calculated. The total content of all the PLFA molecules (PLFAT) was used as an indicator of the total microbial biomass.
2.8. Statistical Analyses
Data analysis was carried out with the help of software R, version 3.6.3. [23], together with the additional package “ggplot2” [24] for creating advanced statistical graphs and “factoextra” [25] and “FactoMineR” [26] for a principal component analysis (PCA).
For the characterization of the relation between the soil microbial properties and different treatments (with 4 replicates), a one-way analysis of variance (ANOVA), a Dunnett–Tukey–Kramer pair-wise multiple comparison test from the package “agricolae” [27], and PCA were implemented. The Pearson’s correlation coefficient was applied for measuring the statistical relation between the soil microbial properties; the chart of a correlation matrix was created with help of the additional package “PerformanceAnalytics” [27]. The partial eta-squared (ηp2) from the package “BaylorEdPsych” [28] was employed for the measuring of the effect size.
3. Results
3.1. Dependences of Microbial Indexes on the Treatment Applied
A summary of all the measured soil parameters is given in the following Table 2. Furthermore, Figure 2 and Figure 3 summarize information on the interactions of the individual soil parameters. The measured values (Table 1, Figure 2 and Figure 3) confirmed not only the relation and potentially mutual influence between the individual parameters, but also the obvious influence of the common application of bentonite and manure. The strongest positive correlation was confirmed between BPLFA and 16S AOB rDNA (Pearson’s r(24) = 0.96 (95% CI[0.91, 0.98]), p < 0.001) and between DHA and 18S rDNA (Pearson’s r(24) = 0.93 (95% CI[0.84, 0.97]), p < 0.001).

Table 2.
Basic descriptive statistics for the soil microbial properties.
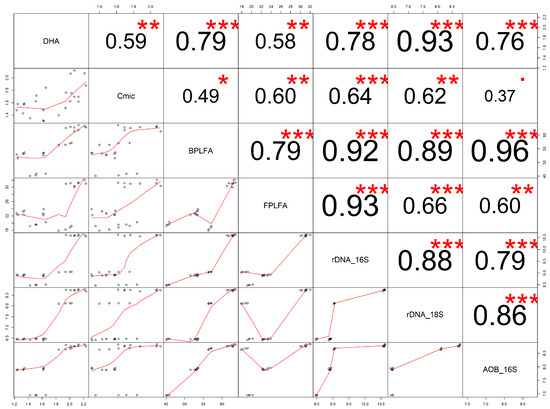
Figure 2.
Pearson’s correlation matrix of the soil microbial properties.
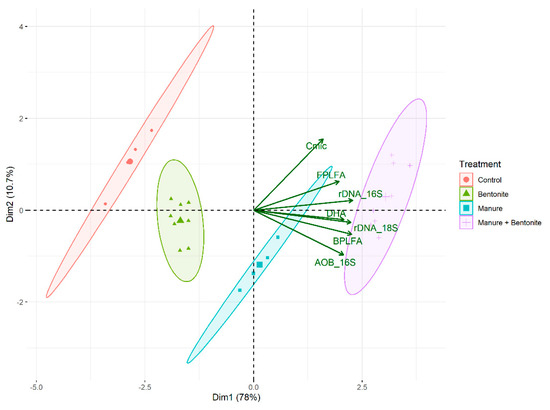
Figure 3.
Rohlf biplot for the characterization of the relation between the soil microbial properties with dependence on the treatments.
Based on the results of the PCA, it can be concluded that approximately 89% of the variation was clarified by the first two eigenvalues together: the first eigenvalue was 78% and the second one was 11%. In the first dimension, the following soil microbial properties had a large contribution: 16S rDNA (17.1%), BPLFA (16.8%), and 18S rDNA (16.6%). In the second dimension, the following soil microbial properties had a large contribution: Cmic (57.7%) and 16S AOB rDNA (22.6%). The total contribution of the soil microbial properties on PC1 and PC2 together was as follows (in descending order): BPLFA (15.5%), 16S rDNA (15.2%), 16S AOB rDNA (15.2%), 18S rDNA (14.8%), and Cmic (14.4%). Other soil microbial properties did not have a large contribution in accounting for the variability in the first two principal components. The Rohlf biplot (Figure 3) shows clearly this relation between the soil microbial properties and their change with the dependence on the treatments. Mainly in the case of utilizing manure and bentonite together, the values of DHA, FPLFA, BPLFA, 16S rDNA, 18S rDNA, and 16S AOB rDNA demonstrate larger figures than in the control group.
3.2. Dehydrogenase
The results of a one-way ANOVA analysis of the DHA activity values (Figure 4) showed a statistically significant difference between the DHA activity of the respective treatments (F3,20 = 46.27, p < 0.001, ηp2 = 0.87). Significantly increased DHA activity was found in the case of manure (diff = −0.43, p < 0.001) and MB (diff = −0.57, p < 0.001) as compared to the control. The highest value of DHA was found out in the case of MB—on average, 2.10 µg TPF/g/h (95% CI [2.0, 2.19])—and the lowest value of DHA was determined in the case of bentonite, on average 1.42 µg TPF/g//h (95% CI [1.32, 1.51]). The treatments (bentonite, manure) and manure alone showed a maximum increase in DHA activity as compared to the control and bentonite treatment.
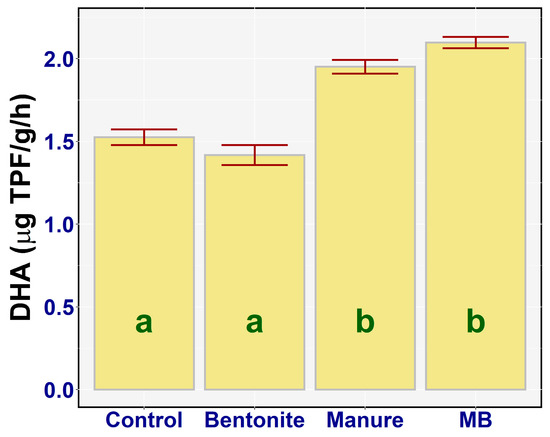
Figure 4.
Dehydrogenase activity (DHA) activity of the treatments: means with SEM (standard error of mean).
Manure (diff = −0.53, p < 0.001) and MB (diff = −0.68, p < 0.001) amendments showed a significant increase in the DHA activity in comparison with bentonite solely.
3.3. Soil Phospholipid Fatty Acid Analysis
A one-way ANOVA revealed a significant difference between the soil fungal community biomass (FPLFA) of the treatments (F3,20 = 582.43, p < 0.001, ηp2 = 0.99). The effect of the applied treatments on the FPLFA was statistically significant in the case of bentonite (diff = −3.23, p < 0.001) and MB (diff = −11.37, p < 0.001), which caused a significant increase in the FPLFA value compared to the control (Figure 5). There was also a statistically significant difference between bentonite and manure (diff = 4.33, p < 0.001), between bentonite and MB (diff = −8.14, p < 0.001), and between manure and MB (diff = −12.47, p < 0.001). The highest value of fungal biomass was recorded for the soil treated with MB—on average, 31.0 nmol/g (95% CI [30.57, 31.43])—and the lowest value for fungal biomass was found in the case of manure, on average 18.53 nmol/g (95% CI [17.92, 19.14]). The addition of bentonite to soils significantly enhanced the soil fungal biomass. While manure alone had no significant effects, when combined with bentonite a strong enhancement of the fungal biomass was observed (Figure 5).
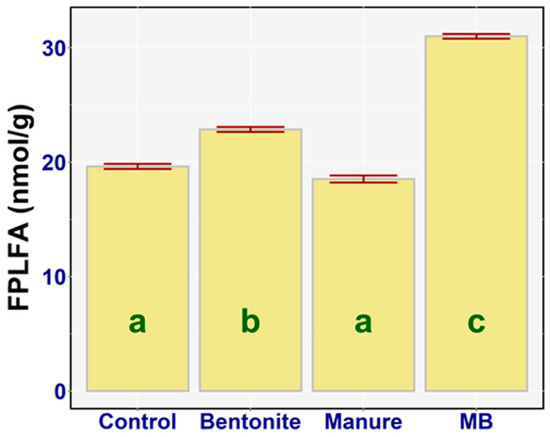
Figure 5.
Amount of FPLFA (nmol/g) in the treatments: means with SEM.
BPLFA showed the similar results to those of FPLFA (Figure 6). A one-way ANOVA indicated that there was a statistically significant difference between the BPLFA values of the treatments (F3,20 = 729.74, p < 0.001, ηp2 = 0.99). The statistically significant increase in the BPLFA value on the addition of bentonite (diff = 7.48, p < 0.001), manure (diff = −11.23, p < 0.001), and MB (diff = −17.13, p < 0.001) was demonstrated. The highest value of bacterial biomass was found in the case of MB—on average, 62.6 nmol/g (95% CI; 62.17, 63.10)—and the lowest value of bacterial biomass was observed in the case of the control, on average 45.5 nmol/g (95% CI; 44.84, 46.16). There was also a statistical difference between bentonite and manure (diff = −3.75, p < 0.001), between bentonite and MB (diff = −9.65, p < 0.001), and between manure and MB (diff = −5.91, p < 0.001). The addition of bentonite and manure to soils significantly enhanced the soil bacterial biomass. Meanwhile, bentonite in combination with manure showed a synergistic effect (Figure 6).
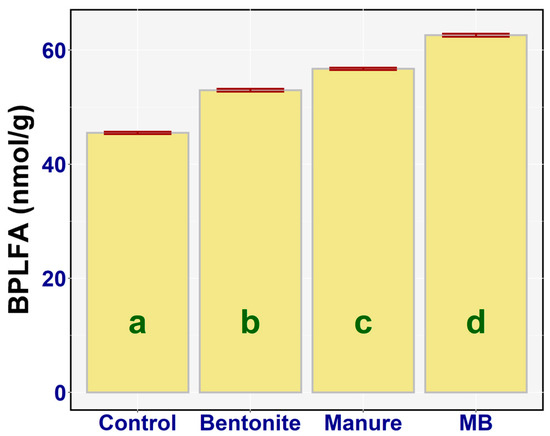
Figure 6.
Amount of BPLFA (nmol/g) in the treatments: means with SEM.
3.4. Microbial Biomass Carbon
Cmic is one of the main indicators of soil fertility, which is more sensitive to soil treatment practices than bulk organic matter. The results of a one-way ANOVA denoted statistically significant differences between the Cmic of the treatments (F3,20 = 7.67, p = 0.001, ηp2 = 0.54). There was a large statistically significant difference between bentonite and MB (diff = −0.39, p < 0.001), and a borderline statistically significant difference between manure and MB (diff = −0.29, p = 0.048). Nevertheless, there were no statistically significant differences between the control and bentonite (diff = −0.16, p = 0.40), manure (diff = 0.06, p = 0.96), and MB (diff = −0.22, p = 0.15). The highest Cmic value was found in the case of MB—on average, 1.86 mg/g (95% CI [1.74, 1.99])—and the lowest value of Cmic was detected in the case of bentonite, on average 1.47 mg/g (95% CI [1.35, 1.59]) Treatments (bentonite, manure) alone or its combination has no effect on the microbial biomass carbon (Figure 7).
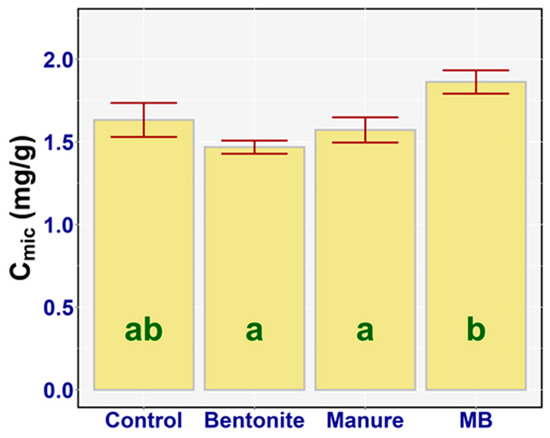
Figure 7.
Cmic (mg/g) of the treatments: means with SEM.
3.5. DNA Extraction and Real-Time qPCR
3.5.1. 16S rDNA
The total amount of bacteria (with no respect to viability) in the soil microbial community was determined as the number of copies of the 16S rDNA (qPCR amplicon from the total soil DNA with targeted oligonucleotide probes—Section 2.6) [17]. The results of bacterial DNA in the soil of all the variants were determined (Figure 8). A one-way ANOVA analysis of variance showed a significant difference between the log 16S rDNA copies of the treatments (F3,20 = 12387, p < 0.001, ηp2 = 0.99). The significantly increased 16S rDNA value was determined in the case of bentonite (diff = −0.42, p < 0.001), manure (diff = −0.55, p < 0.001), and MB (diff = −2.11, p < 0.001) to the control. The highest value of 16S rDNA was found in the case of MB—on average, 10.60 log copies/g (95% CI [10.58, 10.61])—and the lowest value of 16S rDNA was demonstrated in the case of the control, on average 8.49 log copies/g (95% CI [8.47, 8.51]). There was also a large statistical difference between the following treatments (Figure 8): bentonite and manure (diff = −0.13, p < 0.001), bentonite and MB (diff = −1.69, p < 0.001), and manure and MB (diff = −1.55, p < 0.001). The treatments (bentonite, manure) enhanced the bacterial populations, but in particular the effect was strong when they were combined (manure + bentonite).
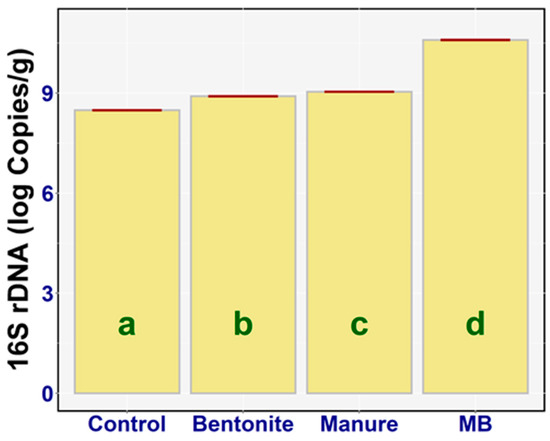
Figure 8.
16S rDNA (log Copies/g) of the treatments: means with SEM.
3.5.2. 18S rDNA
The results of the fungal biomass estimation via the quantification of fungal genomic DNA are in Figure 9. Similarly to the case of bacteria, the total amount of (viable or not) fungi in the soil was determined as the number of copies of the 18S rDNA (qPCR amplicon from the total soil DNA with fungi-targeted oligonucleotide probes—Section 2.6) [18]. The one-way ANOVA also revealed a significant difference between the log 18S rDNA of the treatments (F3,20 = 16,840, p < 0.001, ηp2 = 0.99). Although no significant change in the log 18S rDNA copies was observed in the case of the bentonite treatment (diff = −0.01, p = 0.97) compared to the control, there was a statistically significant difference between manure (diff = −1.67, p < 0.001) and MB compared to the control (diff = −2.29, p < 0.001). The highest value of 18S rDNA was found in the case of MB—on average, 8.74 log copies/g (95% CI [8.72, 8.76])—and the lowest value of 18S rDNA was determined in the case of bentonite, on average 6.45 log copies/g (95% CI [6.43, 6.46]). There was also a large statistical difference between the following treatments (Figure 9): bentonite and manure (diff = −1.67, p < 0.001), bentonite and MB (diff = −2.29, p < 0.001), and manure and MB (diff = −0.62, p < 0.001). The treatments with manure enhanced the fungal populations, but in particular the effect was strong when they were combined (manure + bentonite).
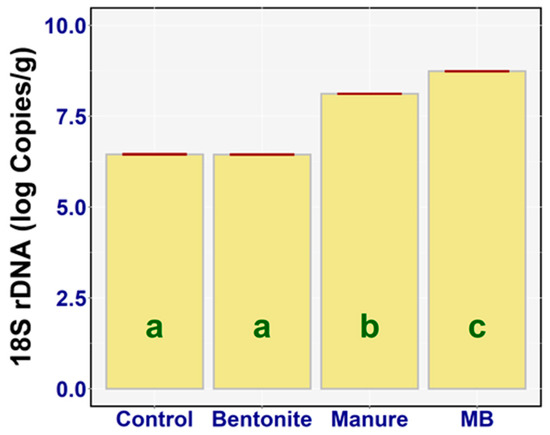
Figure 9.
18S rDNA (log copies/g) of the treatments: means with SEM.
3.5.3. Ammonium Oxidation in Soil (16S AOB-rDNA)
The indicator of nitrification processes in the soil is a community of ammonia-oxidizing bacteria (AOB). The total amount of AOB (viable or not) in the soil samples was determined as the number of copies of the AOB-specific16S rDNA (qPCR amplicon from the total soil DNA with targeted oligonucleotide probes—Section 2.6) [19]. Based on the result of a one-way ANOVA, it can be concluded that there was a statistically significant difference between the 16S AOB rDNA of the treatments (F3,20 = 4403.2, p < 0.001, ηp2 = 0.99). The effect of all the treatments was significant in comparison with the control group: bentonite (diff = 0.97, p < 0.001), manure (diff = −1.71, p < 0.001), and MB (diff = −1.83, p < 0.001). The highest value of 16S AOB rDNA was identified in the case of MB—on average, 8.77 log copies/g (95% CI [8.75, 8.80])—and the lowest value of 16S AOB rDNA was detected in the case of the control group, on average 6.95 log copies/g (95% CI [6.92, 6.98]). There was also a large statistical difference between the following treatments (Figure 10): bentonite and manure (diff = −0.74, p < 0.001), bentonite and MB (diff = −0.86, p < 0.001), and manure and MB (diff = −0.12, p < 0.001). The treatments (bentonite, manure) enhanced the population of ammonium oxidation bacteria, but in particular the effect was strong when they were combined (manure + bentonite).
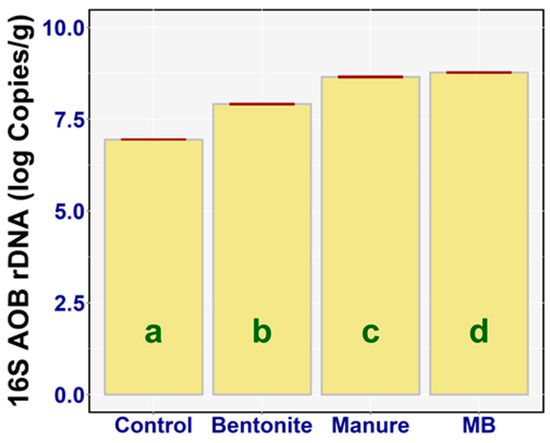
Figure 10.
Ammonia-oxidizing bacterial (AOB) rDNA (log Copies/g) of the treatments: means with SEM.
4. Discussion
4.1. Dehydrogenase
The lowest value of DHA was found for bentonite treatment as compared to all other variants (Figure 4). This decrease in enzyme activity may be due to the addition of bentonite in the pure form. The DHA activity depends on the living cell content of the soil (e.g., genus Pseudomonas). Since it is an intracellular enzyme, dehydrogenase cannot be stored in the soil outside the host cell [29]. The high DHA activity shows a greater number of living bacterial biomass present in the soil [30], whereas low to no DHA activity is seen as the absence of host bacteria. Bentonite is one of the most abundant inorganic colloids and has a strong adsorption power to absorb nutrients from the aqueous solution [31,32]. It has a high surface area and a high CEC and positively affects several soil parameters. However, it interacts with soil nutrients as well as useful soil microbes, and in some cases it results in a negative DHA effect on the soil microflora and rhizosphere [33,34].
On the contrary, significantly increased DHA activity was determined in the case of manure and MB in comparison to the control. The results are consistent with other studies that reported that organic soil amendments could significantly improve soil enzyme activities [35,36]. The manure application provides an additional source of carbon and nitrogen, which increases bacterial activity. In this case, the sustainable and slow release of manure-derived nutrients temporarily immobilized by bentonite for an extended period could be the main reason for the increase in the DHA activity [37,38]. The application of bentonite, which was shown to increase soil moisture [39], improved the soil enzyme activity when amended with manure. There are studies coupling the elevated levels of organic agriculture pollutants with the induced DHA activity [40], which may indicate that the higher DHA activity (in the soil with manure and bentonite) is an assumption for a higher decontamination potential.
4.2. Soil Phospholipid Fatty Acid Analysis
PLFA is a rapid and sensitive method used to detect living microbial types and their abundance in the soil. Soil mix with bentonite contains a large surface area for microbial growth [41,42], and small pores provide extra protection for soil microorganisms [43,44,45]. This is the reason for the higher FPLFA and BPLFA values for the samples treated both with bentonite solely and in combination with manure. However, the bentonite-derived increase in microbial abundance was contradictory to the results of the DHA measurement. Although microbial biomass was identified to correlate with the rate of soil organic carbon decomposition, it could be mainly attributed to respiration [42], whereas DHA strongly increases under anaerobic conditions. This finding is in agreement with a bentonite-referred role in the retention of organic matter decomposition [43,46,47]. An interaction of soil microflora and bentonite changes the SOM quality, and SOM increase with the clay content (due to physical and chemical bindings) was not coupled with a significant increase in its readily mineralizable portion [48]. A similar result for the bentonite effect of SOM decomposition was presented by other scientists [45,49]. Manure showed the lowest FPLFA value; this might be due to the significantly lower soil pH value in the incriminated manure variant (5.64, in contrast to pH 7.40 of the control). It was reported that the fungal/bacterial PLFA ratio tends to increase with an increasing soil pH [50].
4.3. Microbial Biomass Carbon
Cmic is a measure of the carbon contained in an active component of SOM. Its formation is contributed by mostly bacteria and fungi, which transform plant residues and soil organism debris in the products ready for plant assimilation. Cmic is primarily used to evaluate the soil quality; it is an essential driver of ecosystem functioning [51,52]. Figure 7 shows the measured values of the soil Cmic within the individual variants. The soils treated with bentonite showed the lowest value of Cmic. It can be assumed (in agreement with other authors, e.g., [53]) that a clay fraction of the soil was enriched with the soil organic carbon (in comparison with the bulk soils). This might be due to the montmorillonite type of sorbent, which was referred to show a slower mineralization rate (compared to other clay minerals), which was in line with the smaller microbial biomass in this soil [54]. These authors also evidenced the retarded decomposition of various soil organic compounds due to the adsorption to clay sorbents [55,56,57], which may clarify the lowered Cmic in comparison with the control. Another explanation for the decrease in the Cmic value is that bentonite absorbs water from the surrounding and swells up, and the swelling of bentonite substantially reduces the microbial activity in the soil [58,59]. A small pore size on the bentonite surface and the low free water could be other reasons for the decline in the microbial activity. Some authors have proved that a minimum of 15% water content is necessary for microbial growth in a 50:50 mixture of sand and bentonite [60,61]. A significant increase in the Cmic was observed compared to bentonite (Figure 7). Presumably, the known positive effect of manure on the water holding capacity [62] and the surplus access of organic carbon might have alleviated both putative negative effects of bentonite on the Cmic value.
4.4. DNA Extraction and Real-time qPCR
4.4.1. 16S rDNA
Bacteria represent a dominant fraction of the soil microflora, which largely contributes to the majority of processes in the soil—e.g., to the soil aggregation and structure, metabolism and nutrient fluxes, and plant health and growth. A significant increase in the 16S rDNA value has been observed for the bentonite variant in comparison to the control. It has been claimed the presumed beneficial effect of the bentonite amendment to the soil quality is due to the increased microbial biomass—see Figure 2, Figure 3 and Figure 8. There was also reported a significant increase in bacterial 16S rDNA genes upon the bentonite treatment [63].
In contrast to the results of the Cmic, the amendment of manure led to a significantly increased bacterial (16S rDNA) abundance compared to both the control and bentonite variants. The more significant positive contribution of manure to bacterial biomass in comparison with the bentonite effect was expected [64], and was in conformity with the results of the BPLFA measurement (Section 4.2). Finally, the combined treatment with bentonite and manure together resulted in the highest numbers of bacteria in the soil (compared to all the other variants). The evidenced additional enhancement of bacterial colonization of a small-sized clay fraction of the soil (which may be attributed to bentonite) due to the manure amendment has also been reported [65].
4.4.2. 18S rDNA
Bentonite gives a place to live to beneficial microbes (bacteria, fungi, protozoa, nematodes, etc.) [66,67]. The course of the measured values of the 18S rDNA are thus partially similar to those in the case of 16S rDNA (Figure 8 and Figure 9). The soil with a content of clay minerals provides a niche for fungal mycelial growth; however, it was suggested that fungal ones are more likely associated with macroaggregates, whereas bacterial ones dominate in microaggregates, which prevail in clay sorbents such as bentonite [68,69]. This may be an explanation for the lack of significant difference in the log 18S rDNA copies of the bentonite treatment as compared to the control. It has been proven that the soil receiving farmyard manure exerts increased the fungal biomass [70]—the significant difference in the 18S rDNA values between the manure and control correlates with these findings.
Due to high porosity and surface area, bentonite absorbs nutrients from manure and provides a good environment for microbial growth. Up to 25% of the biological mass is made up of fungi [71], of which microscopic fungi are the largest component (75%). Microscopic fungi easily colonize the surface of the bentonite–manure mixture and are also protected from predators thanks to the manure-mediated formation of macroaggregates [72]. This could be the reason why the sample treated with the combination of manure and bentonite showed a significant increase in the log 18S rDNA copies compared to all the treatments, including the control. These results thus confirm the necessity of a common application of bentonite and organic matter (in this case manure) to achieve the desired positive effect on the microbial community in the soil.
4.4.3. 16S AOB-rDNA
Bentonite can provide a consistent source of exchangeable NH4+ ions [73] that support populations of nitrifiers on the clay surface [74]. Additionally, other authors have reported that clay amendments (i.e., bentonite) may cause a slight increase (up to 25%) in the potential nitrification in some soils (at application rates of up to 5%) [34]. The same was evidenced here, with a significantly higher 16S AOB-rDNA value of the bentonite variant as compared to the control (Figure 10). Further, the AOB content was significantly improved with the manure treatment as compared to bentonite, and this was in agreement with previously reported results [75]. The highest value of 16S AOB rDNA was found in the case of MB (on average, 8.77 log copies.g−1). and demonstrated that the soil containing the bentonite and manure mixture can fix ammonia from manure due to the presence of expanding interlayers in bentonite and similar clays [76,77].
A mutual interaction between bentonite and manure significantly modifies the physico-chemical properties of bentonite on the grounds of the adsorption of manure-derived organic compounds on the surface of bentonite; as a result, the bentonite surface becomes enriched with manure-born microorganisms [78].
4.5. Potential for the Reclamation of Polluted Soils
Soil pollution from agriculture caused by various substances has become a growing concern in most developed and developing countries worldwide due to enhanced urbanization, agricultural practices, and especially industrialization [79]. The results of this experiment show the theoretical potential of the combined use of the bentonite amendment with manure for the elimination of soil pollutants in agricultural soils. From other studies, it is evident that microorganisms immobilized on mineral carriers changed the chemical composition of manure [80]; thus, they may possess significant biological activity. In a similar way, the immobilization of microorganism on the bentonite surface causes an alteration in the bentonite properties that facilitates the bioconversion of adsorbed polluting compounds towards decomposition products that are non-toxic in the soil and environment [81,82]. This proves the environmental-decontaminating potential of the manure-bentonite combined amendment described in this study. However, further research is needed.
5. Conclusions
In conclusion, these research findings suggest that the application of solely bentonite or its combination with manure increases fungal and bacterial biomass in comparison with the control variant, as evidenced by the increased values of BPLFA and FPLFA. The increased fungal and bacterial biomass upon the bentonite and manure treatment positively correlates with the 16S rDNA copies and 18S rDNA copies. The decrease in the values of the soil Cmic and DHA activity was identified for the soil samples treated with bentonite only, but, on the contrary, these values reached the maximum for the MB treatment. The AOB population was also increased with the application of bentonite solely, and reached its maximum value when bentonite was applied in combination with manure. Similar trends are seen for BPLFA, FPLFA, and 16S rDNA.
Author Contributions
Conceptualization, M.B., R.D., and J.H.; methodology, M.B., O.L., J.H., M.R.; software, T.B.; validation, M.B., J.H., P.S., J.E., R.D., A.K.; formal analysis, T.H., V.P., P.S.; investigation, M.B., J.H., L.B., T.V.; resources, M.B., J.H., O.L.; data curation, T.H., V.P., J.E., T.B., A.K.; writing—original draft preparation, R.D., J.H.; writing—review and editing, R.D., M.B., T.V., L.B., M.R., V.P., J.H.; visualization, J.E., J.H.; supervision, O.L., T.V., P.S., L.B., M.R., S.D., M.Z.-u.-H.; project administration, M.B., O.L.; funding acquisition, M.B, O.L. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the project of Technology Agency of the Czech Republic TH02030169: “Effect of biologically transformed organic matter and biochar application on the stability of productive soil properties and reduction of environmental risks”.
Acknowledgments
The work was supported by the project of Technology Agency of the Czech Republic TH02030169: “Effect of biologically transformed organic matter and biochar application on the stability of productive soil properties and reduction of environmental risks”; and by the project of Ministry of Education, Youth and Sports of the Czech Republic FCH-S-20-6446.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Foley, J.A. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Hernández, T.; Chocano, C.; Moreno, J.-L.; García, C. Use of compost as an alternative to conventional inorganic fertilizers in intensive lettuce (Lactuca sativa L.) crops—Effects on soil and plant. Soil Tillage Res. 2016, 160, 14–22. [Google Scholar] [CrossRef]
- Davies, J.E.D.; Jabeen, N. The adsorption of herbicides and pesticides on clay minerals and soils. Part 1. Isoproturon. J. Incl. Phenom. Macrocycl. Chem. 2002, 43, 329–336. [Google Scholar] [CrossRef]
- Genç, N.; Dogan, E.C. Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalin. Water Treat. 2013, 53, 785–793. [Google Scholar] [CrossRef]
- Baker, L.R.; White, P.M.; Pierzynski, G.M. Changes in microbial properties after manure, lime, and bentonite application to a heavy metal-contaminated mine waste. Appl. Soil Ecol. 2011, 48, 1–10. [Google Scholar] [CrossRef]
- Li, J.; Sun, X.; Li, S. Effects of Garden Waste Compost and Bentonite on Muddy Coastal Saline Soil. Sustainability 2020, 12, 3602. [Google Scholar] [CrossRef]
- Myasnikov, S.K.; Tikhonov, A.Y.; Chipryakova, A.P.; Kulov, N.N. Removal of heavy metal ions from water by an combined sorption–crystallization process using activated clays. Theor. Found. Chem. Eng. 2016, 50, 366–382. [Google Scholar] [CrossRef]
- Bouabid, R.; Badraoui, M.; Bloom, P.R. Potassium Fixation and Charge Characteristics of Soil Clays. Soil Sci. Soc. Am. J. 1991, 55, 1493–1498. [Google Scholar] [CrossRef]
- Sequi, P. Le funzioni agronomiche della sostanza organica. In Chimica del suolo; Patron editore: Bologna, Italy, 1989; pp. 279–292. [Google Scholar]
- Paustian, K.; Larson, E.; Kent, J.; Marx, E.; Swan, A. Soil C Sequestration as a Biological Negative Emission Strategy. Available online: https://www.frontiersin.org/articles/10.3389/fclim.2019.00008/full (accessed on 15 July 2020).
- Wacal, C.; Ogata, N.; Basalirwa, D.; Sasagawa, D.; Ishigaki, T.; Handa, T.; Kato, M.; Tenywa, M.M.; Masunaga, T.; Yamamoto, S.; et al. Imbalanced Soil Chemical Properties and Mineral Nutrition in Relation to Growth and Yield Decline of Sesame on Different Continuously Cropped Upland Fields Converted Paddy. Agronomy 2019, 9, 184. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.J.; Zhang, Z.; Shen, F.; Zhang, G.; Qin, R.; Li, X.; Xiao, R. Nutrient transformations during composting of pig manure with bentonite. Bioresour. Technol. 2012, 121, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Coal fly ash and farmyard manure amendments in dry-land paddy agriculture field: Effect on N-dynamics and paddy productivity. Appl. Soil Ecol. 2011, 47, 133–140. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982. [Google Scholar] [CrossRef]
- Casida, L.E.J.; Klein, D.A.; Santoro, D. Soil Dehydrogenase Activity. Soil Sci. Annu. 1964, 98, 371–378. [Google Scholar] [CrossRef]
- Amann, R.I.; Ludwig, W.; Schleifer, K.H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 1995, 59, 143–169. [Google Scholar] [CrossRef] [PubMed]
- Vainio, E.; Hantula, J. Direct analysis of wood-inhabiting fungi using denaturing gradient gel electrophoresis of amplified ribosomal DNA. Mycol. Res. 2000, 104, 927–936. [Google Scholar] [CrossRef]
- Hermansson, A.; Lindgren, P.-E. Quantification of Ammonia-Oxidizing Bacteria in Arable Soil by Real-Time PCR. Appl. Environ. Microbiol. 2001, 67, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A RAPID METHOD OF TOTAL LIPID EXTRACTION AND PURIFICATION. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Oravecz, O.; Elhottova, D.; Kristufek, V.; Sustr, V.; Frouz, J.; Triska, J.; Marialigeti, K. Application of ARDRA and PLFA analysis in characterizing the bacterial communities of the food, gut and excrement of saprophagous larvae of Penthetria holosericea (Diptera: Bibionidae): A pilot study. Folia Microbiol. 2004, 49, 83–93. [Google Scholar] [CrossRef]
- R_CORE_TEAM. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Wickham, H. Ggplot2: Elegant graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: https://rpkgs.datanovia.com/factoextra/ (accessed on 15 July 2020).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Mendiburu, d.F. Agricolae: Statistical Procedures for Agricultural Research. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 15 July 2020).
- Beaujean, A.A. R Package for Baylor University Educational Psychology Quantitative Courses. Available online: https://rdrr.io/cran/BaylorEdPsych/ (accessed on 15 July 2020).
- Ukaoma, A.A.; Ukaoma, V.O.; Opara, F.N.; Osuala, F.O.U. Inhibition of Dehydrogenase Activity in Pathogenic Bacteria Isolates by Aqueous Extract of Curcuma Longa (Turmeric) Rhizome. J. Phytopharm. 2013, 2, 9–17. [Google Scholar]
- Walls-Thumma, D. Dehydrogenase Activity in Soil Bacteria. Available online: https://www.intechopen.com/books/dehydrogenases/dehydrogenase-activity-in-the-soil-environment (accessed on 15 July 2020).
- Haderlein, S.B.; Weissmahr, K.W.; Schwarzenbach, R.P. Specific Adsorption of Nitroaromatic Explosives and Pesticides to Clay Minerals. Environ. Sci. Technol. 1996, 30, 612–622. [Google Scholar] [CrossRef]
- Chaerun, S.K.; Tazaki, K. How kaolinite plays an essential role in remediating oil-polluted seawater. In ENERGYO; De Gruyter: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Makádi, M.; Tomócsik, A.; Orosz, V.; Bogdányi, Z.; Biró, B. Effect of a biogas-digestate and bentonite on some enzyme activities of the amended soils. Cereal Res. Commun. 2007, 35, 741–744. [Google Scholar] [CrossRef]
- Sarkar, B.; Megharaj, M.; Shanmuganathan, D.; Naidu, R. Toxicity of organoclays to microbial processes and earthworm survival in soils. J. Hazard. Mater. 2013, 261, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Lin, X.G.; Fujii, T.; Morimoto, S.; Yagi, K.; Hu, J.L.; Zhang, J.B. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol. Biochem. 2007, 39, 2971–2976. [Google Scholar] [CrossRef]
- Tejada, M.; Hernandez, M.T.; Garcia, C. Application of two organic amendments on soil restoration: Effects on the soil biological properties. J. Environ. Qual. 2006, 35, 1010–1017. [Google Scholar] [CrossRef]
- Lu, Q.; Feng, X.; Sun, K.; Liao, Z. Study on the use of polymer/bentonite composites for controlled release. Plant Nutr. Fertil. Sci. 2005, 2, 183. [Google Scholar]
- Wangwang, L.; Chengcheng, H.; Huixing, X.; Yafan, B. Study on the Release Property of Nitrogen in Sustained-release Fertilizer with Carrier of Bentonite. In Proceedings of the 2013 Third International Conference on Intelligent System Design and Engineering Applications, Hong Kong, China, 16–18 January 2013. [Google Scholar]
- Li, H.F.; Chen, M.Q.; Fu, B.A.; Liang, B. Evaluation on the thermal and moisture diffusion behavior of sand/bentonite. Appl. Therm. Eng. 2019, 151, 55–65. [Google Scholar] [CrossRef]
- Kizilkaya, R.; Akça, İ.; Aşkın, T.; Olekhov, V.; Samofalova, I.; Mudrykh, N. Effect of Soil contamination with azadirachtin on dehydrogenase and catalase activity of soil. Eurasian J. Soil Sci. 2012, 1, 93–108. [Google Scholar]
- Bach, E.M.; Baer, S.G.; Meyer, C.K.; Six, J. Soil texture affects soil microbial and structural recovery during grassland restoration. Soil Biol. Biochem. 2010, 42, 2182–2191. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Pronk, G.J.; Heister, K.; Koegel-Knabner, I.; Martens, R.; Tebbe, C.C. Artificial soil studies reveal domain-specific preferences of microorganisms for the colonisation of different soil minerals and particle size fractions. FEMS Microbiol. Ecol. 2014, 90, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Lutzow, M.v.; Kogel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Müller, T.; Höper, H. Soil organic matter turnover as a function of the soil clay content: Consequences for model applications. Soil Biol. Biochem. 2004, 36, 877–888. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Zhang, X.; Liang, W. Contributions of soil biota to C sequestration varied with aggregate fractions under different tillage systems. Soil Biol. Biochem. 2013, 62, 147–156. [Google Scholar] [CrossRef]
- Oades, J.M. The retention of organic matter in soils. Biogeochemistry 1988, 5, 35–70. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Haney, R.L.; Hons, F.M.; Zuberer, D.A. Active fractions of organic matter in soils with different texture. Soil Biol. Biochem. 1996, 28, 1367–1372. [Google Scholar] [CrossRef]
- Dilustro, J.J.; Collins, B.; Duncan, L.; Crawford, C. Moisture and soil texture effects on soil CO2 efflux components in southeastern mixed pine forests. For. Ecol. Manag. 2005, 204, 87–97. [Google Scholar] [CrossRef]
- Baath, E.; Anderson, T.H. Comparison of soil fungal/bacterial ratios in a pH gradient using physiological and PLFA-based techniques. Soil Biol. Biochem. 2003, 35, 955–963. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Harris, J.A. Measurements of the soil microbial community for estimating the success of restoration. Eur. J. Soil Sci. 2003, 54, 801–808. [Google Scholar] [CrossRef]
- Alekseev, A.; Alekseeva, T.; Kalinin, P.; Hajnos, M. Soils response to the land use and soil climatic gradients at ecosystem scale: Mineralogical and geochemical data. Soil Tillage Res. 2018, 180, 38–47. [Google Scholar] [CrossRef]
- Vogel, C.; Heister, K.; Buegger, F.; Tanuwidjaja, I.; Haug, S.; Schloter, M.; Kogel-Knabner, I. Clay mineral composition modifies decomposition and sequestration of organic carbon and nitrogen in fine soil fractions. Biol. Fertil. Soils 2015, 51, 427–442. [Google Scholar] [CrossRef]
- Gonod, L.V.; Jones, D.L.; Chenu, C. Sorption regulates the fate of the amino acids lysine and leucine in soil aggregates. Eur. J. Soil Sci. 2006, 57, 320–329. [Google Scholar] [CrossRef]
- Morrissey, E.M.; McHugh, T.A.; Preteska, L.; Hayer, M.; Dijkstra, P.; Hungate, B.A.; Schwartz, E. Dynamics of extracellular DNA decomposition and bacterial community composition in soil. Soil Biol. Biochem. 2015, 86, 42–49. [Google Scholar] [CrossRef]
- Spence, A.; Robinson, C.; Hanson, R.E. The effects of microstructural changes on montmorillonite–microbial interactions. J. Mol. Struct. 2014, 1056–1057, 157–165. [Google Scholar] [CrossRef]
- Yang, H.; Tong, M.; Kim, H. Influence of Bentonite Particles on Representative Gram Negative and Gram Positive Bacterial Deposition in Porous Media. Environ. Sci. Technol. 2012, 46, 11627–11634. [Google Scholar] [CrossRef]
- Černá, K.; Ševců, A.; Steinová, J.; Burkartová, K. Microbial Diversity in Aged Bentonites; Technical University of Liberec: Liberec, Czechia, 2018. [Google Scholar]
- Stroes-Gascoyne, S.; Haveman, S.A.; Hamon, C.J.; Delaney, T.-L.; Pedersen, K.; Arlinger, J.; Ekendahl, S.; Hallbeck, L.; Jahromi, N.; Dekeyser, K.; et al. Occurrence and identification of microorganisms in compacted clay-based buffer material designed for use in a nuclear fuel waste disposal vault. Can. J. Microbiol. 1997, 43, 1133–1146. [Google Scholar] [CrossRef]
- Stroes-Gascoyne, S.; West, J.M. An overview of microbial research related to high-level nuclear waste disposal with emphasis on the Canadian concept for the disposal of nuclear fuel waste. Can. J. Microbiol. 1996, 42, 349–366. [Google Scholar] [CrossRef]
- Haynes, R.J.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosyst. 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Engel, K.; Ford, S.E.; Coyotzi, S.; McKelvie, J.; Diomidis, N.; Slater, G.; Neufeld, J.D. Stability of Microbial Community Profiles Associated with Compacted Bentonite from the Grimsel Underground Research Laboratory. MSphere 2019, 4, e00601–e00619. [Google Scholar] [CrossRef] [PubMed]
- Marschner, P.; Kandeler, E.; Marschner, B. Structure and function of the soil microbial community in a long-term fertilizer experiment. Soil Biol. Biochem. 2003, 35, 453–461. [Google Scholar] [CrossRef]
- Poll, C.; Thiede, A.; Wermbter, N.; Sessitsch, A.; Kandeler, E. Micro-scale distribution of microorganisms and microbial enzyme activities in a soil with long-term organic amendment. Eur. J. Soil Sci. 2003, 54, 715–724. [Google Scholar] [CrossRef]
- Moosavi, M. Bentonite Clay as a Natural Remedy: A Brief Review. Iran. J. Public Health 2017, 46, 1176–1183. [Google Scholar] [PubMed]
- Rättö, M.; Itävaara, M.; Nol, E. Microbial Activity in Bentonite Buffers Literature Study; Technical University of Liberec: Liberec, Czechia, 2012. [Google Scholar]
- Schutter, M.; Dick, R. Microbial Community Profiles and Activities among Aggregates of Winter Fallow and Cover-Cropped Soil. Soil Sci. Soc. Am. J. SSSAJ 2002, 66, 142–153. [Google Scholar] [CrossRef]
- Tisdall, J.; Oades, J. Organic Matter and Water-stable Aggregates in Soils. J. Soil Sci. 2006, 33, 141–163. [Google Scholar] [CrossRef]
- Birkhofer, K.; Bezemer, T.M.; Bloem, J.; Bonkowski, M.; Christensen, S.; Dubois, D.; Ekelund, F.; Fliessbach, A.; Gunst, L.; Hedlund, K.; et al. Long-term organic farming fosters below and aboveground biota: Implications for soil quality, biological control and productivity. Soil Biol. Biochem. 2008, 40, 2297–2308. [Google Scholar] [CrossRef]
- Miller, J.D. Fungi as contaminants in indoor air. Atmos. Environ. Part A. Gen. Top. 1992, 26, 2163–2172. [Google Scholar] [CrossRef]
- Whalen, J.; Chang, C. Macroaggregate Characteristics in Cultivated Soils after 25 Annual Manure Applications. Soil Sci. Soc. Am. J. 2002, 66, 1637–1647. [Google Scholar] [CrossRef]
- Sawhney, B.L. Selective Sorption and Fixation of Cations by Clay Minerals: A Review. Clays Clay Miner. 1972, 20, 93–100. [Google Scholar] [CrossRef]
- Plaza, C.; Giannetta, B.; Fernández, J.M.; López-de-Sá, E.G.; Polo, A.; Gascó, G.; Méndez, A.; Zaccone, C. Response of different soil organic matter pools to biochar and organic fertilizers. Agric. Ecosyst. Environ. 2016, 225, 150–159. [Google Scholar] [CrossRef]
- Hallin, S.; Jones, C.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, M.I. Soil Clay Mineralogies in Relation to Fertility Management: Effect of Clay Mineral Types on Ammonium Fixation under Conditions of Wetland Rice Culture1. Agron. J. 1907, 74, 143–144. [Google Scholar] [CrossRef]
- Meunier, A.; Velde, B.D. Illite-Origins, Evolution and Metamorphism, 1st ed.; Springer: Berlin, Germany, 2004. [Google Scholar] [CrossRef]
- Barthod, J. Innovative Waste Treatment by Composting with Minerals and Worms: Effects on Carbon Storage, Soil Properties and Plant Growth. Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 2017. [Google Scholar]
- Skála, J.; Vácha, R.; Hofman, J.; Horváthová, V.; Sáňka, M.; Čechmánková, J. Spatial differentiation of ecosystem risks of soil pollution in floodplain areas of the Czech Republic. Soil Water Res. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Gutarowska, B.; Matusiak, K.; Borowski, S.; Rajkowska, A.; Brycki, B. Removal of odorous compounds from poultry manure by microorganisms on perlite-bentonite carrier. J. Environ. Manag. 2014, 141, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Biswas, B.; Sarkar, B.; Rusmin, R.; Naidu, R. Bioremediation of PAHs and VOCs: Advances in clay mineral-microbial interaction. Environ. Int. 2015, 85, 168–181. [Google Scholar] [CrossRef]
- Briones, R.M.; Sarmah, A.K. Insight into the sorption mechanism of metformin and its transformation product guanylurea in pastoral soils and model sorbents. Sci. Total Environ. 2018, 645, 1323–1333. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).