Soil–Atmosphere GHG Fluxes in Cacao Agroecosystems on São Tomé Island, Central Africa: Toward Climate-Smart Practices
Abstract
1. Introduction
2. Materials and Methods
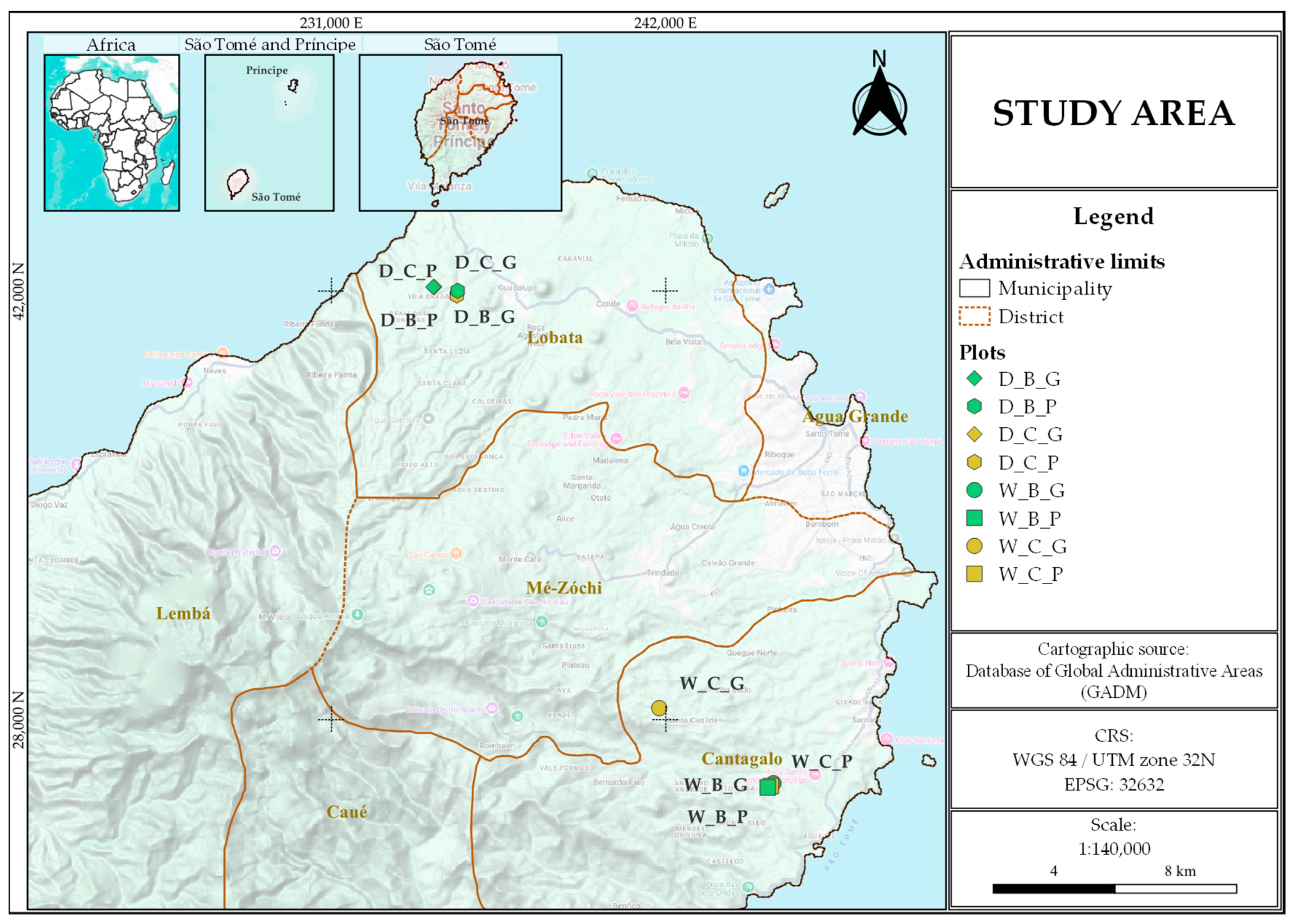
2.1. Study Sites
2.2. Sampling Design
2.3. Flux Measurements
2.4. Environmental and Carbon Stock Measurements
2.5. Statistical Analysis
3. Results
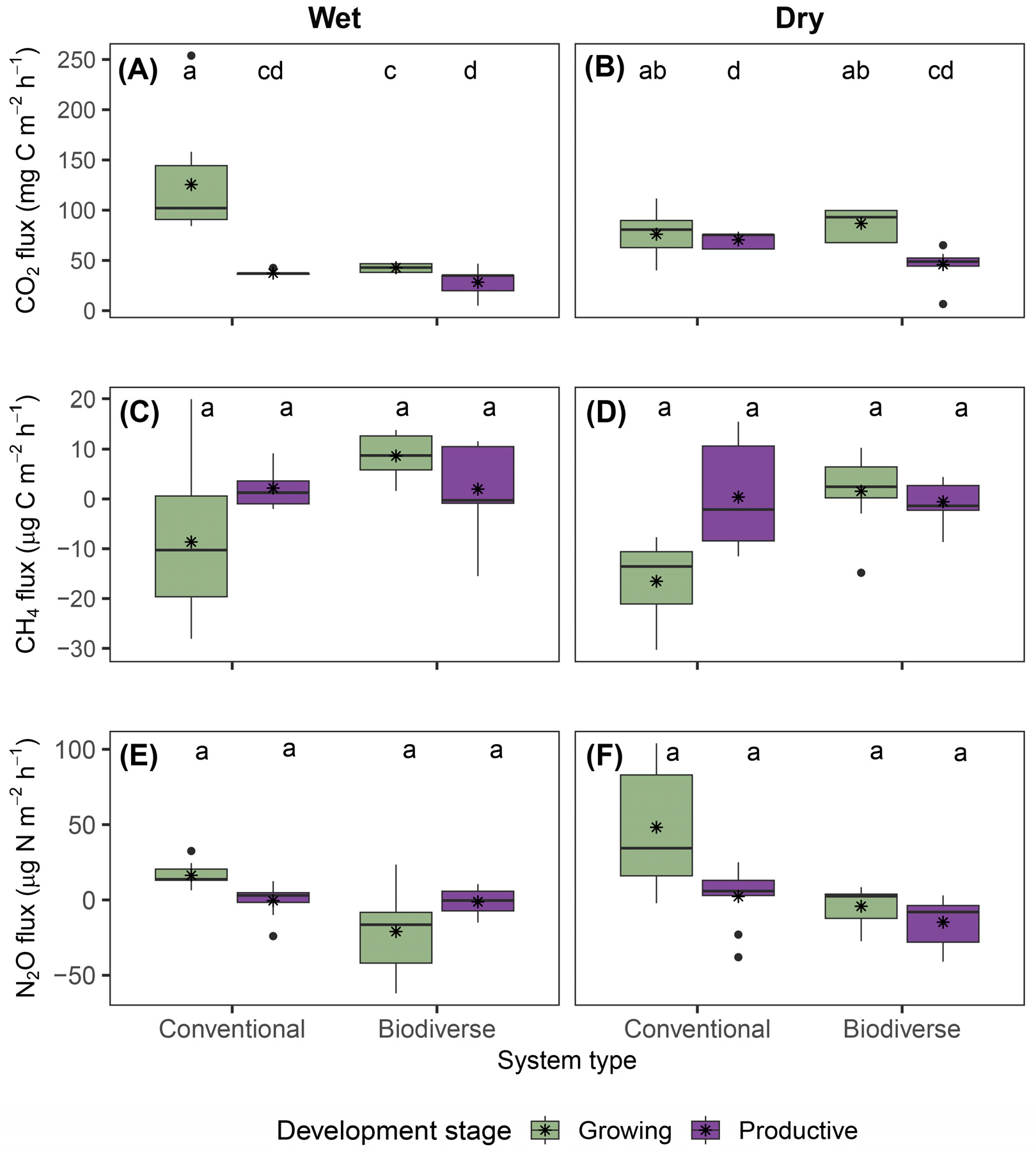
3.1. Soil CO2, CH4, and N2O Fluxes
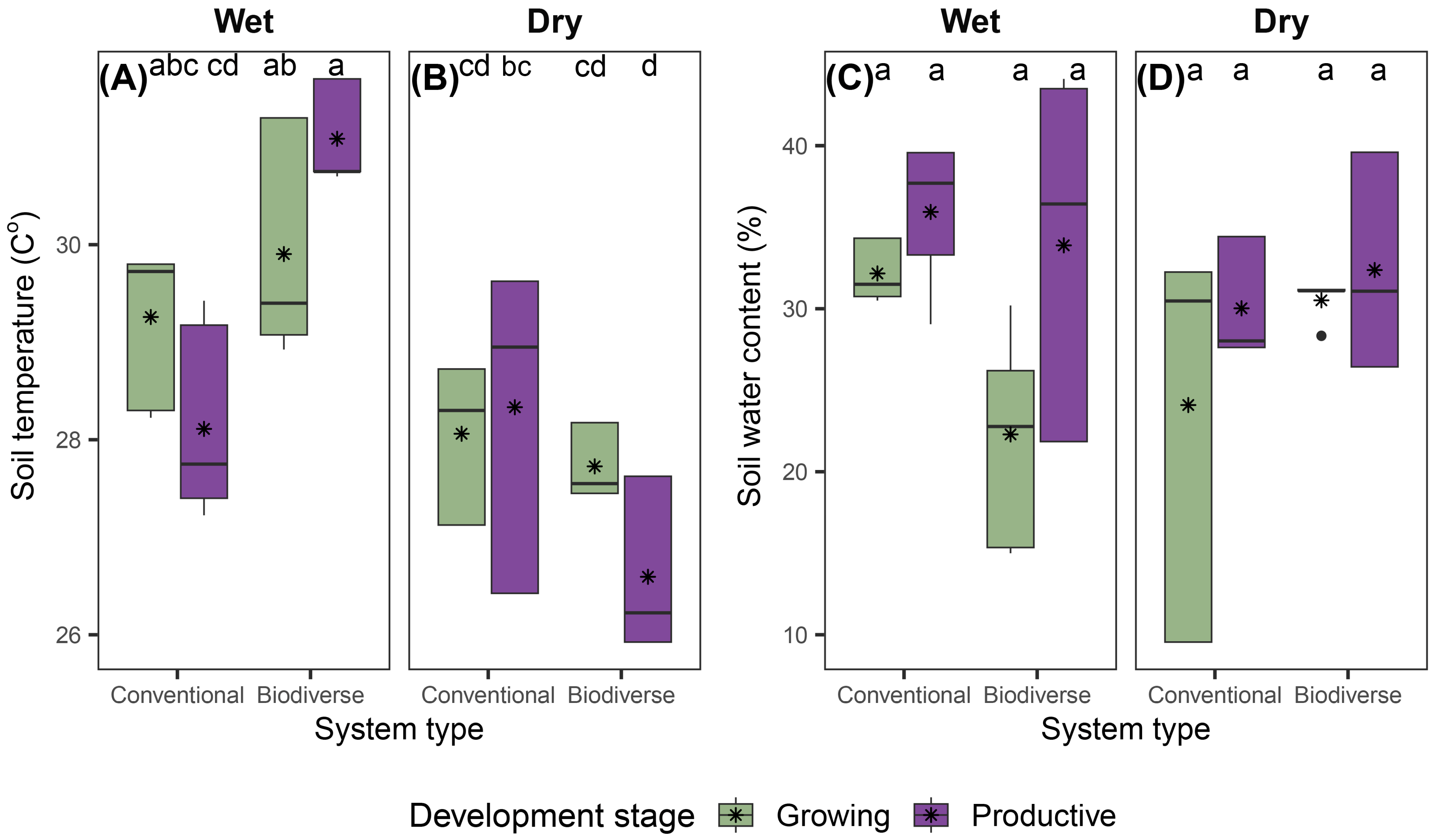
3.2. Soil Temperature and Water Content
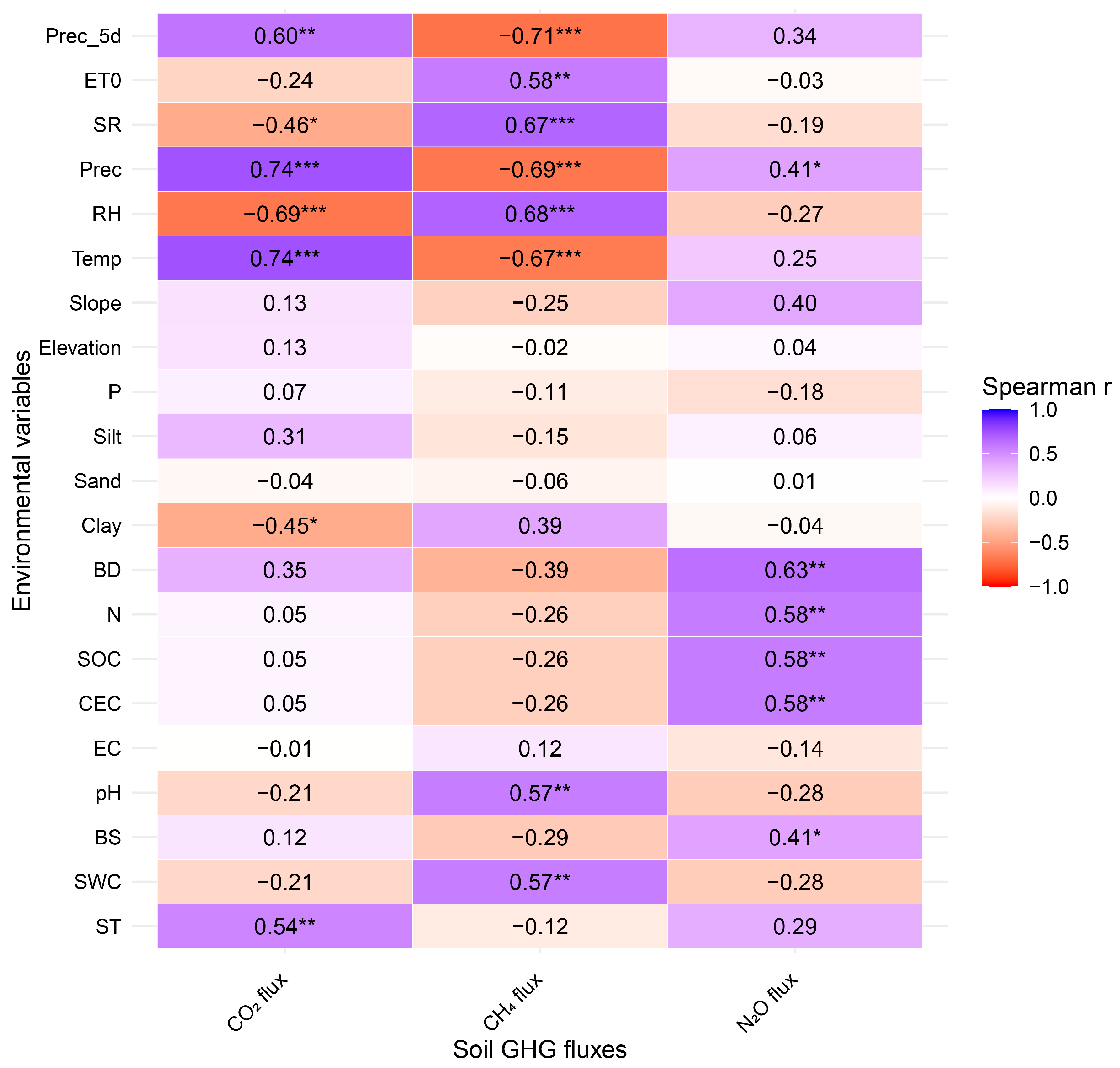
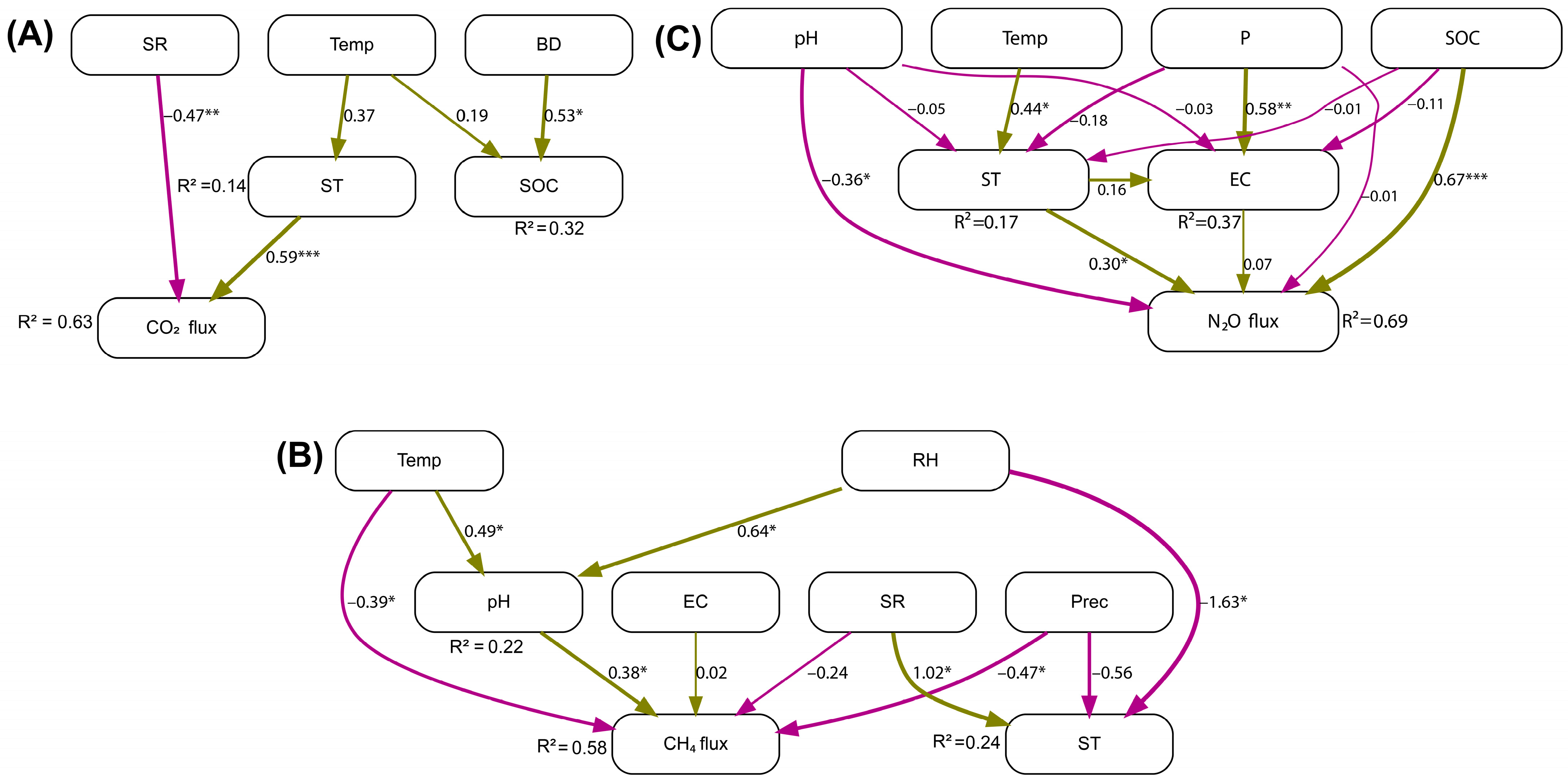
3.3. Environmental Drivers of Soil GHG Fluxes
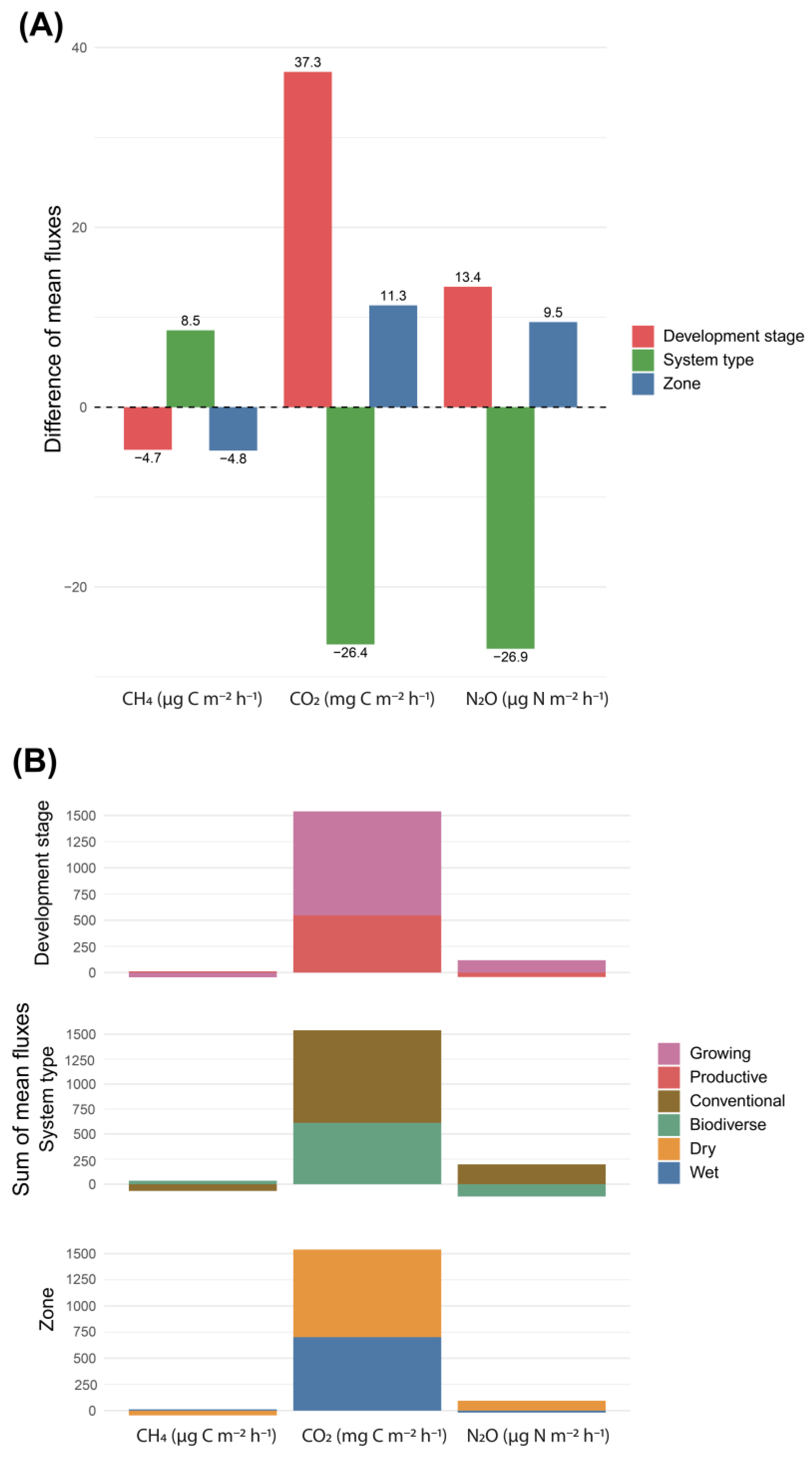
3.4. Global Warming Potential (GWP)
4. Discussion
4.1. CO2 Fluxes Are Higher in Conventional Systems and During the Growing Stage
4.2. Net CH4 Emissions in Biodiverse Systems and Greater Uptake in Conventional Systems
4.3. Higher N2O Emissions in Conventional Systems and During Growing Stages
4.4. Global Warming Potential (GWP) and the Role of Climate-Smart Practices
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Cocoa Organization—ICCO Quarterly Bulletin of Cocoa Statistics (Vol. LI, No. 2). Revised Estimates of World Cocoa Production, Grindings and Stocks for the 2023/24 Cocoa Year. 2025. Available online: https://www.icco.org/may-2025-quarterly-bulletin-of-cocoa-statistics/ (accessed on 10 April 2025).
- Vervuurt, W.; Slingerland, M.A.; Pronk, A.A.; Van Bussel, L.G.J. Modelling Greenhouse Gas Emissions of Cacao Production in the Republic of Côte d’Ivoire. Agrofor. Syst. 2022, 96, 417–434. [Google Scholar] [CrossRef]
- Hawkins, J.W.; Gallagher, E.J.; van der Haar, S.; Sevor, M.K.E.; Weng, X.; Rufino, M.C.; Schoneveld, G.C. Low-Emissions and Profitable Cocoa through Moderate-Shade Agroforestry: Insights from Ghana. Agric. Ecosyst. Environ. 2024, 367, 108961. [Google Scholar] [CrossRef]
- Anokye, J.; Abunyewa, A.A.; Jørgensen, U.; Kaba, J.S.; Twum-Ampofo, K.; Dawoe, E.; Barnes, V.R.; Plauborg, F.; Pedersen, S.M.; Berg, T.R.; et al. Mitigation of Greenhouse Gas Emissions through Shade Systems and Climate-Smart Soil Fertility Interventions in Cocoa Landscapes in the Semi-Deciduous Ecological Zone of Ghana. Soil Adv. 2024, 1, 100001. [Google Scholar] [CrossRef]
- Iddris, N.A.-A.; Corre, M.D.; Yemefack, M.; van Straaten, O.; Veldkamp, E. Stem and Soil Nitrous Oxide Fluxes from Rainforest and Cacao Agroforest on highly Weathered Soils in the Congo Basin. Biogeosciences 2020, 17, 5377–5397. [Google Scholar] [CrossRef]
- Da Costa, E.N.D.; Landim de Souza, M.F.; Lima Marrocos, P.C.; Lobão, D.; Lopes da Silva, D.M. Soil Organic Matter and CO2 Fluxes in Small Tropical Watersheds under Forest and Cacao Agroforestry. PLoS ONE 2018, 13, e0200550. [Google Scholar] [CrossRef] [PubMed]
- São Tomé y Príncipe—STP. Third National Communication of São Tomé and Príncipe to the United Nations Framework Convention on Climate Change; Ministry of Public Works, Infrastructures, Natural Resources and the Environment: São Tomé, São Tomé and Príncipe, 2019.
- São Tomé and Príncipe—STP. Nationally Determined Contributions (NDC-STP) Updated; Ministry of Infrastructures and Natural Resources: São Tomé, São Tomé and Príncipe, 2021.
- IPCC Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Tian, H.; Lu, C.; Ciais, P.; Michalak, A.M.; Canadell, J.G.; Saikawa, E.; Huntzinger, D.N.; Gurney, K.R.; Sitch, S.; Zhang, B.; et al. The Terrestrial Biosphere as a Net Source of Greenhouse Gases to the Atmosphere. Nature 2016, 531, 225–228. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse Gases Emissions and Global Climate Change: Examining the Influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Peng, C.; Wang, X.; Zhang, H.; Zhang, S. Soil-Atmosphere Exchange of Carbon Dioxide, Methane and Nitrous Oxide in Temperate Forests along an Elevation Gradient in the Qinling Mountains, China. Plant Soil 2023, 488, 325–342. [Google Scholar] [CrossRef]
- Bārdule, A.; Butlers, A.; Spalva, G.; Ivanovs, J.; Meļņiks, R.N.; Līcīte, I.; Lazdiņš, A. The Surface-to-Atmosphere GHG Fluxes in Rewetted and Permanently Flooded Former Peat Extraction Areas Compared to Pristine Peatland in Hemiboreal Latvia. Water 2023, 15, 1954. [Google Scholar] [CrossRef]
- Kamyab, H.; SaberiKamarposhti, M.; Hashim, H.; Yusuf, M. Carbon Dynamics in Agricultural Greenhouse Gas Emissions and Removals: A Comprehensive Review. Carbon. Lett. 2024, 34, 265–289. [Google Scholar] [CrossRef]
- Mapanda, F.; Mupini, J.; Wuta, M.; Nyamangara, J.; Rees, R.M. A Cross-ecosystem Assessment of the Effects of Land Cover and Land Use on Soil Emission of Selected Greenhouse Gases and Related Soil Properties in Zimbabwe. Eur. J. Soil Sci. 2010, 61, 721–733. [Google Scholar] [CrossRef]
- Ntamwira, J.; Ocimati, W.; Blomme, G.; Lubobo, A.K.; Mwarabu Lolonga Pyame, D.; Dhed’a Djailo, B. Innovative Agroecological Practices Can Restore Degraded Farmlands and Revive Crop Yields. Front. Sustain. Food Syst. 2023, 7, 1017341. [Google Scholar] [CrossRef]
- Auffret, M.D.; Karhu, K.; Khachane, A.; Dungait, J.A.J.; Fraser, F.; Hopkins, D.W.; Wookey, P.A.; Singh, B.K.; Freitag, T.E.; Hartley, I.P.; et al. The Role of Microbial Community Composition in Controlling Soil Respiration Responses to Temperature. PLoS ONE 2016, 11, e0165448. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.-J.; Yu, G.-R.; Cheng, S.-L.; Mo, J.-M.; Yan, J.-H.; Li, S. 13C Abundance, Water-Soluble and Microbial Biomass Carbon as Potential Indicators of Soil Organic Carbon Dynamics in Subtropical Forests at Different Successional Stages and Subject to Different Nitrogen Loads. Plant Soil 2009, 320, 243–254. [Google Scholar] [CrossRef]
- Whitaker, J.; Ostle, N.; Nottingham, A.T.; Ccahuana, A.; Salinas, N.; Bardgett, R.D.; Meir, P.; McNamara, N.P. Microbial Community Composition Explains Soil Respiration Responses to Changing Carbon Inputs along an ANdes-to-AMazon Elevation Gradient. J. Ecol. 2014, 102, 1058–1071. [Google Scholar] [CrossRef]
- Bréchet, L.; Ponton, S.; Roy, J.; Freycon, V.; Coûteaux, M.-M.; Bonal, D.; Epron, D. Do Tree Species Characteristics Influence Soil Respiration in Tropical Forests? A Test Based on 16 Tree Species Planted in Monospecific Plots. Plant Soil 2009, 319, 235–246. [Google Scholar] [CrossRef]
- Epron, D.; Bosc, A.; Bonal, D.; Freycon, V. Spatial Variation of Soil Respiration across a Topographic Gradient in a Tropical Rain Forest in French Guiana. J. Trop. Ecol. 2006, 22, 565–574. [Google Scholar] [CrossRef]
- Luizão, R.C.C.; Luizão, F.J.; Paiva, R.Q.; Monteiro, T.F.; Sousa, L.S.; Kruijt, B. Variation of Carbon and Nitrogen Cycling Processes along a Topographic Gradient in a Central Amazonian Forest. Glob. Change Biol. 2004, 10, 592–600. [Google Scholar] [CrossRef]
- Courtois, E.A.; Stahl, C.; Van den Berge, J.; Bréchet, L.; Van Langenhove, L.; Richter, A.; Urbina, I.; Soong, J.L.; Peñuelas, J.; Janssens, I.A. Spatial Variation of Soil CO2, CH4 and N2O Fluxes Across Topographical Positions in Tropical Forests of the Guiana Shield. Ecosystems 2018, 21, 1445–1458. [Google Scholar] [CrossRef]
- Dutaur, L.; Verchot, L.V. A Global Inventory of the Soil CH4 Sink. Glob. Biogeochem. Cycles 2007, 21, GB4013. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Daniel, W.; Stahl, C.; Burban, B.; Goret, J.-Y.; Cazal, J.; Richter, A.; Janssens, I.A.; Bréchet, L.M. Tree Stem and Soil Methane and Nitrous Oxide Fluxes, but Not Carbon Dioxide Fluxes, Switch Sign along a Topographic Gradient in a Tropical Forest. Plant Soil 2023, 488, 533–549. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Zhu, Q.; Peng, C.; Wu, N.; Yang, G.; Zhu, D.; Tian, J.; Tian, L.; Kang, X.; et al. Soil Methane Uptake by Grasslands and Forests in China. Soil Biol. Biochem. 2014, 74, 70–81. [Google Scholar] [CrossRef]
- Bowden, R.D.; Rullo, G.; Stevens, G.R.; Steudler, P.A. Soil Fluxes of Carbon Dioxide, Nitrous Oxide, and Methane at a Productive Temperate Deciduous Forest. J. Environ. Qual. 2000, 29, 268–276. [Google Scholar] [CrossRef]
- Davidson, E.A.; de Carvalho, C.J.R.; Figueira, A.M.; Ishida, F.Y.; Ometto, J.P.H.B.; Nardoto, G.B.; Sabá, R.T.; Hayashi, S.N.; Leal, E.C.; Vieira, I.C.G.; et al. Recuperation of Nitrogen Cycling in Amazonian Forests Following Agricultural Abandonment. Nature 2007, 447, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Kaba, J.S.; Agyei, E.K.; Avilineni, M.K.C.; Yamoah, F.A.; Issahaku, I.; Ntiamoah, P.; Acquah, E.; Mas-Ud, M. Agroforestry as an Old Approach to a New Challenge of Combating Climate Change: A Critical Analysis of the Cocoa Sector. Discov. Agric. 2024, 2, 92. [Google Scholar] [CrossRef]
- Becker, A.; Wegner, J.D.; Dawoe, E.; Schindler, K.; Thompson, W.J.; Bunn, C.; Garrett, R.D.; Castro, F.; Hart, S.P.; Blaser-Hart, W.J. The Unrealized Potential of Agroforestry for an Emissions-Intensive Agricultural Commodity. Nat. Sustain. 2025, 8, 994–1003. [Google Scholar] [CrossRef]
- Belay, I.G.; Tanaka, R.; Kitagawa, H.; Kobayashi, K.; Nakamura, E. Origin of Ocean Island Basalts in the West African Passive Margin without Mantle Plume Involvement. Nat. Commun. 2019, 10, 3022. [Google Scholar] [CrossRef]
- Burgess, N.; Hales, J.D.; Underwood, E.; Dinerstein, E.; Olson, D.; Itoua, I.; Schipper, J.; Ricketts, T.; Newman, K. Terrestrial Ecoregions of Africa and Madagascar: A Conservation Assessment; Island Press: Washington, DC, USA, 2004. [Google Scholar]
- Dauby, G.; Stévart, T.; Barberá, P.; Benitez, L.; do Céu Madureira, M.; Soares, F.C.; Viennois, G.; de Lima, R.F. Classification, Distribution, and Biodiversity of Terrestrial Ecosystems in the Gulf of Guinea Oceanic Islands. In Biodiversity of the Gulf of Guinea Oceanic Islands; Springer International Publishing: Cham, Switzerland, 2022; pp. 37–69. [Google Scholar]
- Soares, F.C.; Panisi, M.; Sampaio, H.; Soares, E.; Santana, A.; Buchanan, G.M.; Leal, A.I.; Palmeirim, J.M.; de Lima, R.F. Land-use Intensification Promotes Non-native Species in a Tropical Island Bird Assemblage. Anim. Conserv. 2020, 23, 573–584. [Google Scholar] [CrossRef]
- Chou, S.C.; de Arruda Lyra, A.; Gomes, J.L.; Rodriguez, D.A.; Alves Martins, M.; Costa Resende, N.; da Silva Tavares, P.; Pereira Dereczynski, C.; Lopes Pilotto, I.; Martins, A.M.; et al. Downscaling Projections of Climate Change in Sao Tome and Principe Islands, Africa. Clim. Dyn. 2020, 54, 4021–4042. [Google Scholar] [CrossRef]
- Ministry of Infrastructure Natural Resources and Environment. São Tomé and Príncipe National Biodiversity Strategy And Action Plan 2015-2020 (NBSAP II). In National Commission for Monitoring and Evaluation; Ministry of Infrastructure Natural Resources and Environment: São Tomé, São Tomé and Príncipe, 2015. [Google Scholar]
- Lima, D.; de Oliveira, R.P. Caracterização Dos Recursos Hídricos, Dos Serviços de Água de São Tomé e Principe, Dos Cenários de Alteração Climática e Dos Seus Impactos. In Proceedings of the SILUSBA—Simpósio de Hidráulica e Recursos Hídricos dos Paises de Expressão Portuguesa, Brasilia, Brazil, 13–15 September 2017. [Google Scholar]
- Contreras, B.V.; Keese, A. The Art of Running Away: Escapes and Flight Movements During the Great Depression in São Tomé e Príncipe, 1930–1936. Int. Rev. Soc. Hist. 2021, 66, 357–388. [Google Scholar] [CrossRef]
- Pavelka, M.; Acosta, M.; Kiese, R.; Altimir, N.; Bruemmer, C.; Crill, P.; Darenova, E.; Fuß, R.; Gielen, B.; Graf, A.; et al. Standardisation of Chamber Technique for CO2, N2O and CH4 Fluxes Measurements from Terrestrial Ecosystems. Int. Agrophys. 2018, 32, 569–587. [Google Scholar] [CrossRef]
- Fuss, R. Gasfluxes: Greenhouse Gas Flux Calculation from Chamber Measurements. CRAN: Contributed Packages. 2024. Available online: https://cran.r-project.org/web/packages/gasfluxes/index.html (accessed on 10 May 2025).
- Parkin, T.B.; Venterea, R.T.; Hargreaves, S.K. Calculating the Detection Limits of Chamber-Based Soil Greenhouse Gas Flux Measurements. J. Environ. Qual. 2012, 41, 705–715. [Google Scholar] [CrossRef]
- Pedersen, A.R.; Petersen, S.O.; Schelde, K. A Comprehensive Approach to Soil-Atmosphere Trace-Gas Flux Estimation with Static Chambers. Eur. J. Soil Sci. 2010, 61, 888–902. [Google Scholar] [CrossRef]
- Hüppi, R.; Felber, R.; Krauss, M.; Six, J.; Leifeld, J.; Fuß, R. Restricting the Nonlinearity Parameter in Soil Greenhouse Gas Flux Calculation for More Reliable Flux Estimates. PLoS ONE 2018, 13, e0200876. [Google Scholar] [CrossRef]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Geneva, Switzerland, 2019; ISBN 978-4-88788-232-4. [Google Scholar]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved Allometric Models to Estimate the Aboveground Biomass of Tropical Trees. Glob. Change Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef]
- Nascimento, H.E.M.; Laurance, W.F. Total Aboveground Biomass in Central Amazonian Rainforests: A Landscape-Scale Study. Ecol. Manag. 2002, 168, 311–321. [Google Scholar] [CrossRef]
- Morán-Villa, V.L.; Monterroso-Rivas, A.I.; Mata-González, R.; Márquez-Berber, S.R.; Abdallah, M.A.B.; Valdes-Velarde, E.; Hernández-Sánchez, R. Above-Ground Biomass Estimation by Developing Allometric Equations for Theobroma Cacao in Tabasco, Mexico. Agrofor. Syst. 2024, 98, 537–549. [Google Scholar] [CrossRef]
- Hairiah, K.; Dewi, S.; Agus, F.; Velarde, S.; Ekadinata, A.; Rahayu, S.; van Noordwijk, M. Measuring Carbon Stocks Across Land Use Systems: A Manual; World Agroforestry Centre (ICRAF), SEA Regional Office: Bogor, Indonesia, 2011; ISBN 978-979-3198-55-2. [Google Scholar]
- Maia, S.M.F.; Ogle, S.M.; Cerri, C.C.; Cerri, C.E.P. Changes in Soil Organic Carbon Storage under Different Agricultural Management Systems in the Southwest Amazon Region of Brazil. Soil Tillage Res. 2010, 106, 177–184. [Google Scholar] [CrossRef]
- Soil Survey Staff. Kellogg Soil Survey Laboratory Methods Manual, Soil Survey Investigations Report No. 42, Version 6.0.; Department of Agriculture: Lincoln, NE, USA, 2022.
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2025. [Google Scholar]
- RStudio. RStudio Version 2025.05.0. 2025. Available online: https://posit.co/blog/rstudio-2025-05-0-whats-new/ (accessed on 10 May 2025).
- Hollister, J.; Shah, T.; Nowosad, J.; Robitaille, A.L.; Beck, M.W.; Johnson, M. Elevatr: Access Elevation Data from Various APIs. CRAN: Contributed Packages. 2023. Available online: https://cran.r-project.org/web/packages/elevatr/index.html (accessed on 10 May 2025).
- Hijmans, R.J. Terra: Spatial Data Analysis. CRAN: Contributed Packages. 2025. Available online: https://cran.r-project.org/web/packages/terra/index.html (accessed on 10 May 2025).
- Sparks, A. Nasapower: A NASA POWER Global Meteorology, Surface Solar Energy and Climatology Data Client for R. J. Open Source Softw. 2018, 3, 1035. [Google Scholar] [CrossRef]
- Hargreaves, G.H.; Samani, Z.A. Reference Crop Evapotranspiration from Temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-131.1. 2018. Available online: https://CRAN.R-project.org/package=nlme (accessed on 30 April 2025).
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat Versión 2020. 2020. Available online: https://www.infostat.com.ar/ (accessed on 30 April 2025).
- Pebesma, E.; Graeler, B. Gstat: Spatial and Spatio-Temporal Geostatistical Modelling, Prediction and Simulation. V. 2.1-4. CRAN: Contributed Packages. 2025. Available online: https://cran.r-project.org/web/packages/gstat/index.html (accessed on 10 May 2025).
- Harrell, F.E., Jr. Hmisc: Harrell Miscellaneous. Version: 5.1-3. CRAN: Contributed Packages. 2024. Available online: https://cran.r-project.org/web/packages/Hmisc/index.html (accessed on 10 May 2025).
- Worldwide, R.C.T. and contributors Package The R Stats Package Version 4.3.3. 2024. Available online: https://stat.ethz.ch/R-manual/R-devel/library/stats/html/00Index.html (accessed on 30 April 2025).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Package ‘Ggplot2’: Create Elegant Data Visualisations Using the Grammar of Graphics Version 3.3.3. 2020. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 10 May 2025).
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48. [Google Scholar] [CrossRef]
- Iannone, R.; Roy, O. DiagrammeR: Graph/Network Visualization. Version: 1.0.11. CRAN: Contributed Packages. 2024. Available online: https://cran.r-project.org/web/packages/DiagrammeR/index.html (accessed on 10 May 2025).
- Luo, Y.; Zhou, X. Soil Respiration and the Environment. Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780120887828. [Google Scholar]
- Kouadio, K.A.L.; Kouakou, A.T.M.; Zanh, G.G.; Jagoret, P.; Bastin, J.-F.; Barima, Y.S.S. Floristic Structure, Potential Carbon Stocks, and Dynamics in Cocoa-Based Agroforestry Systems in Côte d’Ivoire (West Africa). Agrofor. Syst. 2024, 99, 12. [Google Scholar] [CrossRef]
- Wolf, S.; Paul-Limoges, E. Drought and Heat Reduce Forest Carbon Uptake. Nat. Commun. 2023, 14, 6217. [Google Scholar] [CrossRef] [PubMed]
- Sousa Neto, E.; Carmo, J.B.; Keller, M.; Martins, S.C.; Alves, L.F.; Vieira, S.A.; Piccolo, M.C.; Camargo, P.; Couto, H.T.Z.; Joly, C.A.; et al. Soil-Atmosphere Exchange of Nitrous Oxide, Methane and Carbon Dioxide in a Gradient of Elevation in the Coastal Brazilian Atlantic Forest. Biogeosciences 2011, 8, 733–742. [Google Scholar] [CrossRef]
- Li, T.; Lu, L.; Kang, Z.; Li, H.; Li, H. Warming Enhances Soil Microbial Respiration through Divergent Mechanisms in a Tropical Forest and a Temperate Forest. Geoderma 2025, 459, 117380. [Google Scholar] [CrossRef]
- Gui, W.; You, Y.; Yang, F.; Zhang, M. Soil Bulk Density and Matric Potential Regulate Soil CO2 Emissions by Altering Pore Characteristics and Water Content. Land 2023, 12, 1646. [Google Scholar] [CrossRef]
- Kohl, T.; Niether, W.; Abdulai, I. Impact of Common Shade Tree Species on Microclimate and Cocoa Growth in Agroforestry Systems in Ghana. Agrofor. Syst. 2024, 98, 1579–1590. [Google Scholar] [CrossRef]
- Raich, J.W.; Schlesinger, W.H. The Global Carbon Dioxide Flux in Soil Respiration and Its Relationship to Vegetation and Climate. Tellus B Chem. Phys. Meteorol. 1992, 44, 81. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature Sensitivity of Soil Carbon Decomposition and Feedbacks to Climate Change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Martiny, J.B.H. Resistance, Resilience, and Redundancy in Microbial Communities. Proc. Natl. Acad. Sci. USA 2008, 105, 11512–11519. [Google Scholar] [CrossRef]
- Conrad, R. Microbial Ecology of Methanogens and Methanotrophs. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2007; Volume 96, pp. 1–63. ISSN 0065-2113. [Google Scholar]
- Le Mer, J.; Roger, P. Production, Oxidation, Emission and Consumption of Methane by Soils: A Review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Malghani, S.; Han, M.; Cao, P.; Sun, J.; Xu, X.; Xu, X.; Wang, W. Effects of Soil Moisture and Temperature on Microbial Regulation of Methane Fluxes in a Poplar Plantation. Forests 2021, 12, 407. [Google Scholar] [CrossRef]
- Miharza, T.; Wijayanto, N.; Roshetko, J.M.; Siregar, I.Z. Carbon Stocks and Footprints of Smallholder Cacao Systems in Polewali Mandar, West Sulawesi. Front. Environ. Sci. 2023, 11, 680984. [Google Scholar] [CrossRef]
- Dawoe, E.; Asante, W.; Acheampong, E.; Bosu, P. Shade Tree Diversity and Aboveground Carbon Stocks in Theobroma Cacao Agroforestry Systems: Implications for REDD+ Implementation in a West African Cacao Landscape. Carbon. Balance Manag. 2016, 11, 17. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, X.; Xia, W.; Li, Z.; Wang, L.; Chen, Z.; Ge, S. Effect of Extreme PH Conditions on Methanogenesis: Methanogen Metabolism and Community Structure. Sci. Total Environ. 2023, 877, 162702. [Google Scholar] [CrossRef]
- Butterbach-Bahl, K.; Baggs, E.M.; Dannenmann, M.; Kiese, R.; Zechmeister-Boltenstern, S. Nitrous Oxide Emissions from Soils: How Well Do We Understand the Processes and Their Controls? Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20130122. [Google Scholar] [CrossRef]
- Davidson, E.A.; Verchot, L.V. Testing the Hole-in-the-Pipe Model of Nitric and Nitrous Oxide Emissions from Soils Using the TRAGNET Database. Glob. Biogeochem. Cycles 2000, 14, 1035–1043. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of Greenhouse Gases between Soil and Atmosphere: Interactions of Soil Physical Factors and Biological Processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef]
- Chen, Q.; Chang, L.; Khan, K.S.; Chai, S.; Chai, Y.; Han, F. The Impact of the Soil Environment and Surface Mulching on N2O Emissions from Farmland. Sustainability 2025, 17, 2502. [Google Scholar] [CrossRef]
- Wang, C.; Amon, B.; Schulz, K.; Mehdi, B. Factors That Influence Nitrous Oxide Emissions from Agricultural Soils as Well as Their Representation in Simulation Models: A Review. Agronomy 2021, 11, 770. [Google Scholar] [CrossRef]
- Ju, O.; Kang, N.; Soh, H.; Park, J.-S.; Choi, E.; Jeong, H. Nitrous Oxide Emissions during Cultivation and Fallow Periods from Rice Paddy Soil under Urea Fertilization. Atmosphere 2024, 15, 143. [Google Scholar] [CrossRef]
- Wang, L.; Hao, D.-C.; Fan, S.; Xie, H.; Bao, X.; Jia, Z.; Wang, L. N2O Emission and Nitrification/Denitrification Bacterial Communities in Upland Black Soil under Combined Effects of Early and Immediate Moisture. Agriculture 2022, 12, 330. [Google Scholar] [CrossRef]
- Žurovec, O.; Wall, D.P.; Brennan, F.P.; Krol, D.J.; Forrestal, P.J.; Richards, K.G. Increasing Soil PH Reduces Fertiliser Derived N2O Emissions in Intensively Managed Temperate Grassland. Agric. Ecosyst. Environ. 2021, 311, 107319. [Google Scholar] [CrossRef]
- Turner, P.A.; Griffis, T.J.; Lee, X.; Baker, J.M.; Venterea, R.T.; Wood, J.D. Indirect Nitrous Oxide Emissions from Streams within the US Corn Belt Scale with Stream Order. Proc. Natl. Acad. Sci. USA 2015, 112, 9839–9843. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Miah, M.A.; Hassan, M.M.; Mobin, M.N.; Baten, M.A. Textural Influence on Surface and Subsurface Soil Temperatures under Various Conditions. J. Environ. Sci. Nat. Resour. 2016, 8, 147–151. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Bhagwat, S.A.; Buchori, D.; Faust, H.; Hertel, D.; Hölscher, D.; Juhrbandt, J.; Kessler, M.; Perfecto, I.; et al. Multifunctional Shade-Tree Management in Tropical Agroforestry Landscapes—A Review. J. Appl. Ecol. 2011, 48, 619–629. [Google Scholar] [CrossRef]
- Somarriba, E.; Cerda, R.; Orozco, L.; Cifuentes, M.; Dávila, H.; Espin, T.; Mavisoy, H.; Ávila, G.; Alvarado, E.; Poveda, V.; et al. Carbon Stocks and Cocoa Yields in Agroforestry Systems of Central America. Agric. Ecosyst. Environ. 2013, 173, 46–57. [Google Scholar] [CrossRef]
- Gockowski, J.; Sonwa, D. Cocoa Intensification Scenarios and Their Predicted Impact on CO2 Emissions, Biodiversity Conservation, and Rural Livelihoods in the Guinea Rain Forest of West Africa. Environ. Manag. 2011, 48, 307–321. [Google Scholar] [CrossRef]
- Ruf, F.; Schroth, G.; Doffangui, K. Climate Change, Cocoa Migrations and Deforestation in West Africa: What Does the Past Tell Us about the Future? Sustain. Sci. 2015, 10, 101–111. [Google Scholar] [CrossRef]
- Vaast, P.; Somarriba, E. Trade-Offs between Crop Intensification and Ecosystem Services: The Role of Agroforestry in Cocoa Cultivation. Agrofor. Syst. 2014, 88, 947–956. [Google Scholar] [CrossRef]
- Quiñones, C.M.O.; Veldkamp, E.; Lina, S.B.; Bande, M.J.M.; Arribado, A.O.; Corre, M.D. Soil Greenhouse Gas Fluxes from Tropical Vegetable Farms, Using Forest as a Reference. Nutr. Cycl. Agroecosystems 2022, 124, 59–79. [Google Scholar] [CrossRef]
- Cooper, H.V.; Evers, S.; Aplin, P.; Crout, N.; Dahalan, M.P.B.; Sjogersten, S. Greenhouse Gas Emissions Resulting from Conversion of Peat Swamp Forest to Oil Palm Plantation. Nat. Commun. 2020, 11, 407. [Google Scholar] [CrossRef]
- Lestari, I.; Murdiyarso, D.; Taufik, M. Rewetting Tropical Peatlands Reduced Net Greenhouse Gas Emissions in Riau Province, Indonesia. Forests 2022, 13, 505. [Google Scholar] [CrossRef]
- Ishikura, K.; Darung, U.; Inoue, T.; Hatano, R. Variation in Soil Properties Regulate Greenhouse Gas Fluxes and Global Warming Potential in Three Land Use Types on Tropical Peat. Atmosphere 2018, 9, 465. [Google Scholar] [CrossRef]
- Baah-Acheamfour, M.; Carlyle, C.N.; Lim, S.-S.; Bork, E.W.; Chang, S.X. Forest and Grassland Cover Types Reduce Net Greenhouse Gas Emissions from Agricultural Soils. Sci. Total Environ. 2016, 571, 1115–1127. [Google Scholar] [CrossRef]
| Attribute | Development Stage | Dry | Wet | ||
|---|---|---|---|---|---|
| Biodiverse | Conventional | Biodiverse | Conventional | ||
| Location (DMS) | Growing | 0°22′51.00″ N, 6°36′48.00″ E | 0°22′51.25″ N, 6°36′49.21″ E | 0°14′3.45″ N, 6°42′50.04″ E | 0°15′23.26″ N, 6°40′48.49″ E |
| Productive | 0°22′47.00″ N, 6°37′14.00″ E | 0°22′42.02″ N, 6°37′13.13″ E | 0°13′59.00″ N, 6°42′44.00″ E | 0°14′0.50″ N, 6°42′47.30″ E | |
| Elevation (m a.s.l.) | Growing | 50.00 | 50.00 | 114.00 | 343.00 |
| Productive | 214.00 | 214.00 | 114.00 | 114.00 | |
| Slope (°) | Growing | 4.44 | 6.36 | 5.55 | 20.59 |
| Productive | 11.07 | 12.06 | 10.95 | 12.05 | |
| Age (year) | Growing | 2.00 | 3.00 | 3.50 | 4.00 |
| Productive | 15.00 | 17.00 | 14.00 | 22.00 | |
| Dominant species | Growing | T. cacao, Ananas comosus, Manihot esculenta, Cocos nucifera, Xanthosoma sagittifolium, Musa acuminata | T. cacao, Musa acuminata, Artocarpus heterophyllus, Erythrina sp. | T. cacao, Xanthosoma sagittifolium, Manihot esculenta, Musa acuminata, Arecaceae spp., Artocarpus altilis, Cedrela odorata | T. cacao, Xanthosoma sagittifolium, Musa acuminata, Artocarpus heterophyllus |
| Productive | T. cacao, Citrus sinensis, Erythrina sp., Musa acuminata, Psidium guajava, Dacryodes edulis | T. cacao, Manihot esculenta, Xanthosoma sagittifolium, Cedrela odorata | T. cacao, Manihot esculenta, Xanthosoma sagittifolium, Spondias mombin, Annona muricata, Persea americana | T. cacao, Xanthosoma sagittifolium, Musa acuminata | |
| DH30/DBH (cm) Cacao | Growing | 1.32 | 1.75 | 2.96 | 3.20 |
| Productive | 14.55 | 13.87 | 14.88 | 15.12 | |
| Height (m) Cacao | Growing | 2.01 | 1.44 | 1.56 | 1.57 |
| Productive | 4.38 | 4.24 | 4.53 | 4.48 | |
| SOC (Mg ha−1) | Growing | 73.52 | 55.71 | 67.41 | 62.88 |
| Productive | 75.87 | 70.87 | 75.04 | 58.76 | |
| AGBc (Mg C ha−1) | Growing | 13.61 | 12.20 | 14.50 | 11.80 |
| Productive | 23.00 | 19.19 | 28.74 | 24.92 | |
| Zone | System Type | Development Stage | GWP (Mg CO2-eq ha−1 Year−1) |
|---|---|---|---|
| Dry | Biodiverse | Growing | 27.74 ± 3.00 |
| Dry | Biodiverse | Productive | 14.19 ± 0.46 |
| Dry | Conventional | Growing | 26.19 ± 5.04 |
| Dry | Conventional | Productive | 22.71 ± 1.43 |
| Wet | Biodiverse | Growing | 12.98 ± 0.62 |
| Wet | Biodiverse | Productive | 9.05 ± 2.77 |
| Wet | Conventional | Growing | 40.90 ± 6.23 |
| Wet | Conventional | Productive | 11.99 ± 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sterling, A.; Suárez-Córdoba, Y.D.; Orlandi, F.d.B.; Rodríguez-León, C.H. Soil–Atmosphere GHG Fluxes in Cacao Agroecosystems on São Tomé Island, Central Africa: Toward Climate-Smart Practices. Land 2025, 14, 1918. https://doi.org/10.3390/land14091918
Sterling A, Suárez-Córdoba YD, Orlandi FdB, Rodríguez-León CH. Soil–Atmosphere GHG Fluxes in Cacao Agroecosystems on São Tomé Island, Central Africa: Toward Climate-Smart Practices. Land. 2025; 14(9):1918. https://doi.org/10.3390/land14091918
Chicago/Turabian StyleSterling, Armando, Yerson D. Suárez-Córdoba, Francesca del Bove Orlandi, and Carlos H. Rodríguez-León. 2025. "Soil–Atmosphere GHG Fluxes in Cacao Agroecosystems on São Tomé Island, Central Africa: Toward Climate-Smart Practices" Land 14, no. 9: 1918. https://doi.org/10.3390/land14091918
APA StyleSterling, A., Suárez-Córdoba, Y. D., Orlandi, F. d. B., & Rodríguez-León, C. H. (2025). Soil–Atmosphere GHG Fluxes in Cacao Agroecosystems on São Tomé Island, Central Africa: Toward Climate-Smart Practices. Land, 14(9), 1918. https://doi.org/10.3390/land14091918









