Carbon Stocks and Microbial Activity in the Low Arctic Tundra of the Yana–Indigirka Lowland, Russia
Abstract
1. Introduction
2. Materials and Methods
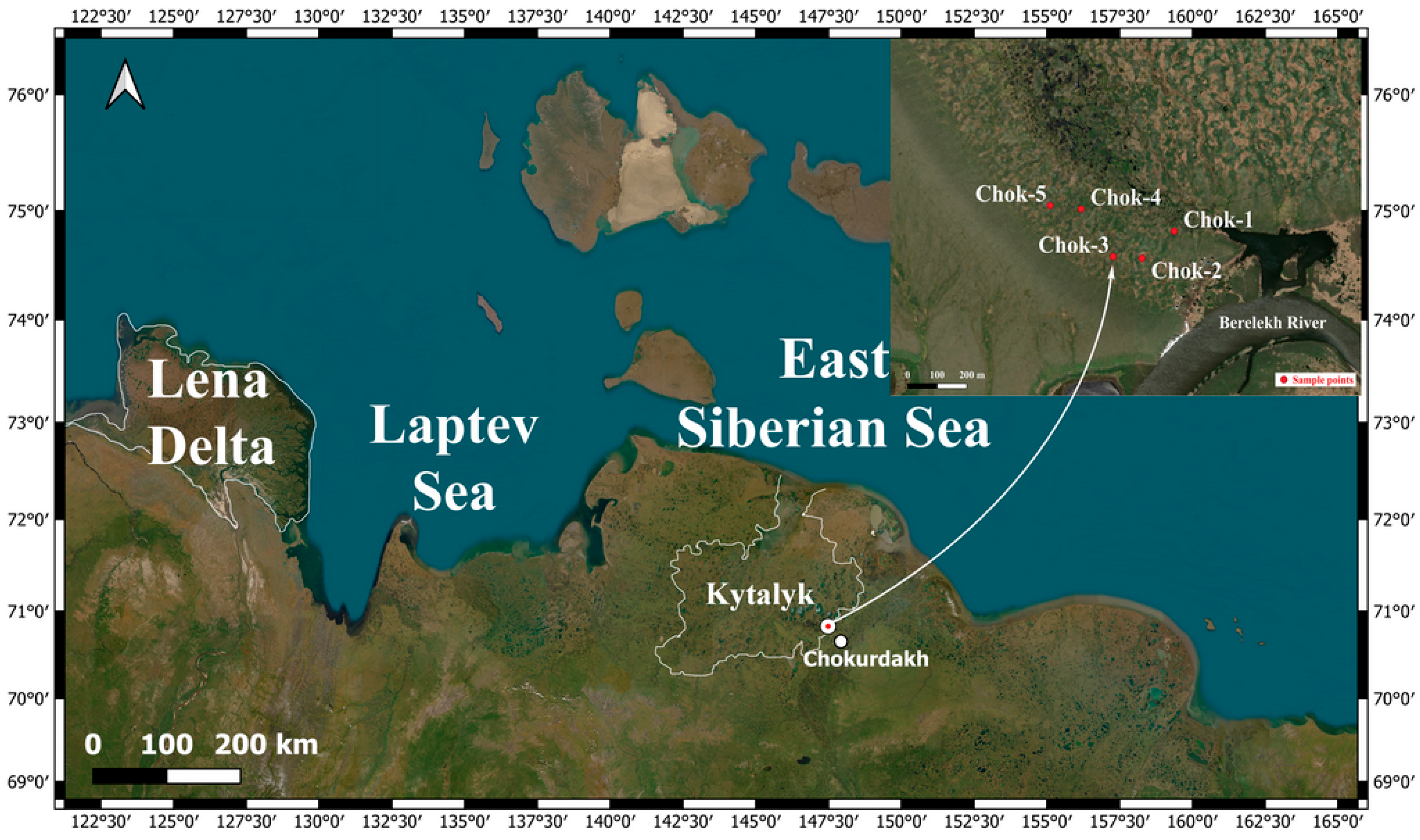
2.1. Study Area Characteristics
2.2. Methods for Assessing Carbon Stocks and Microbial Activity
- Total C and N in plants, necromass, and soil were determined by dry combustion using an ECS 8020 elemental analyzer (NC Technologies, Bussero, Italy).
- The pH of an aqueous extract was measured in the organic, peat, and mineral soil horizons. The pH was determined potentiometrically in a soil: water suspension using a 1:25 ratio for organic horizons and a 1:2.5 ratio for mineral horizons.
- The particle size distribution of the parent material was determined in accordance with ISO 11277:2020 [36].
2.3. Permafrost and Landscape Conditions
2.4. Cryogenic Processes and Their Impact on Soil Carbon
2.5. Morphological and Physicochemical Characteristics of the Soil Cover
3. Results
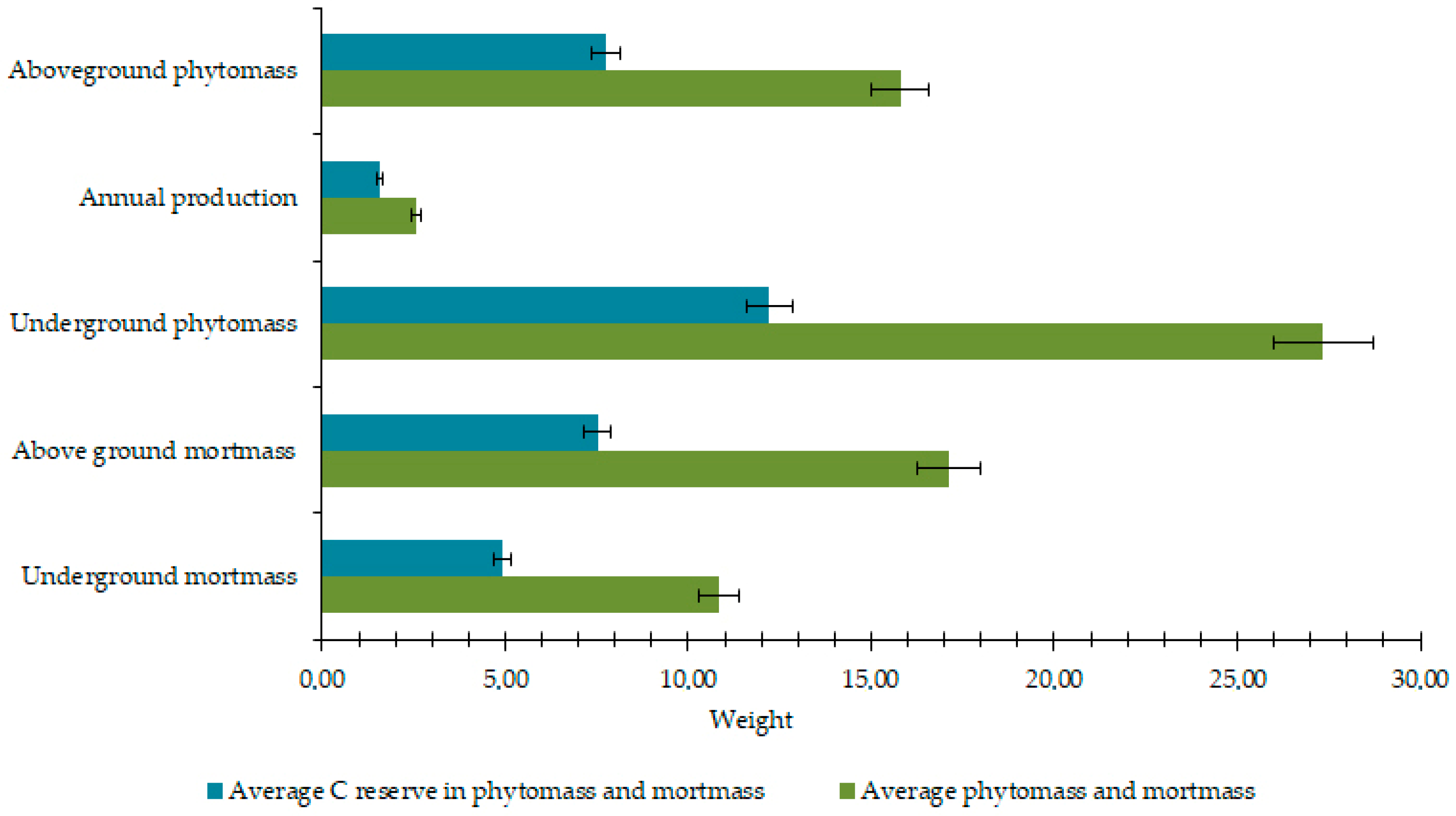
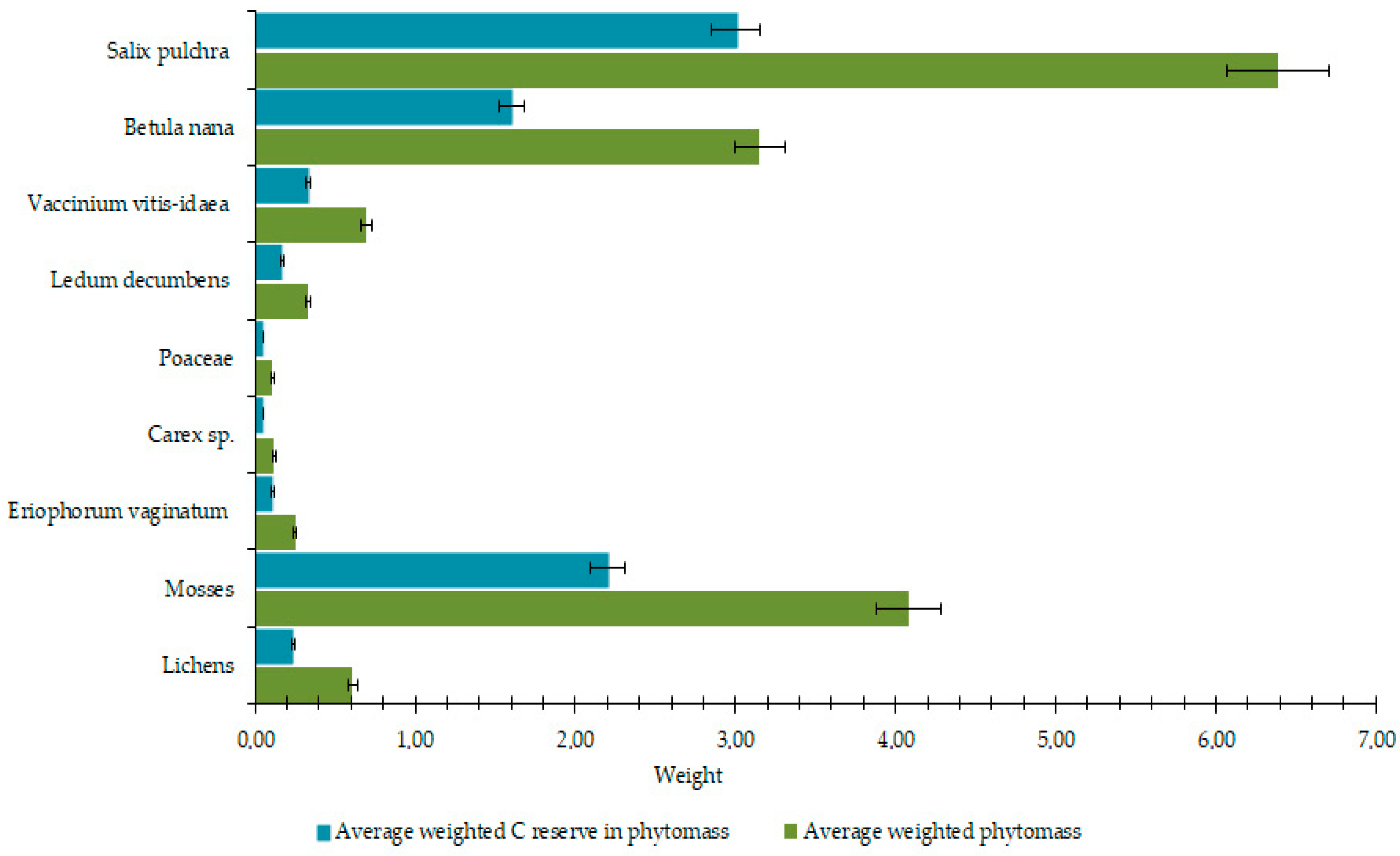
3.1. Geobotanical Analysis and Content of Biogenic Elements in Biomass and Necromass
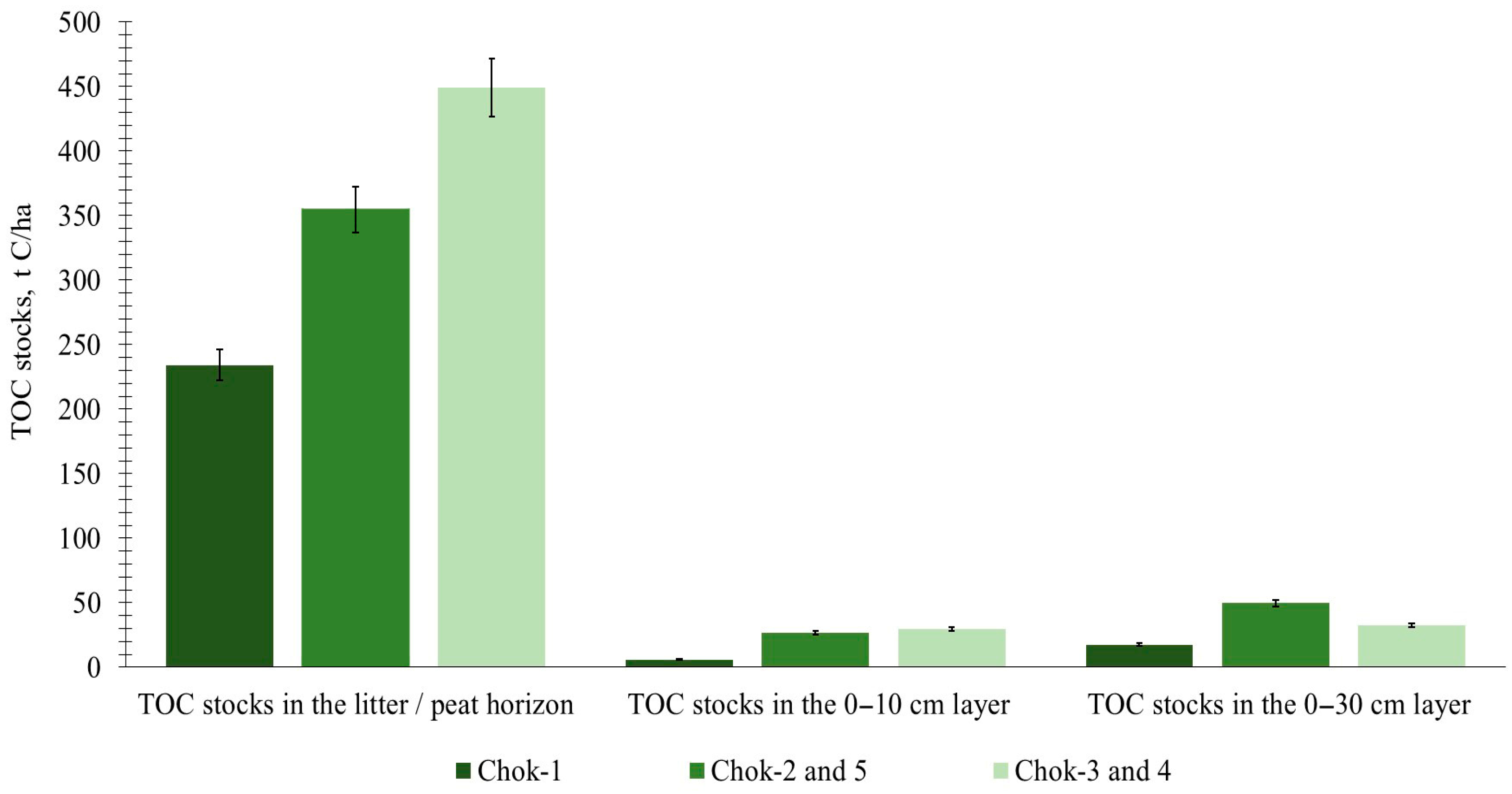
3.2. Carbon Pools and Stocks by Tundra Type
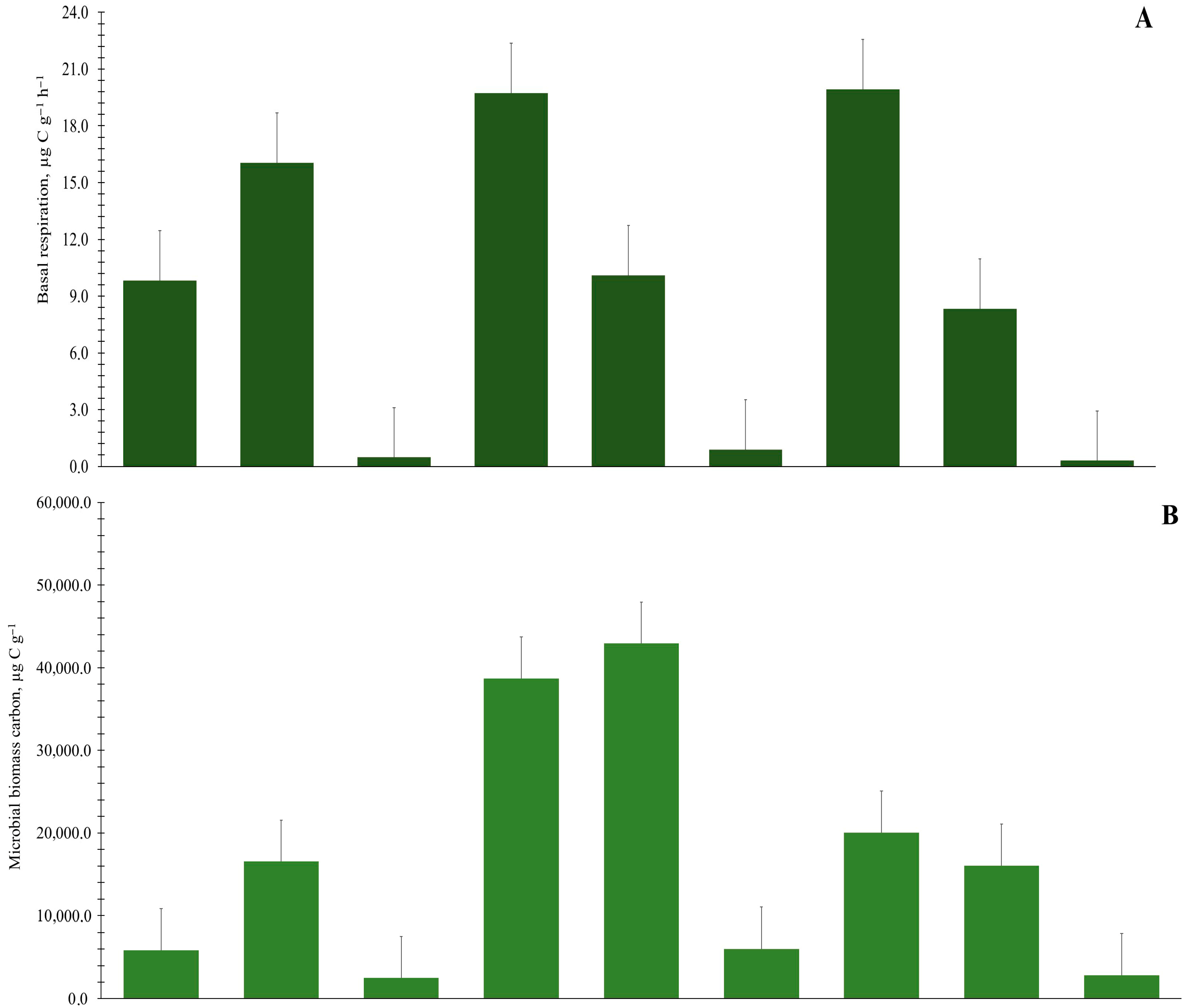
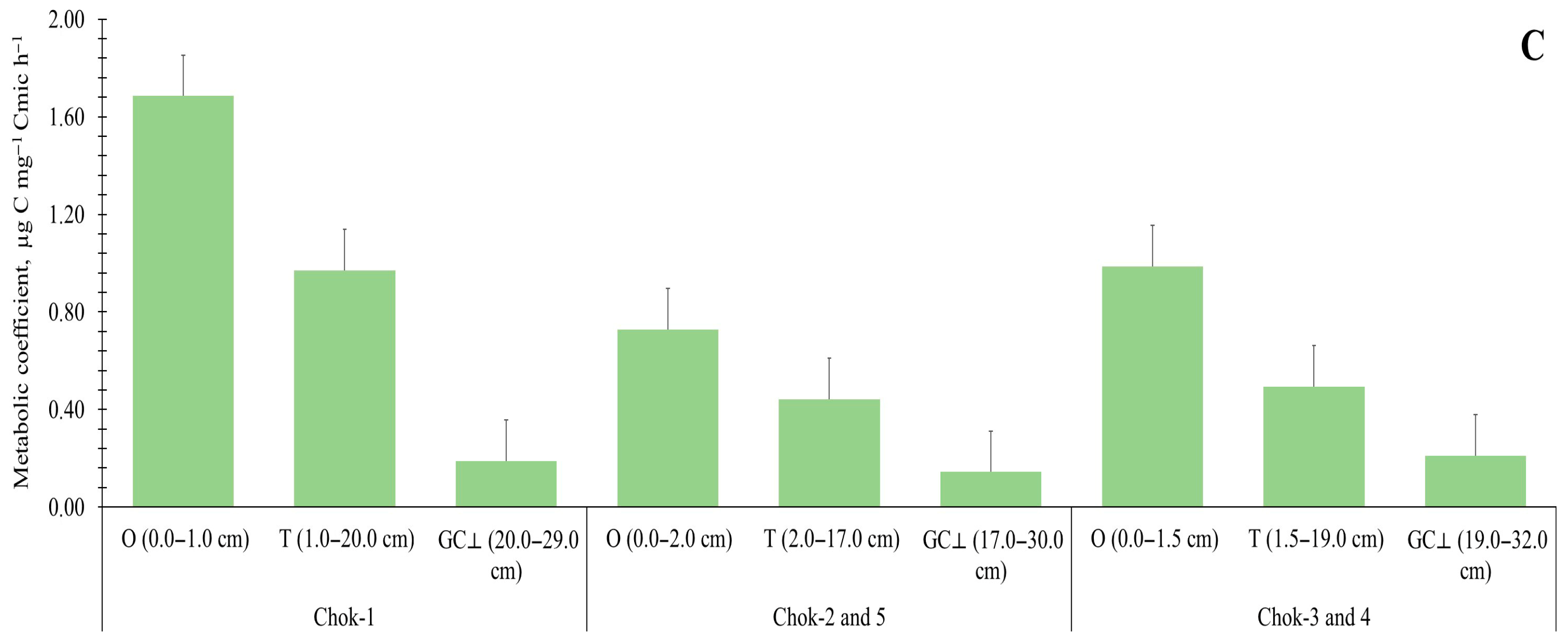
3.3. Heterotrophic Activity of the Organic and Mineral Soil Horizons
4. Discussion
4.1. Assessment of Above- and Below-Ground Biomass and Necromass in the Context of Southern Tundra Ecology and a Changing Climate
4.2. Influence of Permafrost on Biogenic Elements and Microbial Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edelgeriev, R.S.-K.; Ivanov, A.L. (Eds.) Global Climate and Soil Cover of Russia: Arctic Zone, Permafrost Soils—The Future of Russia (Agriculture and Forestry); National Report; Dokuchaev Soil Science Institute: Moscow, Russia, 2024; Volume 4, 672p. (In Russian) [Google Scholar]
- Akentyeva, E.M.; Anisimov, O.A.; Bardin, M.Y.; Zhuravlev, S.A.; Kattsov, V.M.; Kiselev, A.A.; Klyueva, M.V.; Konstantinov, P.I.; Korotkov, V.N.; Kostyanoy, A.G.; et al. The Third Assessment Report on Climate Change and its Consequences in the Russian Federation. General Summary; Science-Intensive Technologies: St. Petersburg, Russia, 2022; 124p. (In Russian) [Google Scholar]
- Walker, M.D.; Wahren, C.H.; Hollister, R.D.; Henry, G.H.R.; Ahlquist, L.E.; Alatalo, J.M.; Bret-Harte, M.S.; Calef, M.P.; Callaghan, T.V.; Carroll, A.B.; et al. Plant community responses to experimental warming across the tundra biome. Proc. Natl. Acad. Sci. USA 2006, 103, 1342–1346. [Google Scholar] [CrossRef]
- Tape, K.; Sturm, M.; Racine, C. The evidence for shrub expansion in northern Alaska and the pan-Arctic. Glob. Change Biol. 2006, 12, 686–702. [Google Scholar] [CrossRef]
- Bret-Harte, M.S.; Mack, M.C.; Goldsmith, G.R.; Sloan, D.B.; DeMarco, J.; Shaver, G.R.; Ray, P.M.; Biesinger, Z.; Chapin, F.S., III. Plant functional types do not predict biomass responses to removal and fertilization in Alaskan tussock tundra. J. Ecol. 2008, 96, 713–726. [Google Scholar] [CrossRef]
- Forbes, B.C.; Fauria, M.M.; Zetterberg, P. Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Glob. Change Biol. 2010, 16, 1542–1554. [Google Scholar] [CrossRef]
- Blok, D.; Schaepman-Strub, G.; Bartholomeus, H.; Heijmans, M.M.P.D.; Maximov, T.C.; Berendse, F. The response of Arctic vegetation to the summer climate: Relation between shrub cover, NDVI, surface albedo and temperature. Environ. Res. Lett. 2011, 6, 035502. [Google Scholar] [CrossRef]
- Blok, D.; Sass-Klaassen, U.; Schaepman-Strub, G.; Heijmans, M.M.P.D.; Sauren, P.; Berendse, F. What are the main climate drivers for shrub growth in Northeastern Siberian tundra? Biogeosciences 2011, 8, 1169–1179. [Google Scholar] [CrossRef]
- Myers-Smith, I.H.; Forbes, B.C.; Wilmking, M.; Hallinger, M.; Lantz, T.; Blok, D.; Tape, K.D.; Macias-Fauria, M.; Sass-Klaassen, U.; Levesque, E.; et al. Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environ. Res. Lett. 2011, 6, 045509. [Google Scholar] [CrossRef]
- Tishkov, A.A.; Belonovskaya, E.A.; Vaisfeld, M.A.; Glazov, P.M.; Krenke, A.N.; Tertitsky, G.M. “The greening” of the tundra as a driver of the modern dynamics of arctic biota. Arct. Ecol. Econ. 2018, 2, 31–44. (In Russian) [Google Scholar] [CrossRef]
- Bazilevich, N.I. Biological Productivity of Ecosystems of Northern Eurasia; Nauka: Moscow, Russia, 1993; 293p. (In Russian) [Google Scholar]
- Wang, J.; Wilson, R.S.; Aristilde, L. Electrostatic coupling and water bridging in adsorption hierarchy of biomolecules at water–clay interfaces. Proc. Natl. Acad. Sci. USA 2024, 121, e2316569121. [Google Scholar] [CrossRef] [PubMed]
- Bjorkman, A.D.; Myers-Smith, I.H.; Elmendorf, S.C.; Normand, S.; Rüger, N.; Beck, P.S.A.; Blach-Overgaard, A.; Blok, D.; Cornelissen, J.H.C.; Forbes, B.C.; et al. Plant functional trait change across a warming tundra biome. Nature 2018, 562, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Schuur, E.A.G.; Mack, M.C. Ecological Response to Permafrost Thaw and Consequences for Local and Global Ecosystem Services. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 279–301. [Google Scholar] [CrossRef]
- Schuur, E.A.G.; Abbott, B.W.; Commane, R.; Ernakovich, J.; Euskirchen, E.; Hugelius, G.; Grosse, G.; Jones, M.; Koven, C.; Leshyk, V.; et al. Permafrost and Climate Change: Carbon Cycle Feedbacks from the Warming Arctic. Annu. Rev. Environ. Resour. 2022, 47, 343–371. [Google Scholar] [CrossRef]
- Natali, S.M.; Holdren, J.P.; Rogers, B.M.; Treharne, R.; Duffy, P.B.; Pomerance, R.; MacDonald, E. Permafrost carbon feedbacks threaten global climate goals. Proc. Natl. Acad. Sci. USA 2021, 118, e2100163118. [Google Scholar] [CrossRef]
- Chestnykh, O.V.; Grabovskiy, V.I.; Zamolodchikov, D.G. Estimate of the Soil Carbon Stock of Forested Regions in Russia Using Databases of Soil Properties. Contemp. Probl. Ecol. 2022, 15, 731–740. [Google Scholar] [CrossRef]
- Hugelius, G.; Bockheim, J.G.; Camill, P.; Elberling, B.; Grosse, G.; Harden, J.W.; Johnson, K.; Jorgenson, T.; Koven, C.D.; Kuhry, P.; et al. A new data set for estimating organic carbon storage to 3 m depth in soils of the northern circumpolar permafrost region. Earth Syst. Sci. Data 2013, 5, 393–402. [Google Scholar] [CrossRef]
- Gentsch, N.; Mikutta, R.; Shibistova, O.; Wild, B.; Schnecker, J.; Richter, A.; Urich, T.; Gittel, A.; Šantrůčková, H.; Bárta, J.; et al. Properties and bioavailability of particulate and mineral-associated organic matter in Arctic permafrost soils, Lower Kolyma Region, Russia. Eur. J. Soil Sci. 2015, 66, 722–734. [Google Scholar] [CrossRef]
- Sun, T.; Ocko, I.B.; Hamburg, S.P. The value of early methane mitigation in preserving Arctic summer sea ice. Environ. Res. Lett. 2022, 17, 044001. [Google Scholar] [CrossRef]
- Strauss, J.; Schirrmeister, L.; Mangelsdorf, K.; Eichhorn, L.; Wetterich, S.; Herzschuh, U. Organic-matter quality of deep permafrost carbon—A study from Arctic Siberia. Biogeosciences 2015, 12, 2227–2245. [Google Scholar] [CrossRef]
- Walter Anthony, K.M.; Zimov, S.A.; Grosse, G.; Jones, M.C.; Anthony, P.M.; Chapin, F.S., III; Finlay, J.C.; Mack, M.C.; Davydov, S.; Frenzel, P.; et al. A shift of thermokarst lakes from carbon sources to sinks during the Holocene epoch. Nature 2014, 511, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Hugelius, G.; Strauss, J.; Zubrzycki, S.; Schuur, E.A.G.; Ping, C.-L.; Schirrmeister, L.; Grosse, G.; Michaelson, G.J.; Koven, C.D.; O’Donnell, J.A.; et al. Estimated stocks of circumpolar permafrost carbon with quantified uncertainty ranges and identified data gaps. Biogeosciences 2014, 11, 6573–6593. [Google Scholar] [CrossRef]
- Harden, J.W.; Koven, C.D.; Ping, C.-L.; Hugelius, G.; McGuire, A.D.; Camill, P.; Jorgenson, T.; Kuhry, P.; Michaelson, G.J.; O’Donnell, J.A.; et al. Field information links permafrost carbon to physical vulnerabilities of thawing. Geophys. Res. Lett. 2012, 39, L15704. [Google Scholar] [CrossRef]
- Kuhry, P.; Bárta, J.; Blok, D.; Elberling, B.; Faucherre, S.; Hugelius, G.; Jørgensen, C.J.; Richter, A.; Šantrůčková, H.; Weiss, N. Lability classification of soil organic matter in the northern permafrost region. Biogeosciences 2020, 17, 361–379. [Google Scholar] [CrossRef]
- Weiss, N.; Blok, D.; Elberling, B.; Hugelius, G.; Jörgensen, C.J.; Siewert, M.B.; Kuhry, P. Thermokarst dynamics and soil organic matter characteristics controlling initial carbon release from permafrost soils in the Siberian Yedoma region. Sediment. Geol. 2016, 340, 38–48. [Google Scholar] [CrossRef]
- Palmtag, J.; Obu, J.; Kuhry, P.; Richter, A.; Siewert, M.B.; Weiss, N.; Westermann, S.; Hugelius, G. A high spatial resolution soil carbon and nitrogen dataset for the northern permafrost region based on circumpolar land cover upscaling. Earth Syst. Sci. Data 2022, 14, 4095–4110. [Google Scholar] [CrossRef]
- Ogureeva, G.N.; Leonova, N.B.; Miklyaeva, I.M.; Bocharnikov, M.V.; Fedosov, V.E.; Muchnik, E.E.; Urbanavičius, G.P.; Emelyanova, L.G.; Khlyap, L.A.; Rumyantsev, V.Y.; et al. Biodiversity of Russian Biomes. Plain Biomes; Ogureeva, G.N., Ed.; IGKE: Moscow, Russia, 2020; 623p. (In Russian) [Google Scholar]
- Azizova, L.I.; Andreev, V.N.; Androsov, N.E.; Barashkova, E.S.; Bolshev, V.M.; Bochkareva, L.V.; Bukovskaya, Z.I.; Vorovskaya, V.N.; Gavrilyev, E.V.; Galaktionova, T.F.; et al. Atlas of Agriculture of the Yakut ASSR; Main Directorate of Geodesy and Cartography: Moscow, Russia, 1989; 115p. (In Russian) [Google Scholar]
- Bazilevich, N.I.; Titlyanova, A.A.; Smirnov, V.V.; Rodin, L.E.; Nechaeva, N.T.; Levin, F.I. Methods of Studying Biological Circulation in Various Natural Zones; Mysl: Moscow, Russia, 1978; 185p. (In Russian) [Google Scholar]
- Scurlock, J.M.O.; Johnson, K.; Olson, R.J. Estimating net primary productivity from grassland biomass dynamics measurements. Glob. Change Biol. 2002, 8, 736–753. [Google Scholar] [CrossRef]
- Schmidt, I.K.; Ostonen, I.; Blume-Werry, G. Belowground plant biomass. In The Handbook for Standardised Field and Laboratory Measurements in Terrestrial Climate Change Experiments and Observational Studies; Halbritter, A.H., Ed.; ClimEx: Naarden, The Netherlands, 2020; pp. 63–76. [Google Scholar]
- Perfilyeva, V.I.; Teterina, L.V.; Karpov, N.S. The Vegetation Cover of Yakutia Zonal Tundras; YSC SD RAS: Yakutsk, Russia, 1991; 192p. (In Russian) [Google Scholar]
- Reinsch, S.; Linstädter, A.; Beil, I.; Berauer, B.; Kröel-Dulay, G.; Stuart-Haëntjens, E.; Schmidt, I.K. Aboveground plant biomass. In The Handbook for Standardised Field and Laboratory Measurements in Terrestrial Climate Change Experiments and Observational Studies; Halbritter, A.H., Ed.; ClimEx: Naarden, The Netherlands, 2020; pp. 46–63. [Google Scholar]
- Walker, D.; Breen, A.; Druckenmiller, L.; Wirth, L.W.; Fisher, W.; Raynolds, M.K.; Sibik, J.; Walker, M.D.; Hennekens, S.; Boggs, K.; et al. The Alaska Arctic vegetation archive (AVA-AK). Phytocoenologia 2016, 46, 221–229. [Google Scholar] [CrossRef]
- ISO 11277:2020; Soil Quality—Determination of Particle Size Distribution in Mineral Soil Material—Method by Sieving and Sedimentation. ISO: Geneva, Switzerland, 2020.
- Ananyeva, N.D.; Susyan, E.A.; Gavrilenko, E.G. Determination of the soil microbial biomass carbon using the method of substrate-induced respiration. Eurasian Soil Sci. 2011, 44, 1215–1221. [Google Scholar] [CrossRef]
- Anderson, J.P.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- ISO 16072:2002; Soil Quality—Laboratory Methods for Determination of Micro-Bial Soil Respiration. International Organization for Standardization: Geneva, Switzerland, 2002.
- Geocryology of USSR. Eastern Siberia and Far East; Ershov, E.D., Ed.; Nedra: Moscow, Russia, 1989; 515p. (In Russian) [Google Scholar]
- Dubikov, G.I.; Baulin, V.V. History of development of permafrost of the Eurasia; Nauka: Moscow, Russia, 1981; 197p. (In Russian) [Google Scholar]
- Gvozdetsky, N.A.; Mikhailov, N.I. Physical Geography of the USSR (Asian Part); Geografgiz: Moscow, Russia, 1963; 572p. (In Russian) [Google Scholar]
- Fedorov, A.N. Permafrost Landscapes of Yakutia: Identification Methods and Mapping Issues; Permafrost Institute: Yakutsk, Russia, 1991; 140p. (In Russian) [Google Scholar]
- Fedorov, A.N.; Vasilyev, N.F.; Torgovkin, Y.I.; Shestakova, A.A.; Varlamov, S.P.; Zheleznyak, M.N.; Shepelev, V.V.; Konstantinov, P.Y.; Kalinicheva, S.V.; Basharin, N.I.; et al. Permafrost-landscape map of the Republic of Sakha (Yakutia) at scale 1:1,500,000. Geosciences 2018, 8, 465. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences: Vienna, Austria, 2022. [Google Scholar]
- Urusevskaya, I.S.; Alyabina, I.O.; Shoba, S.A. Map of Soil-Ecological Zoning of the Russian Federation. Scale 1: 8,000,000. Explanatory Text and Map Legend; MAKS Press: Moscow, Russia, 2020; 100p. (In Russian) [Google Scholar]
- Karavaeva, N.A. Tundra Soils of Northern Yakutia; Nauka: Moscow, Russia, 1969; 205p. (In Russian) [Google Scholar]
- Kuznetsova, L.V.; Zakharova, V.I. Conspectus of the Flora of Yakutia: Vascular Plants; Nauka: Novosibirsk, Russia, 2012; 272p. (In Russian) [Google Scholar]
- van der Molen, M.K.; van Huissteden, J.; Parmentier, F.J.W.; Petrescu, A.M.R.; Dolman, A.J.; Maximov, T.C.; Kononov, A.V.; Karsanaev, S.V.; Suzdalov, D.A. The seasonal cycle of the greenhouse gas balance of a continental tundra site in the Indigirka lowlands, NE Siberia. Biogeosci. Discuss. 2007, 4, 2329–2384. [Google Scholar] [CrossRef]
- Becklin, K.M.; Anderson, J.T.; Gerhart, L.M.; Wadgymar, S.M.; Wessinger, C.A.; Ward, J.K. Examining plant physiological responses to climate change through an evolutionary lens. Plant Physiol. 2016, 172, 635–649. [Google Scholar] [CrossRef]
- Belonovskaya, E.A.; Tishkov, A.A.; Vaisfeld, M.A.; Glazov, P.M.; Krenke, A.N.; Morozova, O.V.; Pokrovskaya, I.V.; Tsarevskaya, N.G.; Tertitskii, G.M. “Greening” of the Russian Arctic and the Modern Trends of Transformation of Its Biota. Izv. Ross. Akad. Nauk. Seriya Geograficheskaya. 2016, 3, 28–39. (In Russian) [Google Scholar] [CrossRef]
- Frost, G.V.; Bhatt, U.S.; Macander, M.J.; Berner, L.T.; Walker, D.A.; Raynolds, M.K.; Magnússon, R.Í.; Bartsch, A.; Bjerke, J.W.; Epstein, H.E.; et al. The changing face of the Arctic: Four decades of greening and implications for tundra ecosystems. Front. Environ. Sci. 2025, 13, 1525574. [Google Scholar] [CrossRef]
- Walker, D.A.; Epstein, H.E.; Raynolds, M.K.; Kuss, P.; Kopecky, M.A.; Frost, G.V.; Daniels, F.J.A.; Leibman, M.O.; Moskalenko, N.G.; Matyshak, G.V.; et al. Environment, vegetation and greenness (NDVI) along the North America and Eurasia Arctic transects. Environ. Res. Lett. 2012, 7, 015504. [Google Scholar] [CrossRef]
- Lavrinenko, I.A.; Lavrinenko, O.V. The impact of climate change on the plant cover of the Barents Sea islands. Proc. Karelian Res. Cent. RAS 2013, 6, 5–16. (In Russian) [Google Scholar]
- Epstein, H.E.; Raynolds, M.K.; Walker, D.A.; Bhatt, U.S.; Tucker, C.J.; Pinzon, J.E. Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the past three decades. Environ. Res. Lett. 2012, 7, 015506. [Google Scholar] [CrossRef]
- Shevtsova, I.; Heim, B.; Kruse, S.; Schröder, J.; Troeva, E.I.; Pestryakova, L.A.; Zakharov, E.S.; Herzschuh, U. Strong shrub expansion in tundra-taiga, tree infilling in taiga and stable tundra in central Chukotka (north-eastern Siberia) between 2000 and 2017. Environ. Res. Lett. 2020, 15, 085006. [Google Scholar] [CrossRef]
- Magnússon, R.Í.; Groten, F.; Bartholomeus, H.; van Huissteden, K.; Heijmans, M.M.P.D. Tundra browning in the Indigirka Lowlands (North-eastern Siberia) explained by drought, floods and small-scale vegetation shifts. J. Geophys. Res. Biogeosci. 2023, 128, e2022JG007330. [Google Scholar] [CrossRef]
- Rietze, N.; Heim, R.J.; Troeva, E.; Schaepman-Strub, G.; Assmann, J.J. Pre-fire vegetation conditions and topography shape burn mosaics of Siberian tundra fire scars. J. Geophys. Res. Biogeosci. 2025, 130, e2024JG008608. [Google Scholar] [CrossRef]
- Koroleva, T.M.; Gogoleva, P.A.; Petrovsky, V.V.; Zverev, A.A.; Troeva, E.I. Monitoring of local flora in the vicinity of the village of Chokurdakh (north-east of Yakutia). Bot. J. 2019, 104, 1386–1420. (In Russian) [Google Scholar] [CrossRef]
- Information Portal «Weather and Climate». Available online: http://www.pogodaiklimat.ru/climate/21946.htm (accessed on 11 May 2025).
- Petrov, R.E.; Maximov, T.C.; Karsanaev, S.V. Studies of interannual and seasonal variability of balance of carbon and permafrost rock mass in typical tundra ecosystem in Northeast of Russia. Arct. Subarct. Nat. Resour. 2018, 23, 89–96. (In Russian) [Google Scholar] [CrossRef]
- Petrov, R.E.; Karsanaev, S.V.; Grigoriev, M.R.; Maximov, T.C. Dynamics of CO2 fluxes in northeastern Russia: Contribution to understanding the carbon cycle in the Arctic. The Arctic is a territory of strategic scientific research. In Proceedings of the II Arctic Congress, Yakutsk, Russia, 20–22 September 2024; pp. 314–317. (In Russian). [Google Scholar]
- Wahren, C.-H.A.; Walker, M.D.; Bret-Harte, M.S. Vegetation responses in Alaskan arctic tundra after 8 years of a summer warming and winter snow manipulation experiment. Glob. Change Biol. 2005, 11, 537–552. [Google Scholar] [CrossRef]
- Blok, D.; Heijmans, M.M.P.D.; Schaepman-Strub, G.; Kononov, A.V.; Maximov, T.C.; Berendse, F. Shrub expansion may reduce summer permafrost thaw in Siberian tundra. Glob. Change Biol. 2010, 16, 1296–1305. [Google Scholar] [CrossRef]
- Magnússon, R.Í.; Sass-Klaassen, U.; Limpens, J.; Karsanaev, S.V.; Ras, S.; van Huissteden, K.; Blok, D.; Heijmans, M.M.P.D. Spatiotemporal variability in precipitation-growth association of Betula nana in the Siberian lowland tundra. J. Ecol. 2023, 111, 1882–1904. [Google Scholar] [CrossRef]
- Nauta, A.L.; Heijmans, M.M.; Blok, D.; Limpens, J.; Elberling, B.; Gallagher, A.; Li, B.; Petrov, R.E.; Maximov, T.C.; Van Huissteden, J. Permafrost collapse after shrub removal shifts tundra ecosystem to a methane source. Nat. Clim. Change 2015, 5, 67–70. [Google Scholar] [CrossRef]
- Andreev, V.N.; Galaktionova, T.F.; Govorov, P.M.; Zakharova, V.I.; Neustroeva, A.I.; Savinov, D.D.; Torgovkina, E.E. Seasonal and Year-to-Year Dynamics of Phytomass in Subarctic Tundra [Sezonnaya i Pogodovaya Dinamika Fitomassy v Subarkticheskoy Tundre]; Nauka: Novosibirsk, Russia, 1978; 191p. (In Russian) [Google Scholar]
- Gotovtsev, S.P.; Kopyrina, L.I.; Efimova, A.P.; Zakharova, V.I.; Nogovitsyn, D.D.; Poryadina, L.N.; Zabolotnik, P.S.; Syromyatnikov, I.I.; Ivanova, A.Z.; Egorov, N.N.; et al. Cryoecosystems of the Alazeya River Basin; Isaev, A.P., Klimovsky, I.V., Eds.; Geo: Novosibirsk, Russia, 2018; 211p. (In Russian) [Google Scholar]
- Andreev, V.I. Tundra Studies; Nauka: Novosibirsk, Russia, 2017; 312p. (In Russian) [Google Scholar]
- Li, B.; Heijmans, M.M.P.D.; Blok, D.; Wang, P.; Karsanaev, S.V.; Maximov, T.C.; van Huissteden, J.; Berendse, F. Thaw pond development and initial vegetation succession in experimental plots at a Siberian lowland tundra site. Plant Soil 2017, 420, 147–162. [Google Scholar] [CrossRef]
- Wang, P.; Heijmans, M.M.P.D.; Mommer, L.; Van Ruijven, J.; Berendse, F.; Maximov, T.C. Belowground plant biomass allocation in tundra ecosystems and its relationship with temperature. Environ. Res. Lett. 2016, 11, 055003. [Google Scholar] [CrossRef]
- Petrov, R.E.; Karsanaev, S.V.; Maximov, T.C. The stabilizing role of the shrub layer of the tundra biogeocenoses of the north-east of Russia. Probl. Reg. Ecol. 2022, 1, 89–95. (In Russian) [Google Scholar] [CrossRef]
- Beringer, J.; Lynch, A.H.; Chapin, F.S., III; Mack, M.; Bonan, G.B. The representation of arctic soils in the Land Surface Model: The importance of mosses. J. Clim. 2001, 14, 3324. [Google Scholar] [CrossRef]
- Andreev, V.N.; Galaktionova, T.F. Productivity of Forage Plants of the Yakut Tundra; YSC SB RAS: Yakutsk, Russia, 1994; 193p. (In Russian) [Google Scholar]
- Veretennikova, E.E.; Kurina, I.V.; Dyukarev, E.A.; Golovatskaya, E.A.; Smirnov, S.V. Geochemical Features of Peat Deposits at Oligotrophic Bogs in the Southern Taiga Subzone of West Siberia. Geochem. Int. 2021, 59, 618–631. [Google Scholar] [CrossRef]
- Bazilevich, N.I.; Tishkov, A.A. Live and dead reserves and primary production in polar desert, tundra and forest tundra of the former Soviet Union. In Ecosystems of the World 3. Polar and Alpine Tundra; Wielgolaski, F.E., Ed.; Elsevier: Amsterdam, The Netherlands; Lausanne, Switzerland; New York, NY, USA; Oxford, UK; Shannon, Ireland; Singapore; Tokyo, Japan, 1997; pp. 509–539. [Google Scholar]
- Siewert, M.B.; Hanisch, J.; Weiss, N.; Kuhry, P.; Maximov, T.C.; Hugelius, G. Comparing carbon storage of Siberian tundra and taiga permafrost ecosystems at very high spatial resolution. Geophys. Res. Lett. Biogeosci. 2015, 120, 1973–1994. [Google Scholar] [CrossRef]
- Walker, D.A.; Raynolds, M.K.; Daniëls, F.J.A.; Einarsson, E.; Elvebakk, A.; Gould, W.A.; Katenin, A.E.; Kholod, S.S.; Markon, C.J.; Melnikov, E.; et al. The circumpolar Arctic vegetation map. J. Veg. Sci. 2005, 16, 267–282. [Google Scholar] [CrossRef]
- Wang, P.; van Ruijven, J.; Heijmans, M.M.P.D.; Berendse, F.; Maksimov, A.; Maximov, T.C.; Mommer, L. Short-term root and leaf decomposition of two dominant plant species in a Siberian tundra. Pedobiologia 2017, 65, 68–76. [Google Scholar] [CrossRef]
- van Huissteden, J.; Teshebaeva, K.; Cheung, Y.; Magnússon, R.Í.; Noorbergen, H.; Karsanaev, S.V.; Maximov, T.C.; Dolman, A.J. Geomorphology and InSAR-Tracked Surface Displacements in an Ice-Rich Yedoma Landscape. Front. Earth Sci. 2021, 9, 680565. [Google Scholar] [CrossRef]
- Tumskoy, V.E.; Tarasov, A.I. New stage of study of Quaternary deposits of the lower reach of the Indigirka river (2022–2024). Relief Quat. Depos. Arct. Subarct. North-West Russ. 2024, 11, 375–384. (In Russian) [Google Scholar] [CrossRef]
- Serna-Chavez, H.M.; Fierer, N.; van Bodegom, P.M. Global drivers and patterns of microbial abundance in soil. Glob. Ecol. Biogeogr. 2013, 22, 1162–1172. [Google Scholar] [CrossRef]
- Wang, P.; Limpens, J.; Mommer, L.; van Ruijven, J.; Nauta, A.L.; Berendse, F.; Schaepman-Strub, G.; Blok, D.; Maximov, T.C.; Heijmans, M.M.P.D. Above- and below-ground responses of four tundra plant functional types to deep soil heating and surface soil fertilization. J. Ecol. 2017, 105, 947–957. [Google Scholar] [CrossRef]
- Grodnitskaya, I.D.; Sorokin, N.D.; Evgrafova, S.Y.; Antonov, G.I.; Syrtsov, S.N.; Alexandrov, D.E.; Trusova, M.Y.; Koroban, N.V. Microbial Transformation of Carbon CH4 and CO2 in Permafrost-Affected Soils in Tundra and Forest Ecosystems in Siberia. Russ. J. For. Sci. 2017, 2, 111–127. (In Russian) [Google Scholar] [CrossRef]
- Blaud, A.; Lerch, T.Z.; Phoenix, G.K.; Osborn, A.M. Arctic soil microbial diversity in a changing world. Res. Microbiol. 2015, 166, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.D.; McCann, C.M.; Christgen, B.; Ahammad, S.Z.; Roberts, J.A.; Graham, D.W. Soil geochemistry confines microbial abundances across an arctic landscape; implications for net carbon exchange with the atmosphere. Biogeochemistry 2014, 120, 307–317. [Google Scholar] [CrossRef]
- Grodnitskaya, I.D.; Trusova, M.Y.; Syrtsov, S.N.; Koroban, N.V. Structure of microbial communities of peat soils in two bogs in Siberian tundra and forest zones. Microbiology 2018, 87, 89–102. [Google Scholar] [CrossRef]
- Parmentier, F.J.W.; van der Molen, M.K.; van Huissteden, J.; Karsanaev, S.A.; Kononov, A.V.; Suzdalov, D.A.; Maximov, T.C.; Dolman, A.J. Longer growing seasons do not increase net carbon uptake in the northeastern Siberian tundra. J. Geophys. Res. 2011, 116, G04013. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Chabot, C.L.; Liebner, S.; Holm, S.; Snyder, M.W.; Dillon, M.; Dudgeon, S.R.; Douglas, T.A.; Leewis, M.-C.; Walter Anthony, K.M.; et al. Permafrost microbial communities and functional genes are structured by latitudinal and soil geochemical gradients. ISME J. 2023, 17, 1224–1235. [Google Scholar] [CrossRef]
- Marsh, G.; Chernikhova, D.; Thiele, S.; Altshuler, I. Microbial dynamics in rapidly transforming Arctic proglacial landscapes. PLoS Clim. 2024, 3, e0000337. [Google Scholar] [CrossRef]
- Danilova, A.A. Microbial landscapes of permafrost soils in degraded pasture exclusion. Probl. Reg. Ecol. 2018, 6, 118–121. (In Russian) [Google Scholar] [CrossRef]
- Taş, N.; Prestat, E.; Wang, S.; Wu, Y.; Ulrich, C.; Kneafsey, T.; Tringe, S.G.; Torn, M.S.; Hubbard, S.S.; Jansson, J.K. Landscape topography structures the soil microbiome in arctic polygonal tundra. Nat. Commun. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Bobrik, A.A.; Goncharova, O.Y.; Matyshak, G.V.; Tarkhov, M.O.; Petrzhik, N.M.; Drozdov, D.S.; Ponomareva, O.E. Spatial Distribution of Soil Carbon Cycle Components and Environmental Factors in Southern Tundra Ecosystems of the Taz Peninsula. Earth’s Cryosphere 2018, 22, 41–48. [Google Scholar] [CrossRef]


| Site (PSP) | Horizon (cm) | Soil Type (WRB, 2022 [45]) | Soil Profile | Total N (%) | TOC (%) | C:N | Bulk Density (g cm−3) | pH (H2O) | Sand (2–0.063 mm, %) | Silt (0.063–0.002 mm, %) | Clay (<0.002 mm, %) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chok-1 | Litter O (0–1) | Gleysol |  | 0.78 ± 0.16 | 39.62 ± 4.16 | 51.0 ± 10.31 | 0.31 ± 0.01 | 5.20 ± 0.15 | - | - | - |
| Peat T (1–20) | 1.84 ± 0.12 | 12.74 ± 6.29 | 6.9 ± 4.33 | 0.92 ± 0.02 | 5.20 ± 0.09 | - | - | - | |||
| Sandy loam GC⊥ (20–29) | 0.11 ± 0.01 | 1.34 ± 0.17 | 12.0 ± 3.04 | 1.37 ± 0.02 | 6.0 ± 0.25 | 11.22 ± 2.97 | 74.29 ± 2.85 | 14.49 ± 0.44 | |||
| Chok-2 | Litter O (0–2) | Gleysol |  | 0.62 ± 0.10 | 46.60 ± 6.37 | 75.0 ± 14.28 | 0.28 ± 0.01 | 5.10 ± 0.12 | - | - | - |
| Peat T (2–17) | 1.53 ± 0.15 | 31.60 ± 3.24 | 21.0 ± 8.40 | 0.87 ± 0.02 | 4.90 ± 0.05 | - | - | - | |||
| Sandy loam GC⊥ (17–30) | 0.06 ± 0.01 | 0.96 ± 0.12 | 17.0 ± 3.57 | 1.41 ± 0.02 | 5.60 ± 0.30 | 14.59 ± 3.26 | 72.90 ± 3.00 | 12.51 ± 0.58 | |||
| Chok-3 | Litter O (0–1.5) | Gleysol |  | 1.30 ± 0.17 | 23.8 ± 2.34 | 18.0 ± 7.02 | 0.26 ± 0.01 | 5.50 ± 0.12 | - | - | - |
| Peat T (1.5–19) | 1.32 ± 0.10 | 36.80 ± 4.05 | 28.0 ± 3.07 | 0.90 ± 0.02 | 5.30 ± 0.07 | - | - | - | |||
| Sandy loam GC⊥ (19–32) | 0.10 ± 0.01 | 1.91 ± 0.18 | 18.0 ± 4.02 | 1.47 ± 0.02 | 5.10 ± 0.19 | 22.24 ± 1.32 | 64.66 ± 1.61 | 13.10 ± 0.52 | |||
| Chok-4 | Litter O (0–3) | Gleysol |  | 0.79 ± 0.10 | 46.50 ± 6.05 | 59.0 ± 11.63 | 0.28 ± 0.01 | 5.80 ± 0.14 | - | - | - |
| Peat T (3–12) | 1.81 ± 0.23 | 46.80 ± 3.77 | 26.0 ± 6.82 | 0.84 ± 0.02 | 5.40 ± 0.11 | - | - | - | |||
| Sandy loam GC⊥ (12–24) | 0.09 ± 0.01 | 1.46 ± 0.11 | 16.0 ± 3.70 | 1.37 ± 0.02 | 6.10 ± 0.29 | 26.80 ± 3.56 | 59.40 ± 3.93 | 13.80 ± 0.37 | |||
| Chok-5 | Litter O (0–1.1) | Gleysol |  | 1.45 ± 0.19 | 39.90 ± 4.64 | 28.0 ± 6.33 | 0.24 ± 0.01 | 5.80 ± 0.14 | - | - | - |
| Peat T (1.1–17) | 1.96 ± 0.22 | 17.10 ± 4.61 | 8.7 ± 3.51 | 0.96 ± 0.02 | 5.10 ± 0.08 | - | - | - | |||
| Sandy loam GC⊥ (17–41) | 0.08 ± 0.01 | 1.38 ± 0.17 | 17.0 ± 3.11 | 1.41 ± 0.02 | 4.80 ± 0.13 | 24.33 ± 3.73 | 63.65 ± 3.99 | 12.02 ± 0.53 |
| Plant Organs and Fragments | Mean C Content, % | Mean N Content, % |
|---|---|---|
| Salix pulchra | ||
| Woody stems | 49.68 ± 4.51 | 0.53 ± 0.12 |
| Woody twigs | 48.52 ± 2.33 | 0.65 ± 0.10 |
| Current shoots | 48.21 ± 1.89 | 1.01 ± 0.27 |
| Leaves | 46.94 ± 1.38 | 1.78 ± 0.38 |
| Underground rooting shoots | 45.85 ± 1.81 | 0.65 ± 0.11 |
| Betula nana | ||
| Woody stems | 50.31 ± 3.56 | 0.51 ± 0.05 |
| Woody twigs | 52.45 ± 3.21 | 0.57 ± 0.13 |
| Current shoots | 53.56 ± 3.04 | 1.17 ± 0.23 |
| Leaves | 49.42 ± 0.99 | 1.78 ± 0.24 |
| Underground rooting shoots | 47.45 ± 1.50 | 0.87 ± 0.12 |
| Ledum decumbens | ||
| Woody stems | 54.49 ± 2.51 | 0.52 ± 0.11 |
| Woody twigs | 54.48 ± 2.49 | 0.52 ± 0.09 |
| Current shoots | 46.13 ± 1.25 | 0.78 ± 0.12 |
| Current leaves | 48.37 ± 1.12 | 1.25 ± 0.20 |
| Perennial leaves | 47.93 ± 1.32 | 0.89 ± 0.15 |
| Underground rooting shoots | 47.14 ± 1.27 | 0.53 ± 0.13 |
| Vaccinium vitis-idaea | ||
| Perennial shoots | 48.36 ± 1.42 | 0.50 ± 0.07 |
| Current shoots | 48.58 ± 1.32 | 0.57 ± 0.10 |
| Current leaves | 49.08 ± 1.98 | 0.50 ± 0.06 |
| Perennial leaves | 49.63 ± 3.87 | 0.72 ± 0.06 |
| Poaceae | ||
| Vegetative annual shoots | 44.83 ± 0.33 | 1.19 ± 0.01 |
| Carex | ||
| Vegetative annual shoots | 43.30 ± 1.87 | 1.74 ± 0.42 |
| Eriophorum vaginatum | ||
| Vegetative annual shoots | 42.78 ± 2.11 | 1.18 ± 0.22 |
| Mosses | ||
| Aulacomnium palustre | ||
| Current leafy shoots | 41.42 ± 2.10 | 0.84 ± 0.30 |
| Perennial leafy shoots | 44.13 ± 1.13 | 0.76 ± 0.22 |
| Aulacomnium turgidum | ||
| Current leafy shoots | 36.85 ± 1.15 | 1.21 ± 0.50 |
| Perennial leafy shoots | 39.28 ± 1.17 | 1.13 ± 0.45 |
| Sphagnum warnstorfii | ||
| Current leafy shoots | 39.54 ± 1.20 | 0.85 ± 0.31 |
| Perennial leafy shoots | 39.45 ± 1.19 | 0.83 ± 0.35 |
| Lichens | ||
| Thallus | 32.71 ± 1.30 | 0.96 ± 0.81 |
| Flavocetraria cucullata | ||
| Thallus | 31.65 ± 0.72 | 0.24 ± 0.04 |
| Mean C Stocks, t C ha−1 | Chok-1 | Chok-2 | Chok-3 | Chok-4 | Chok-5 |
|---|---|---|---|---|---|
| In above-ground living biomass | 3.62 ± 1.06 | 5.59 ± 1.31 | 6.66 ± 1.33 | 7.75 ± 1.54 | 11.93 ± 1.51 |
| In below-ground living biomass | 9.68 ± 2.69 | 9.34 ± 3.56 | 10.88 ± 4.00 | 10.76 ± 3.01 | 10.26 ± 4.61 |
| In necromass | 7.08 ± 1.61 | 7.64 ± 1.73 | 12.30 ± 1.29 | 12.22 ± 1.30 | 20.14 ± 3.81 |
| Correlation Coefficient * | TOC Stocks in Ground and Soil Cover | ||||
|---|---|---|---|---|---|
| Chok-1 | Chok-2 | Chok-3 | Chok-4 | Chok-5 | |
| TOC in above-ground living biomass | 0.77 | 0.99 | 0.93 | 0.90 | 0.99 |
| TOC in below-ground living biomass | 0.86 | 0.86 | 0.92 | 0.89 | 0.89 |
| TOC in necromass | 0.73 | 0.73 | 0.87 | 0.89 | 0.88 |
| Tundra Type | Soil Horizon (cm) | Basal Respiration (BR), μg C g−1 h−1 | Microbial Biomass Carbon (MBC), μg C g−1 | Metabolic Quotient (qCO2), μg C mg−1 Cmic h−1 | Relationship Between Microbiological Parameters and TOC Stocks | ||
|---|---|---|---|---|---|---|---|
| BR | MBC | qCO2 | |||||
| Chok-1 | O (0.0–1.0) | 9.82 ± 2.02 | 5827.23 ± 896.71 | 1.69 ± 0.30 | 0.40 | 0.57 | 0.52 |
| T (1.0–20.0) | 16.05 ± 2.91 | 10,173.98 ± 3344.22 | 1.65 ± 0.32 | ||||
| GC⟂ (20.0–29.0) | 0.48 ± 0.11 | 2515.04 ± 367.68 | 0.19 ± 0.03 | ||||
| Chok-2 | O (0.0–2.0) | 17.6 ± 0.94 | 23,745.00 ± 2043.27 | 0.74 ± 0.02 | 0.58 | 0.70 | 0.93 |
| T (2.0–17.0) | 8.19 ± 0.58 | 70,627.77 ± 18,983.98 | 0.12 ± 0.02 | ||||
| GC⟂ (17.0–30.0) | 1.32 ± 0.36 | 6292.76 ± 307.76 | 0.21 ± 0.06 | ||||
| Chok-3 | O (0.0–1.5) | 18.2 ± 1.52 | 13,175.99 ± 4619.50 | 1.50 ± 0.57 | 0.73 | 0.45 | 0.97 |
| T (1.5–19.0) | 4.79 ± 1.67 | 15,554.83 ± 3591.38 | 0.31 ± 0.07 | ||||
| GC⟂ (19.0–32.0) | 0.64 ± 0.04 | 946.81 ± 93.76 | 0.68 ± 0.06 | ||||
| Chok-4 | O (0.0–3.0) | 20.52 ± 2.91 | 22,332.51 ± 5654.38 | 0.94 ± 0.12 | 0.54 | 0.42 | 0.91 |
| T (3.0–12.0) | 9.52 ± 1.80 | 16,214.92 ± 1931.95 | 0.58 ± 0.04 | ||||
| GC⟂ (12.0–24.0) | 0.20 ± 0.08 | 3476.42 ± 1389.86 | 0.06 ± 0.00 | ||||
| Chok-5 | O (0.0–1.1) | 21.86 ± 1.00 | 53,644.28 ± 2271.74 | 0.41 ± 0.03 | 0.46 | 0.72 | 0.60 |
| T (1.1–17.0) | 11.99 ± 1.10 | 15,245.78 ± 643.65 | 0.79 ± 0.05 | ||||
| GC⟂ (17.0–41.0) | 0.44 ± 0.11 | 2829.54 ± 244.57 | 0.16 ± 0.04 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepelev, A.G.; Efimova, A.P.; Maximov, T.C. Carbon Stocks and Microbial Activity in the Low Arctic Tundra of the Yana–Indigirka Lowland, Russia. Land 2025, 14, 1839. https://doi.org/10.3390/land14091839
Shepelev AG, Efimova AP, Maximov TC. Carbon Stocks and Microbial Activity in the Low Arctic Tundra of the Yana–Indigirka Lowland, Russia. Land. 2025; 14(9):1839. https://doi.org/10.3390/land14091839
Chicago/Turabian StyleShepelev, Andrei G., Aytalina P. Efimova, and Trofim C. Maximov. 2025. "Carbon Stocks and Microbial Activity in the Low Arctic Tundra of the Yana–Indigirka Lowland, Russia" Land 14, no. 9: 1839. https://doi.org/10.3390/land14091839
APA StyleShepelev, A. G., Efimova, A. P., & Maximov, T. C. (2025). Carbon Stocks and Microbial Activity in the Low Arctic Tundra of the Yana–Indigirka Lowland, Russia. Land, 14(9), 1839. https://doi.org/10.3390/land14091839








