Contaminated Land by Wildfire Effect on Ultramafic Soil and Associated Human Health and Ecological Risk
Abstract
:1. Introduction
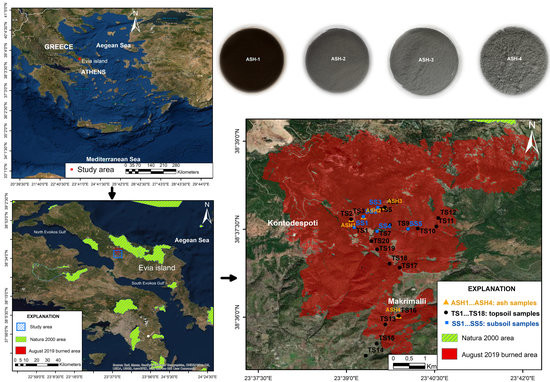
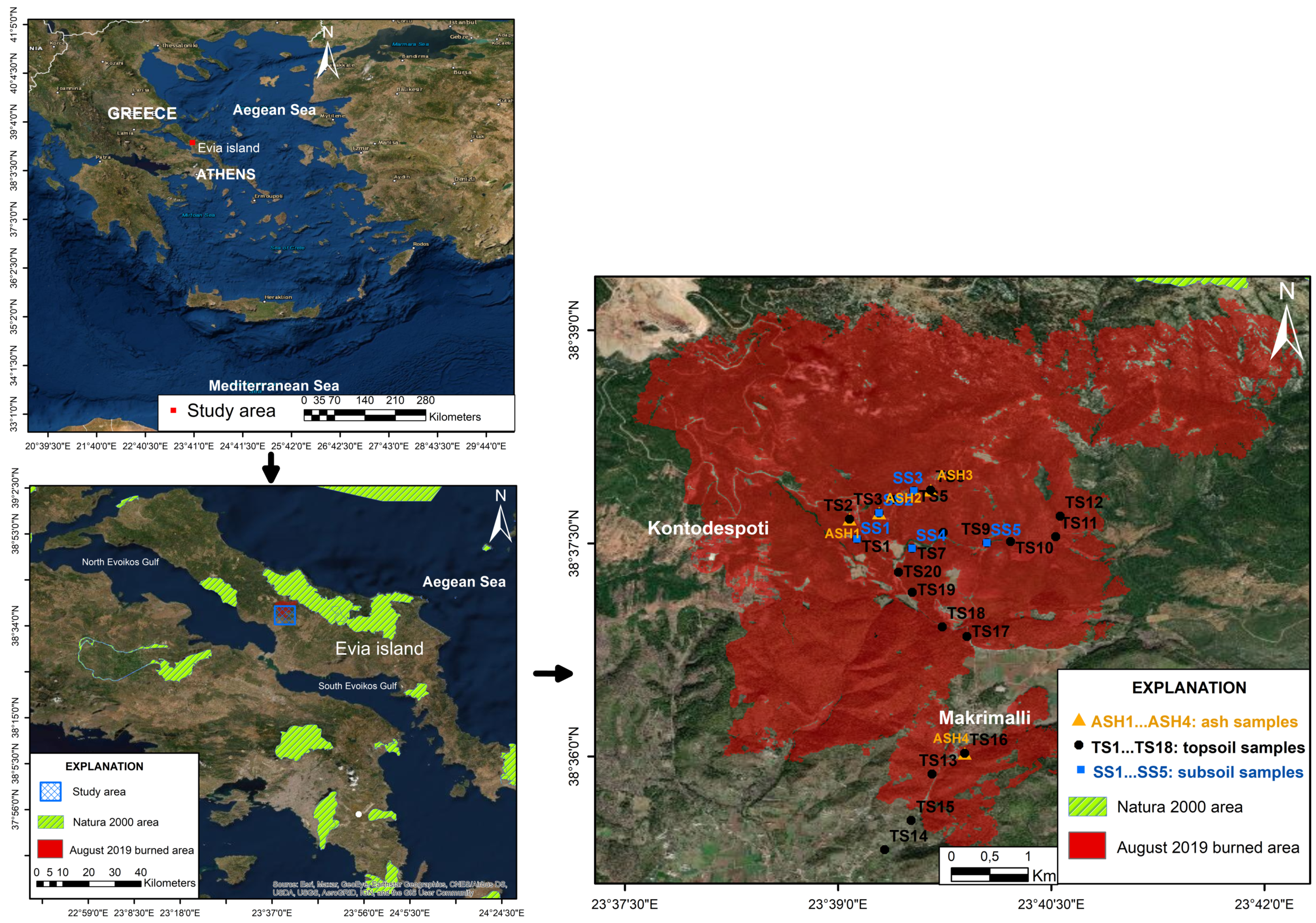
2. Materials and Methods
3. Results and Discussion
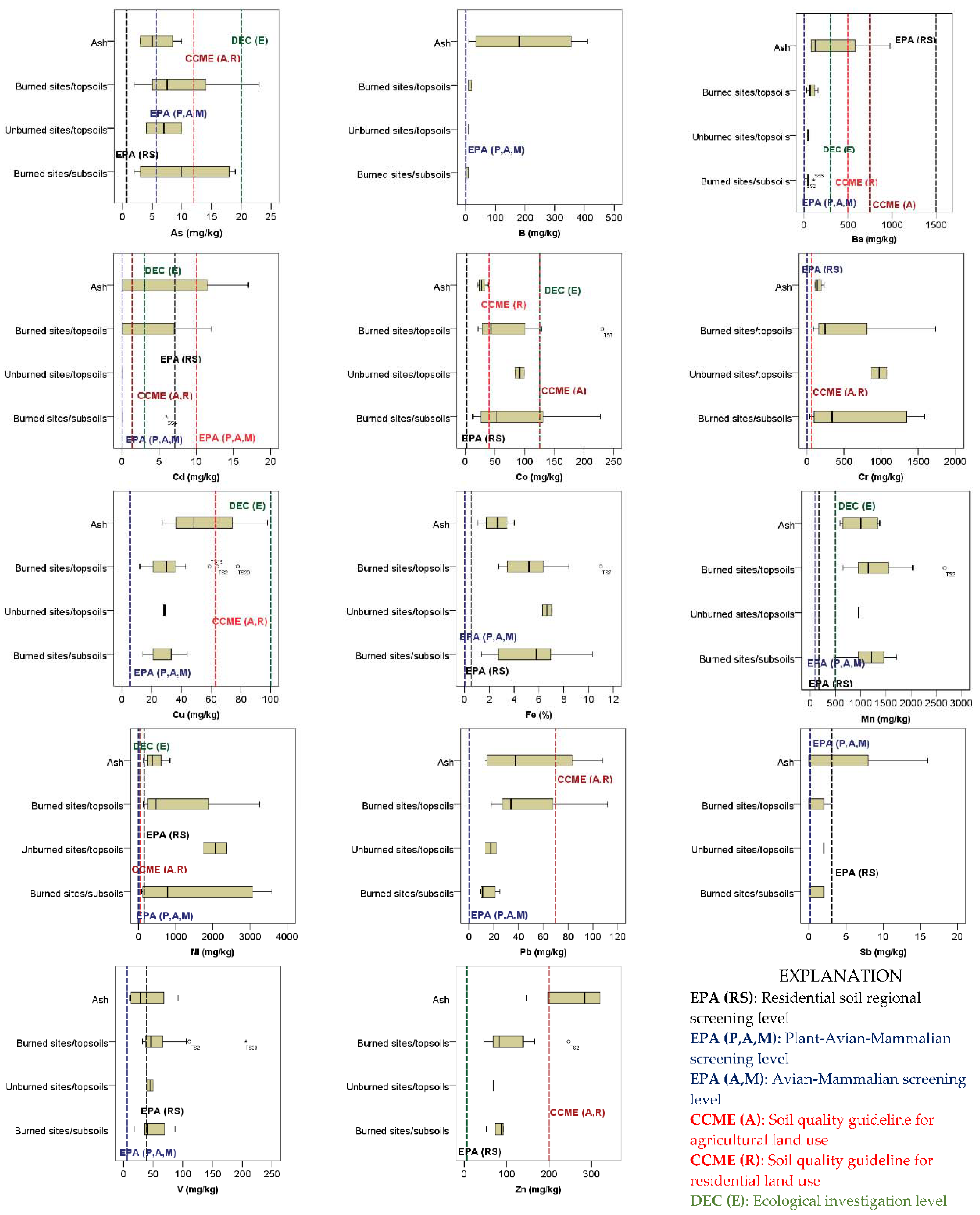
3.1. Element Contents of Ash and Soil
3.2. Estimation of the Combustion Temperature and Fire Severity
3.3. Quantification of Net Elemental Changes to the Ash
3.4. Human Health and Ecological Risk Assessment
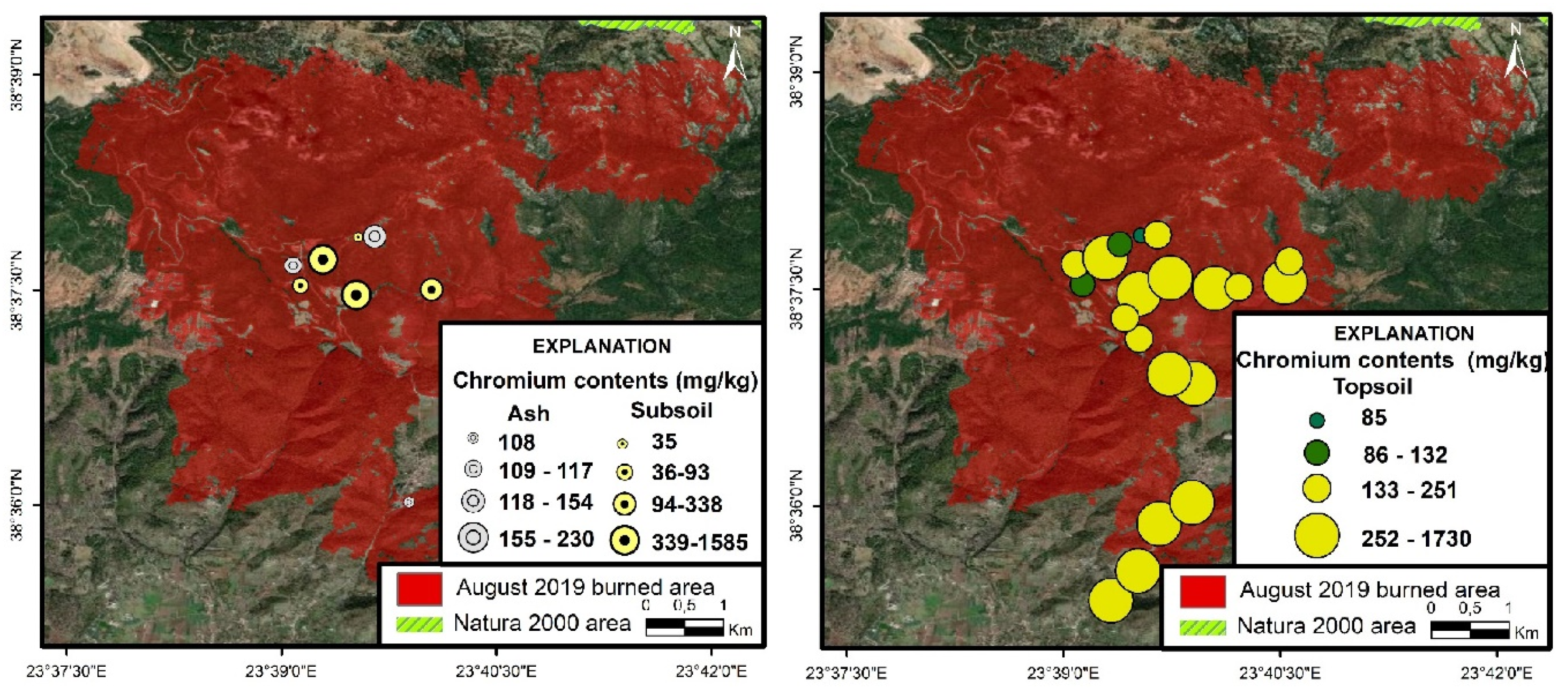
3.4.1. Chromium
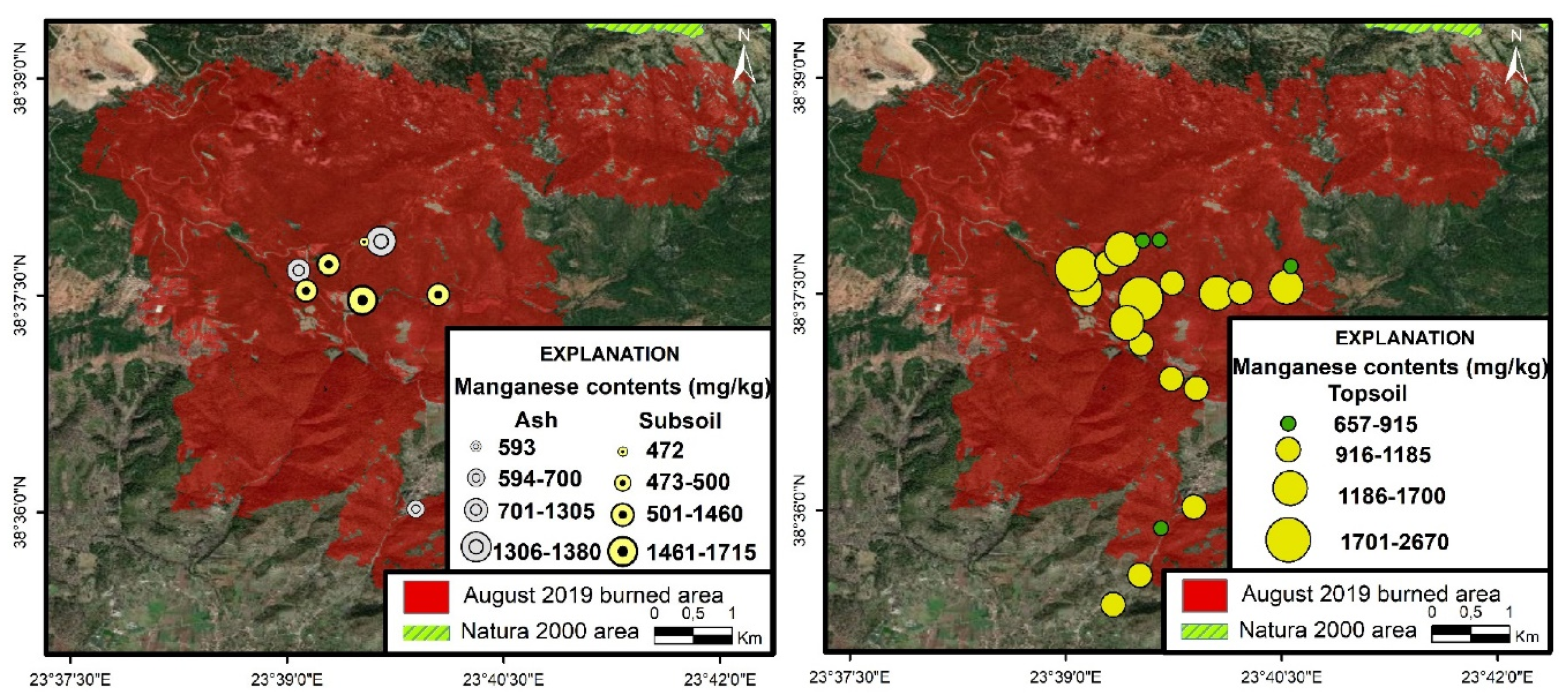
3.4.2. Manganese
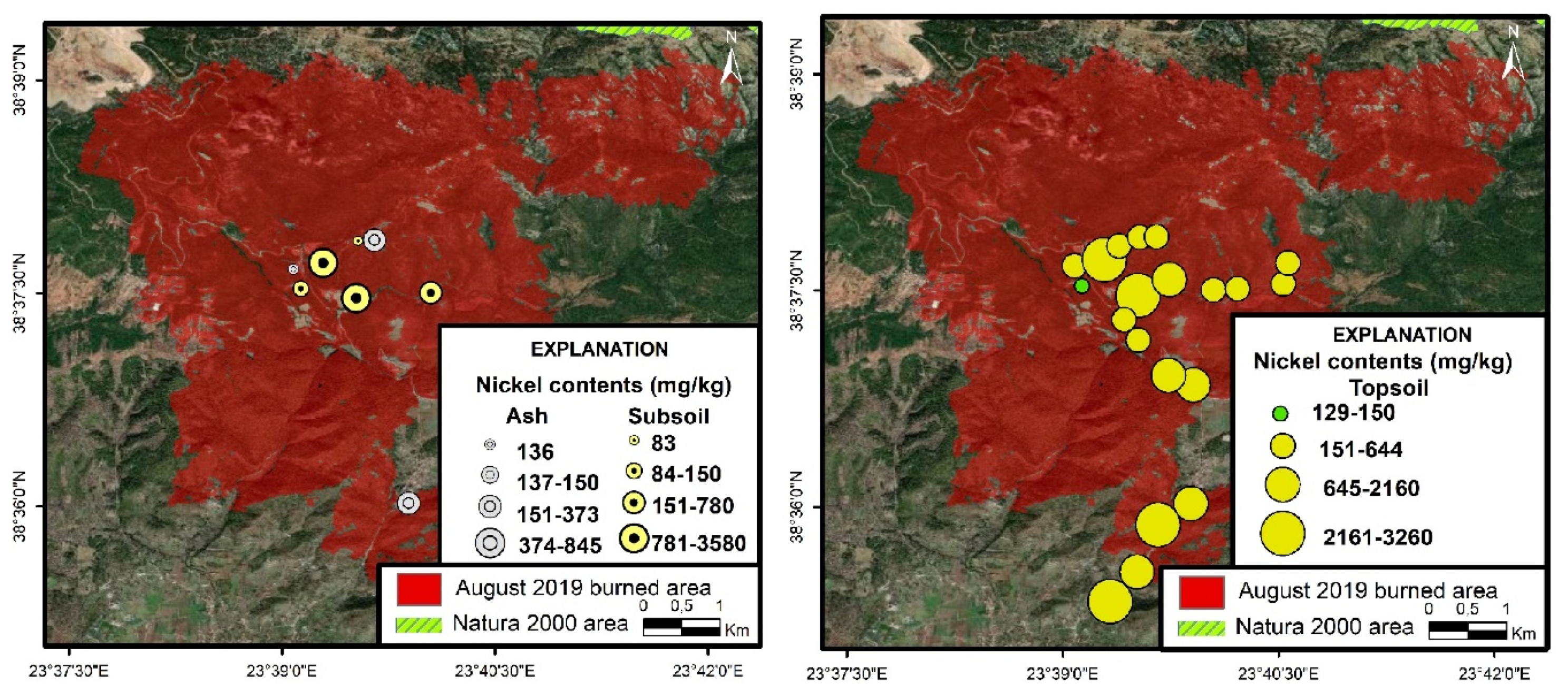
3.4.3. Nickel
3.4.4. Zinc
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- IPCC (Intergovernmental Panel on Climate Change). Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Harden, J.W.; Neff, J.C.; Sandberg, D.V.; Turetsky, M.R.; Ottmar, R.; Gleixner, G.; Fries, T.L.; Manies, K.L. Chemistry of burning the forest floor during the frostfire experimental burn, interior Alaska, 1999. Glob. Biochem. Cyc. 2004, 18, GB3014. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, W.H.; Dobson, M.; Kane, D.M.; Johnson, N.D. Toxic trace elements associated with airborne particulate matter: A review. JAPCA 1987, 37, 1267–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexakis, D.; Gamvroula, D.; Theofili, E. Environmental availability of potentially toxic elements in an agricultural Mediterranean site. Environ. Eng. Geosci. 2019, 25, 169–178. [Google Scholar] [CrossRef]
- Da Silva, E.B.; Gao, P.; Xu, M.; Guan, D.; Tang, X. Background concentrations of trace metals As, Ba, Cd, Co, Cu, Ni, Pb, Se, and Zn in 214 Florida urban soils: Different cities and land uses. Environ. Pollut. 2020, 264, 114737. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Surya, B.; Saleh, H.; Suriani, S.; Sakti, H.H.; Hadijah, H.; Idris, M. Environmental pollution control and sustainability management of slum settlements in Makassar City, South Sulawesi, Indonesia. Land 2020, 9, 279. [Google Scholar] [CrossRef]
- Vandecasteele, B.; Quataert, P.; Piesschaert, F.; Lettens, S.; De Vos, B.; Du Laing, G. Translocation of Cd and Mn from bark to leaves in willows on contaminated sediments: Delayed budburst is related to high Mn concentrations. Land 2015, 4, 255–280. [Google Scholar] [CrossRef]
- Odigie, K.O.; Khanis, E.; Hibdon, S.A.; Jana, P.; Araneda, A.; Urrutia, R.; Flegal, A.R. Remobilization of trace elements by forest fire in Patagonia, Chile. Reg. Environ. Change 2016, 16, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Alexakis, D. Suburban areas in flames: Dispersion of potentially toxic elements from burned vegetation and buildings. Estimation of the associated ecological and human health risk. Environ. Res. 2020, 183, 109153. [Google Scholar] [CrossRef]
- Alexakis, D.; Kokmotos, I.; Gamvroula, D.; Varelidis, G. Wildfire effects on soil quality: Application on a suburban area of West Attica (Greece). Geosci. J. 2020. [Google Scholar] [CrossRef]
- Bodí, M.; Martin, D.A.; Santín, C.; Balfour, V.; Doerr, S.H.; Pereira, P.; Cerdà, A.; Mataix- Solera, J. Wildland fire ash: Production, composition and eco-hydro-geomorphic effects. Earth Sci. Rev. 2014, 130, 103–127. [Google Scholar] [CrossRef]
- Campos, I.; Abrantes, N.; Keizer, J.J.; Vale, C.; Pereira, P. Major and trace elements in soil and ashes of eucalypt and pine forest plantations in Portugal following a wildfire. Sci. Total Environ. 2016, 572, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.R.; Calvão, A.R.; Aranha, J. Linking wildfire effects on soil and water chemistry of the Marão River watershed, Portugal, and biomass changes detected from Landsat imagery. Appl. Geochem. 2014, 44, 93–102. [Google Scholar] [CrossRef]
- Panichev, N.; Mabasa, W.; Ngobeni, P.; Mandiwana, K.; Panicheva, S. The oxidation of Cr(III) to Cr(VI) in the environment by atmospheric oxygen during the bush fires. J. Hazard. Mat. 2008, 153, 937–941. [Google Scholar] [CrossRef]
- Parra, J.G.; Rivero, V.C.; Lopez, T.I. Forms of Mn in soils affected by a forest fire. Sci. Total Environ. 1996, 181, 231–236. [Google Scholar] [CrossRef]
- Bockheim, J.G. (Ed.) Ultramafic soils. In Soil Geography of the USA, 1st ed.; Springer: Cham, Switzerland, 2014; Volume 1, pp. 267–281. [Google Scholar] [CrossRef]
- Abraham, J.; Dowling, K. The unquantified risk of post-fire metal concentration in soil: A Review. Water Air Soil Pollut. 2017, 228, 175. [Google Scholar] [CrossRef]
- Shcherbov, B.L. The role of forest floor in migration of metals and artificial nuclides during forest fires in Siberia. Contemp. Probl. Ecol. 2012, 5, 191–199. [Google Scholar] [CrossRef]
- Young, D.; Jan, T.-K. Fire fallout of metals off California. Mar. Poll. Bul. 1977, 8, 109–112. [Google Scholar] [CrossRef]
- Alexakis, D. Human health risk assessment associated with Co, Cr, Mn, Ni and V contents in agricultural soils from a Mediterranean site. Arch. Agron. Soil Sci. 2016, 62, 359–373. [Google Scholar] [CrossRef]
- Alexakis, D.; Gamvroula, D. Arsenic, chromium, and other potentially toxic elements in the rocks and sediments of Oropos-Kalamos basin, Attica, Greece. Appl. Environ. Soil Sci. 2014. [Google Scholar] [CrossRef] [Green Version]
- European Environment Agency. Natura 2000 Network Viewer. European Environment Agency, Copenhagen. 2020. Available online: http://natura2000.eea.europa.eu/# (accessed on 1 June 2020).
- Copernicus EMS-Copernicus Emergency Management Service, 2019. Makrimalli-Greece Wildfire-Situation as of 21/08/2019, Activation ID: EMSR380, Product N.:01 MAKRIMALLI, v.2, English. 2019. Available online: https://emergency.copernicus.eu/mapping/ems/forest-fire-evia-island-greece-1 (accessed on 17 May 2020).
- IGME (Institute of Geology and Mineral Exploration). Geological Map of Greece. Psachna-Pilion Sheet (1:50.000 Scale); Institute of Geology and Mineral Exploration: Athens, Greece, 1981. [Google Scholar]
- Google Earth. Kontodespoti-Makrimalli area. 38°36′23.31″ Ν, 23°40′37.74″ E, Eye Alt 19.8 km, World Imagery. Imagery date 31 August 2018. Source: Esri, Maxar, GeoEye, Earthstar Geographics, CNES/Airbus DS, USDA, USGS, AeroGRID, IGN, and the GIS User Community. Available online: https://earth.google.com/web (accessed on 1 June 2020).
- Vardaki, C.; Kelepertsis, A. Environmental impact of heavy metals (Fe, Ni, Cr, Co) in soils waters and plants of Triada in Euboea from ultrabasic rocks and nickeliferous mineralization. Environ. Geochem. Health 1999, 21, 211–226. [Google Scholar] [CrossRef]
- Munsell, A.H. Soil Color Chart Handbook; U.S. Department of Agriculture: Baltimore, MD, USA, 1975; p. 58.
- White, E.M.; Thompson, W.W.; Gartner, F.R. Heat effects on nutrient release from soils under ponderosa pine. J. Range Manag. 1973, 26, 22–24. [Google Scholar] [CrossRef]
- Úbeda, X.; Pereira, P.; Outeiro, L.; Martin, D.A. Effects of fire temperature on the physical and chemical characteristics of the ash from two plots of cork oak (Quercus suber). Land Degrad. Develop. 2009, 20, 589–608. [Google Scholar] [CrossRef] [Green Version]
- White, R.H.; Zipperer, W.C. Testing and classification of individual plants for fire behavior: Plant selection for the wildland–urban interface. Int. J. Wildl. Fire 2010, 19, 213–227. [Google Scholar] [CrossRef]
- Brimhall, G.H.; Chadwick, O.A.; Lewis, C.J.; Compston, W.; Williams, I.S.; Danti, K.J.; Dietrich, W.E.; Power, M.E.; Hendricks, D.; Bratt, J. Deformational mass transport and invasive processes in soil evolution. Science 1992, 255, 695–702. [Google Scholar] [CrossRef]
- White, A.F.; Blum, A.E.; Schulz, M.S.; Bullen, T.D.; Harden, J.W.; Peterson, M.L. Chemical weathering rates of a soil chronosequence on granitic alluvium: I. Quantification of mineralogical and surface area changes and calculation of primary silicate reaction rates. Geochim. Cosmochim. Acta 1996, 60, 2533–2550. [Google Scholar] [CrossRef]
- Peijnenburg, W.J.G.M.; Zablotskaja, M.; Vijver, M.G. Monitoring metals in terrestrial environments within a bioavailability framework and a focus on soil extraction. Ecotox. Environ. Safety 2007, 67, 163–179. [Google Scholar] [CrossRef]
- EPA (Environmental Protection Agency) Cleanup Regulations and Standards. 2011. Available online: http://www.epa.gov/cleanup/regs.htm (accessed on 17 July 2020).
- CCME (Canadian Council of Ministers of the Environment). 1996. Available online: http://st-ts.ccme.ca (accessed on 17 July 2020).
- DEC (Department of Environment and Conservation). Contaminated Sites Management Series: Assessment Levels for Soil, Sediment and Water. 2010. Available online: http://www.dec.wa.gov.au/contaminatedsites (accessed on 17 July 2020).
- Odigie, K.O.; Flegal, A.R. Trace metal inventories and lead isotopic composition chronicle a forest fire’s remobilization of industrial contaminants deposited in the Angeles National Forest. PLoS ONE 2014, 9, e107835. [Google Scholar] [CrossRef]
- Bytnerowicz, A.; Arbaugh, M.; Riebau, A.; Andersen, C. Wildland fires and air pollution. In Developments in Environmental Science 8, 1st ed.; Krupa, S.V., Ed.; Elsevier: Budapest, Hungary, 2008; Volume 8, p. 688. Available online: https://www.fs.fed.us/psw/publications/4451/psw_2009_4451-001.pdf (accessed on 27 October 2020).
- Pereira, P.; Jordán, A.; Cerdà, A.; Martin, D. Editorial: The role of ash in fire-affected ecosystems. Catena 2014, 135, 337–339. [Google Scholar] [CrossRef]
- Goforth, B.R.; Graham, R.C.; Hubebrt, K.R.; Zanner, C.W.; Minnich, R.A. Spatial distribution and properties of ash and thermally altered soils after high severity forest fire southern California. Int. J. Wildl. Fire 2005, 14, 343–354. [Google Scholar] [CrossRef]
- Stone, E.L. Boron deficiency and excess in forest trees: A review. For. Ecol. Manag. 1990, 37, 49–75. [Google Scholar] [CrossRef]
- Bogunovic, I.; Kisic, I.; Jurisic, A. Influence of wildfire and fire suppression by seawater on soil properties. Appl. Ecol. Environ. Res. 2015, 13, 1157–1169. [Google Scholar] [CrossRef]
- Hem, J. Study and Interpretation of the Chemical Characteristics of Natural Water; Reprinted from the 1970 Edition; University Press of the Pacific: Honolulu, HI, USA, 2005. [Google Scholar]
- Nzihou, A.; Stanmore, B. The fate of heavy metals during combustion and gasification of contaminated biomass-A brief review. J. Hazard Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.J.; Chiou, H.Y.; Chiang, M.H.; Lin, L.J.; Tai, T.Y. Dose-response relationship between ischemic heart disease and long term arsenic exposure. Arterioscl. Thromb. Vasc. Biol. 1996, 16, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Centeno, J.A.; Tseng, C.H.; Van der Voet, G.B.; Finkelman, R.B. Global impacts of geogenic arsenic: A medical geology research case. Ambio A J. Hum. Environ. 2007, 36, 78–81. [Google Scholar] [CrossRef]
- Mazumdar, G. Chronic arsenic toxicity and human health. Ind. J. Med. Res. 2008, 128, 436–447. [Google Scholar]
- Scholz, V.; Ellerbrock, R. The growth productivity and environmental impact of cultivation of energy crop on sandy soil in Germany. Biom. Bioenergy 2002, 23, 81–92. [Google Scholar] [CrossRef]
- ATSDR (Agency for Toxic Substance and Disease Registry). 2020. Available online: https://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=3 (accessed on 7 August 2020).
- Bergqvist, C.; Herbert, R.; Persson, I.; Greger, M. Plants influence on arsenic availability and speciation in the rhizosphere, roots and shoots of three different vegetables. Environ. Pollut. 2014, 184, 540–546. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Otto, M. Determination of nickel and cobalt in reference plant materials by carbon slurry sampling GFAAS technique after their simultaneous preconcentration onto modified activated carbon. J. Food Compos. Anal. 2012, 26, 58–65. [Google Scholar] [CrossRef]
- Basu, N.; Abare, M.; Buchanan, S.; Cryderman, D.; Nam, D.H.; Sirkin, S.; Schmitt, S.; Hu, H. A combined ecological and epidemiologic investigation of metal exposures amongst indigenous peoples near the Marlin Mine in Western Guatemala. Sci. Total Environ. 2010, 409, 70–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Rizo, O.; Hernández-Merlo, M.; Echeverría-Castillo, F.; Arado-López, J.O. Assessment of metal pollution in soils from a former Havana (Cuba) solid waste open dump. Bull. Environ. Contam. Toxicol. 2012, 88, 182–186. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Effect of cobalt on environment and living organisms—A review. Appl. Ecol. Environ. Res. 2019, 17, 11419–11449. [Google Scholar] [CrossRef]
- Cork, S.C. Iron storage diseases in birds. Avian Pathol. 2000, 29, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Bulte, J.W.M.; Miller, G.F.; Vymazal, J.; Brooks, R.A.; Frank, J.A. Hepatic hemosiderosis in non-human primates: Quantification of liver using different field strengths. Magn. Reason. Med 1997, 37, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Hamid, N.; Bukhari, N.; Jawaid, F. Physiological responses of Phaseolus vulgaris to different lead concentrations. Pak. J. Bot. 2010, 42, 239–246. [Google Scholar]
- Jin, H.; Xu, C.X.; Lim, H.T.; Park, S.J.; Shin, J.Y.; Chung, Y.S.; Park, S.C.; Chang, S.H.; Youn, H.J.; Lee, K.H.; et al. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, M.; Razzaque, M.S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. Fed. Am. Soc. Exp. Biol. 2010, 24, 3562–3571. [Google Scholar] [CrossRef] [Green Version]
- Razzaque, M.S. Phosphate toxicity: New insights into an old problem. Clin. Sci. 2011, 120, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R.R.; Lisehora, G.R.; Braxton, M., Jr.; Barcia, P.J. Fatal poisoning from sodium phosphate enema. Case report and experimental study. J. Am. Med. Assoc. 1987, 257, 2190–2192. [Google Scholar] [CrossRef]
- Rim, K.-T.; Koo, K.-H.; Park, J.-S. Toxicological evaluations of rare earths and their health impacts to workers: A literature review. Saf. Health Work 2013, 4, 12–26. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, L.G.; Bedinger, G.M.; Piatak, N.M.; Titanium, T.; Schulz, K.J.; DeYoung, J.H., Jr.; Seal, R.R.; Bradley, D.C. (Eds.) U.S. Geological Survey Professional Paper 1802. In Critical Mineral Resources of the United States-Economic and Environmental Geology and Prospects for Future Supply; U.S. Geological Survey: Reston, VA, USA, 2017; pp. T1–T23. [Google Scholar] [CrossRef]
- Pratt, P.F. Vanadium. In Diagnostic Criteria for Plants and Soils; Chapman, H.D., Ed.; University of California, Division of Agricultural Science: Riverside, CA, USA, 1966; pp. 480–483. [Google Scholar]
- Schmidt, J.A.; Andren, A.W. The atmospheric chemistry of nickel. In Nickel in the Environment; Nriagu, J.O., Ed.; Wiley-Interscience: New York, NY, USA, 1980; pp. 93–136. [Google Scholar]
- Outridge, P.M.; Scheuhammer, A.M. Bioaccumulation and toxicology of nickel: Implications for wild mammals and birds. Environ. Rev. 1993, 1, 172–197. [Google Scholar] [CrossRef]
- Outridge, P.M.; Scheuhammer, A.M. Bioaccumulation and toxicology of chromium: Implications for wildlife. Reviews of Environmental Contamination and Toxicology. Rev. Environ. Contam. Toxicol. 1993, 130, 31–77. [Google Scholar] [CrossRef]
- Gad, S.C. Acute and chronic systemic chromium toxicity. Sci. Total Environ. 1989, 86, 149–157. [Google Scholar] [CrossRef]
- Custer, T.W.; Franson, J.C.; Moore, J.F.; Myers, J.E. Reproductive success and heavy metal contamination in Rhode Island common terns. Environ. Pollut. 1986, A41, 33–52. [Google Scholar] [CrossRef]
- Eisler, R. Chromium Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review; United States Fish and Wildlife Service: Washington, DC, USA, 1986.
- Taylor, F.G.; Parr, P.D. Distribution of chromium in vegetation and small mammals adjacent to cooling towers. J. Tenn. Acad. Sci. 1978, 53, 87–91. [Google Scholar]
- Reichrtova, E.; Takac, L.; Kovacikova, Z.; Foltinova, J.; Bencko, V.; Kranerova, J. Bioaccumulation of metals from nickel smelter waste in P and Fl generations of exposed animals. I. Dynamics of metals distribution in the organs and AM activity. J. Hyg. Epidemiol. Microbiol. ImmunoI. 1989, 33, 1–10. [Google Scholar]
- Costa, M.; Klein, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef]
- Martin, S.; Griswold, W. Human health effects of heavy metals. Environ. Sci. Tech. Briefs Citiz. 2009, 15, 1–6. [Google Scholar]
- Levy, B.S.; Nassetta, W.J. Neurologic effects of manganese in humans: A review. Int. J. Occup. Environ. Health 2003, 9, 153–163. [Google Scholar] [CrossRef]
- Shenker, M.; Plessner, O.E.; Tel-Or, E. Manganese nutrition effects on tomato growth, chlorophyll concentration, and superoxide dismutase activity. J. Plant Physiol. 2004, 161, 197–202. [Google Scholar] [CrossRef]
- Arya, S.K.; Roy, B.K. Manganese induced changes in growth, chlorophyll content and antioxidants activity in seedlings of broad bean (Vicia faba L.). J. Environ. Biol. 2011, 32, 707–711. [Google Scholar] [PubMed]
- Howe, P.D.; Malcolm, H.M.; Dobson, S. Manganese and Its Compounds: Environmental Aspects. Concise International Chemical Assessment Document 63. Centre for Ecology & Hydrology, Monks Wood, United Kingdom. World Health Organization, Geneva. 2004. Available online: https://www.who.int/ipcs/publications/cicad/cicad63_rev_1.pdf?ua=1 (accessed on 27 October 2020).
- Das, K.; Das, S.; Dhundasi, S. Nickel, its adverse health effects & oxidative stress. Ind. J. Med. Res. 2008, 128, 412–425. [Google Scholar]
- Athar, M.; Vohora, S. Heavy Metals and Environment; New Age International: New Delhi, India, 1995. [Google Scholar]
- Kasprazak, K.S.; Sunderman, F.W.; Salnikow, K. Nickel carcinogenesis. Mut. Res/Fund. Molec. Mechan. Mutage. 2003, 533, 67–97. [Google Scholar] [CrossRef]
- Salnikow, K.; Li, X.; Lippmann, M. Effect of nickel and iron co-exposure on human lung cells. Toxicol. Appl. Pharmacol. 2004, 196, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006, 53, 257–277. [Google Scholar] [CrossRef]
- Li, H.-B.; Wang, J.-Y.; Chen, X.-Q.; Li, Y.-P.; Fan, J.; Ren, J.-H.; Luo, X.-S.; Juhasz, A.; Ma, L. Geogenic nickel exposure from food consumption and soil ingestion: A bioavailability based assessment. Environ. Pollut. 2020, 265, 114873. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, S.; Stoynova, Z.; Velikova, V. Influence of succinate on zinc toxicity of pea plants. J. Plant Nutr. 2001, 24, 789–804. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexakis, D.E. Contaminated Land by Wildfire Effect on Ultramafic Soil and Associated Human Health and Ecological Risk. Land 2020, 9, 409. https://doi.org/10.3390/land9110409
Alexakis DE. Contaminated Land by Wildfire Effect on Ultramafic Soil and Associated Human Health and Ecological Risk. Land. 2020; 9(11):409. https://doi.org/10.3390/land9110409
Chicago/Turabian StyleAlexakis, Dimitrios E. 2020. "Contaminated Land by Wildfire Effect on Ultramafic Soil and Associated Human Health and Ecological Risk" Land 9, no. 11: 409. https://doi.org/10.3390/land9110409