The Landscape of Fear and Wild Boar (Sus scrofa) Spatial Use in a Peri-Urban Area from West-Central Spain
Abstract
1. Introduction
2. Materials and Methods
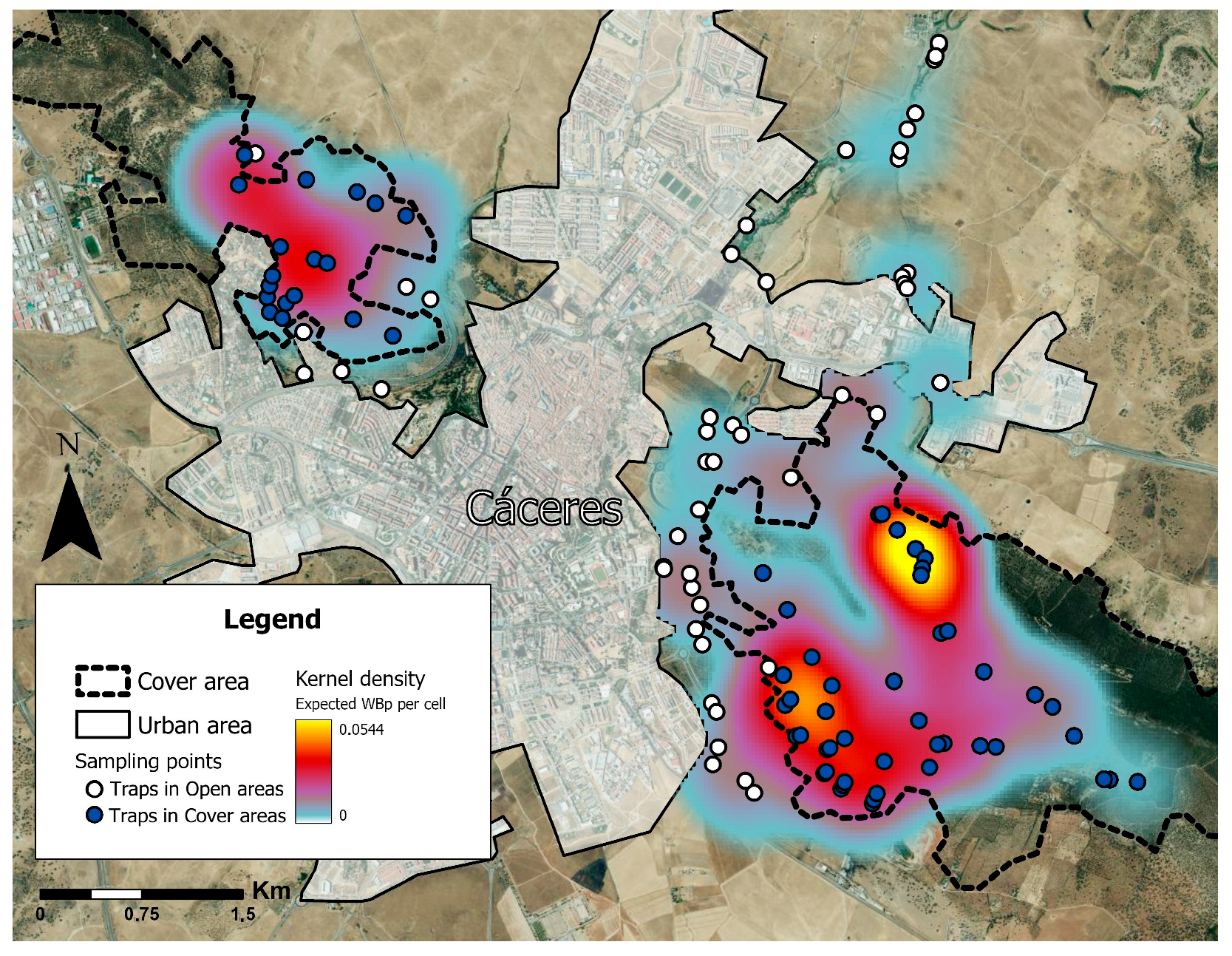
2.1. Study Area
2.2. Wild Boar Presence
2.3. Landscape of Fear Variables
2.4. Spatial and Statistical Analyses
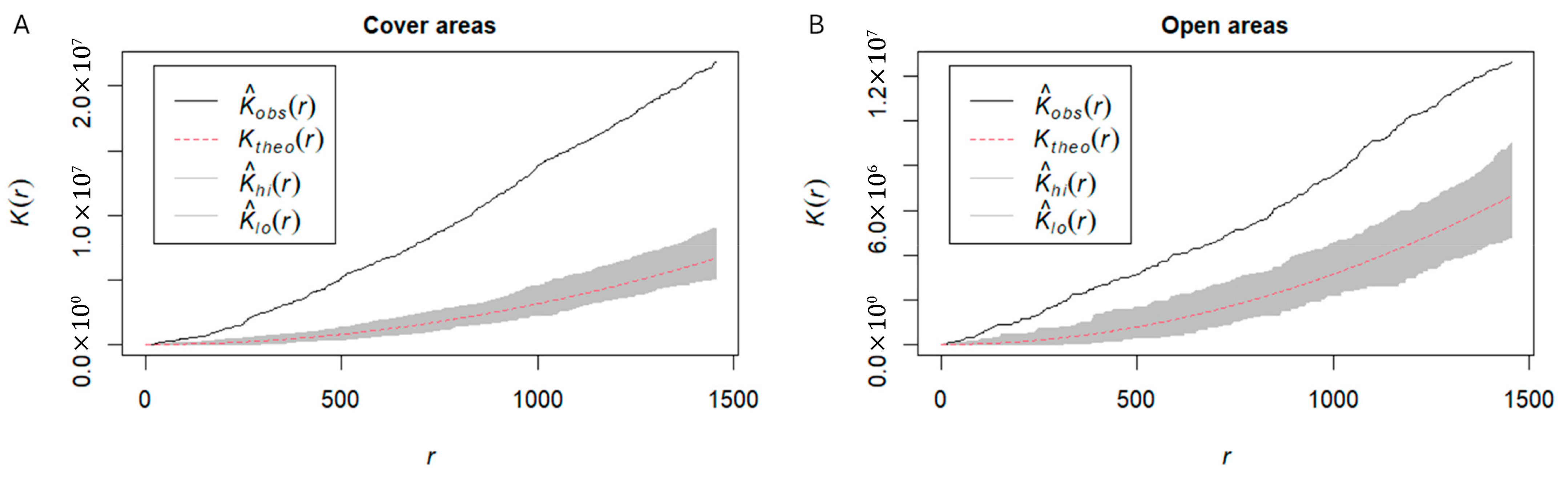
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowlishaw, G. Refuge use and predation risk in a desert baboon population. Anim. Behav. 1997, 54, 241–253. [Google Scholar] [CrossRef]
- Fortin, D.; Beyer, H.L.; Boyce, M.S.; Smith, D.W.; Duchesne, T.; Mao, J.S. Wolves influence elk movements: Behavior shapes a trophic cascade in Yellowstone National Park. Ecology 2005, 86, 1320–1330. [Google Scholar] [CrossRef]
- Bleicher, S.S. The landscape of fear conceptual framework: Definition and review of current applications and misuses. PeerJ 2017, 5, e3772. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Brown, J.S.; Middleton, A.D.; Power, M.E.; Brashares, J.S. Landscapes of fear: Spatial patterns of risk perception and response. Trends Ecol. Evol. 2019, 34, 355–368. [Google Scholar] [CrossRef]
- Laundré, J.W.; Hernández, L.; Ripple, W.J. The landscape of fear: Ecological implications of being afraid. Open Ecol. J. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Doherty, J.F.; Ruehle, B. An integrated landscape of fear and disgust: The evolution of avoidance behaviors amidst a myriad of natural enemies. Front. Ecol. Evol. 2020, 8, 564343. [Google Scholar] [CrossRef]
- Creel, S.; Schuette, P.; Christianson, D. Effects of predation risk on group size, vigilance, and foraging behavior in an African ungulate community. Behav. Ecol. 2014, 25, 773–784. [Google Scholar] [CrossRef]
- Déry, F.; Hamel, S.; Côté, S.D. Linking proximate drivers and fitness returns of vigilance in a large ungulate. Oikos 2025, 2025, e10879. [Google Scholar] [CrossRef]
- Lima, S.L.; Bednekoff, P.A. Temporal variation in danger drives antipredator behavior: The predation risk allocation hypothesis. Am. Nat. 1999, 153, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Descalzo, E.; Tobajas, J.; Villafuerte, R.; Mateo, R.; Ferreras, P. Plasticity in daily activity patterns of a key prey species in the Iberian Peninsula to reduce predation risk. Wildl. Res. 2021, 48, 481–490. [Google Scholar] [CrossRef]
- Creel, S.; Becker, M.S.; Goodheart, B.; de Merkle, J.R.; Dröge, E.; M’soka, J.; Rosenblatt, E.; Mweetwa, T.; Mwape, H.; Vinks, M.A.; et al. Habitat shifts in response to predation risk are constrained by competition within a grazing guild. Front. Ethol. 2023, 2, 1231780. [Google Scholar] [CrossRef]
- Liao, W.; Zanca, T.; Niemelä, J. Predation risk modifies habitat use and habitat selection of diving beetles (Coleoptera: Dytiscidae) in an Urban Pondscape. Glob. Ecol. Conserv. 2024, 49, e02801. [Google Scholar] [CrossRef]
- Coleman, B.T.; Hill, R.A. Living in a landscape of fear: The impact of predation, resource availability and habitat structure on primate range use. Anim. Behav. 2014, 88, 165–173. [Google Scholar] [CrossRef]
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Beale, C.M.; Monaghan, P. Human disturbance: People as predation-free predators? J. Appl. Ecol. 2004, 41, 335–343. [Google Scholar] [CrossRef]
- Ortiz-Jimenez, C.A.; Michelangeli, M.; Pendleton, E.; Sih, A.; Smith, J.E. Behavioural correlations across multiple stages of the antipredator response: Do animals that escape sooner hide longer? Anim. Behav. 2022, 185, 175–184. [Google Scholar] [CrossRef]
- Lasky, M.; Bombaci, S. Human-induced fear in wildlife: A review. J. Nat. Conserv. 2023, 74, 126448. [Google Scholar] [CrossRef]
- Knapp, C.R.; Hines, K.N.; Zachariah, T.T.; Perez-Heydrich, C.; Iverson, J.B.; Buckner, S.D.; Halach, S.C.; Lattin, C.R.; Romero, L.M. Physiological effects of tourism and associated food provisioning in an endangered iguana. Conserv. Physiol. 2013, 1, cot032. [Google Scholar] [CrossRef]
- French, S.S.; Neuman-Lee, L.A.; Terletzky, P.A.; Kiriazis, N.M.; Taylor, E.N.; DeNardo, D.F. Too much of a good thing? Human disturbance linked to ecotourism has a “dose-dependent” impact on innate immunity and oxidative stress in marine iguanas, Amblyrhynchus cristatus. Biol. Conserv. 2017, 210, 37–47. [Google Scholar] [CrossRef]
- Montgomery, R.A.; Macdonald, D.W.; Hayward, M.W.; Sandercock, B. The inducible defences of large mammals to human lethality. Funct. Ecol. 2020, 34, 2426–2441. [Google Scholar] [CrossRef]
- Tolon, V.; Dray, S.; Loison, A.; Zeileis, A.; Fischer, C.; Baubet, E. Responding to spatial and temporal variations in predation risk: Space use of a game species in a changing landscape of fear. Can. J. Zool. 2009, 87, 1129–1137. [Google Scholar] [CrossRef]
- Takada, H.; Nakamura, K. Effects of human harvesting, residences, and forage abundance on deer spatial distribution. Animals 2024, 14, 1924. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Wintarren, P.S.; Williams, N.S.G.; Clilliers, S.; Clarkson, B.; et al. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Mainwaring, M.C.; Song, G.; Zhang, S. Urban biodiversity in the Anthropocene. Sci. Rep. 2024, 14, 27851. [Google Scholar] [CrossRef]
- Yiu, S.W.; Suraci, J.P.; Norbury, G.; Glen, A.S.; Peace, J.E.; Garvey, P.M. Problematic cats in urban reserves: Implications for native biodiversity and urban cat management. Glob. Ecol. Conserv. 2025, 60, e03584. [Google Scholar] [CrossRef]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Fairbairn, A.J.; Meyer, S.T.; Mühlbauer, M.; Jung, K.; Apfelbeck, B.; Berthon, K.; Frank, A.; Guthmann, L.; Jokisch, J.; Kerler, K.; et al. Urban biodiversity is affected by human-designed features of public squares. Nat. Cities 2024, 1, 706–715. [Google Scholar] [CrossRef]
- Contesse, P.; Hegglin, D.; Gloor, S.; Bontadina, F.; Deplazes, P. The diet of urban foxes (Vulpes vulpes) and the availability of anthropogenic food in the city of Zurich, Switzerland. Mamm. Biol. 2004, 69, 81–95. [Google Scholar] [CrossRef]
- O’Leary, R.; Jones, D.N. The use of supplementary foods by Australian magpies Gymnorhina tibicen: Implications for wildlife feeding in suburban environments. Austral Ecol. 2006, 31, 208–216. [Google Scholar] [CrossRef]
- Herr, J.; Schley, L.; Engel, E.; Roper, T.J. Den preferences and denning behaviour in urban stone martens (Martes foina). Mamm. Biol. 2010, 75, 138–145. [Google Scholar] [CrossRef]
- Williams, J.J.; Newbold, T. Local climatic changes affect biodiversity responses to land use: A review. Divers. Distrib. 2020, 26, 76–92. [Google Scholar] [CrossRef]
- Gámez, S.; Potts, A.; Mills, K.L.; Allen, A.A.; Holman, A.; Randon, P.M.; Linson, O.; Harris, N.C. Downtown diet: A global meta-analysis of increased urbanization on the diets of vertebrate predators. Proc. R. Soc. B Biol. Sci. 2022, 289, 20212487. [Google Scholar] [CrossRef]
- Stillfried, M.; Gras, P.; Börner, K.; Göritz, F.; Painer, J.; Röllig, K.; Wenzler, M.; Hofer, H.; Ortmann, S.; Kramer-Schadt, S. Secrets of success in a landscape of fear: Urban wild boar adjust risk perception and tolerate disturbance. Front. Ecol. Evol. 2017, 5, 157. [Google Scholar] [CrossRef]
- Ardila-Villamizar, M.; Alarcón-Nieto, G.; Maldonado-Chaparro, A.A. Fear in urban landscapes: Conspecific flock size drives escape decisions in tropical birds. R. Soc. Open Sci. 2022, 9, 221344. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, A.V.; Wood, E.M. Urban parks are a refuge for birds in park-poor areas. Front. Ecol. Evol. 2022, 10, 958572. [Google Scholar] [CrossRef]
- Berger, J.L.; Daum, S.N.; Hartlieb, M. Simply the green: Urban refuges. Basic Appl. Ecol. 2024, 80, 108–119. [Google Scholar] [CrossRef]
- Greenspoon, L.; Krieger, E.; Sender, R.; Rosenberg, Y.; Bar-On, Y.M.; Moran, U.; Antman, T.; Meiri, S.; Roll, U.; Noor, E.; et al. The global biomass of wild mammals. Proc. Natl. Acad. Sci. USA 2023, 120, e2204892120. [Google Scholar] [CrossRef]
- Baubet, E.; Bonenfant, C.; Brandt, S. Diet of the wild boar in the French Alps. Galemys 2004, 16, 101–113. [Google Scholar] [CrossRef]
- Massei, G.; Genov, P.V. The environmental impact of wild boar. Galemys 2004, 16, 135–145. [Google Scholar] [CrossRef]
- Acevedo, P.; Quiros-Fernandez, F.; Casal, J.; Vicente, J. Spatial distribution of wild boar population abundance: Basic information for spatial epidemiology and wildlife management. Ecol. Indic. 2014, 36, 594–600. [Google Scholar] [CrossRef]
- Brook, R.K.; van Beest, F.M. Feral wild boar distribution and perceptions of risk on the central Canadian prairies. Wildl. Soc. Bull. 2014, 38, 486–494. [Google Scholar] [CrossRef]
- Stegeman, L.R.C. The European wild boar in the Cherokee national forest, Tennessee. J. Mammal. 1938, 19, 279–290. [Google Scholar] [CrossRef]
- Genov, P. Food composition of wild boar in north-eastern and western Poland. Acta Theriol. 1981, 26, 185–205. [Google Scholar] [CrossRef]
- Bengsen, A.J.; Gentle, M.N.; Mitchell, J.L.; Pearson, H.E.; Saunders, G.R. Impacts and management of wild pigs S us scrofa in Australia. Mammal Rev. 2014, 44, 135–147. [Google Scholar] [CrossRef]
- Salvador, C.H.; Fernandez, F. Biological invasion of wild boar and feral pigs Sus scrofa (Suidae) in South America: Review and mapping with implications for conservation of peccaries (Tayassuidae). In Ecology, Conservation and Management of Wild Pigs and Peccaries; Melletti, M., Meijard, E., Eds.; Cambridge University Press: Cambridge, UK, 2017; pp. 313–324. [Google Scholar]
- Markov, N.; Economov, A.; Hjeljord, O.; Rolandsen, C.M.; Bergqvist, G.; Danilov, P.; Dolinin, V.; Kambalin, V.; Kondratov, A.; Krasnoshapka, N.; et al. The wild boar Sus scrofa in northern Eurasia: A review of range expansion history, current distribution, factors affecting the northern distributional limit, and management strategies. Mammal Rev. 2022, 52, 519–537. [Google Scholar] [CrossRef]
- Bongi, P.; Tomaselli, M.; Petraglia, A.; Tintori, D.; Carbognani, M. Wild boar impact on forest regeneration in the northern Apennines (Italy). For. Ecol. Manag. 2017, 391, 230–238. [Google Scholar] [CrossRef]
- Barrios-Garcia, M.N.; Gonzalez-Polo, M.; Simberloff, D.; Classen, A.T. Wild boar rooting impacts soil function differently in different plant community types. Biol. Invasions 2023, 25, 583–592. [Google Scholar] [CrossRef]
- Carpio, A.J.; Guerrero-Casado, J.; Tortosa, F.S.; Vicente, J. Predation of simulated red-legged partridge nests in big game estates from South Central Spain. Eur. J. Wildl. Res. 2014, 60, 391–394. [Google Scholar] [CrossRef][Green Version]
- Oja, R.; Soe, E.; Valdmann, H.; Saarma, U. Non-invasive genetics outperforms morphological methods in faecal dietary analysis, revealing wild boar as a considerable conservation concern for ground-nesting birds. PLoS ONE 2017, 12, e0179463. [Google Scholar] [CrossRef] [PubMed]
- Lombardini, M.; Meriggi, A.; Fozzi, A. Factors influencing wild boar damage to agricultural crops in Sardinia (Italy). Curr. Zool. 2017, 63, 507–514. [Google Scholar] [CrossRef]
- Rutten, A.; Casaer, J.; Strubbe, D.; Leirs, H. Agricultural and landscape factors related to increasing wild boar agricultural damage in a highly anthropogenic landscape. Wildl. Biol. 2020, 2020, 1–11. [Google Scholar] [CrossRef]
- Sáenz-de-Santa-María, A.; Tellería, J.L. Wildlife-vehicle collisions in Spain. Eur. J. Wildl. Res. 2015, 61, 399–406. [Google Scholar] [CrossRef]
- Naranjo, V.; Gortazar, C.; Vicente, J.; de La Fuente, J. Evidence of the role of European wild boar as a reservoir of Mycobacterium tuberculosis complex. Vet. Microbiol. 2008, 127, 1–9. [Google Scholar] [CrossRef]
- Risco, D.; Cuesta, J.M.; Fernández-Llario, P.; Salguero, F.J.; Gonçalves, P.; García-Jiménez, W.L.; Martínez, R.; Velarde, R.; de Mendoza, M.H.; Gómez, L.; et al. Pathological observations of porcine respiratory disease complex (PRDC) in the wild boar (Sus scrofa). Eur. J. Wildl. Res. 2015, 61, 669–679. [Google Scholar] [CrossRef]
- Pepin, K.M.; Golnar, A.J.; Abdo, Z.; Podgórski, T. Ecological drivers of African swine fever virus persistence in wild boar populations: Insight for control. Ecol. Evol. 2020, 10, 2846–2859. [Google Scholar] [CrossRef] [PubMed]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef]
- Barasona, J.A.; Carpio, A.; Boadella, M.; Gortazar, C.; Pineiro, X.; Zumalacárregui, C.; Viñuela, J. Expansion of native wild boar populations is a new threat for semi-arid wetland areas. Ecol. Indic. 2021, 125, 107563. [Google Scholar] [CrossRef]
- Murray, M.; Cembrowski, A.; Latham, A.D.M.; Lukasik, V.M.; Pruss, S.; St Clair, C.C. Greater consumption of protein-poor anthropogenic food by urban relative to rural coyotes increases diet breadth and potential for human-wildlife conflict. Ecography 2015, 38, 1235–1242. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Mentaberre, G.; Aguilar, X.F.; Conejero, C.; Colom-Cadena, A.; Ráez-Bravo, A.; López-Olvera, J.R. Wild boar in the city: Phenotypic responses to urbanisation. Sci. Total Environ. 2021, 773, 145593. [Google Scholar] [CrossRef]
- Bateman, P.W.; Fleming, P.A. Big city life: Carnivores in urban environments. J. Zool. 2012, 287, 1–23. [Google Scholar] [CrossRef]
- Castillo-Contreras, R.; Carvalho, J.; Serrano, E.; Mentaberre, G.; Fernández-Aguilar, X.; Colom, A.; González-Crespo, C.; Lavín, S.; López-Olvera, J.R. Urban wild boars prefer fragmented areas with food resources near natural corridors. Sci. Total Environ. 2018, 615, 282–288. [Google Scholar] [CrossRef]
- Amendolia, S.; Lombardini, M.; Pierucci, P.; Meriggi, A. Seasonal spatial ecology of the wild boar in a peri-urban area. Mammal Res. 2019, 64, 387–396. [Google Scholar] [CrossRef]
- Marin, C.; Werno, J.; Le Campion, G.; Couderchet, L. Navigating discreetly: Spatial ecology of urban wild boar in Bordeaux City’s landscape of fear, France. Sci. Total Environ. 2024, 954, 176436. [Google Scholar] [CrossRef]
- Collins, M.K.; Magle, S.B.; Gallo, T. Global trends in urban wildlife ecology and conservation. Biol. Conserv. 2021, 261, 109236. [Google Scholar] [CrossRef]
- Ramírez-Valiente, J.A.; Santos del Blanco, L.; Alía, R.; Robledo-Arnuncio, J.J.; Climent, J. Adaptation of Mediterranean forest species to climate: Lessons from common garden experiments. J. Ecol. 2022, 110, 1022–1042. [Google Scholar] [CrossRef]
- Laguna, E.; Barasona, J.A.; Vicente, J.; Keuling, O.; Acevedo, P. Differences in wild boar spatial behaviour among land uses and management scenarios in Mediterranean ecosystems. Sci. Total Environ. 2021, 796, 148966. [Google Scholar] [CrossRef] [PubMed]
- Boitani, L.; Mattei, L.; Nonis, D.; Corsi, F. Spatial and activity patterns of wild boars in Tuscany, Italy. J. Mammal. 1994, 75, 600–612. [Google Scholar] [CrossRef]
- Spitz, F.; Janeau, G. Daily selection of habitat in wild boar (Sus scrofa). J. Zool. 1995, 237, 423–434. [Google Scholar] [CrossRef]
- Meriggi, A.; Sacchi, O. Habitat requirements of wild boars in the northern Apennines (N Italy): A multi-level approach. Ital. J. Zool. 2001, 68, 47–55. [Google Scholar] [CrossRef]
- Sodeikat, G.; Pohlmeyer, K. Impact of drive hunts on daytime resting site areas of wild boar family groups (Sus scrofa L.). Wildl. Biol. Pract. 2007, 3, 28–38. [Google Scholar] [CrossRef]
- Scillitani, L.; Monaco, A.; Toso, S. Do intensive drive hunts affect wild boar (Sus scrofa) spatial behaviour in Italy? Some evidences and management implications. Eur. J. Wildl. Res. 2010, 56, 307–318. [Google Scholar] [CrossRef]
- Saïd, S.; Tolon, V.; Brandt, S.; Baubet, E. Sex effect on habitat selection in response to hunting disturbance: The study of wild boar. Eur. J. Wildl. Res. 2012, 58, 107–115. [Google Scholar] [CrossRef]
- Johann, F.; Handschuh, M.; Linderoth, P.; Dormann, C.F.; Arnold, J. Adaptation of wild boar (Sus scrofa) activity in a human-dominated landscape. BMC Ecol. 2020, 20, 4. [Google Scholar] [CrossRef]
- Baddeley, A.; Rubak, E.; Turner, R. Spatial Point Patterns: Methodology and Applications with R; Chapman and Hall/CRC Press: Boca Raton, FL, USA, 2015; Available online: http://www.crcpress.com/Spatial-Point-Patterns-Methodology-and-Applications-with-R/Baddeley-Rubak-Turner/9781482210200/ (accessed on 1 July 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 15 January 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Rousset, F.; Ferdy, J.B. Testing environmental and genetic effects in the presence of spatial autocorrelation. Ecography 2014, 37, 781–790. [Google Scholar] [CrossRef]
- Cahill, S.; Llimona, F.; Cabañeros, L.; Calomardo, F. Characteristics of wild boar (Sus scrofa) habituation to urban areas in the Collserola Natural Park (Barcelona) and comparison with other locations. Anim. Biodivers. Conserv. 2012, 35, 221–233. [Google Scholar] [CrossRef]
- Fulgione, D.; Buglione, M. The boar war: Five hot factors unleashing boar expansion and related emergency. Land 2022, 11, 887. [Google Scholar] [CrossRef]
- Conejero, C.; González-Crespo, C.; Fatjó, J.; Castillo-Contreras, R.; Serrano, E.; Lavín, S.; Mentaberre, G.; López-Olvera, J.R. Between conflict and reciprocal habituation: Human-wild boar coexistence in urban areas. Sci. Total Environ. 2024, 936, 173258. [Google Scholar] [CrossRef]
- Faltusová, M.; Cukor, J.; Linda, R.; Silovský, V.; Kušta, T.; Ježek, M. Wild Boar Proves High Tolerance to Human-Caused Disruptions: Management Implications in African Swine Fever Outbreaks. Animals 2024, 14, 2710. [Google Scholar] [CrossRef] [PubMed]
- Brogi, R.; Apollonio, M.; Grignolio, S.; Cossu, A.; Luccarini, S.; Brivio, F. Behavioural responses to temporal variations of human presence: Insights from an urban adapter. J. Zool. 2023, 321, 215–224. [Google Scholar] [CrossRef]
- Fu, Y.; Tan, M.; Gong, Y.; Zhao, G.; Ge, J.; Yang, H.; Feng, L. Wild boar survives in a landscape that prohibits anthropogenic persecution. Front. Ecol. Evol. 2022, 10, 820915. [Google Scholar] [CrossRef]
- Olejarz, A.; Augustsson, E.; Kjellander, P.; Ježek, M.; Podgórski, T. Experience shapes wild boar spatial response to drive hunts. Sci. Rep. 2024, 14, 19930. [Google Scholar] [CrossRef] [PubMed]
- Snow, N.P.; Jarzyna, M.A.; VerCauteren, K.C. Interpreting and predicting the spread of invasive wild pigs. J. Appl. Ecol. 2017, 54, 2022–2032. [Google Scholar] [CrossRef]
- Fischer, J.W.; Snow, N.P.; Wilson, B.E.; Beckerman, S.F.; Jacques, C.N.; VanNatta, E.H.; Kay, S.L.; VerCauteren, K.C. Factors and costs associated with removal of a newly established population of invasive wild pigs in Northern US. Sci. Rep. 2020, 10, 11528. [Google Scholar] [CrossRef] [PubMed]
- Gentle, M.; Wilson, C.; Cuskelly, J. Feral pig management in Australia: Implications for disease control. Aust. Vet. J. 2022, 100, 492. [Google Scholar] [CrossRef]


| Estimate | SE | z | p | |
|---|---|---|---|---|
| Intercept | −0.073 | 0.161 | −0.454 | 0.650 |
| Habitat (Open) | −1.043 | 0.247 | −4.223 | <0.001 |
| Urbanization gradient | 0.080 | 0.088 | 0.908 | 0.364 |
| Season (AAP) | −0.461 | 0.168 | −2.752 | 0.006 |
| Season (FAP) | −0.514 | 0.170 | −3.018 | 0.003 |
| (A). Cover Areas | (B). Open Areas | |
|---|---|---|
| N | 189 | 147 |
| Number of groups (X + Y) | 63 | 49 |
| Intercept (Estimate ± SE) | −0.069 ± 0.178 (t = −0.388) | −0.984 ± 0.379 (t = −2.599) * |
| Season AAP (Estimate ± SE and t value) | −0.473 ± 0.197 (t = −2.401) * | −0.427 ± 0.335 (t = −1.275) |
| Season FAP (Estimate ± SE and t value) | −0.427 ± 0.194 (t = −2.200) * | −0.833 ± 0.383 (t = −2.177) * |
| Spatial variance (λ) | 0.463 | 0.618 |
| Matérn smoothness (ν) | 0.599 | 2.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo-Toledo, S.P.; Pérez-González, J.; Hidalgo-de-Trucios, S.J. The Landscape of Fear and Wild Boar (Sus scrofa) Spatial Use in a Peri-Urban Area from West-Central Spain. Land 2025, 14, 1845. https://doi.org/10.3390/land14091845
Hidalgo-Toledo SP, Pérez-González J, Hidalgo-de-Trucios SJ. The Landscape of Fear and Wild Boar (Sus scrofa) Spatial Use in a Peri-Urban Area from West-Central Spain. Land. 2025; 14(9):1845. https://doi.org/10.3390/land14091845
Chicago/Turabian StyleHidalgo-Toledo, Sebastián P., Javier Pérez-González, and Sebastián J. Hidalgo-de-Trucios. 2025. "The Landscape of Fear and Wild Boar (Sus scrofa) Spatial Use in a Peri-Urban Area from West-Central Spain" Land 14, no. 9: 1845. https://doi.org/10.3390/land14091845
APA StyleHidalgo-Toledo, S. P., Pérez-González, J., & Hidalgo-de-Trucios, S. J. (2025). The Landscape of Fear and Wild Boar (Sus scrofa) Spatial Use in a Peri-Urban Area from West-Central Spain. Land, 14(9), 1845. https://doi.org/10.3390/land14091845






