Response Patterns of Soil Organic Carbon Fractions and Storage to Vegetation Types in the Yellow River Wetland
Abstract
1. Introduction
2. Materials and Methods
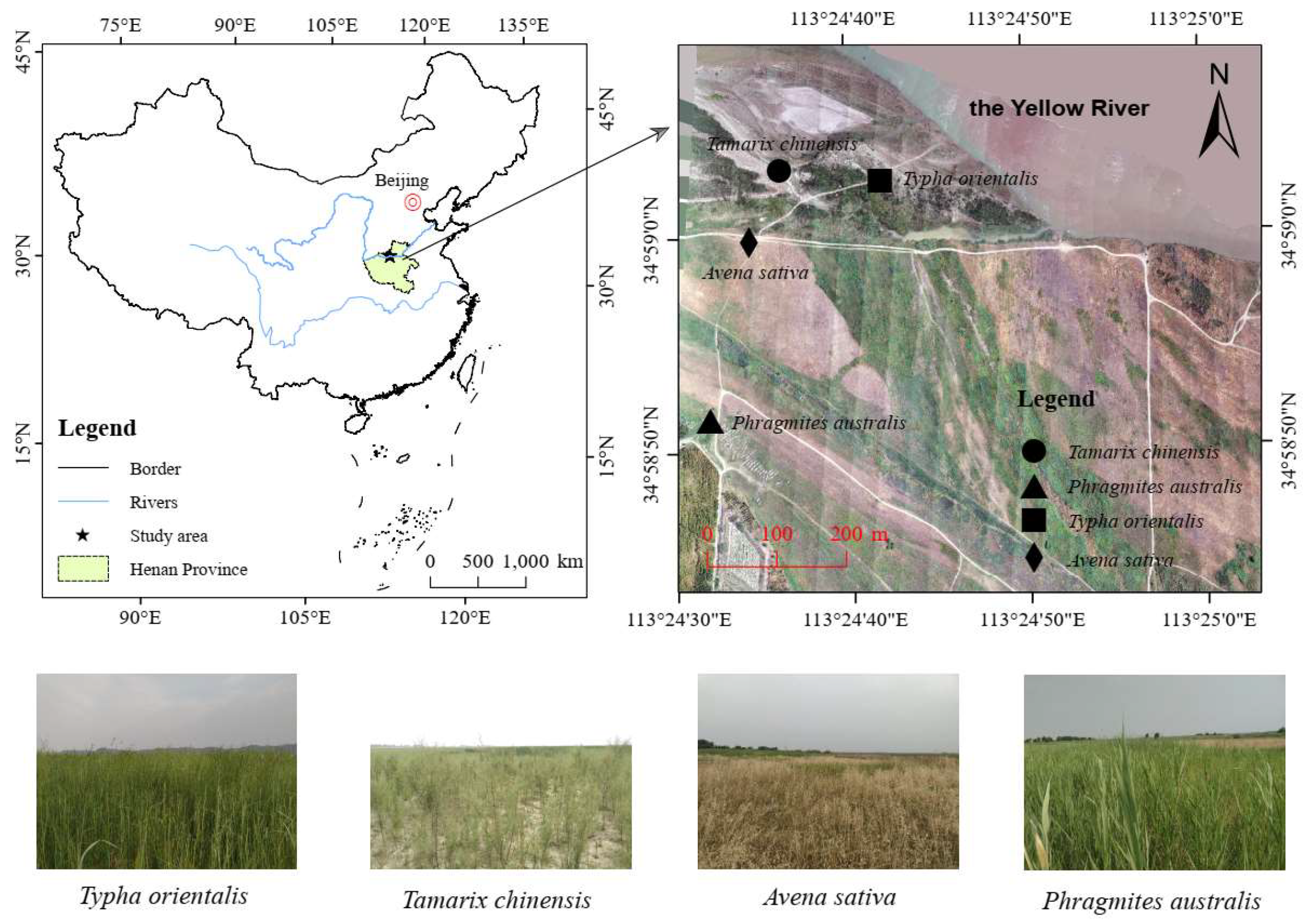
2.1. Study Area
2.2. Sampling Methods
2.3. Soil Property Measurement
2.4. Data Analyses
3. Results
3.1. The Variations in Soil Physicochemical Properties Among Vegetation Types
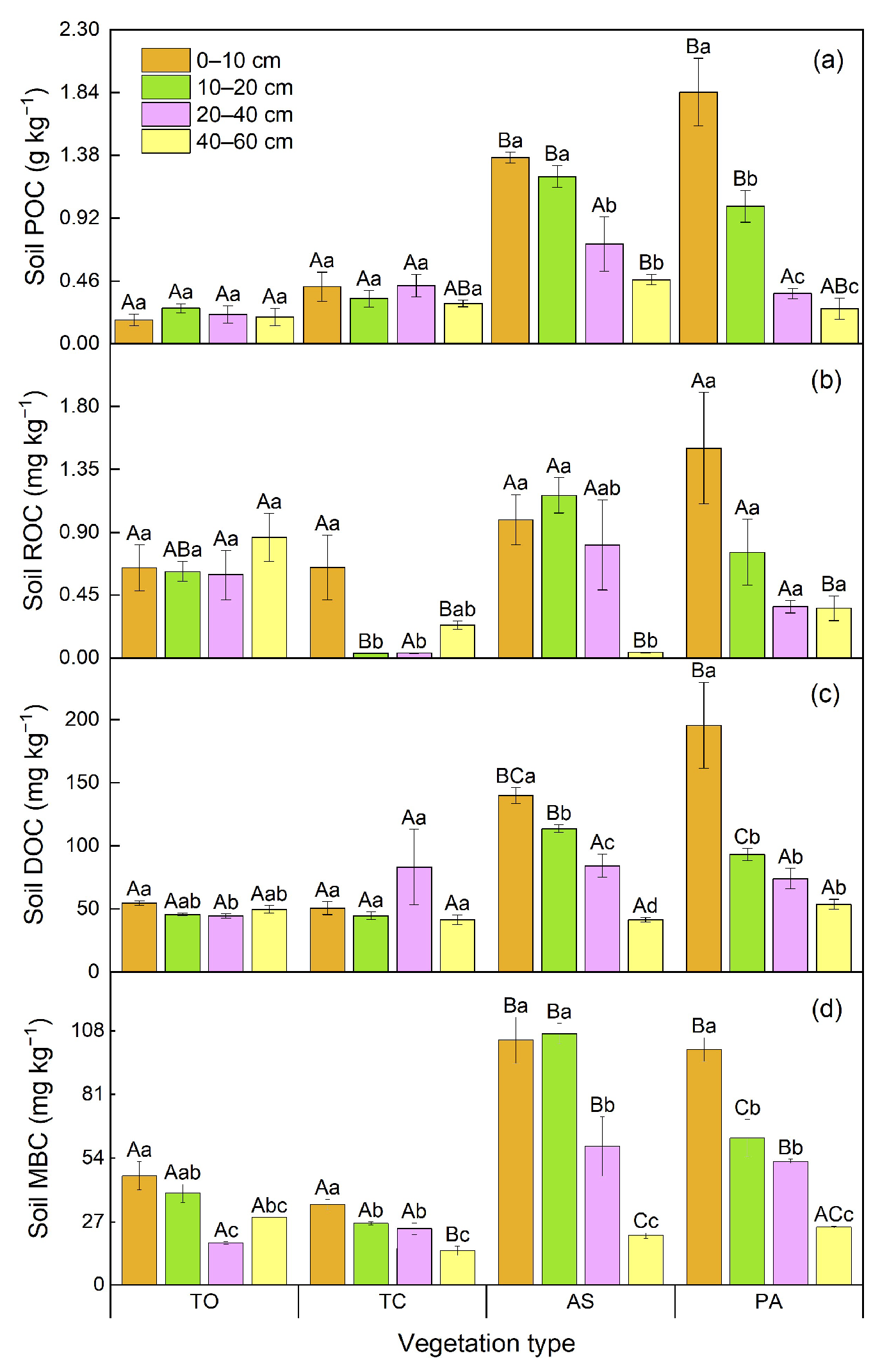
3.2. Variations of Soil Organic C Fractions Among Various Vegetation Types
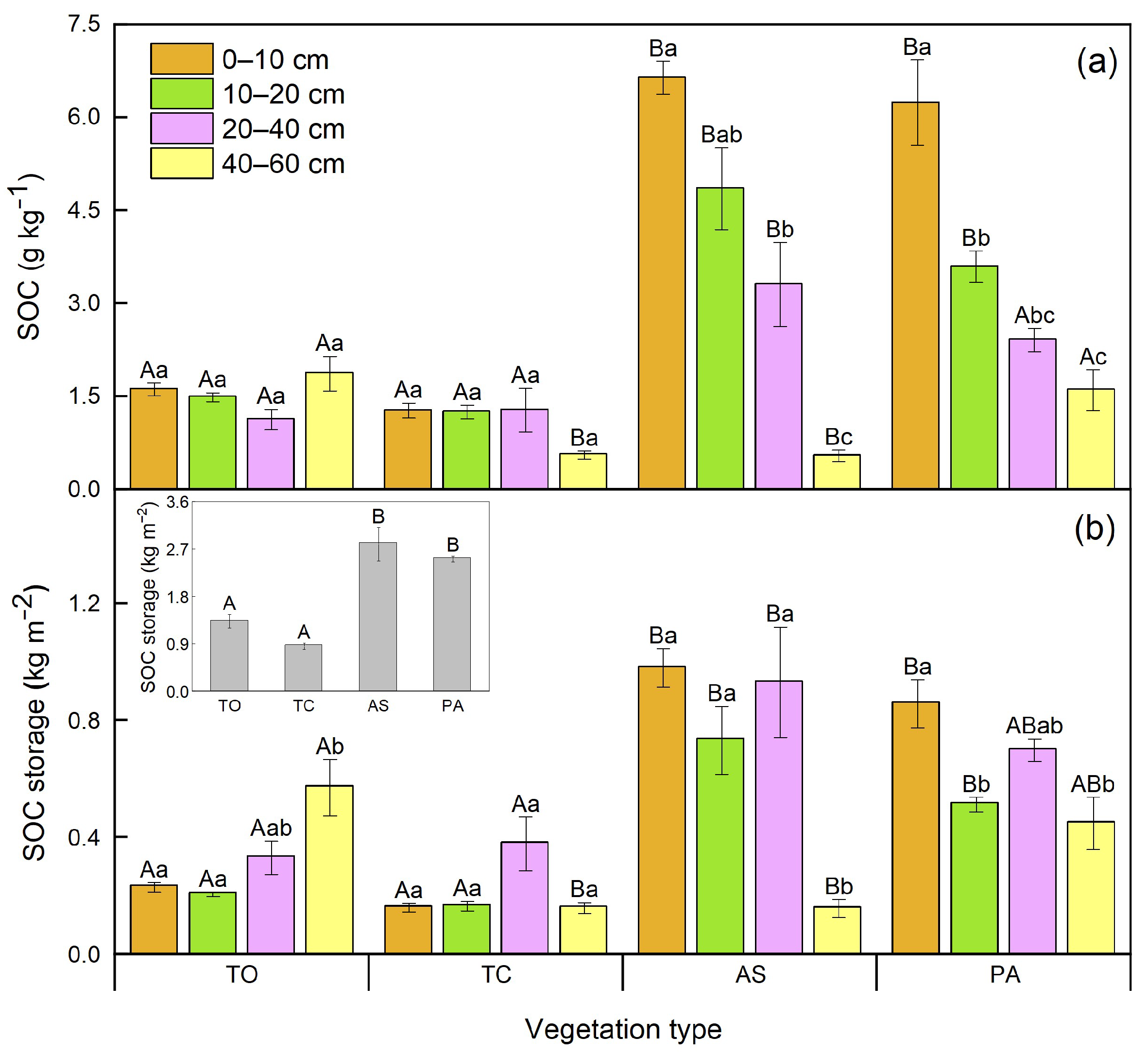
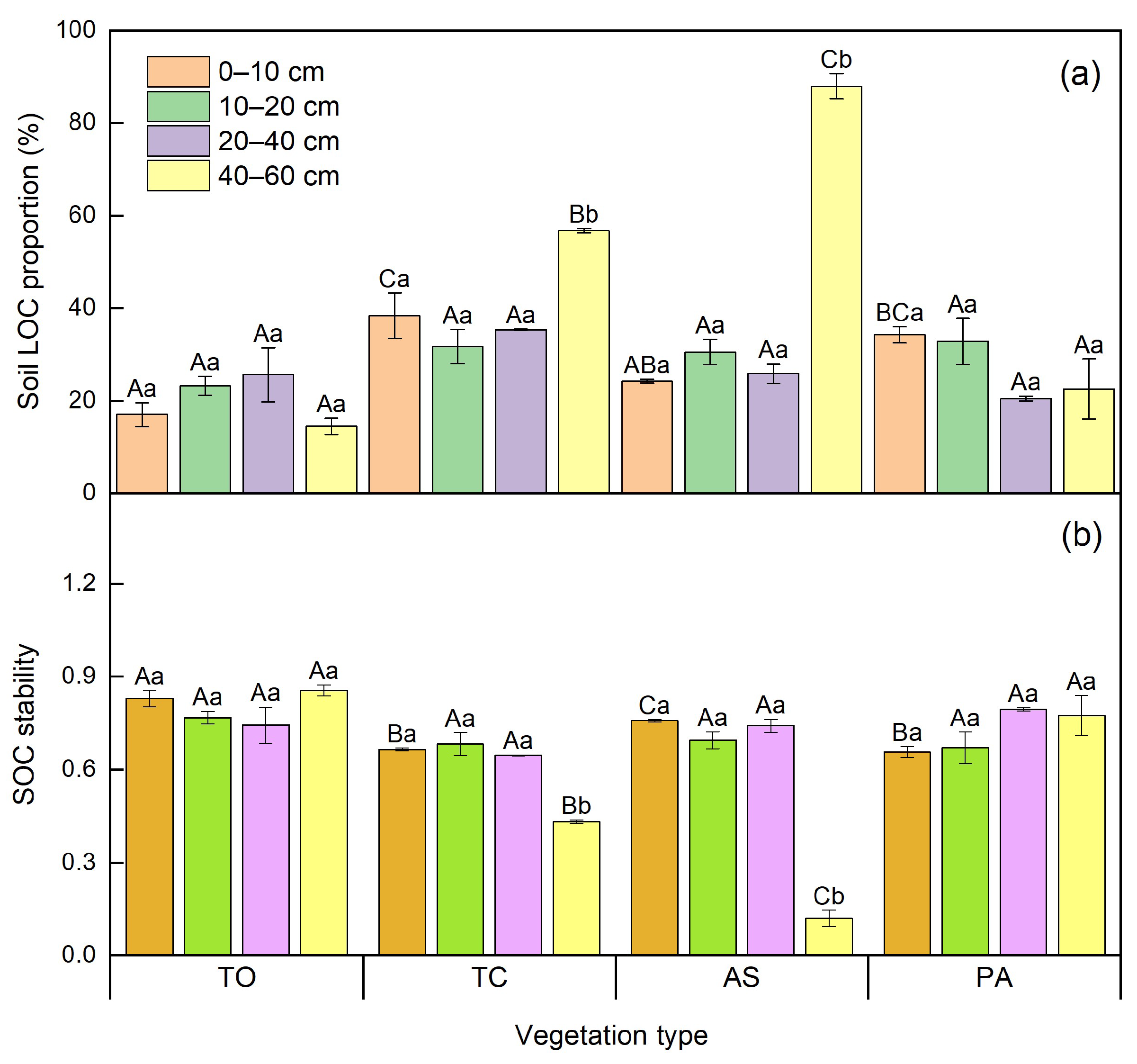
3.3. Differences of Soil Organic C Stability and Storage Among Diverse Vegetation Types
3.4. The Influences of Soil Physicochemical Properties on Soil Organic C Fractions, Stability, and Storage
4. Discussion
4.1. The Responses of Soil Organic C Fractions to Vegetation Types in the Yellow River Wetland
4.2. Vegetation Types Can Alter Soil Organic C Stability in the Yellow River Wetland
4.3. Soil Organic C Storage Varied with Vegetation Types in the Yellow River Wetland
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SOC | Soil organic C |
| CST | Soil organic C stability |
| CS | Soil organic C storage |
| POC | Particulate organic C |
| ROC | Readily oxidized organic C |
| DOC | Dissolved organic C |
| MBC | Microbial biomass C |
| LOC | Labile organic C |
| EC | Electrical conductivity |
| SWC | Soil water content |
| BD | Bulk density |
| RDA | Redundancy analysis |
References
- IPCC. Climate Change—The Physical Science Basis; IPCC: Cambridge, UK, 2007; pp. 131–234. [Google Scholar]
- Lane, R.R.; Mack, S.K.; Day, J.W.; DeLaune, R.D.; Madison, M.J.; Precht, P.R. Fate of soil organic carbon during wetland loss. Wetlands 2016, 36, 1167–1181. [Google Scholar] [CrossRef]
- Villa, J.A.; Bernal, B. Carbon sequestration in wetlands, from science to practice: An overview of the biogeochemical process, measurement methods, and policy framework. Ecol. Eng. 2018, 114, 115–128. [Google Scholar] [CrossRef]
- Adame, M.F.; Santini, N.S.; Tovilla, C.; Vázquez-Lule, A.; Castro, L.; Guevara, M. Carbon stocks and soil sequestration rates of tropical riverine wetlands. Biogeosciences 2015, 12, 3805–3818. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhao, D.; Zhang, C.; Cao, Q.Q.; Fang, J.H.; Yang, R.R.; Ji, S.P.; Li, C.C.; Zhao, R.Q.; Liu, J. Factors controlling organic carbon distributions in a riverine wetland. Environ. Sci. Pollut. R. 2020, 27, 34529–34540. [Google Scholar] [CrossRef]
- Shen, R.C.; Yang, H.; Rinklebe, J.; Bolan, N.; Hu, Q.W.; Huang, X.Y.; Wen, X.T.; Zheng, B.F.; Shi, L. Seasonal flooding wetland expansion would strongly affect soil and sediment organic carbon storage and carbon-nutrient stoichiometry. Sci. Total Environ. 2022, 828, 154427. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, H.W. Distribution characteristic of soil organic carbon fraction in different types of wetland in Hongze Lake of China. Sci. World J. 2014, 2014, 487961. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.B.; Bai, J.H.; Gao, H.F.; Xiao, R.; Liu, P.P.; Chen, B. Soil organic carbon content and storage of raised field wetlands in different functional zones of a typical shallow freshwater lake, China. Soil. Res. 2012, 50, 664–671. [Google Scholar] [CrossRef]
- Sun, D.B.; Li, Y.Z.; Yu, J.B.; Li, B.Q.; Guan, B.; Zhou, D.; Wang, X.H.; Yang, J.S.; Ma, Y.Q.; Zhang, X.; et al. Spatial distribution of soil quality under different vegetation types in the Yellow River Delta wetland. Front. Ecol. Evol. 2022, 10, 977899. [Google Scholar] [CrossRef]
- Qu, W.D.; Han, G.X.; Wang, J.; Li, J.Y.; Zhao, M.L.; He, W.J.; Li, X.G.; Wei, S.Y. Short-term effects of soil moisture on soil organic carbon decomposition in a coastal wetland of the Yellow River Delta. Hydrobiologia 2021, 848, 3259–3271. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Bai, J.H.; Zhang, G.L.; Jia, J.; Wang, W.; Wang, X. Effects of water and salinity regulation measures on soil carbon sequestration in coastal wetlands of the Yellow River Delta. Geoderma 2018, 319, 219–229. [Google Scholar] [CrossRef]
- Zhong, R.; Pu, L.J.; Xie, J.Y.; Yao, J.M.; Qie, L.; He, G.L.; Wang, X.Q.; Zhang, R.; Zhai, J.H.; Gong, Z.S.; et al. Carbon storage in typical ecosystems of coastal wetlands in Jiangsu, China: Spatiotemporal patterns and mechanisms. Catena 2025, 254, 108882. [Google Scholar] [CrossRef]
- Yao, L.; Adame, M.F.; Chen, C.R. Resource stoichiometry, vegetation type and enzymatic activity control wetlands soil organic carbon in the Herbert River catchment, North-east Queensland. J. Environ. Manag. 2021, 296, 113183. [Google Scholar] [CrossRef]
- Knelman, J.E.; Legg, T.M.; O’Neill, S.P.; Washenberger, C.L.; González, A.; Cleveland, C.C.; Nemergut, D.R. Bacterial community structure and function change in association with colonizer plants during early primary succession in a glacier forefield. Soil Biol. Biochem. 2012, 46, 172–180. [Google Scholar] [CrossRef]
- Zhou, N.; Li, H.G.; Wang, B.L.; Rengel, Z.; Li, H.B. Differential root nutrient-acquisition strategies underlie biogeochemical niche separation between grasses and forbs across grassland biomes. Funct. Ecol. 2024, 38, 2286–2299. [Google Scholar] [CrossRef]
- Huang, K.L.; De Long, J.R.; Yan, X.B.; Wang, X.Y.; Wang, C.L.; Zhang, Y.W.; Zhang, Y.Y.; Wang, P.; Du, G.Z.; van Kleunen, M.; et al. Why are graminoid species more dominant? Trait-mediated plant–soil feedbacks shape community composition. Ecology 2024, 105, e4295. [Google Scholar] [CrossRef]
- Arnosti, C.; Bell, C.; Moorhead, D.L.; Sinsabaugh, R.L.; Steen, A.D.; Stromberger, M.; Wallenstein, M.; Weintraub, M.N. Extracellular enzymes in terrestrial, freshwater, and marine environments: Perspectives on system variability and common research needs. Biogeochemistry 2014, 117, 5–21. [Google Scholar] [CrossRef]
- Xia, S.P.; Song, Z.L.; Li, Q.; Guo, L.D.; Yu, C.X.; Singh, B.P.; Fu, X.L.; Chen, C.M.; Wang, Y.D.; Wang, H.L. Distribution, sources, and decomposition of soil organic matter along a salinity gradient in estuarine wetlands characterized by C:N ratio, δ13C- δ15N, and lignin biomarker. Glob. Change Biol. 2020, 27, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Song, C.C.; Yang, G.S.; Wang, X.W.; Wang, L.L.; Li, Y.C.; Hou, C.C. Variation of soil enzyme activities and correlations with labile organic carbon fractions in different types of wetlands in the sanjiang plain, northeast China. Fresenius Environ. Bull. 2013, 22, 86–94. [Google Scholar]
- Wang, H.Y.; Wu, J.Q.; Li, G.; Yan, L.J. Changes in soil carbon fractions and enzyme activities under different vegetation types of the northern Loess Plateau. Ecol. Evol. 2020, 10, 12211–12223. [Google Scholar] [CrossRef]
- Wu, L.L.; Song, Z.L.; Wu, Y.T.; Xia, S.P.; Kuzyakov, Y.; Hartley, I.P.; Fang, Y.Y.; Yu, C.X.; Wang, Y.D.; Chen, J.; et al. Organic matter composition and stability in estuarine wetlands depending on soil salinity. Sci. Total Environ. 2024, 945, 173861. [Google Scholar] [CrossRef]
- Ji, H.; Han, J.G.; Xue, J.M.; Hatten, J.A.; Wang, M.H.; Guo, Y.H.; Li, P.P. Soil organic carbon pool and chemical composition under different types of land use in wetland: Implication for carbon sequestration in wetlands. Sci. Total Environ. 2020, 716, 136996. [Google Scholar] [CrossRef]
- Wang, X.L.; Xu, L.G.; Wan, R.G.; Chen, Y.W. Seasonal variations of soil microbial biomass within two typical wetland areas along the vegetation gradient of Poyang Lake, China. Catena 2016, 137, 483–493. [Google Scholar] [CrossRef]
- Ren, Q.; Yuan, J.H.; Wang, J.P.; Liu, X.; Ma, S.L.; Zhou, L.Y.; Miao, L.J.; Zhang, J.C. Water level has higher influence on soil organic carbon and microbial community in Poyang Lake wetland than vegetation type. Microorganisms 2022, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Were, D.; Kansiime, F.; Fetahi, T.; Hein, T. Soil organic carbon storage in a tropical freshwater wetland: The influence of vegetation type. Afr. J. Aquat. Sci. 2021, 46, 161–172. [Google Scholar] [CrossRef]
- Mao, H.R.; Cotrufo, M.F.; Hart, S.C.; Sullivan, B.W.; Zhu, X.F.; Zhang, J.C.; Liang, C.; Zhu, M.Q. Dual role of silt and clay in the formation and accrual of stabilized soil organic carbon. Soil. Biol. Biochem. 2024, 192, 109390. [Google Scholar] [CrossRef]
- Qiu, S.Y.; Wang, Z.Y.; Xu, J.L.; Cui, E.R.; Yan, L.M. Influence of vegetation dynamics on soil organic carbon and its fractions in a coastal wetland. Ecosyst. Health Sustain. 2023, 9, 0016. [Google Scholar] [CrossRef]
- Qian, L.W.; Yan, J.F.; Hu, Y.; Gao, L.Y.; Wu, P.F.; Wang, L. Spatial distribution patterns of annual soil carbon accumulation and carbon storage in the Jiuduansha wetland of the Yangtze River estuary. Environ. Monit. Assess. 2019, 191, 750. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, J.J.; Wang, P.; Zhu, B. The direct and legacy effects of drying-rewetting cycles on active and relatively resistant soil carbon decomposition. Land Degrad. Dev. 2023, 34, 2124–2135. [Google Scholar] [CrossRef]
- Pendall, E.; Osanai, Y.; Williams, A.L.; Hovenden, M.J. Soil carbon storage under simulated climate change is mediated by plant functional type. Glob. Change Biol. 2011, 17, 505–514. [Google Scholar] [CrossRef]
- Zhang, K.L.; Feng, R.R.; Han, J.N.; Zhang, Z.C.; Zhang, H.J.; Liu, K. Temporal and spatial differentiation characteristics of ecosystem service values based on the ecogeographical division of China: A case study in the Yellow River Basin, China. Environ. Sci. Pollut. R. 2022, 30, 8317–8337. [Google Scholar] [CrossRef]
- Liang, G.F.; Ding, S.Y. Impacts of human activity and natural change on the wetland landscape pattern along the Yellow River in Henan Province. J. Geogr. Sci. 2004, 14, 339–348. [Google Scholar] [CrossRef]
- Li, X.F.; Ding, C.X.; Bu, H.; Han, L.L.; Ma, P.; Su, D.R. Effects of submergence frequency on soil C:N:P ecological stoichiometry in riparian zones of Hulunbuir steppe. J. Soils Sed. 2020, 20, 1480–1493. [Google Scholar] [CrossRef]
- You, C.M.; Wu, F.Z.; Gan, Y.M.; Yang, W.Q.; Hu, Z.M.; Xu, Z.F.; Tan, B.; Liu, L.; Ni, X.Y. Grass and forbs respond differently to nitrogen addition: A meta-analysis of global grassland ecosystems. Sci. Rep.-UK 2017, 7, 1563. [Google Scholar] [CrossRef]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Yin, S.; Bai, J.H.; Wang, W.; Zhang, G.L.; Jia, J.; Cui, B.S.; Liu, X.H. Effects of soil moisture on carbon mineralization in floodplain wetlands with different flooding frequencies. J. Hydrol. 2019, 574, 1074–1084. [Google Scholar] [CrossRef]
- Mavi, M.S.; Marschner, P.; Chittleborough, D.J.; Cox, J.W.; Sanderman, J. Salinity and sodicity affect soil respiration and dissolved organic matter dynamics differentially in soils varying in texture. Soil Biol. Biochem. 2012, 45, 8–13. [Google Scholar] [CrossRef]
- Wang, L.X.; Nie, X.D.; Li, J.Q.; Liu, Y.J.; Wang, H.; Li, Y.Z.; Li, Z.W. Erosion-induced recovery CO2 sink offset the horizontal soil organic carbon removal at the basin scale. Sci. China Earth Sci. 2024, 67, 2019–2033. [Google Scholar] [CrossRef]
- Van Oost, K.; Six, J. Reconciling the paradox of soil organic carbon erosion by water. Biogeosciences 2023, 20, 635–646. [Google Scholar] [CrossRef]
- Liu, S.; Huang, X.J.; Gan, L.; Zhang, Z.B.; Dong, Y.; Peng, X.H. Drying-wetting cycles affect soil structure by impacting soil aggregate transformations and soil organic carbon fractions. Catena 2024, 243, 108188. [Google Scholar] [CrossRef]
- Chai, Y.N.; Schachtman, D.P. Root exudates impact plant performance under abiotic stress. Trends Plant Sci. 2022, 27, 80–91. [Google Scholar] [CrossRef]
- Shabtai, I.A.; Hafner, B.D.; Schweizer, S.A.; Höschen, C.; Possinger, A.; Lehmann, J.; Bauerle, T. Root exudates simultaneously form and disrupt soil organo-mineral associations. Commun. Earth Environ. 2024, 5, 699. [Google Scholar] [CrossRef]
- Bi, X.Q.; Chu, H.; Fu, M.M.; Xu, D.D.; Zhao, W.Y.; Zhong, Y.J.; Wang, M.; Li, K.; Zhang, Y.n. Distribution characteristics of organic carbon (nitrogen) content, cation exchange capacity, and specific surface area in different soil particle sizes. Sci. Rep.-UK 2023, 13, 12242. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.D.; Li, J.Y.; Han, G.X.; Wu, H.T.; Song, W.M.; Zhang, X.S. Effect of salinity on the decomposition of soil organic carbon in a tidal wetland. J. Soils Sed. 2019, 19, 609–617. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Mayes, M.A.; Heal, K.R.; Brandt, C.C.; Phillips, J.R.; Jardine, P.M. Relation between soil order and sorption of dissolved organic carbon in temperate subsoils. Soil Sci. Soc. Am. J. 2012, 76, 1027–1037. [Google Scholar] [CrossRef]
- Hartley, I.P.; Hill, T.C.; Chadburn, S.E.; Hugelius, G. Temperature effects on carbon storage are controlled by soil stabilisation capacities. Nat. Commun. 2021, 12, 6713. [Google Scholar] [CrossRef]
- Tang, H.; Li, Q.; Bao, Q.; Tang, B.; Li, K.; Ding, Y.; Luo, X.J.; Zeng, Q.S.; Liu, S.Z.; Shu, X.Y.; et al. Alpine wetland litter decomposition under wet and dry conditions: A comparative study of native vs. standardized litter. Ecol. Indic. 2024, 161, 111982. [Google Scholar] [CrossRef]
- Rakhsh, F.; Golchin, A.; Al Agha, A.B.; Nelson, P.N. Mineralization of organic carbon and formation of microbial biomass in soil: Effects of clay content and composition and the mechanisms involved. Soil. Biol. Biochem. 2020, 151, 108036. [Google Scholar] [CrossRef]
- Boeddinghaus, R.S.; Nunan, N.; Berner, D.; Marhan, S.; Kandeler, E. Do general spatial relationships for microbial biomass and soil enzyme activities exist in temperate grassland soils? Soil Biol. Biochem. 2015, 88, 430–440. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Jia, J.C.; Zhang, P.P.; Yang, X.F.; Zhang, X.C. Feldspathic sandstone addition and its impact on hydraulic properties of sandy soil. Can. J. Soil Sci. 2018, 98, 399–406. [Google Scholar] [CrossRef]
- Ren, W.T.; Zhang, D.S.; Zhou, Y.; Wang, H.; Xia, L.M.; Tan, C.S.; Guo, F.; Zhang, X.X.; Yang, R.L.; Yu, Y. Lignin’s complex role in lignocellulosic biomass Recalcitrance: A case study on bamboo. Chem. Eng. J. 2024, 490, 151422. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barre, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Bernal, B.; Mitsch, W.J. Comparing carbon sequestration in temperate freshwater wetland communities. Glob. Change Biol. 2012, 18, 1636–1647. [Google Scholar] [CrossRef]
- Zhang, Q.J.; Wang, Z.S.; Xia, S.X.; Zhang, G.S.; Li, S.X.; Yu, D.K.; Yu, X.B. Hydrologic-induced concentrated soil nutrients and improved plant growth increased carbon storage in a floodplain wetland over wet-dry alternating zones. Sci. Total Environ. 2022, 822, 153512. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Rengasamy, P.; Smith, E.; McDonald, G.K. The effect of cation-anion interactions on soil pH and solubility of organic carbon. Eur. J. Soil Sci. 2015, 66, 1054–1062. [Google Scholar] [CrossRef]
- Chen, Z.; Li, H.B.; Zhang, W.H.; Wang, B.L. The roles of stomatal morphologies in transpiration and nutrient transportation between grasses and forbs in a temperate steppe. Ann. Bot. 2023, 132, 229–239. [Google Scholar] [CrossRef]
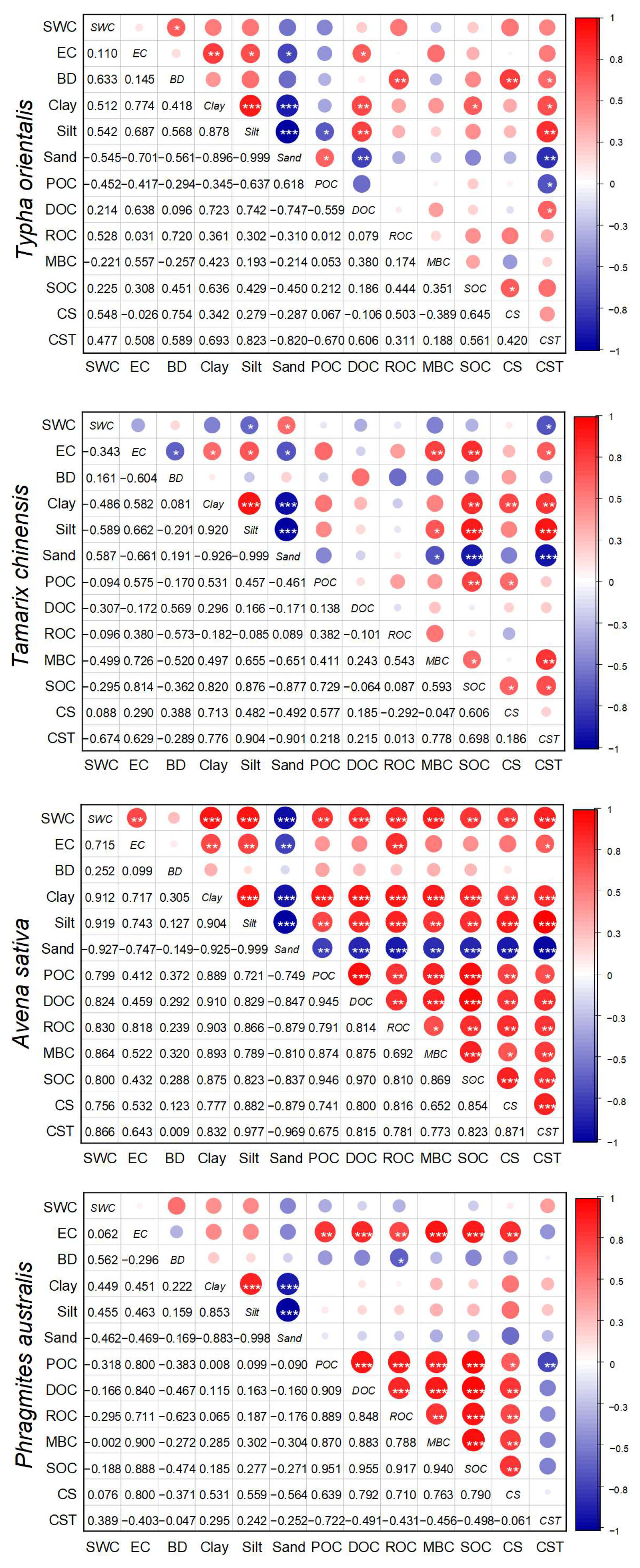
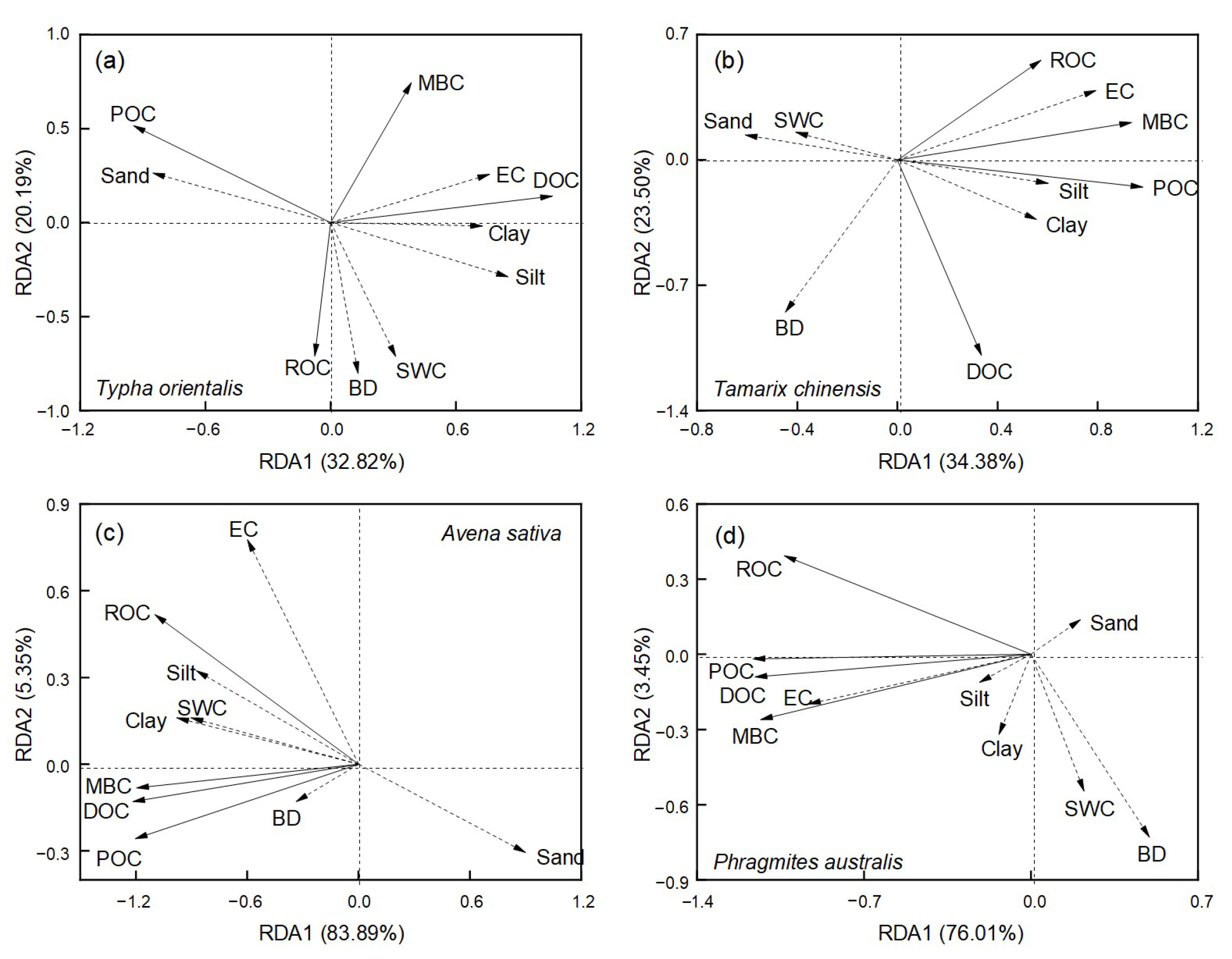
| Vegetation Types | Soil Layer (cm) | BD (kg m−3) | SWC (%) | pH | EC (μs cm−1) | Clay (%) | Silt (%) | Sand (%) |
|---|---|---|---|---|---|---|---|---|
| TO | 0–10 | 1443.47 ± 13.70 Aa | 29.84 ± 0.76 Aa | 9.25 ± 0.06 Aa | 158.97 ± 5.92 Aa | 3.38 ± 0.12 ABa | 72.54 ± 2.95 Aa | 24.08 ± 3.06 Aa |
| 10–20 | 1407.10 ± 14.92 ABa | 27.67 ± 2.06 Aa | 9.29 ± 0.06 Aa | 104.30 ± 0.15 Ab | 2.07 ± 0.10 Ab | 58.97 ± 1.62 Aa | 38.96 ± 1.71 Aa | |
| 20–40 | 1466.53 ± 65.06 Aa | 29.90 ± 1.31 Aa | 9.14 ± 0.21 Aa | 110.00 ± 3.72 Ab | 2.13 ± 0.14 Ab | 62.43 ± 4.77 ABa | 35.44 ± 4.91 Aa | |
| 40–60 | 1519.63 ± 32.01 Aa | 32.16 ± 0.91 Aa | 9.01 ± 0.06 Aa | 127.33 ± 11.34 Ab | 3.02 ± 0.19 Aa | 68.03 ± 0.70 Aa | 28.95 ± 0.88 Aa | |
| TC | 0–10 | 1279.90 ± 19.65 Ba | 1.32 ± 0.27 Ba | 9.21 ± 0.07 Aa | 121.87 ± 7.02 Aa | 1.81 ± 0.06 Ba | 42.72 ± 0.49 Ba | 55.47 ± 0.44 Ba |
| 10–20 | 1340.03 ± 14.66 Bab | 2.09 ± 0.14 Ba | 9.27 ± 0.06 Aa | 98.10 ± 1.96 Aab | 1.87 ± 0.06 Aa | 46.64 ± 4.64 Ba | 51.49 ± 4.68 Ba | |
| 20–40 | 1514.30 ± 53.58 Abc | 2.08 ± 0.90 Ba | 9.33 ± 0.09 Aab | 98.50 ± 13.51 Aab | 2.51 ± 0.24 Ab | 48.06 ± 6.09 Ba | 49.44 ± 6.33 Aa | |
| 40–60 | 1428.37 ± 13.99 Ac | 4.98 ± 1.72 Ba | 9.62 ± 0.03 Bb | 77.23 ± 2.58 Ab | 0.75 ± 0.04 Bc | 8.50 ± 0.90 Bb | 90.75 ± 0.87 Bb | |
| AS | 0–10 | 1477.00 ± 49.86 Aa | 7.95 ± 0.51 Ca | 8.55 ± 0.01 Ba | 142.03 ± 11.78 Aa | 9.23 ± 1.19 Cab | 78.38 ± 4.68 Aa | 12.39 ± 5.73 Aa |
| 10–20 | 1507.30 ± 33.65 Aa | 10.03 ± 0.32 Cb | 8.58 ± 0.02 Ba | 234.53 ± 46.30 Bab | 10.52 ± 0.45 Ba | 82.52 ± 1.77 Ca | 6.96 ± 1.43 Ca | |
| 20–40 | 1410.10 ± 32.08 Aa | 7.29 ± 0.67 Ba | 8.92 ± 0.12 Ab | 194.20 ± 24.44 Aab | 6.28 ± 1.24 Bb | 79.60 ± 3.12 Aa | 14.12 ± 4.30 Ba | |
| 40–60 | 1455.87 ± 58.78 Aa | 2.06 ± 0.44 Bc | 9.61 ± 0.09 Bc | 78.40 ± 1.14 Ab | 0.51 ± 0.17 Bc | 11.59 ± 1.72 Bb | 87.90 ± 1.88 Bb | |
| PA | 0–10 | 1383.10 ± 19.91 ABa | 17.58 ± 1.73 Da | 8.44 ± 0.03 Ba | 1030.00 ± 23.54 Ba | 4.78 ± 0.05 Aab | 73.13 ± 0.90 Aa | 22.10 ± 0.93 Aa |
| 10–20 | 1440.63 ± 32.30 ABa | 16.98 ± 3.10 Da | 8.57 ± 0.08 Bab | 728.33 ± 38.95 Cb | 5.02 ± 0.23 Cab | 74.96 ± 1.88 Ca | 20.02 ± 2.09 Da | |
| 20–40 | 1458.10 ± 33.89 Aa | 21.99 ± 4.46 Aa | 8.73 ± 0.08 Ab | 677.67 ± 86.58 Bb | 6.11 ± 0.57 Ba | 78.56 ± 0.33 Aa | 15.34 ± 0.87 Ba | |
| 40–60 | 1403.43 ± 15.94 Aa | 17.94 ± 2.60 Ca | 8.81 ± 0.05 Ab | 487.00 ± 88.39 Bb | 3.57 ± 0.79 Ab | 64.88 ± 10.11 Aa | 31.54 ± 10.88 Aa |
| Index | Variables | SOC | POC | ROC | DOC | MBC | CST | CS |
|---|---|---|---|---|---|---|---|---|
| F | V | 62.43 | 51.21 | 7.36 | 21.02 | 75.22 | 44.55 | 36.82 |
| D | 46.76 | 32.00 | 7.91 | 18.53 | 69.62 | 32.97 | 9.96 | |
| V × D | 14.83 | 12.29 | 3.79 | 7.67 | 12.19 | 29.43 | 9.01 | |
| p value | V | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| D | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| V × D | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
| Vegetation Types | Partial Correlation Coefficients | F Value | p Value | R2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| SWC | pH | EC | Clay | Silt | Sand | ||||
| Typha orientalis | ns | −0.832 | ns | 0.786 | ns | ns | 6.708 | <0.05 | 0.675 |
| Tamarix chinensis | 0.633 | ns | ns | 0.821 | ns | ns | 12.400 | 0.002 | 0.757 |
| Avena sativa | ns | ns | ns | ns | 0.870 | ns | 35.140 | <0.001 | 0.756 |
| Phragmites australis | ns | ns | 0.777 | ns | ns | ns | 17.810 | 0.002 | 0.604 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yan, C.; Zhu, M.; Yan, S.; Wang, J.; Qian, F. Response Patterns of Soil Organic Carbon Fractions and Storage to Vegetation Types in the Yellow River Wetland. Land 2025, 14, 1785. https://doi.org/10.3390/land14091785
Li S, Yan C, Zhu M, Yan S, Wang J, Qian F. Response Patterns of Soil Organic Carbon Fractions and Storage to Vegetation Types in the Yellow River Wetland. Land. 2025; 14(9):1785. https://doi.org/10.3390/land14091785
Chicago/Turabian StyleLi, Shuangquan, Chuang Yan, Mengke Zhu, Shixin Yan, Jingxu Wang, and Fajun Qian. 2025. "Response Patterns of Soil Organic Carbon Fractions and Storage to Vegetation Types in the Yellow River Wetland" Land 14, no. 9: 1785. https://doi.org/10.3390/land14091785
APA StyleLi, S., Yan, C., Zhu, M., Yan, S., Wang, J., & Qian, F. (2025). Response Patterns of Soil Organic Carbon Fractions and Storage to Vegetation Types in the Yellow River Wetland. Land, 14(9), 1785. https://doi.org/10.3390/land14091785







