Analysis of Vegetation Phytosociological Characteristics and Soil Physico-Chemical Conditions in Harishin Rangelands of Eastern Ethiopia
Abstract
:1. Introduction
2. Material and Method
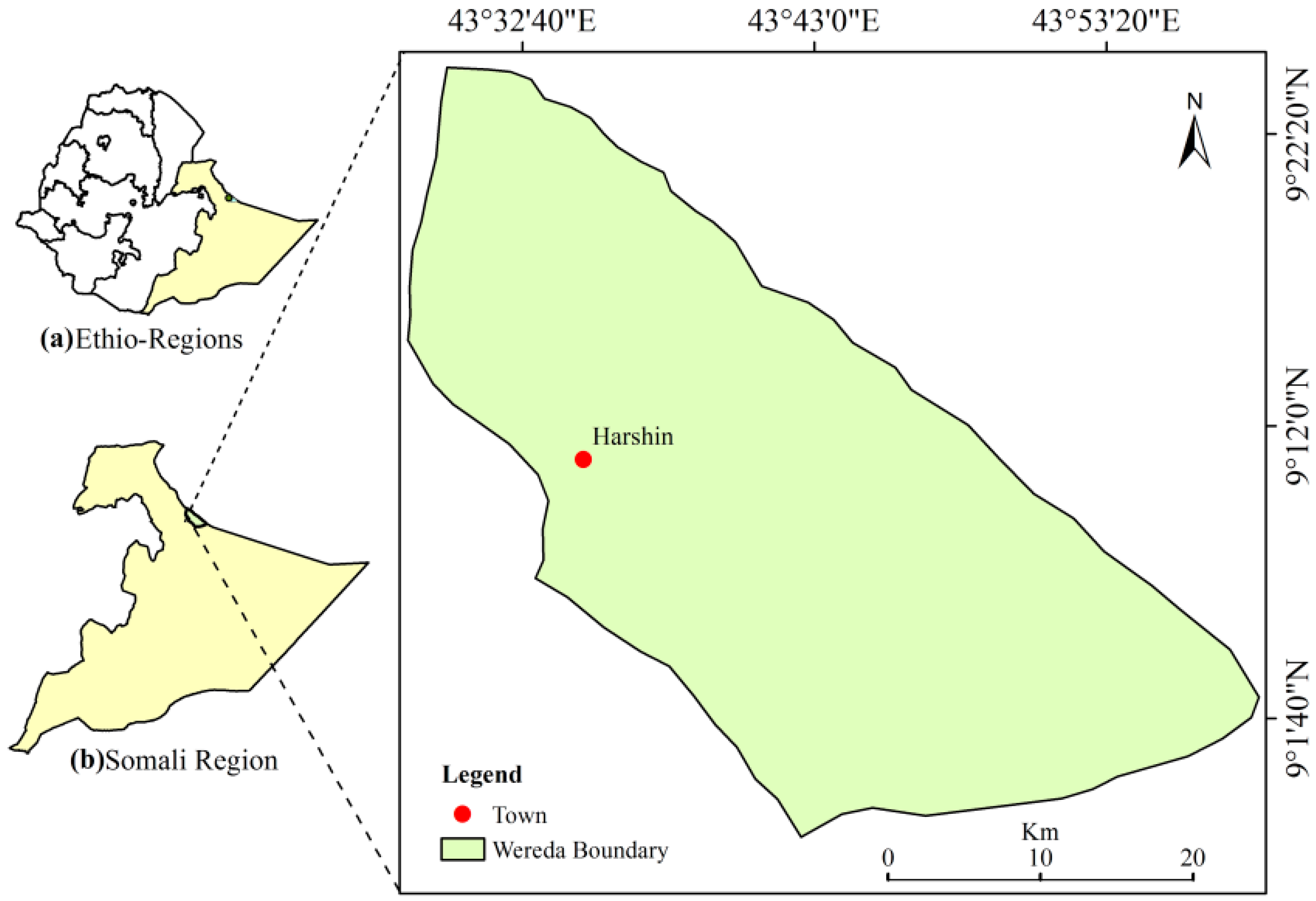
2.1. Study Area
2.2. Site Selection
2.3. Herbaceous and Woody Vegetation Sampling
2.4. Soil Sampling and Analysis
2.5. Data Analysis
3. Results
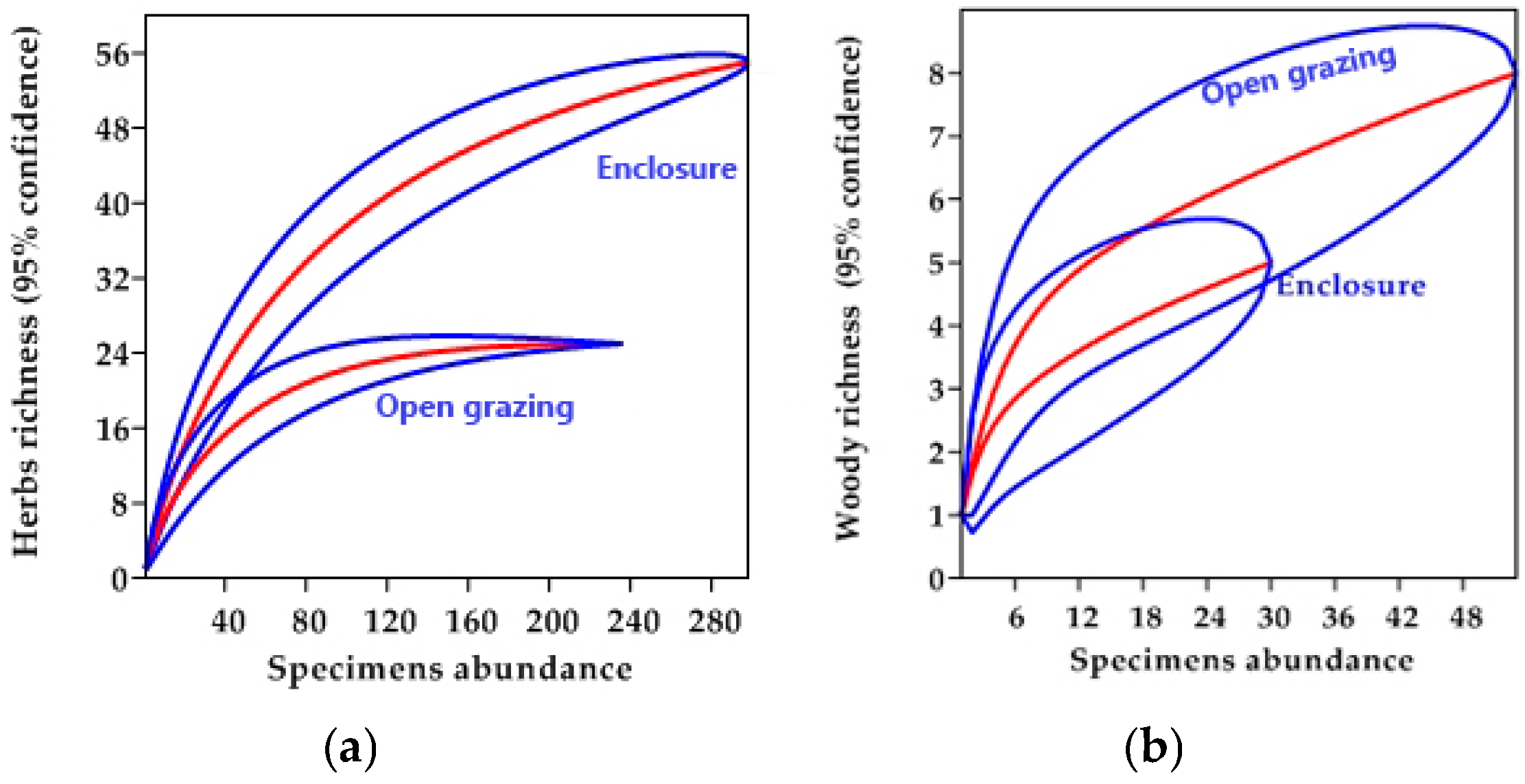
3.1. Species Similarity between Management Practices
3.2. Species Diversity and Distribution
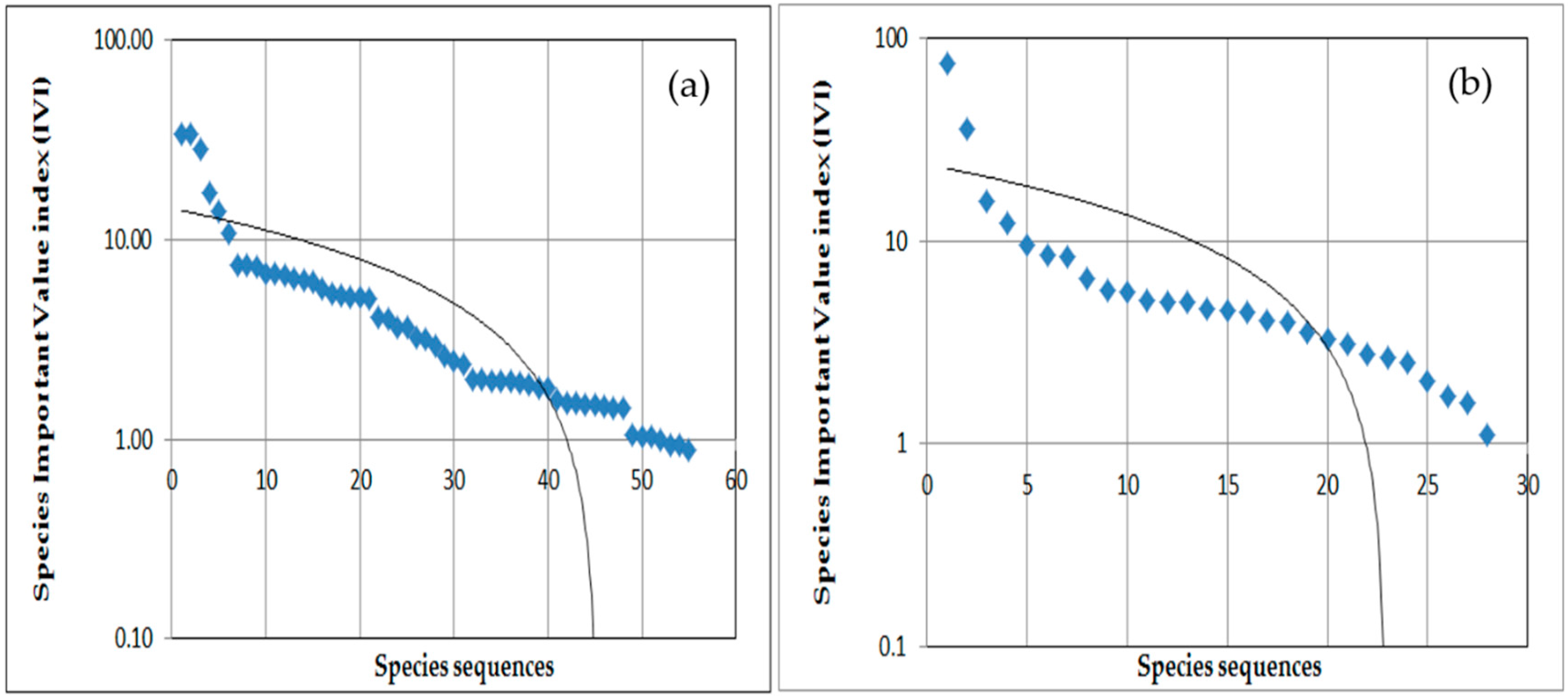
3.2.1. Diversity and Dominance (IVI) of Herb Species in Open Grazing Area
3.2.2. Diversity and Dominance (IVI) of Herb Species in Enclosure Areas
3.2.3. Diversity and Dominance (IVI) of Woody Species in the Open Grazing Areas
3.2.4. Diversity and Dominance (IVI) of Woody Species in Enclosure Areas
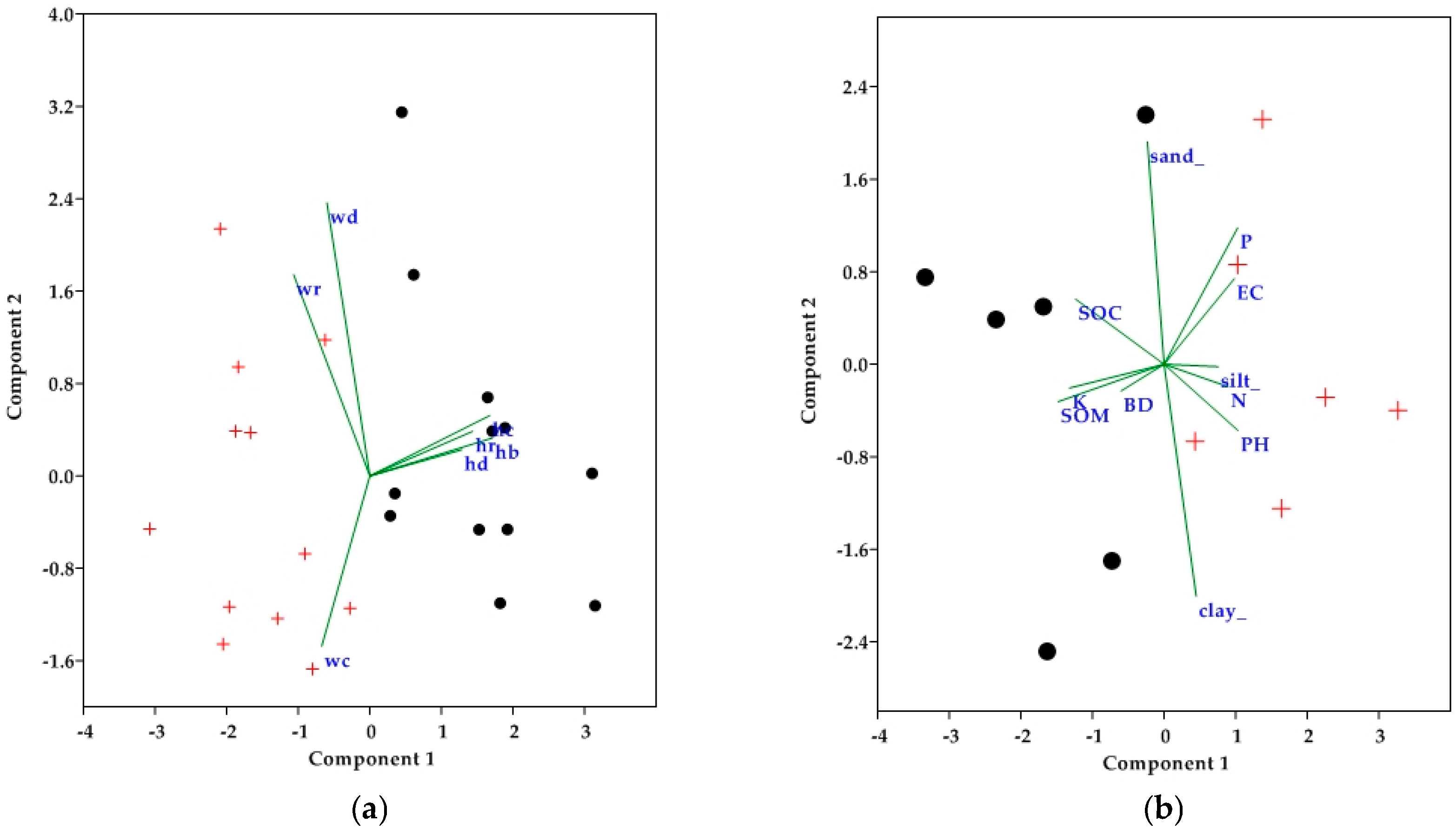
3.3. Vegetation Attributes Ordination and Analysis
3.4. Soil Physico-Chemical Attribute Ordination and Analysis
4. Discussion
4.1. Species Phytosociological Characteristics
4.2. Interaction of Management and Vegetation Attributes
4.3. Soil Physico-Chemical Attributes
5. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Derner, J.D.; Boutton, T.W.; Briske, D.D. Grazing and ecosystem carbon storage in the North American Great plains. Plant Soil J. 2006, 280, 77–90. [Google Scholar] [CrossRef]
- Tennigkeit, T.; Wilkes, A. Carbon finance in rangelands: An assessment of the potential for carbon finance in rangelands. In Proceedings of the International Council for Research in Agroforestry, World Agroforestry Center, Kunming, China, 30 September 2008; p. 29.
- Rutherford, M.C.; Mucina, L.; Lotter, M.C.; Bredenkamap, G.J.; Simit, J.H.T.; Scott-Shaw, C.R.; Hoare, D.B.; Goodman, P.S.; Bezuidwnhout, H.; Scott, L.; et al. The Vegetation of South Africa, Lestho and Swaziland. Strelitzia 19; South Africa National Biodiversity Institute: Pretoria, South Africa, 2006; pp. 439–567. [Google Scholar]
- Coppock, D.L.; Tezera, S.; Desta, S.; Gebru, G. Achieving real development impact among pastoralists: Lessons from Ethiopia. In Proceedings of the IX International Rangeland Congress (IX IRC), Diverse Rangelands for a Sustainable Society, Rosario, Argentina, 2–8 April 2011; pp. 679–684.
- Tessema, Z.K.; de Boer, W.F.; Baars, R.M.T.; Prins, H.H.T. Changes in soil nutrients, vegetation structure and herbaceous biomass in response to grazing in a semiarid savanna in Ethiopia. J. Arid Environ. 2011, 75, 662–670. [Google Scholar] [CrossRef]
- Flintan, F.; Behnke, R.; Neely, C. Natural Resource Management in the Dry Lands in the Horn of Africa: Brief Prepared by a Technical Consortium Hosted by CGIAR in Partnership with the FAO Investment Centre, Technical Consortium Brief 1; International Livestock Research Institute: Nairobi, Kenya, 2013. [Google Scholar]
- Abule, E.; Snyman, H.A.; Smit, G.N. Rangeland evaluation in the middle of Ethiopia: I. Herbaceous vegetation cover. J. Arid Environ. 2007, 70, 253–271. [Google Scholar] [CrossRef]
- Kassahun, A.; Snyman, H.A.; Smit, G.N. Impact of rangeland degradation on pastoral production systems, livelihoods and perceptions of the Somali pastoralists in eastern Ethiopia. J. Arid Environ. 2008, 72, 1265–1281. [Google Scholar] [CrossRef]
- Angassa, A.; Sheleme, B.; Oba, G.; Treydte, A.C.; Linstödter, A.; Sauerborn, J. Savanna land use and its effect on soil characteristics in southern Ethiopia. J. Arid Environ. 2012, 81, 67–76. [Google Scholar] [CrossRef]
- Haftay, H.; Yayneshet, T.; Animut, G.; Treydte, C. Rangeland vegetation responses to traditional enclosure management in eastern Ethiopia. Rangel. J. 2013, 35, 29–36. [Google Scholar] [CrossRef]
- Napier, A.; Desta, S. Review of Pastoral Rangeland Enclosures in Ethiopia; Feinstein International Center, Tufts University: Medford, MA, USA, 2011. [Google Scholar]
- Fekadu, B. Exploring incentives for rangeland enclosures among pastoral and agro-pastoral households in eastern Ethiopia. Glob. Environ. Chang. 2009, 19, 494–502. [Google Scholar] [CrossRef]
- Hogg, R. Changing land use and resources conflict among Somali pastoralists in the Haud of South-east Ethiopia. In Pastoralists, Ethnicity and the State in Ethiopia; Hogg, R., Ed.; HAAN publishing: London, UK, 1997; pp. 105–122. [Google Scholar]
- Sugule, J.; Walker, R. Changing Pastorialism in the Ethiopian Somali National Regional State; South East Rangelands Project and UNDP Emergencies unit for Ethiopia: Addis Ababa, Ethiopia, 1998. [Google Scholar]
- Aerts, R.; Nyssen, J.; Haile, M. On the difference between “exclosures” and “enclosures” in ecology and the environment. J. Arid Environ. 2009, 73, 762–763. [Google Scholar] [CrossRef]
- Gairola, S.; Sharma, C.M.; Suyal, S.; Ghildiyal, S.K. Composition and diversity of five major forest types in moist temperate climate of the western Himalayas. For. Stud. China 2011, 13, 139–153. [Google Scholar] [CrossRef]
- Khoshoo, T.N. Plant Diversity in the Himalaya: Conservation and Utilization; Govind Ballabh Pant Institute of Himalayan Environment and Development: Almora, India, 1992. [Google Scholar]
- Odum, E.P. Fundamentals of Ecology, 3rd ed.; Saunders: Philadelphia, PA, USA, 1971. [Google Scholar]
- Warger, M.J.A.; Morrel, V.E. Plant species and plant communities: Some conclusion. In Proceedings of the International Symposium, Nijmegen, The Netherlands, 11–12 November 1976; pp. 167–175.
- Mandal, J.; Joshi, S.P. Analysis of vegetation dynamics and phytodiversity from three dry deciduous forests of Doon Valley, Western Himalaya, India. J. Asia Pac. Biodivers. 2014, 7, 292–304. [Google Scholar] [CrossRef]
- Brand, M.D.; Goetz, H. Vegetation of exclosures in Southwestern North Dakota. J. Range Manag. 1986, 39, 434–437. [Google Scholar] [CrossRef]
- Descheemaeker, K.; Raes, D.; Nyssen, J.; Poesen, J.; Haile, M.; Deckers, J. Changes in water flows and water productivity upon vegetation regeneration on degraded hill slopes in northern Ethiopia: A water balance modeling exercise. Rangel. J. 2009, 31, 237–249. [Google Scholar] [CrossRef]
- Yayneshet, T.; Eik, L.O.; Moe, S.R. The effects of exclosures in restoring degraded semi-arid vegetation in communal grazing lands in northern Ethiopia. J. Arid Environ. 2009, 73, 542–549. [Google Scholar] [CrossRef]
- Bakker, J.D.; Rudebusch, F.; Moore, M.M. Effects of long-term livestock grazing and habitat on understory vegetation. West. N. Am. Nat. 2010, 70, 334–344. [Google Scholar] [CrossRef]
- Allington, G.R.H.; Valone, T.J. Long-term livestock exclusion in arid grassland alters vegetation and soil. Rangel. Ecol. Manag. 2011, 64, 424–428. [Google Scholar] [CrossRef]
- Le Houérou, H.N.; Corra, M. Some browse plants of Ethiopia. In Browse in Africa: The Current State of Knowledge; International Livestock Centre for Africa: Addis Ababa, Ethiopia, 1980; pp. 109–114. [Google Scholar]
- Greig-Smith, P. Quantitative Plant Ecology, 3rd ed.; Blackwell Scientific: Oxford, UK, 1983. [Google Scholar]
- Hedberg, I.; Edwards, S. Flora of Ethiopia; The National Herbarium: Addis Ababa, Ethiopia, 1995. [Google Scholar]
- Lewis, J.; Freitas, F. Physical and Chemical Methods of Soil and Water Analysis; Food and Agriculture Organization (FAO): Rome, Italy, 1984; p. 275. [Google Scholar]
- Chopra, S.L.; Kanwar, K. Analytical Agricultural Chemistry; Kalyani University Publishers: New Delhi, India, 1976. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall: Englewood Cliffs, NJ, USA, 1970; p. 498. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soil, 12th ed.; Print Ice Hall: Upper Saddle River, NJ, USA, 1999. [Google Scholar]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture (USDA): Washington, DC, USA, 1954; pp. 1–19.
- Gee, G.W.; Bauder, J.W. Particle size analysis by hydrometer: A simplified method for routine textural analysis and a sensitivity test ofmeasurement parameters. J. Soil Sci. 1979, 43, 1004–1007. [Google Scholar] [CrossRef]
- Blake, G.R.; Hartage, K.H. Bulk density. In Method of Soil Analysis, Part 1: Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1986; pp. 12–15. [Google Scholar]
- Whittaker, R.H. Communities and Ecosystems; Macmillan: London, UK, 1970; p. 158. [Google Scholar]
- Krebs, C.J. Ecological Methodology; University of British Colombia: Vancouver, BC, Canada, 1999. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontola Electron. 2001, 4, 9. [Google Scholar]
- Nie, N.H.; Bent, D.H.; Hull, C.H. SPSS: Statistical Package for Social Sciences; McGraw-Hill: New York City, NY, USA, 1970. [Google Scholar]
- Parthasarathy, N.; Karthikeyan, R. Biodiversity and population density of woody species in a tropical evergreen forest in Courtallum reserve forest, Western Ghats, India. Trop. Ecol. 1997, 38, 297–306. [Google Scholar]
- McGranahan, D.A.; David, M.E.; Samuel, D.F.; James, R.M.; Diane, M.D. Multivariate analysis of rangeland vegetation and soil organic carbon describes degradation, informs restoration and conservation. Land 2013, 2, 328–350. [Google Scholar] [CrossRef]
- Banda, T.; Schwartz, M.W.; Caro, T. Woody vegetation structure and composition along a protection gradient in a miombo ecosystem of western Tanzania. For. Ecol. Manag. 2006, 230, 179–185. [Google Scholar] [CrossRef]
- Gandiwa, E.; Tupulu, N.; Zisadza-Gandiwa, P.; Muvengwi, J. Structure and composition of woody vegetation around permanent-artificial and phemeral-natural water points in northern Gonarezhou National Park, Zimbabwe. Trop. Ecol. 2012, 53, 169–175. [Google Scholar]
- Muposhi, V.K.; Ndlovu, M.; Gandiwa, E.; Muvengwi, J.; Muboko, N. Vegetation dynamics prior to wildlife reintroductions in southern Umfurudzi Park, Zimbabwe. J. Anim. Plant Sci. 2014, 24, 1680–1690. [Google Scholar]
- Grime, J.D. Control of species diversity in herbaceous vegetation. J. Environ. Manag. 1973, 1, 151–167. [Google Scholar]
- McNaughton, S.J.; Wolf, L.L. Dominance and the niche in ecological systems. Science 1970, 167, 131–139. [Google Scholar] [CrossRef] [PubMed]
- May, R.M. Patterns of species abundance and diversity. In Ecology and Evolution of Communities; Cody, M.L., Diamond, J.M., Eds.; Harvard University Press: Cambridge, MA, USA, 1975; pp. 81–120. [Google Scholar]
- Whittaker, R.H. Dominance and diversity in land plant communities. Science 1965, 147, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Misra, M.K. Floristic and structure of the herbaceous vegetation of four recovering forest stands in the Eastern Ghats of India. Biodivers. Conserv. 2006, 15, 2263–2285. [Google Scholar] [CrossRef]
- Tripathi, O.P.; Pandey, H.N.; Tripathi, R.S. Distribution, community characteristic and tree population structure of subtropical pine forest of Meghalaya, northeast India. Int. J. Ecol. Environ. Sci. 2004, 30, 207–214. [Google Scholar]
- Singhal, R.M.; Soni, S. Quantitative ecological analysis of some woody species of Mussoorie Himalayas. Indian For. 1989, 115, 327–336. [Google Scholar]
- Bilotta, G.S.; Brazier, R.E.; Haygarth, P.M. The impacts of grazing animals on the quality of soils, vegetation, and surface waters in intensively managed grasslands. Adv. Agron. 2007, 94, 237–250. [Google Scholar]
- Mekuria, W.; Veldkamp, E.; Haile, M.; Nyssen, J.; Muys, B.; Gebrehiwot, K. Effectiveness of exclosures to restore degraded soils as a result of overgrazing in Tigray, Ethiopia. J. Arid Environ. 2007, 69, 270–284. [Google Scholar] [CrossRef]
- Belsky, A.J.; Amundson, R.G.; Duxbury, J.M.; Riha, S.J.; Ali, A.R.; Mwonga, S.M. The effects of trees on their physical, chemical, and biological environment in a semi-arid savanna in Kenya. J. Appl. Ecol. 1989, 26, 1005–1024. [Google Scholar] [CrossRef]
- Treydte, A.C.; Looringh van Beeck, F.; Ludwig, F.; Heitkönig, I.M.A. Improved beneath-crown grass quality in South African savannas varying locally and over seasons. J. Veg. Sci. 2008, 19, 663–670. [Google Scholar] [CrossRef]
- Richter, C.G.F.; Snyman, H.A.; Smit, G.N. The influence of tree density on the grass layer of three semiarid savanna types of Southern Africa. Afr. J. Range Forage Sci. 2001, 18, 103–109. [Google Scholar] [CrossRef]
- Reeder, J.D.; Schuman, G.E. Influence of livestock grazing on C sequestration in semi-arid mixed-grass and short-grass rangelands. Environ. Pollut. 2002, 116, 457–463. [Google Scholar] [CrossRef]
- Han, G.; Hao, X.; Zhao, M.; Wang, M.; Ellert, B.H.; Willms, W.; Wang, M. Effect of grazing intensity on carbon and nitrogen in soil and vegetation in a meadow steppe in Inner Mongolia. Agric. Ecosyst. Environ. 2008, 125, 21–32. [Google Scholar] [CrossRef]
- Tefera, S.; Snyman, H.A.; Smit, G.N. Rangeland dynamics in southern Ethiopia: (1) Botanical composition of grasses and soil characteristics in relation to land-use and distance from water in semi-arid Borana rangelands. J. Environ. Manag. 2007, 85, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Murage, E.W.; Karanja, N.K.; Smithson, P.C.; Woomer, P.L. Diagnostic indicator of soil quality in productive and non-productive smallholders’ fields of Kenya’s Central Highlands. Agric. Ecosyst. Environ. 2000, 79, 1–8. [Google Scholar] [CrossRef]
- Du Preez, C.C.; Snyman, H.A. Organic matter content of a soil in a semi-arid climate with long-standing veld conditions. Afr. J. Range Forage Sci. 1993, 19, 108–110. [Google Scholar] [CrossRef]
- Yates, C.J.; Norton, D.A.; Hobbs, R.J. Grazing effect on plant cover, soil in fragmented woodlands in southern-western Australia: Implications for restoration. Austral Ecol. 2000, 25, 36–47. [Google Scholar] [CrossRef]
- Wang, J.; Fu, B.; Qiu, Y.; Chen, L. Soil nutrients in relation to land-use and landscape position in the semi-arid small catchments on the Loess Plateau in China. J. Arid Environ. 2001, 48, 537–555. [Google Scholar] [CrossRef]
| Species Scientific Name | Abundance | Density | Frequency | A/F | IVI |
|---|---|---|---|---|---|
| High Dominance Herbs | |||||
| Eragorostis aspera | 67.70 | 39.49 | 58.33 | 1.16 | 75.29 |
| Eragrostis cilianensis | 43.31 | 14.44 | 33.33 | 1.30 | 36.11 |
| Cynodon dactylon | 9.77 | 5.70 | 58.33 | 0.17 | 15.82 |
| Medium Dominance Herbs | |||||
| Tragus racemosus | 7.83 | 3.92 | 50.00 | 0.16 | 12.30 |
| Cenchurus ciliaris | 5.07 | 2.96 | 58.33 | 0.09 | 10.99 |
| Panicum atrosanguineum | 4.89 | 2.44 | 50.00 | 0.10 | 9.54 |
| Tragus berteronianus | 3.90 | 1.95 | 50.00 | 0.08 | 8.62 |
| Hibiscus aponeurus | 4.97 | 2.07 | 41.67 | 0.12 | 8.34 |
| Eragrostis ciliaris | 8.64 | 2.16 | 25.00 | 0.35 | 8.25 |
| Crotalaria laburnifolia | 2.99 | 1.49 | 50.00 | 0.06 | 7.76 |
| Solanum incanum | 2.01 | 1.01 | 50.00 | 0.04 | 6.85 |
| Ocimum lamiifolium | 2.95 | 1.23 | 41.67 | 0.07 | 6.63 |
| Eragrostis superba | 7.08 | 1.18 | 16.67 | 0.42 | 5.75 |
| Chrysopogon aucheri | 4.81 | 1.20 | 25.00 | 0.19 | 5.69 |
| Solanum somalense | 2.56 | 0.85 | 33.33 | 0.08 | 5.25 |
| Dactyloctenium scindicum | 3.92 | 0.98 | 25.00 | 0.16 | 5.09 |
| Indigofera articulate | 5.92 | 0.99 | 16.67 | 0.36 | 5.07 |
| Aristida adscencionis | 3.78 | 0.94 | 25.00 | 0.15 | 5.00 |
| Heliotropium somalense | 1.83 | 0.61 | 33.33 | 0.05 | 4.69 |
| Gomphocarpus fruticosur | 5.00 | 0.83 | 16.67 | 0.30 | 4.54 |
| Sida sp. | 2.97 | 0.74 | 25.00 | 0.12 | 4.46 |
| Abutilon fruticasum | 4.25 | 0.71 | 16.67 | 0.26 | 4.11 |
| Heliotropium zeylonicum | 6.58 | 0.55 | 8.33 | 0.79 | 4.04 |
| Ocimum urticifolium | 2.31 | 0.58 | 25.00 | 0.09 | 4.02 |
| Blepharis ciliaris | 1.67 | 0.42 | 25.00 | 0.07 | 3.59 |
| Sporobolus marginatus | 3.13 | 0.52 | 16.67 | 0.19 | 3.46 |
| Least Important Herbs | |||||
| Aristida adoensis | 5.08 | 0.42 | 8.33 | 0.61 | 3.31 |
| Balenites aegyptiaca | 5.08 | 0.42 | 8.33 | 0.61 | 3.31 |
| Achyranthus aspera | 2.50 | 0.42 | 16.67 | 0.15 | 3.10 |
| Senna occidentalis | 0.44 | 0.11 | 25.00 | 0.02 | 2.78 |
| Eragrostis sp. | 3.75 | 0.31 | 8.33 | 0.45 | 2.66 |
| Eragrostis schlueinfurthii | 3.58 | 0.30 | 8.33 | 0.43 | 2.58 |
| Setaria verticillata | 3.50 | 0.29 | 8.33 | 0.42 | 2.54 |
| Leucas microphylla | 0.67 | 0.11 | 16.67 | 0.04 | 2.04 |
| Parthenium hysterophrus | 2.42 | 0.20 | 8.33 | 0.29 | 2.01 |
| Lactuca sativa | 1.83 | 0.15 | 8.33 | 0.22 | 1.72 |
| Dactiloctenium aegyptium | 1.58 | 0.13 | 8.33 | 0.19 | 1.60 |
| Eriachloa colonum | 0.58 | 0.05 | 8.33 | 0.07 | 1.11 |
| Species Scientific Name | Abundance | Density | Frequency | A/F | IVI |
|---|---|---|---|---|---|
| High Dominance Herbs | |||||
| Cucumis ficiofolius | 0.83 | 0.07 | 8.33 | 0.10 | 33.96 |
| Eragorostis aspera | 10.14 | 2.53 | 25.00 | 0.41 | 33.75 |
| Bothriochlea insulpta | 1.38 | 0.23 | 16.67 | 0.08 | 28.78 |
| Chloris radiota | 4.35 | 1.45 | 33.33 | 0.13 | 17.29 |
| Aristida adoensis | 22.33 | 1.86 | 8.33 | 2.68 | 13.84 |
| Medium Dominance Herbs | |||||
| Dactyloctenium aegyptium | 22.33 | 1.86 | 8.33 | 2.68 | 10.89 |
| Ageratum conyzoides | 12.92 | 2.15 | 16.67 | 0.77 | 7.42 |
| Cynodon dactylon | 36.41 | 24.27 | 66.67 | 0.55 | 7.42 |
| Leucas microphylla | 1.08 | 0.18 | 16.67 | 0.06 | 7.4 |
| Crotalaria albicaulis | 6.90 | 2.30 | 33.33 | 0.21 | 6.8 |
| Oxygonum atriplicifolium | 5.50 | 0.46 | 8.33 | 0.66 | 6.76 |
| Enteropogon elegans | 1.00 | 0.08 | 8.33 | 0.12 | 6.62 |
| Commicarpus verticillatus | 4.08 | 0.34 | 8.33 | 0.49 | 6.42 |
| Flaveria terinervia | 0.67 | 0.06 | 8.33 | 0.08 | 6.27 |
| Achyranthus aspera | 1.38 | 0.23 | 16.67 | 0.08 | 6.21 |
| Panicum atrosanguineum | 5.38 | 2.24 | 41.67 | 0.13 | 5.67 |
| Indigofera articulate | 2.83 | 0.24 | 8.33 | 0.34 | 5.4 |
| Sonchus oleraceus | 1.08 | 0.09 | 8.33 | 0.13 | 5.3 |
| Parthenium hysterophorus | 7.08 | 1.18 | 16.67 | 0.43 | 5.15 |
| Cenchurus ciliaris | 23.63 | 19.69 | 83.33 | 0.28 | 5.14 |
| Tagetes minuta | 0.67 | 0.06 | 8.33 | 0.08 | 5.1 |
| Panicum sp. | 16.50 | 1.38 | 8.33 | 1.98 | 4.08 |
| Setaria acromelaena | 1.33 | 0.22 | 16.67 | 0.08 | 4 |
| Heliotropium somalense | 9.33 | 2.33 | 25.00 | 0.37 | 3.68 |
| Aristida adscencionis | 26.81 | 6.70 | 25.00 | 1.07 | 3.64 |
| Tephrosia emeroides | 6.64 | 1.66 | 25.00 | 0.27 | 3.24 |
| Crotalaria natalitia | 2.67 | 0.22 | 8.33 | 0.32 | 3.18 |
| Ocimum lamiifolium | 3.15 | 1.84 | 58.33 | 0.05 | 2.98 |
| Setaria verticillata | 4.11 | 1.03 | 25.00 | 0.16 | 2.63 |
| Eragrostis cilianensis | 72.72 | 18.18 | 25.00 | 2.91 | 2.46 |
| Ocimum urficifolium | 4.08 | 0.68 | 16.67 | 0.25 | 2.39 |
| Blepharis ciliaris | 0.88 | 0.15 | 16.67 | 0.05 | 1.99 |
| Verbesina encelioides | 0.50 | 0.04 | 8.33 | 0.06 | 1.99 |
| Senna occidentalis | 1.21 | 0.20 | 16.67 | 0.07 | 1.97 |
| Chrysopogon aucheri | 14.76 | 9.84 | 66.67 | 0.22 | 1.96 |
| Hibiscus aspera | 2.58 | 0.22 | 8.33 | 0.31 | 1.96 |
| Senna obtusifolia | 2.50 | 0.21 | 8.33 | 0.30 | 1.92 |
| Launea intyybacea | 2.42 | 0.20 | 8.33 | 0.29 | 1.88 |
| Abutilon fruticasum | 3.58 | 0.30 | 8.33 | 0.43 | 1.81 |
| Asparagus asiaticus | 3.28 | 0.82 | 25.00 | 0.13 | 1.8 |
| Hypoestes forskafii | 2.33 | 0.19 | 8.33 | 0.28 | 1.59 |
| Crotalaria laburnifolia | 7.67 | 2.56 | 33.33 | 0.23 | 1.54 |
| Hibiscus aponeurus | 6.00 | 1.00 | 16.67 | 0.36 | 1.51 |
| Least Important Herbs | |||||
| Sansevieria abyssinica | 14.75 | 1.23 | 8.33 | 1.77 | 1.49 |
| Sansevieria sp. | 2.50 | 0.21 | 8.33 | 0.30 | 1.49 |
| Lactuca sativa | 7.33 | 1.83 | 25.00 | 0.29 | 1.46 |
| Hibiscus trionum | 4.08 | 0.34 | 8.33 | 0.49 | 1.44 |
| Tephrosia interrupta | 4.79 | 0.80 | 16.67 | 0.29 | 1.44 |
| Sida sp. | 0.94 | 0.24 | 25.00 | 0.04 | 1.06 |
| Dactyloctenium scindicum | 10.14 | 5.07 | 50.00 | 0.20 | 1.04 |
| Tragus berteronionus | 2.33 | 0.19 | 8.33 | 0.28 | 1.04 |
| Crotolaria radiota | 8.17 | 0.68 | 8.33 | 0.98 | 0.99 |
| Eragrostis sp. | 5.75 | 0.48 | 8.33 | 0.69 | 0.94 |
| Sporobolus marginatus | 2.82 | 1.17 | 41.67 | 0.07 | 0.94 |
| Tragus racemosus | 1.00 | 0.08 | 8.33 | 0.12 | 0.89 |
| Species Scientific Name | Abundance | Density | Frequency | A/F | IVI |
|---|---|---|---|---|---|
| Acacia busie | 8.11 | 12.67 | 75 | 0.11 | 58.13 |
| Acacia nilotica | 7.82 | 9.83 | 75 | 0.1 | 51.23 |
| Acacia etbica | 11.1 | 9.42 | 58.33 | 0.19 | 49.62 |
| Acacia toritilis | 27 | 1.08 | 25 | 1.08 | 39.55 |
| Acacia melifera | 3.69 | 6.5 | 58.33 | 0.06 | 34.48 |
| Acacia abyssinica | 22.44 | 1 | 25 | 0.9 | 34.2 |
| Acacia oerfota | 7.38 | 0.25 | 16.67 | 0.44 | 13.26 |
| Balenites aegyptiaca | 0.19 | 1.58 | 25 | 0.01 | 10.41 |
| Securinega virosa | 0.76 | 0.75 | 25 | 0.03 | 9.13 |
| Species Scientific Name | Abundance | Density | Frequency | A/F | IVI |
|---|---|---|---|---|---|
| Acacia nilotica | 10.19 | 10.17 | 100 | 0.1 | 94.44 |
| Acacia etbica | 8.45 | 12.75 | 66.67 | 0.13 | 88.06 |
| Acacia bussei | 13.19 | 2.58 | 58.33 | 0.23 | 62.39 |
| Acacia melifera | 3.58 | 1.17 | 33.33 | 0.11 | 24.22 |
| Securinega virosa | 0.76 | 1.42 | 16.67 | 0.05 | 12.33 |
| Acacia nubica | 1.31 | 0.33 | 16.67 | 0.08 | 9.99 |
| Grewia ferruginea | 0.76 | 0.33 | 16.67 | 0.05 | 8.56 |
| Variable | Enclosure | Open | T | Sig.2 Tailed |
|---|---|---|---|---|
| Herbs Diversity (Exp. (H’)) | 6.55 + 0.54 | 4.51 + 0.75 | 2.2 | 0.038 |
| Woody Diversity (Exp. (H’)) | 2.39 + 0.26 | 3.17 + 0.26 | −2.42 | 0.047 |
| Herbs Biomass (g/m2) | 383.61 + 24.68 | 167.9984 + 12.26 | 7.83 | 0.000 |
| Herbs Basal cover (%) | 85.70 + 1.69 | 31.49 + 2.70 | 17.00 | 0.000 |
| Woody basal cover (/400 m2 ) | 13.42 + 5.76 | 31.23 + 4.19 | 2.49 | 0.020 |
| Variable | Enclosure | Open | T | Sig.2 Tailed |
|---|---|---|---|---|
| Total N (Ppm) | 0.10 ± 0.01 | 0.16 ± 0.04 | −1.27 | 0.23 |
| P mg/kg | 4.55 ± 0.83 | 6.93 ± 0.90 | −1.95 | 0.80 |
| K | 2.09 ± 0.11 | 1.67 ± 0.04 | 3.49 | 0.006 |
| PH | 7.43 ± 0.01 | 7.65 ± 0.01 | −3.57 | 0.005 |
| SOM % | 2.46 ±0.1 | 1.58 ± 0.13 | 5.31 | 0.000 |
| SOC % | 2.46 ± 0.10 | 1.00 ± 0.12 | 2.26 | 0.047 |
| EC (mmhos/cm) | 0.16 ± 0.01 | 0.35 ± 0.09 | −2.03 | 0.063 |
| Bulk density | 1.20 ± 0.03 | 1.2 ± 0.01 | 0.12 | 0.93 |
| Clay % | 16.32 ± 1.54 | 16.91 ± 1.23 | −0.29 | 0.777 |
| Sand % | 47.63 ± 1.73 | 46.93 ± 0.63 | 0.38 | 0.761 |
| Silt % | 34.67 ± 0.80 | 36.67 ± 0.71 | −1.83 | 0.092 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hailu, H. Analysis of Vegetation Phytosociological Characteristics and Soil Physico-Chemical Conditions in Harishin Rangelands of Eastern Ethiopia. Land 2017, 6, 4. https://doi.org/10.3390/land6010004
Hailu H. Analysis of Vegetation Phytosociological Characteristics and Soil Physico-Chemical Conditions in Harishin Rangelands of Eastern Ethiopia. Land. 2017; 6(1):4. https://doi.org/10.3390/land6010004
Chicago/Turabian StyleHailu, Haftay. 2017. "Analysis of Vegetation Phytosociological Characteristics and Soil Physico-Chemical Conditions in Harishin Rangelands of Eastern Ethiopia" Land 6, no. 1: 4. https://doi.org/10.3390/land6010004
APA StyleHailu, H. (2017). Analysis of Vegetation Phytosociological Characteristics and Soil Physico-Chemical Conditions in Harishin Rangelands of Eastern Ethiopia. Land, 6(1), 4. https://doi.org/10.3390/land6010004





