Do Soil Methanotrophs Really Remove About 5% of Atmospheric Methane?
Abstract
1. Introduction
2. Methodology
3. Microbial-Driven Methane Oxidation: Current Status and Limitations
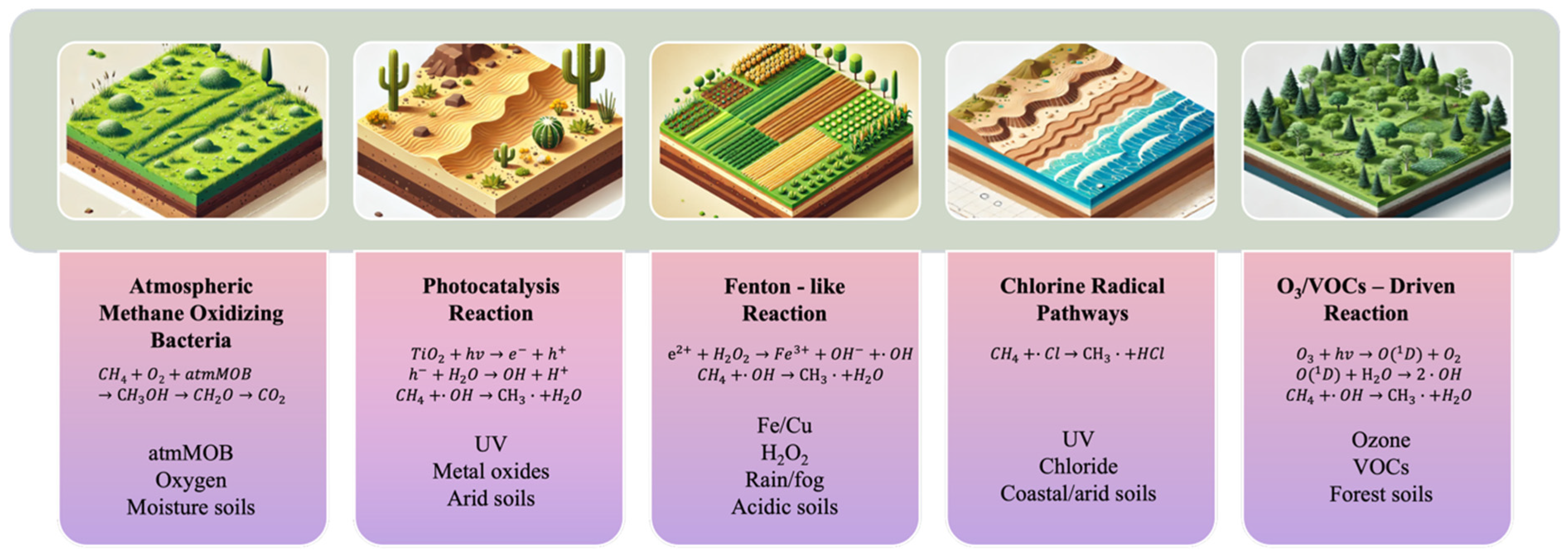
4. Potential Chemical Mechanisms
4.1. Photocatalysis
4.2. Fenton-like Reactions
- Chemical reaction mechanisms involved:
4.3. Chlorine Radical Pathway
- Chemical reaction mechanisms involved:
4.4. Ozone/VOCs Driven Reaction
4.5. Free Radicals
4.6. Coexistence and Integration with Microbial Processes
5. Discussion
- Radiotracer experiments
- Temperature variations and its effects
- Soils of arid or desert regions and rain effects
- Soil pH modification
- Kinetic isotope effect
- Diffusion rates in soils
- Diffusion rates in subsurface environments
- Models and AMR attribution to methanotrophic activity
- The scale of global air-soil exchange
6. Conclusions and Future
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Academies of Sciences, Engineering, and Medicine. A Research Agenda Toward Atmospheric Methane Removal; The National Academies Press: Washington, DC, USA, 2024. [Google Scholar] [CrossRef]
- Lan, X.; Thoning, K.; Dlugokencky, E. Trends in Globally-Averaged CH4, N2O, and SF6 Determined from NOAA Global Monitoring Laboratory Measurements. Available online: https://gml.noaa.gov/ccgg/trends_ch4/ (accessed on 22 February 2025).
- Saunois, M.; Martinez, A.; Poulter, B.; Zhang, Z.; Raymond, P.A.; Regnier, P.; Canadell, J.G.; Jackson, R.B.; Patra, P.K.; Bousquet, P.; et al. Global Methane Budget 2000–2020. Earth Syst. Sci. Data 2025, 17, 1873–1958. [Google Scholar] [CrossRef]
- Roslev, P.; Iversen, N.; Henriksen, K. Oxidation and assimilation of atmospheric methane by soil methane oxidizers. Appl. Environ. Microbiol. 1997, 63, 874–880. [Google Scholar] [CrossRef]
- Steudler, P.A.; Bowden, R.D.; Melillo, J.M.; Aber, J.D. Influence of nitrogen fertilization on methane uptake in temperate forest soils. Nature 1989, 341, 314–316. [Google Scholar] [CrossRef]
- Tveit, A.T.; Hestnes, A.G.; Robinson, S.L.; Schintlmeister, A.; Dedysh, S.N.; Jehmlich, N.; von Bergen, M.; Herbold, C.; Wagner, M.; Richter, A.; et al. Widespread soil bacterium that oxidizes atmospheric methane. Proc. Natl. Acad. Sci. USA 2019, 116, 8515–8524. [Google Scholar] [CrossRef]
- Schmider, T.; Hestnes, A.G.; Brzykcy, J.; Schmidt, H.; Schintlmeister, A.; Roller, B.R.K.; Teran, E.J.; Söllinger, A.; Schmidt, O.; Polz, M.F.; et al. Physiological basis for atmospheric methane oxidation and methanotrophic growth on air. Nat. Commun. 2024, 15, 4151. [Google Scholar] [CrossRef]
- Davidson, E.A.; Monteverde, D.R.; Semrau, J.D. Viability of enhancing methanotrophy in terrestrial ecosystems exposed to low concentrations of methane. Commun. Earth Environ. 2024, 5, 487. [Google Scholar] [CrossRef]
- Tveit, A.T.; Dumont, M.G.; Schmider, T. Physiology of atmospheric methane-oxidizing bacteria. Curr. Opin. Microbiol. 2025, 88, 102656. [Google Scholar] [CrossRef]
- King, G. Responses of atmospheric methane consumption by soils to global climate change. Glob. Change Biol. 1997, 3, 351–362. [Google Scholar] [CrossRef]
- Song, H.; Peng, C.; Zhu, Q.; Chen, Z.; Blanchet, J.-P.; Liu, Q.; Li, T.; Li, P.; Liu, Z. Quantification and uncertainty of global upland soil methane sinks: Processes, controls, model limitations, and improvements. Earth-Sci. Rev. 2024, 252, 104758. [Google Scholar] [CrossRef]
- Striegl, R.G. Diffusional limits to the consumption of atmospheric methane by soils. Chemosphere 1993, 26, 715–720. [Google Scholar] [CrossRef]
- Whalen, S.C.; Reeburgh, W.S. Consumption of atmospheric methane by tundra soils. Nature 1990, 346, 160–162. [Google Scholar] [CrossRef]
- Fernandez-Cortes, A.; Cuezva, S.; Alvarez-Gallego, M.; Garcia-Anton, E.; Pla, C.; Benavente, D.; Jurado, V.; Saiz-Jimenez, C.; Sanchez-Moral, S. Subterranean atmospheres may act as daily methane sinks. Nat. Commun. 2015, 6, 7003. [Google Scholar] [CrossRef] [PubMed]
- Olszyna, K.J.; Meagher, J.F.; Bailey, E.M. Gas-phase, cloud and rain-water measurements of hydrogen peroxide at a high-elevation site. Atmos. Environ. (1967) 1988, 22, 1699–1706. [Google Scholar] [CrossRef]
- Kok, G.L. Measurements of hydrogen peroxide in rainwater. Atmos. Environ. (1967) 1980, 14, 653–656. [Google Scholar] [CrossRef]
- McElroy, W. Sources of hydrogen peroxide in cloudwater. Atmos. Environ. (1967) 1986, 20, 427–438. [Google Scholar] [CrossRef]
- Heindel, J.P.; Hao, H.; LaCour, R.A.; Head-Gordon, T. Spontaneous formation of hydrogen peroxide in water microdroplets. J. Phys. Chem. Lett. 2022, 13, 10035–10041. [Google Scholar] [CrossRef]
- Mehrgardi, M.A.; Mofidfar, M.; Zare, R.N. Sprayed water microdroplets are able to generate hydrogen peroxide spontaneously. J. Am. Chem. Soc. 2022, 144, 7606–7609. [Google Scholar] [CrossRef]
- Krushinski, L.E.; Dick, J.E. Direct electrochemical evidence suggests that aqueous microdroplets spontaneously produce hydrogen peroxide. Proc. Natl. Acad. Sci. USA 2024, 121, e2321064121. [Google Scholar] [CrossRef]
- Qiu, L.; Cooks, R.G. Spontaneous oxidation in aqueous microdroplets: Water radical cation as primary oxidizing agent. Angew. Chem. 2024, 136, e202400118. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Liu, F.; Xiao, S.; Xiao, F.; Dong, X.; Shan, S. Water microdroplets: A catalyst-free source of reactive oxygen species for pollutants removal. J. Clean. Prod. 2023, 420, 138444. [Google Scholar] [CrossRef]
- Zhou, K.; Su, H.; Gao, J.; Li, H.; Liu, S.; Yi, X.; Zhang, Z.; Wang, W. Deciphering the Kinetics of Spontaneous Generation of H2O2 in Individual Water Microdroplets. J. Am. Chem. Soc. 2024, 146, 2445–2451. [Google Scholar] [CrossRef]
- Leung, P.M.; Bay, S.K.; Meier, D.V.; Chiri, E.; Cowan, D.A.; Gillor, O.; Woebken, D.; Greening, C.; Stegen, J.C. Energetic basis of microbial growth and persistence in desert ecosystems. mSystems 2020, 5. [Google Scholar] [CrossRef]
- Riley, W.J.; Subin, Z.M.; Lawrence, D.M.; Swenson, S.C.; Torn, M.S.; Meng, L.; Mahowald, N.M.; Hess, P. Barriers to predicting changes in global terrestrial methane fluxes: Analyses using CLM4Me, a methane biogeochemistry model integrated in CESM. Biogeosciences 2011, 8, 1925–1953. [Google Scholar] [CrossRef]
- Murguia-Flores, F.; Arndt, S.; Ganesan, A.L.; Murray-Tortarolo, G.; Hornibrook, E.R.C. Soil Methanotrophy Model (MeMo v1.0): A process-based model to quantify global uptake of atmospheric methane by soil. Geosci. Model Dev. 2018, 11, 2009–2032. [Google Scholar] [CrossRef]
- Striegl, R.G.; McConnaughey, T.A.; Thorstenson, D.C.; Weeks, E.P.; Woodward, J.C. Consumption of atmospheric methane by desert soils. Nature 1992, 357, 145–147. [Google Scholar] [CrossRef]
- Curry, C.L. Modeling the soil consumption of atmospheric methane at the global scale. Glob. Biogeochem. Cycles 2007, 21, GB4012. [Google Scholar] [CrossRef]
- Mosier, A.; Parton, W.; Valentine, D.; Ojima, D.; Schimel, D.; Heinemeyer, O. CH4 and N2O fluxes in the Colorado shortgrass steppe: 2. Long-term impact of land use chang. Glob. Biogeochem. Cycles 1997, 11, 29–42. [Google Scholar] [CrossRef]
- Voigt, C.; Virkkala, A.-M.; Gosselin, G.H.; Bennett, K.A.; Black, T.A.; Detto, M.; Chevrier-Dion, C.; Guggenberger, G.; Hashmi, W.; Kohl, L.; et al. Arctic soil methane sink increases with drier conditions and higher ecosystem respiration. Nat. Clim. Chang. 2023, 13, 1095–1104. [Google Scholar] [CrossRef]
- Lau, M.C.; Stackhouse, B.T.; Layton, A.C.; Chauhan, A.; Vishnivetskaya, T.A.; Chourey, K.; Ronholm, J.; Mykytczuk, N.C.S.; Bennett, P.C.; Lamarche-Gagnon, G.; et al. An active atmospheric methane sink in high Arctic mineral cryosols. ISME J. 2015, 9, 1880–1891. [Google Scholar] [CrossRef]
- Boeckx, P.; Van Cleemput, O.; Villaralvo, I. Methane oxidation in soils with different textures and land use. Nutr. Cycl. Agroecosyst. 1997, 49, 91–95. [Google Scholar] [CrossRef]
- Liu, L.; Estiarte, M.; Peñuelas, J. Soil moisture as the key factor of atmospheric CH4 uptake in forest soils under environmental change. Geoderma 2019, 355, 113920. [Google Scholar] [CrossRef]
- Adamsen, A.P.S.; King, G.M. Methane consumption in temperate and subarctic forest soils: Rates, vertical zonation, and responses to water and nitrogen. Appl. Environ. Microbiol. 1993, 59, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Rafalska, A.; Walkiewicz, A.; Osborne, B.; Klumpp, K.; Bieganowski, A. Variation in methane uptake by grassland soils in the context of climate change–A review of effects and mechanisms. Sci. Total Environ. 2023, 871, 162127. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, W.; Cheng, L.; Smaill, S.J.; Peng, S. Unveiling the impact of soil methane sink on atmospheric methane concentrations in 2020. Glob. Change Biol. 2024, 30, e17381. [Google Scholar] [CrossRef]
- Dörr, H.; Katruff, L.; Levin, I. Soil texture parameterization of the methane uptake in aerated soils. Chemosphere 1993, 26, 697–713. [Google Scholar] [CrossRef]
- Gorgolewski, A.S.; Caspersen, J.P.; Vantellingen, J.; Thomas, S.C. Tree foliage is a methane sink in upland temperate forests. Ecosystems 2023, 26, 174–186. [Google Scholar] [CrossRef]
- Kim, K.; Daly, E.J.; Hernandez-Ramirez, G. Perennial grain cropping enhances the soil methane sink in temperate agroecosystems. Geoderma 2021, 388, 114931. [Google Scholar] [CrossRef]
- Cowan, N.; Maire, J.; Krol, D.; Cloy, J.M.; Hargreaves, P.; Murphy, R.; Carswell, A.; Jones, S.K.; Hinton, N.; Anderson, M.; et al. Agricultural soils: A sink or source of methane across the British Isles? Eur. J. Soil Sci. 2021, 72, 1842–1862. [Google Scholar] [CrossRef]
- D’iMperio, L.; Li, B.-B.; Tiedje, J.M.; Oh, Y.; Christiansen, J.R.; Kepfer-Rojas, S.; Westergaard-Nielsen, A.; Brandt, K.K.; Holm, P.E.; Wang, P.; et al. Spatial controls of methane uptake in upland soils across climatic and geological regions in Greenland. Commun. Earth Environ. 2023, 4, 461. [Google Scholar] [CrossRef]
- Jørgensen, C.J.; Johansen, K.M.L.; Westergaard-Nielsen, A.; Elberling, B. Net regional methane sink in High Arctic soils of northeast Greenland. Nat. Geosci. 2015, 8, 20–23. [Google Scholar] [CrossRef]
- Lee, J.; Oh, Y.; Lee, S.T.; Seo, Y.O.; Yun, J.; Yang, Y.; Kim, J.; Zhuang, Q.; Kang, H. Soil organic carbon is a key determinant of CH4 sink in global forest soils. Nat. Commun. 2023, 14, 3110. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.; Davidson, E.; Verchot, L. Estimation of global biogeochemical controls and seasonality in soil methane consumption. Chemosphere 1996, 32, 2219–2246. [Google Scholar] [CrossRef]
- Kumaraswamy, S.; Rath, A.K.; Ramakrishnan, B.; Sethunathan, N. Wetland rice soils as sources and sinks of methane: A review and prospects for research. Biol. Fertil. Soils 2000, 31, 449–461. [Google Scholar] [CrossRef]
- Castaldi, S.; Costantini, M.; Cenciarelli, P.; Ciccioli, P.; Valentini, R. The methane sink associated to soils of natural and agricultural ecosystems in Italy. Chemosphere 2007, 66, 723–729. [Google Scholar] [CrossRef]
- Dutaur, L.; Verchot, L.V. A global inventory of the soil CH4 sink. Glob. Biogeochem. Cycles 2007, 21, GB4013. [Google Scholar] [CrossRef]
- Lennon, J.T.; Nguyễn-Thùy, D.; Phạm, T.M.; Drobniak, A.; Tạ, P.H.; Phạm, N.Ð.; Streil, T.; Webster, K.D.; Schimmelmann, A. Microbial contributions to subterranean methane sinks. Geobiology 2017, 15, 254–258. [Google Scholar] [CrossRef]
- de_Richter, R.; Caillol, S. Fighting global warming: The potential of photocatalysis against CO2, CH4, N2O, CFCs, tropospheric O3, BC and other major contributors to climate change. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 1–19. [Google Scholar] [CrossRef]
- Wang, P.; Shi, R.; Zhao, J.; Zhang, T. Photodriven methane conversion on transition metal oxide catalyst: Recent progress and prospects. Adv. Sci. 2024, 11, e2305471. [Google Scholar] [CrossRef]
- Shah, S.A.S.; Oh, C.; Park, H.; Hwang, Y.J.; Ma, M.; Park, J.H. Catalytic oxidation of methane to oxygenated products: Recent advancements and prospects for electrocatalytic and photocatalytic conversion at low temperatures. Adv. Sci. 2020, 7, 2001946. [Google Scholar] [CrossRef]
- Lin, X.-Y.; Li, J.-Y.; Qi, M.-Y.; Tang, Z.-R.; Xu, Y.-J. Methane conversion over artificial photocatalysts. Catal. Commun. 2021, 159, 106346. [Google Scholar] [CrossRef]
- Tsopelakou, A.M.; Stallard, J.; Archibald, A.T.; Fitzgerald, S.; Boies, A.M. Exploring the bounds of methane catalysis in the context of atmospheric methane removal. Environ. Res. Lett. 2024, 19, 054020. [Google Scholar] [CrossRef]
- Brenneis, R.J.; Johnson, E.P.; Shi, W.; Plata, D.L. Atmospheric- and low-level methane abatement via an earth-abundant catalyst. ACS Environ. Au 2021, 2, 223–231. [Google Scholar] [CrossRef]
- de Richter, R.; Ming, T.; Davies, P.L.; Liu, W.; Caillol, S. Removal of non-CO2 greenhouse gases by large-scale atmospheric solar photocatalysis. Prog. Energy Combust. Sci. 2017, 60, 68–96. [Google Scholar] [CrossRef]
- Ming, T.; Gui, H.; Shi, T.; Xiong, H.; Wu, Y.; Shao, Y.; Li, W.; Lu, X.; de Richter, R. Solar chimney power plant integrated with a photocatalytic reactor to remove atmospheric methane: A numerical analysis. Sol. Energy 2021, 226, 101–111. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wang, Y.; Bai, Y.; Feng, X.; Zhu, J.; Lu, X.; Mu, L.; Ming, T.; de Richter, R.; et al. Solar Driven Gas Phase Advanced Oxidation Processes for Methane Removal-Challenges and Perspectives. Chem.-A Eur. J. 2022, 28, e202201984. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Ortiz, V.; Rubio, M.A.; Lissi, E.A. Hydrogen peroxide deposition and decomposition in rain and dew waters. Atmos. Environ. 2000, 34, 1139–1146. [Google Scholar] [CrossRef]
- Faust, B.C.; Anastasio, C.; Allen, J.M.; Arakaki, T. Aqueous-phase photochemical formation of peroxides in authentic cloud and fog waters. Science 1993, 260, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Pierotti, D.; Rasmussen, L.E.; Rasmussen, R.A. The Sahara as a possible sink for trace gases. Geophys. Res. Lett. 1978, 5, 1001–1004. [Google Scholar] [CrossRef]
- Rebbert, R.E.; Ausloos, P. Decomposition of N2O over particulate matter. Geophys. Res. Lett. 1978, 5, 761–764. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic production of hydrogen peroxides and organic peroxides in aqueous suspensions of titanium dioxide, zinc oxide, and desert sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Yan, X.; Bain, R.M.; Cooks, R.G. Organic reactions in microdroplets: Reaction acceleration revealed by mass spectrometry. Angew. Chem. Int. Ed. Engl. 2016, 55, 12960–12972. [Google Scholar] [CrossRef]
- Lee, J.K.; Banerjee, S.; Nam, H.G.; Zare, R.N. Acceleration of reaction in charged microdroplets. Q. Rev. Biophys. 2015, 48, 437–444. [Google Scholar] [CrossRef]
- Gauci, V.; Pangala, S.R.; Shenkin, A.; Barba, J.; Bastviken, D.; Figueiredo, V.; Gomez, C.; Enrich-Prast, A.; Sayer, E.; Stauffer, T.; et al. Global atmospheric methane uptake by upland tree woody surfaces. Nature 2024, 631, 796–800. [Google Scholar] [CrossRef]
- Buckley, P.T.; Birks, J.W. Evaluation of visible-light photolysis of ozone-water cluster molecules as a source of atmospheric hydroxyl radical and hydrogen peroxide. Atmos. Environ. 1995, 29, 2409–2415. [Google Scholar] [CrossRef]
- Du, H.; Hu, X.; Huang, Y.; Bai, Y.; Fei, Y.; Gao, M.; Li, Z. A review of copper-based Fenton reactions for the removal of organic pollutants from wastewater over the last decade: Different reaction systems. Environ. Sci. Pollut. Res. 2024, 31, 27609–27633. [Google Scholar] [CrossRef] [PubMed]
- Wittmer, J.; Bleicher, S.; Zetzsch, C. Iron(III)-Induced activation of chloride and bromide from modeled salt pans. J. Phys. Chem. A 2015, 119, 4373–4385. [Google Scholar] [CrossRef]
- Oeste, F.D.; de Richter, R.; Ming, T.; Caillol, S. Climate engineering by mimicking natural dust climate control: The iron salt aerosol method. Earth Syst. Dyn. 2017, 8, 1–54. [Google Scholar] [CrossRef]
- van Herpen, M.M.; Li, Q.; Saiz-Lopez, A.; Liisberg, J.B.; Röckmann, T.; Cuevas, C.A.; Fernandez, R.P.; Mak, J.E.; Natalie Mahowald, N.M.; Hess, P.; et al. Photocatalytic chlorine atom production on mineral dust–sea spray aerosols over the North Atlantic. Proc. Natl. Acad. Sci. USA 2023, 120, e2303974120. [Google Scholar] [CrossRef]
- Li, Y.; Nie, W.; Liu, Y.; Huang, D.; Xu, Z.; Peng, X.; George, C.; Yan, C.; Tham, Y.J.; Yu, C.; et al. Photoinduced production of chlorine molecules from titanium dioxide surfaces containing chloride. Environ. Sci. Technol. Lett. 2020, 7, 70–75. [Google Scholar] [CrossRef]
- Custard, K.D.; Raso, A.R.W.; Shepson, P.B.; Staebler, R.M.; Pratt, K.A. Production and release of molecular bromine and chlorine from the Arctic coastal snowpack. ACS Earth Space Chem. 2017, 1, 142–151. [Google Scholar] [CrossRef]
- Abbatt, J.P.D.; Thomas, J.L.; Abrahamsson, K.; Boxe, C.; Granfors, A.; Jones, A.E.; King, M.D.; Saiz-Lopez, A.; Shepson, P.B.; Sodeau, J.; et al. Halogen activation via interactions with environmental ice and snow in the polar lower troposphere and other regions. Atmos. Chem. Phys. 2012, 12, 6237–6271. [Google Scholar] [CrossRef]
- Lelieveld, J.; Gromov, S.; Pozzer, A.; Taraborrelli, D. Global tropospheric hydroxyl distribution, budget and reactivity. Atmos. Chem. Phys. 2016, 16, 12477–12493. [Google Scholar] [CrossRef]
- Hanisch, F.; Crowley, J.N. Ozone decomposition on Saharan dust: An experimental investigation. Atmos. Meas. Tech. 2003, 3, 119–130. [Google Scholar] [CrossRef]
- Wedow, J.M.; Ainsworth, E.A.; Li, S. Plant biochemistry influences tropospheric ozone formation, destruction, deposition, and response. Trends Biochem. Sci. 2021, 46, 992–1002. [Google Scholar] [CrossRef]
- Pinto, D.M.; Blande, J.D.; Souza, S.R.; Nerg, A.-M.; Holopainen, J.K. Plant volatile organic compounds (VOCs) in ozone (O3) polluted atmospheres: The ecological effects. J. Chem. Ecol. 2010, 36, 22–34. [Google Scholar] [CrossRef]
- Hui, K.; Yuan, Y.; Xi, B.; Tan, W. A review of the factors affecting the emission of the ozone chemical precursors VOCs and NOx from the soil. Environ. Int. 2023, 172, 107799. [Google Scholar] [CrossRef]
- Ganzeveld, L.; Lelieveld, J.; Dentener, F.; Krol, M.; Bouwman, A.; Roelofs, G.J. Global soil-biogenic NOx emissions and the role of canopy processes. J. Geophys. Res. Atmos. 2002, 107, ACH 9-1–ACH 9-17. [Google Scholar] [CrossRef]
- Alvarez, E.G.; Amedro, D.; Afif, C.; Gligorovski, S.; Schoemaecker, C.; Fittschen, C.; Doussin, J.-F.; Wortham, H. Unexpectedly high indoor hydroxyl radical concentrations associated with nitrous acid. Proc. Natl. Acad. Sci. USA 2013, 110, 13294–13299. [Google Scholar] [CrossRef] [PubMed]
- Fahy, W.D.; Gong, Y.; Wang, S.; Zhang, Z.; Li, L.; Peng, H.; Abbatt, J.P. Hydroxyl radical oxidation of chemical contaminants on indoor surfaces and dust. Proc. Natl. Acad. Sci. USA 2024, 121, e2414762121. [Google Scholar] [CrossRef] [PubMed]
- Merzlyak, M.N.; Hendry, G. Free radical metabolism, pigment degradation and lipid peroxidation in leaves during senescence. Proc. R. Soc. Edinburgh. Sect. B Biol. Sci. 1994, 102, 459–471. [Google Scholar] [CrossRef]
- Reichenauer, T.; Goodman, B. Stable free radicals in ozone-damaged wheat leaves. Free Radic. Res. 2001, 35, 93–101. [Google Scholar] [CrossRef]
- Pearce, R.; Edwards, P.; Green, T.; Anderson, P.; Fisher, B.; Carpenter, T.; Hall, L. Immobilized long-lived free radicals at the host–pathogen interface in sycamore (Acer pseudoplatanus L.). Physiol. Mol. Plant Pathol. 1997, 50, 371–390. [Google Scholar] [CrossRef]
- Priestley, D.A.; Werner, B.G.; Leopold, A.C.; McBride, M.B. Organic free radical levels in seeds and pollen: The effects of hydration and aging. Physiol. Plant. 1985, 64, 88–94. [Google Scholar] [CrossRef]
- Steelink, C. Stable Free Radicals in Lignin and Lignin Oxidation Products. In Lignin Structure and Reactions; Advances in Chemistry; ACS Publications: Washington, DC, USA, 1966; Volume 59, pp. 51–64. [Google Scholar]
- Vejerano, E.P.; Ahn, J. Leaves are a source of biogenic persistent free radicals. Environ. Sci. Technol. Lett. 2023, 10, 662–667. [Google Scholar] [CrossRef]
- Steelink, C.; Tollin, G. Stable free radicals in soil humic acid. Biochim. Biophys. Acta 1962, 59, 25–34. [Google Scholar] [CrossRef]
- Tveit, A.T.; Söllinger, A.; Rainer, E.M.; Didriksen, A.; Hestnes, A.G.; Motleleng, L.; Hellinger, H.-J.; Rattei, T.; Svenning, M.M. Thermal acclimation of methanotrophs from the genus Methylobacter. ISME J. 2023, 17, 502–513. [Google Scholar] [CrossRef]
- Flessa, H.; Dörsch, P.; Beese, F. Seasonal variation of N2O and CH4 fluxes in differently managed arable soils in southern Germany. J. Geophys. Res. Atmos. 1995, 100, 23115–23124. [Google Scholar] [CrossRef]
- Sommerfeld, R.A.; Mosier, A.R.; Musselman, R.C. CO2, CH4 and N2O flux through a Wyoming snowpack and implications for global budgets. Nature 1993, 361, 140–142. [Google Scholar] [CrossRef]
- Sullivan, B.W.; Selmants, P.C.; Hart, S.C. Does dissolved organic carbon regulate biological methane oxidation in semiarid soils? Glob. Change Biol. 2013, 19, 2149–2157. [Google Scholar] [CrossRef]
- Angel, R.; Conrad, R. In situ measurement of methane fluxes and analysis of transcribed particulate methane monooxygenase in desert soils. Environ. Microbiol. 2009, 11, 2598–2610. [Google Scholar] [CrossRef]
- Dunfield, P. Chapter 10: The soil methane sink. In Greenhouse Gas Sinks; CABI: Wetherill Park NSW, Australia, 2007; p. 152. ISBN 978 1 84593 189 6. [Google Scholar]
- Oerter, E.; Mills, J.V.; Maurer, G.E.; Lammers, L.N.; Amundson, R. Greenhouse gas production and transport in desert soils of the southwestern united states. Glob. Biogeochem. Cycles 2018, 32, 1703–1717. [Google Scholar] [CrossRef]
- Hütsch, B.W.; Webster, C.P.; Powlson, D.S. Methane oxidation in soil as affected by land use, soil pH and N fertilization. Soil Biol. Biochem. 1994, 26, 1613–1622. [Google Scholar] [CrossRef]
- Saari, A.; Rinnan, R.; Martikainen, P.J. Methane oxidation in boreal forest soils: Kinetics and sensitivity to pH and ammonium. Soil Biol. Biochem. 2004, 36, 1037–1046. [Google Scholar] [CrossRef]
- Page, K.L.; Allen, D.E.; Dalal, R.C.; Slattery, W. Processes and magnitude of CO2, CH4, and N2O fluxes from liming of Australian acidic soils: A review. Soil Res. 2009, 47, 747–762. [Google Scholar] [CrossRef]
- Shaaban, M.; Peng, Q.-A.; Lin, S.; Wu, Y.; Khalid, M.S.; Wu, L.; Mo, Y.; Hu, R. Dolomite application enhances CH4 uptake in an acidic soil. CATENA 2016, 140, 9–14. [Google Scholar] [CrossRef]
- Gropp, J.; Jin, Q.; Halevy, I. Controls on the isotopic composition of microbial methane. Sci. Adv. 2022, 8, eabm5713. [Google Scholar] [CrossRef] [PubMed]
- Haghnegahdar, M.A.; Sun, J.; Hultquist, N.; Hamovit, N.D.; Kitchen, N.; Eiler, J.; Ono, S.; Yarwood, S.A.; Kaufman, A.J.; Dickerson, R.R.; et al. Tracing sources of atmospheric methane using clumped isotopes. Proc. Natl. Acad. Sci. USA 2023, 120, e2305574120. [Google Scholar] [CrossRef]
- Röckmann, T.; Brenninkmeijer, C.A.; Crutzen, P.J.; Platt, U. Short-term variations in the 13C/12C ratio of CO as a measure of Cl activation during tropospheric ozone depletion events in the Arcti. J. Geophys. Res. Atmos. 1999, 104, 1691–1697. [Google Scholar] [CrossRef]
- Cantrell, C.A.; Shetter, R.E.; McDaniel, A.H.; Calvert, J.G.; Davidson, J.A.; Lowe, D.C.; Tyler, S.C.; Cicerone, R.J.; Greenberg, J.P. Carbon kinetic isotope effect in the oxidation of methane by the hydroxyl radical. J. Geophys. Res. Atmos. 1990, 95, 22455–22462. [Google Scholar] [CrossRef]
- Saueressig, G.; Bergamaschi, P.; Crowley, J.N.; Fischer, H.; Harris, G.W. Carbon kinetic isotope effect in the reaction of CH4 with Cl atoms. Geophys. Res. Lett. 1995, 22, 1225–1228. [Google Scholar] [CrossRef]
- Maxfield, P.J.; Evershed, R.P.; Hornibrook, E.R.C. Physical and biological controls on the in situ kinetic isotope effect associated with oxidation of atmospheric CH4 in mineral soils. Environ. Sci. Technol. 2008, 42, 7824–7830. [Google Scholar] [CrossRef]
- Snover, A.K.; Quay, P.D. Hydrogen and carbon kinetic isotope effects during soil uptake of atmospheric methane. Glob. Biogeochem. Cycles 2000, 14, 25–39. [Google Scholar] [CrossRef]
- McCarthy, M.C.; Boering, K.A.; Rice, A.L.; Tyler, S.C.; Connell, P.; Atlas, E. Carbon and hydrogen isotopic compositions of stratospheric methane: 2. Two-dimensional model results and implications for kinetic isotope effects. J. Geophys. Res. Atmos. 2003, 108, 4461. [Google Scholar] [CrossRef]
- Ridgwell, A.J.; Marshall, S.J.; Gregson, K. Consumption of atmospheric methane by soils: A process-based model. Glob. Biogeochem. Cycles 1999, 13, 59–70. [Google Scholar] [CrossRef]
- Ito, A.; Inatomi, M. Use of a process-based model for assessing the methane budgets of global terrestrial ecosystems and evaluation of uncertainty. Biogeosciences 2012, 9, 759–773. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Lu, C.; Xu, X.; Ren, W.; Zhang, B.; Banger, K.; Tao, B.; Pan, S.; Liu, M.; et al. Global methane and nitrous oxide emissions from terrestrial ecosystems due to multiple environmental changes. Ecosyst. Health Sustain. 2015, 1, 11878978. [Google Scholar] [CrossRef]
- Tian, H.; Xu, X.; Liu, M.; Ren, W.; Zhang, C.; Chen, G.; Lu, C. Spatial and temporal patterns of CH4 and N2O fluxes in terrestrial ecosystems of North America during 1979–2008: Application of a global biogeochemistry model. Biogeosciences 2010, 7, 2673–2694. [Google Scholar] [CrossRef]
- Zhuang, Q.; Chen, M.; Xu, K.; Tang, J.; Saikawa, E.; Lu, Y.; Melillo, J.M.; Prinn, R.G.; McGuire, A.D. Response of global soil consumption of atmospheric methane to changes in atmospheric climate and nitrogen deposition. Glob. Biogeochem. Cycles 2013, 27, 650–663. [Google Scholar] [CrossRef]
- Glagolev, M.V.; Suvorov, G.G.; Il’yAsov, D.V.; Sabrekov, A.F.; Terentieva, I.E. What is the maximal possible soil methane uptake? Environ. Dyn. Glob. Clim. Chang. 2022, 13, 123–141. [Google Scholar] [CrossRef]
- Crill, P.M. Seasonal patterns of methane uptake and carbon dioxide release by a temperate woodland soil. Glob. Biogeochem. Cycles 1991, 5, 319–334. [Google Scholar] [CrossRef]
- Le Mer, J.; Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur. J. Soil Biol. 2001, 37, 25–50. [Google Scholar] [CrossRef]
- Webster, K.D.; Drobniak, A.; Etiope, G.; Mastalerz, M.; Sauer, P.E.; Schimmelmann, A. Subterranean karst environments as a global sink for atmospheric methane. Earth Planet. Sci. Lett. 2018, 485, 9–18. [Google Scholar] [CrossRef]
- Cheng, X.; Zeng, Z.; Liu, X.; Li, L.; Wang, H.; Zhao, R.; Bodelier, P.L.; Wang, W.; Wang, Y.; Tuovinen, O.H. Methanotrophs dominate methanogens and act as a methane sink in a subterranean karst cave. Sci. Total Environ. 2023, 892, 164562. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Fernandez-Cortes, A.; Cuezva, S.; Streil, T.; Lennon, J.T.; Wilbanks, E.G. Radiolysis via radioactivity is not responsible for rapid methane oxidation in subterranean air. PLoS ONE 2018, 13, e0206506. [Google Scholar] [CrossRef]
- Bull, I.D.; Parekh, N.R.; Hall, G.H.; Ineson, P.; Evershed, R.P. Detection and classification of atmospheric methane oxidizing bacteria in soil. Nature 2000, 405, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Harriss, R.C.; Sebacher, D.I.; Day, F.P. Methane flux in the Great Dismal Swamp. Nature 1982, 297, 673–674. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, M.; Zheng, X.; Ji, B.; Du, R.; Wang, Y. Effects of environmental factors on N2O emission from and CH4 uptake by the typical grasslands in the Inner Mongolia. Chemosphere 2005, 58, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wei, D.; Qi, Y.-H.; Wang, X.-D. Temperate northern hemisphere dominates the global soil CH4 sink. J. Mt. Sci. 2022, 19, 3051–3062. [Google Scholar] [CrossRef]
- Walter, B.P.; Heimann, M. A process-based, climate-sensitive model to derive methane emissions from natural wetlands: Application to five wetland sites, sensitivity to model parameters, and climate. Glob. Biogeochem. Cycles 2000, 14, 745–765. [Google Scholar] [CrossRef]
- Segers, R.; Kengen, S. Methane production as a function of anaerobic carbon mineralization: A process model. Soil Biol. Biochem. 1998, 30, 1107–1117. [Google Scholar] [CrossRef]
- Zhuang, Q.; Melillo, J.M.; Kicklighter, D.W.; Prinn, R.G.; McGuire, A.D.; Steudler, P.A.; Felzer, B.S.; Hu, S. Methane fluxes between terrestrial ecosystems and the atmosphere at northern high latitudes during the past century: A retrospective analysis with a process-based biogeochemistry model. Glob. Biogeochem. Cycles 2004, 18, GB3010. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Alcott, L.; Maavara, T.; Sun, X.; Zhou, Y.; Planavsky, N.J.; Raymond, P.A. Revisiting the global methane cycle through expert opinion. Earth’s Futur. 2024, 12, e2023EF004234. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Borges, A.V.; Deemer, B.R.; Holgerson, M.A.; Liu, S.; Song, C.; Melack, J.; Raymond, P.A.; Duarte, C.M.; Allen, G.H.; et al. Half of global methane emissions come from highly variable aquatic ecosystem sources. Nat. Geosci. 2021, 14, 225–230. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhang, M.; Hu, Z.; Gao, Y.; Hu, C.; Liu, C.; Liu, S.; Zhang, Z.; Zhao, J.; Xiao, W.; et al. Spatial variations of methane emission in a large shallow eutrophic lake in subtropical climate. J. Geophys. Res. Biogeosci. 2017, 122, 1597–1614. [Google Scholar] [CrossRef]
- Karambelkar, S.; Fischer, M.; Ames, S. Hydropower Reservoir Greenhouse Gas Emissions: State of the Science and Roadmap for Further Research to Improve Emission Accounting and Mitigation. Sustainability 2025, 17, 5794. [Google Scholar] [CrossRef]
- Lehner, B.; Anand, M.; Fluet-Chouinard, E.; Tan, F.; Aires, F.; Allen, G.H.; Bousquet, P.; Canadell, J.G.; Davidson, N.; Ding, M.; et al. Mapping the world’s inland surface waters: An upgrade to the Global Lakes and Wetlands Database (GLWD v2). Earth Syst. Sci. Data 2025, 17, 2277–2329. [Google Scholar] [CrossRef]
- Hines, M.E.; Crill, P.M.; Varner, R.K.; Talbot, R.W.; Shorter, J.H.; Kolb, C.E.; Harriss, R.C. Rapid consumption of low concentrations of methyl bromide by soil bacteria. Appl. Environ. Microbiol. 1998, 64, 1864–1870. [Google Scholar] [CrossRef]
- Wang, D.T.; Welander, P.V.; Ono, S. Fractionation of the methane isotopologues 13CH4, 12CH3D, and 13CH3D during aerobic oxidation of methane by Methylococcus capsulatus (Bath). Geochim. Et. Cosmochim. Acta 2016, 192, 186–202. [Google Scholar] [CrossRef]
- Douglas, P.M.; Stolper, D.A.; Eiler, J.M.; Sessions, A.L.; Lawson, M.; Shuai, Y.; Bishop, A.; Podlaha, O.G.; Ferreira, A.A.; Neto, E.V.S.; et al. Methane clumped isotopes: Progress and potential for a new isotopic tracer. Org. Geochem. 2017, 113, 262–282. [Google Scholar] [CrossRef]
- Lan, X.; Basu, S.; Schwietzke, S.; Bruhwiler, L.M.; Dlugokencky, E.J.; Michel, S.E.; Sherwood, O.A.; Tans, P.P.; Thoning, K.; Etiope, G.; et al. Improved constraints on global methane emissions and sinks using δ13C-CH4. Glob. Biogeochem. Cycles 2021, 35, e2021GB007000. [Google Scholar] [CrossRef] [PubMed]
| Soil Type | Method Type | Removal Amount | Converted | Ref |
|---|---|---|---|---|
| Forest | Field measurement (upscaling) | [37] | ||
| Forest | Field measurement | [34] | ||
| Forest | Field measurement | [5] | ||
| Forest | Field measurement | [38] | ||
| Agriculture | Field measurement | Lack of data for conversion | [39] | |
| Desert | Field measurement | [27] | ||
| Arable | Field measurement | [40] | ||
| Grassland | Field measurement | [40] | ||
| Arctic | Field measurement | Lack of data for conversion | [41] | |
| Arctic | Field measurement | [30] | ||
| Arctic (dry tundra) | Field measurement | [42] | ||
| Arctic (moist tundra) | Field measurement | [42] | ||
| Forest | Modelling | [43] | ||
| Grassland | Modelling | [44] | ||
| Agriculture | Modelling | [45] | ||
| Forest/Arable | Modelling | [46] | ||
| Global Soil | Modelling | [47] | ||
| Forest | Laboratory incubation | Lack of data for conversion | [4] | |
| Forest | Laboratory incubation | [32] | ||
| Arable | Laboratory incubation | [32] | ||
| Grassland | Laboratory incubation | [32] | ||
| Cave/Subterranean | Laboratory incubation | [48] |
| Mechanism | Applicable Soil Types | Necessary Conditions | Potential Contribution | Evidence Sources |
|---|---|---|---|---|
| MOB Oxidation | All aerated soils | O2, ~20% moisture | Major | [3,4,6,7] |
| Photocatalysis | Desert sands, Semi-arid soils | UV light, TiO2/ZnO/WO3 | Low, local (arid regions) | [27,49,50,51,52] |
| Fenton-like Reactions | Forest soils, Desert soils | H2O2 (rain/fog), Fe/Cu, acidic pH | Moderate, wet soils | [18,19,20,21,22,23,38,58,59,60] |
| •Cl Pathways | Coastal soils, Dust aerosols | Chloride (e.g., FeCl3), UV | High, coastal zones | [70,71,72,73] |
| O3/VOC-Driven Reactions | Forest soils, Aerated soils | O3 decomposition, NOx/VOCs | Low, widespread | [67,77,78,79,80,81,82,83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Tao, T.; Li, W.; Ming, T.; de Richter, R. Do Soil Methanotrophs Really Remove About 5% of Atmospheric Methane? Land 2025, 14, 1864. https://doi.org/10.3390/land14091864
Yao X, Tao T, Li W, Ming T, de Richter R. Do Soil Methanotrophs Really Remove About 5% of Atmospheric Methane? Land. 2025; 14(9):1864. https://doi.org/10.3390/land14091864
Chicago/Turabian StyleYao, Xiaokun, Tao Tao, Wei Li, Tingzhen Ming, and Renaud de Richter. 2025. "Do Soil Methanotrophs Really Remove About 5% of Atmospheric Methane?" Land 14, no. 9: 1864. https://doi.org/10.3390/land14091864
APA StyleYao, X., Tao, T., Li, W., Ming, T., & de Richter, R. (2025). Do Soil Methanotrophs Really Remove About 5% of Atmospheric Methane? Land, 14(9), 1864. https://doi.org/10.3390/land14091864







