Abstract
Potatoes are a strategic crop in Poland, particularly important for agriculture in the southern and southeastern parts of the country. Environmental variability makes assessing yield stability and quality traits of varieties crucial for food security. Research Objective and Methodology: This three-year field study (2021–2023) aimed to comprehensively assess the yield stability and quality traits of mid-early potato varieties. The research was conducted in four pedologically diverse locations (rendzinas, brown soils, alluvial soils, and pseudopodzolic soils), according to the COBORU methodology. Key yield parameters (total and marketable tuber yield) and quality traits (dry-matter and starch content and yield) were analyzed. Interregional stability was also assessed. The environmental characteristics were supplemented with detailed analyses of soil physicochemical and biological properties, monitoring of agroclimatic parameters, and an assessment of the impact of geographical location. The collected data was subjected to advanced statistical analyses (ANOVA, correlations, descriptive statistics). Results analyses revealed significant yield variation across soil types, with the highest yields on alluvial soils and the lowest on pseudopodzolic soils. Geographic location significantly influenced yield stability, highlighting the role of local factors. Strong correlations were also found between soil properties and starch content (r = 0.61–0.73), indicating a key influence of the soil matrix on tuber quality. Conclusions and Recommendations: This study provides practical recommendations for selecting potato varieties adapted to specific soil types, precision fertilization strategies, and climate-change-adaptation protocols. Further research should focus on the impact of extreme weather events, optimized water management, and the use of precision agriculture.
Keywords:
geographic locations; potato varieties; yield stability; soil properties; dry mass; starch 1. Introduction
Genotype–environment (G × E) interactions represent one of the key challenges in the breeding and selection of new varieties, including potatoes. This phenomenon, characterized by the differential response of genotypes to changing environmental conditions, directly impacts the commercial success of a variety, which, to be widely adopted, must demonstrate stable and high yields across a wide range of agro-environments. The presence of significant G × E interactions weaken the correlation between phenotype and genotype, significantly hindering the precise assessment of the true genetic potential of individual genotypes. The vegetative propagation of potatoes makes them an exceptionally advantageous species for studying G × E interactions. The same genotype (clone) can be precisely replicated and tested in a variety of environments, minimizing genetic variability within the studied material and allowing for a more precise understanding of the impact of environmental factors on genotype expression. Second, potatoes are cultivated extensively in highly diverse agroecological conditions worldwide. Their growth, yield, and key quality and resistance traits are strongly modulated by environmental factors such as temperature, water availability, nutrient availability, and pathogen pressure. This makes them an excellent model species for observing and analyzing complex G × E interactions. Third, from a breeding perspective, understanding and managing G × E interactions in potatoes is critical to the success of new varieties. Breeders strive to create varieties that demonstrate stable and high productivity across a wide range of environments, which directly translates to their commercial acceptance and food security [1,2,3]. Genotype stability, defined as its ability to maintain consistent levels of productivity and quality across diverse environments, is directly dependent on the severity of G × E interactions. Genotypes that exhibit significant interactions are often classified as unstable. In the analysis of G × E interactions, it is important to distinguish between the genotype × location (G × L) and genotype × year (G × Y) interactions, which together constitute the genotype × environment (G × E) interaction. Studying these interactions enables the identification of stable genotypes, both in the agricultural sense—characterized by high and consistent yields under various conditions [4,5,6,7,8]—and in the biological sense, referring to the predictability of genotype responses to environmental changes [2,9]. Modern data analysis methods, such as AMMI (Additive Main Effects and Multiplicative Interaction) models or the GGE biplot, allow for a deeper understanding of these complex relationships, facilitating the selection of varieties and the identification of so-called “mega-environments” that optimize differentiation between genotypes [9,10].
Understanding these interactions is fundamental to the effective implementation of new, improved varieties that will be able to meet the challenges of modern agriculture, including increasing climatic variability.
The aim of this study was to determine the influence of genotypic (varietal) and environmental factors (locations, years) and their interactions on total and marketable potato tuber yield, as well as on starch and dry-matter content and yield. Additionally, the analysis aimed to identify stable genotypes that demonstrate high productivity and desirable quality under diverse growing conditions in Poland.
Null Hypothesis (H0).
It is assumed that there are no statistically significant genotype–environment interactions (G × E, G × L, G × Y) in the potato yield and tuber quality traits studied. This means that the cultivars respond consistently to different environments, and any observed differences in their stability are due to chance. Consequently, it is impossible to identify genotypes characterized by increased, statistically significant stability of the yield and/or tuber quality under variable environmental conditions.
Alternative Hypothesis (H1).
It is assumed that there are statistically significant genotype–environment interactions (G × E, G × L, G × Y) that significantly modify the phenotypic expression of potato yield and tuber quality traits, leading to varying stability of the studied cultivars across locations and years. Moreover, it is expected that among the analyzed varieties it will be possible to select genotypes characterized by increased yield stability and/or tuber quality in variable environmental conditions.
2. Material and Methods
2.1. Geographical Location and Climatic Conditions
Strict field experiments were conducted in southeastern Poland in 2021–2023 at the Experimental Variety Testing Stations belonging to the Central Research Center for Cultivar Testing in Słupia Wielka. Legends and detailed explanations, including the geographical location and detailed location of the experiments, are available in the Supplementary Materials (Figures S1–S8).
The study was conducted using a randomized complete block design (RCBD), with each cultivar × location × year (plot) combination having three replications. This design is standard in agronomic research and allows for effective control of within-field variability.
2.1.1. Experimental Variety Evaluation Station in Przecław
The station in Przecław (Mielec County, Podkarpackie Voivodeship) is located at 50°11′ N, 21°28′ E, at an elevation of 185 m above sea level, in the southeastern part of the village (Figure 1, Figures S1 and S2 —in the Supplementary Materials).
Figure 1.
Map showing the location of potato field experiments in southeastern Poland; source: own.
Physiography and Geology: Przecław is part of the Sandomierz Basin, on the border of two areas:
Lower Wisłoka Valley: This is comprised of flat terrain, with brown and chernozem soils developed from floodplains. Sandy and sandy–silty soils (poor/medium) predominate here, although better soils with a predominance of silt, clay, and loam occur in the western part [10].
Tarnów Plateau: This is low-undulating terrain west of the Wisłoka Valley, composed of fluvioglacial formations. Poor, sandy soils predominate, with Cambisols or Phaeozems occurring less frequently [10].
The region’s climate is temperate and transitional with continental influences. It is characterized by warm summers and moderately cold winters. Annual rainfall typically ranges from 550 to 700 mm, with the heaviest rainfall occurring in summer. The average annual temperature is 7–8 °C, and the growing season is long, often exceeding 200 days [11]. The primary vegetation type at the Experimental Variety Evaluation Station in Przecław is cultivated vegetation, reflecting the facility’s specific activity. Its immediate surroundings and the regional landscape are dominated by deciduous and mixed forests, as well as wetland vegetation associated with rivers and their valleys [10,11].
2.1.2. Experimental Variety Testing Station in Słupia
Location: The Experimental Variety Testing Station in Słupia is located in the Słupia commune, Jędrzejów County, Świętokrzyskie Voivodeship, at geographical coordinates 50°36′ N, 19°58′ E, at an altitude of 290 m above sea level, (Figure 1, Figures S3 and S4 in the Supplementary Materials).
This area is located within the Lesser Poland Upland, specifically on the border of the Nida Basin and the Kraków-Częstochowa Upland. It is a slightly undulating, upland terrain with minor differences in relative elevation. The terrain is erosional and denudational, shaped primarily by karst processes and water activity [12,13,14].
Geologically, this area is composed primarily of sedimentary rocks of Mesozoic origin—primarily Jurassic limestone, marl, and sandstone. The topsoil consists of Quaternary deposits, including boulder clays, loess, and river sands, which influence the soil composition. The station area is dominated by leached Cambisols and Luvisols, of medium fertility, well-suited for agricultural crops [15]. Rendzina soils associated with a limestone substrate also occur in places. The climate of the area is characterized by a transitional temperate climate with continental influences. Average annual rainfall ranges from 600 to 700 mm, and the average annual temperature is approximately 7.5 to 8.0 °C [10]. The vegetation type at the Experimental Variety Testing Station in Słupia is largely dominated by intensive agricultural activity (arable fields, meadows). However, in the surrounding and less-developed areas, fragments of natural deciduous and mixed forests (hornbeam and oak forests) can be found, as well as regionally specific habitats, such as xerothermic grasslands. The growing season in this region is relatively long, which favors plant growth. Agroclimatic conditions are favorable for conducting field research and testing crop varieties, which facilitates the operation of the experimental station [10,13].
2.1.3. Experimental Variety Testing Station in Uhnin
Location: The Experimental Station is located in the Dębowa Kłoda commune, Parczew County, Lublin Voivodeship, at geographical coordinates 51°34′ N, 23°04′ E, at an elevation of 157 m above sea level [10] Figure 1, [Figures S5 and S6 —in the Supplementary Materials].
Physiographic characteristics: The Dębowa Kłoda commune lies at the junction of the Łęczna-Włodawa Lake District and the Parczew Plain. This area is agricultural and forested, with a predominance of arable fields, meadows, and pastures. The commune is drained by the Piwonia and Zielawa river basins [10].
Geomorphologically, this area is included in the Polesie Lubelskie and the Siedlce Upland [10]. The terrain is characterized by flats and flat relief with local depressions and small elevations, typical of a young glacial landscape and accumulation plains. This area is characterized by relatively small elevation differences, which favor agricultural use [10,12].
Geologically, the Uhnin area is composed primarily of Quaternary sediments, including boulder clays, sands, clays, and fluvial and aeolian silts (loess). The geological substrate is primarily composed of Cretaceous and Tertiary formations, covered by Quaternary layers of variable thickness. Luvisols, Cambisols, and Phaeozems of varying fertility occur here, mostly of medium-to-low fertility, with local complexes of organic soils (Histosols) in the lowlands [15].
The climate of this region is transitional between maritime and continental, resulting in moderate weather variability [10]. This study utilized Woś’s climate classification [10]. According to this system, the study area is characterized by a temperate transitional climate, which represents a transition zone between the influences of oceanic climate from the west and continental climate from the east. It is characterized by variable weather, with warm summers and frosty, snowy winters, as well as a variable distribution of precipitation throughout the year [10]. The average annual air temperature is approximately 7.0–7.5 °C, and the average annual rainfall ranges from 550 to 600 mm. The growing season lasts approximately 200–210 days, allowing for the cultivation of basic agricultural crops. Spring and autumn frosts are relatively frequent, as are episodic periods of summer drought. The dominant vegetation type in the Uhnin area is a mosaic of forests (mainly pine forests, mixed forests, and marsh forests) and extensive peat bogs and other aquatic and marsh communities, with a significant share of agricultural land. This region is part of the unique Polesie Lubelskie region, characterized by high biodiversity, especially of species associated with wetland habitats [10].
2.1.4. Experimental Variety Testing Station in Węgrzce
Location: The Experimental Station is located in the Zielonki commune, Kraków County, Lesser Poland Voivodeship, at geographical coordinates 50°07′ N, 19°59′ E, at an altitude of 285 m above sea level Figure 1, (Figures S7 and S8 are in the Supplementary Materials).
Physiographic Characteristics. The Zielonki Commune borders Kraków to the north and Ojców National Park. Węgrzce lies within the Proszowice Plateau, part of the Vistula Lowland macroregion [12,13]. The terrain is flat or gently undulating, typical of loess areas, with small valleys of watercourses, such as tributaries of the Dłubnia River. The soils in Węgrzce are formed on a loess substrate. Agricultural Significance: The presence of loess makes the Proszowice Plateau, and specifically the area around Węgrzce, one of the most productive agricultural regions in Poland. It strongly influences the types of crops that can be successfully cultivated, favoring demanding species like wheat and sugar beets.
The commune is composed primarily of Mesozoic sediments, particularly Jurassic limestone (primarily Middle and Upper Jurassic), which create characteristic karst formations such as valleys, monadnocks, and caves [13]. Marl, sandstone, and rock formations also occur within the upland elevations, while the ground surface is in places covered by Quaternary formations—primarily loess and silty clay, which play a significant role in shaping the soil cover. The dominant soil types are Cambisols, characterized by high permeability and fertility, and Chernozems in depressions and on loess [15].
Climatic conditions: Węgrzce is located in a transitional temperate climate zone with continental influences. The average annual air temperature is approximately 8.0–8.5 °C, and the average annual rainfall is 650–750 mm, with the heaviest rainfall occurring in the summer months (June–July) [10]. Frequent temperature fluctuations and periodic phenological phenomena, such as late spring frosts and local summer droughts, are also characteristic. Prevailing winds blow from the west and southwest. In winter, cool air masses from the east are possible, while in summer, hot air masses from the south can arrive. The growing season lasts an average of 210–220 days, and it favors the development of agriculture, particularly fruit growing and cereal cultivation [12]. The vegetation type at the Experimental Station in Węgrzce is dominated by arable crops (cereal, rapeseed, beet, and potato fields). The wider area surrounding the station includes deciduous and mixed forests (mainly oak–hornbeam forests), as well as meadows and characteristics of the region, such as xerothermic grasslands in specific habitats [10]. Its location near the Kraków Upland and proximity to the Carpathians and the Sandomierz Basin make the local climate diverse and prone to seasonal changes. Węgrzce’s location in the immediate vicinity of Kraków also causes local microclimate changes, including slightly higher average winter temperatures and possible air pollution due to urban smog [13]. Węgrzce’s location in the immediate vicinity of Krakow does influence local climatic conditions. Krakow’s proximity, as a large urban agglomeration, contributes to the urban heat-island effect. This results in slightly higher average temperatures, especially in winter, compared to areas further from urban development. Chełmnicki [13] confirms thermal variability within the commune, indicating that average annual temperatures on the plateaus are 7.5 °C, while in the valley floors they drop to 6.2 °C. Although these data concern topographical variations, they fit into the overall picture of temperature variability in the region and indirectly confirm that more open areas or those subject to urban influences (such as plateaus close to the agglomeration) may be characterized by higher temperatures. Air pollution (urban smog): The proximity of Krakow, known for its air-quality problems, is associated with the potential occurrence of air pollution, including urban smog. The proximity of a large metropolitan area and the terrain, which favors variable weather conditions (air mass clashes, foehn winds), are factors influencing air quality, which exhibits elevated concentrations of suspended particulate matter (PM10, PM2.5) during the heating season throughout the Krakow metropolitan area and its surroundings [13].
2.2. Detailed Field Experiment Conditions
Strict field experiments were conducted in accordance with the Methodology for the Study of the Economic Value of Varieties (EVV) applicable at the COBORU Experimental Station for Cultivar Testing [14].
2.2.1. Soil Location and Characteristics
Field experiments were conducted in four locations located in four voivodeships of southeastern Poland (voivodeships: Lubelskie, Małopolskie, Podkarpackie, Świętokrzyskie) on four different soil types: Przecław—Brown Alluvial soil; Słupia—Calcaric Cambisols; Uhnin—Haplic Luvisols; Węgrzce—Eutric Cambisols [15].
Soil Suitability Complexes: The soils in the study locations belonged to various agricultural suitability complexes, such as a good wheat complex—Przecław, Słupia, and Węgrzce and a very good rye complex—Uhnin—[16].
The quality classification of the studied soils was conducted according to the Polish classification system [16]. Based on this assessment, the studied soils were assigned to three quality classes: Class II: very good soils, characterized by high fertility and suitability for growing a wide range of plants; Class IIIa: good soils, still highly productive, but which may require slightly more attention to agricultural practices; and Class 4a: medium soils, with satisfactory productivity, but often with certain limitations, such as lower humus content or poor water retention [17].
2.2.2. Crop Rotation, Schedule, and Crop Parameters
The forecrop in Przecław, Słupia, and Węgrzce was winter wheat. In Uhnin, the forecrop was winter triticale. The selection of the forecrop is an important factor in the context of crop rotation and nutrient availability [18].
Agronomic Schedule. Planting: This took place in the second or third decade of April, depending on weather conditions. Tuber harvesting took place in September or the first decade of October each year, allowing for the assessment of the impact of differences in the length of the growing season.
The crop parameters were as follows: Spacing: A constant spacing of 75 × 33 cm was used, ensuring uniform spatial conditions for the plants. A total of 60 potato plants were planted per plot (30 plants in two rows). The plot area was 15.0 m2. Nutrient management: Continuous NPK fertilization was conducted at the following rates: nitrogen (N): 100 kg ha−1; phosphorus (P2O5): 100 kg ha−1; potassium (K2O): 150 kg ha−1.
2.2.3. Plant-Protection Strategies
The weed control of dicots was as follows: Plateen 41.5 WG at a rate of 2.0 kg ha−1 (metribuzin 17.5% + flufenacet 24.0%)—pre-emergence. Monocotyledonous weeds were controlled using Fusilade Super herbicide—1.5 L ha−1 (fluazifop-P-butyl (150 g L−1)).
Disease and pest management: Comprehensive pest and potato disease control was carried out using a broad spectrum of chemical plant-protection products, including insecticides: Mospilan 20SP: 0.08 kg ha−1 (acetamiprid 20%), Carnadine 200 SL: 0.15 L ha−1 (acetamiprid 200 g L−1), and Cyperkil MAX 500 EC: 0.06 L ha−1 (cypermethrin 500 g L−1); and fungicides: Ridomil Gold 67.8 MZ: 2.0 kg ha−1 (metalaxyl-M 3.8% + mancozeb) 64%), Infinito 687.5 SC: 1.6 L ha−1 (propamocarb hydrochloride 625 g L−1 + fluopicolide 62.5 g L−1), Cabrio Duo 112 EC: 2.5 L ha−1 (dimethomorph 72 g L−1 + pyraclostrobin 40 g L−1), and Acrobat MZ 69 WG: 2.0 kg ha−1 (dimethomorph 9% + mancozeb 60%). All preparations were applied at doses consistent with the recommendations of the Institute of Plant Protection—National Research Institute [19].
2.3. Variety Characteristics
Seven mid-early ware potato varieties, each characterized by different tuber morphological characteristics and culinary suitability, were used in the experiment. All tested varieties, i.e., Irmina, Jurek, Laskara, Mazur, Otolia, Satina, and Tajfun, had yellow skin (Table 1).

Table 1.
Description of potato varieties grown in the experiment.
Table 1.
Description of potato varieties grown in the experiment.
| Varieties | Color of Skin | Color of the Flesh | Shape of the Tubers | Depth of the Tuber Eyes at 9° Scale | Taste 9° Scale | Consumer Type |
|---|---|---|---|---|---|---|
| Irmina | yellow | light yellow | round oval | 7.5 | 6.5 | B-BC |
| Jurek | yellow | yellow | round oval | 7.0 | 7.0 | B-BC |
| Laskara | yellow | light yellow | round oval | 7.0 | 6.5 | B-BC |
| Mazur | yellow | light yellow | oval | 6.5 | 6.5 | AB |
| Otolia | yellow | yellow | oval | 8.0 | 7.0 | BC |
| Satina | yellow | yellow | round oval | 7.5 | 7.5 | B |
| Tajfun | yellow | yellow | oval | 7.0 | 7.0 | B-BC |
Eye depth (mm): 9—very shallow, imperceptible to the touch, 8—very shallow, 7—shallow, 6—medium shallow, requiring minor touch-ups after mechanical peeling; 5—medium deep, requiring significant touch-ups after mechanical peeling 4—deep; 3—very deep, with unevenness between the °—very shallow; taste 9° scale: 9, 8—very good, 7—good, 6—quite good, 5—average good with slight eyes 9 taste and smell defects, 3, 2, 1—poor, not suitable for consumption; consumer type: AB—salad; B—general purpose; BC—slightly floury [20].
The potato varieties studied differed in other aspects, such as flesh color: most varieties had yellow flesh (Jurek, Otolia, Satina, Tajfun), while Irmina, Laskara, and Mazur had light yellow flesh. Tuber shape: Two dominant tuber shapes were distinguished among the varieties studied: round-oval (Irmina, Jurek, Laskara, Satina) and oval (Mazur, Otolia, Tajfun). Eye depth (scale 1–9°): The assessment of eye depth, where 1° indicates very deep eyes and 9° indicates very shallow eyes, revealed variation. The Otolia variety had very shallow eyes (8°), a desirable characteristic due to reduced losses during peeling. The Irmina and Satina varieties had shallow eyes (7.5°). The remaining varieties (Jurek, Laskara, Tajfun) had moderately shallow eyes (7°), while Mazur had slightly deeper eyes (6.5°) compared to the other varieties. Taste (scale 1–9°): Taste evaluation, with higher values indicating better flavor, showed that the Satina variety received the highest score (7.5°). The Jurek, Otolia, and Tajfun received a 7° rating, also indicating good flavor. The Irmina, Laskara, and Mazur varieties received a slightly lower, but still good, score of 6.5°.
Culinary Type: In terms of culinary suitability, the varieties were classified as follows: Type B (general use): Satina. Tubers of this type are versatile, slightly floury after cooking, and suitable for most culinary applications; Type B-BC (general use to slightly floury): Irmina, Jurek, Laskara, and Tajfun (these are transitional varieties that perform well in a variety of dishes, with a tendency to be slightly floury); Type BC (slightly floury): Otolia (these tubers become loose and fall apart easily after cooking and are ideal for purees and soups); and Type AB (salad-based, general-purpose): Mazur (this variety holds its shape after cooking, making it suitable for salads, but can also be used in other dishes [20].
This selection of varieties with diverse characteristics allowed for a comprehensive assessment of their response to various environmental conditions in four locations with different soil and meteorological conditions in terms of yield and quality parameters. The selection of varieties with diverse traits was based not solely on their high yield but also on their diverse characteristics. This diversity was crucial because it allowed for a comprehensive assessment of the responses of different varieties to diverse environmental conditions. By considering a range of potato varieties, it was possible to thoroughly analyze their yield and quality parameters under different conditions, allowing for a better understanding of their performance. Indeed, the varieties selected were based on the existing literature, likely providing a representative and relevant sample for the study.
2.4. Determining Yield Structure and Quality
Potatoes were harvested at the stage of potato plant death (99° on the BBCH scale) [21]. During harvest, total tuber yield was determined, and representative tuber samples were then taken from each plot to assess yield structure, starch content, and dry-matter content. For each variety, a representative sample of approximately 50 kg was collected from each 15 m2 plot to determine the yield structure. This standardized sampling was applied across all locations. The total number of samples used for statistical analysis of yield and quality parameters was: 7 (varieties) × 3 (years) × 4 (locations) × 3 (replicates) = 252 samples. This total of 252 samples represents the data points used to evaluate the performance of the studied varieties under all test conditions.
Yield structure is the weight of individual fractions expressed as a percentage of the total sample weight. The collected sample was sorted on sieves to separate and determine individual tuber fractions, then weighed. In accordance with the Polish standard, the following tuber fractions were adopted for edible and starchy varieties harvested after the end of the growing season: up to 35 mm, 36–50 mm, 51–60 mm, and over 60 mm. The marketable yield consisted of tubers over 35 mm in diameter, excluding cracked and deformed tubers and those with initial signs of rot. [14].
2.5. Determination of Starch Content, Dry Matter, and Yield
The sample size for starch-content determination was approximately 12 kg of tubers. Starch content was determined using an electronic scale (Reimann-Parowa) (Producer: AXIS, Sp. z o.o., Gdańsk, Poland) (Figure S9 in the Supplementary Materials) The methodology specified that the starch content in potato tubers was analyzed twice to ensure accuracy [14].
This scale was currently certified. To determine the percentage of starch content, two 5.0 kg samples (tolerance 0.05 kg) were taken from the sample intended for laboratory testing. The tubers were free of soil and undamaged. The water was clean and at a temperature of 17.5 °C. If a difference in starch determination exceeded 0.5%, a third sample was taken, and the two results with a difference of no more than 0.5% were considered correct [14]. Starch yield (expressed in t ha−1) was calculated using the formula:
Therefore, the formula for extrapolation is the tuber yield (Y) was calculated based on the harvest from each plot with an area of 15 m2, and was then converted to yield per hectare (t ha−1) using the following formula:
where M is the mass of tubers harvested from the 15 m2 plot [kg]; 10,000 is the number of square meters in one hectare; and 1000 is the number of kilograms in one ton.
Starch yield [t ha−1] = Tuber yield [t ha−1] × Starch content [%]
After this conversion, the data is typically subjected to statistical analysis, such as analysis of variance (ANOVA), to assess significant differences between varieties or treatments and draw conclusions. The provided formula for starch yield then uses this calculated tuber yield in [t ha−1] to determine the starch yield per hectare.
The dryer method for determining potato dry matter involved drying a potato flesh sample in a laboratory dryer at 105 ± 2 °C for 16–18 h until a constant weight was achieved. Drying equipment brand: The dryer was manufactured by DANLAB with its registered office at ul. Handlowa 6D, 15-399 Białystok, Poland. The difference in sample weight before and after drying allowed for the calculation of the dry-matter content using the following formula:
where DM is the dry-matter content (%); a is the initial weight of the empty container (g); b is the weight of the container with the fresh sample (g); and c is the weight of the container with the dried sample (g). This method is accurate and widely used in laboratory testing and potato quality assessment [22].
2.6. Soil Conditions
2.6.1. Soil Types
Experimental Station for Cultivar Testing in Przecław: Brown Alluvial Soils (Alluvial Soils): The ESCT Przecław is dominated by Brown Alluvial Soils, which, according to the WRB [2022] classification, correspond to Cambisols developed on alluvial sediments (alluvial soils from the Wisłoka River) (Figure S10a in the Supplementary Materials). They are characterized by
- −
- The presence of a diagnostic cambic horizon (Bw), resulting from weathering and browning processes;
- −
- Formation from river sediments, which may influence the variability of their properties.
The profile of these soils includes:
- −
- Humus (A): The surface layer containing organic matter;
- −
- Brownification level (Bw): The level of browning with structural changes.
Parent rock (C): This is the parent rock or unaltered sediment, often with irregularities indicating material deposition [15]. The soils in the Experimental Station for Cultivar Testing in Słupia are Calcaric Cambisols developing on carbonate rocks (limestone, marl, dolomite) (Figure S10b in the Supplementary Materials). They are characterized by:
- -
- High calcium content, resulting in an alkaline or neutral pH and good nutrient availability for plants;
- -
- Profile A-Bw-C, with
- A (humus): a surface layer rich in organic matter;
- Bw (transition to the bedrock): cambic horizon, formed as a result of browning, with visible fragments of parent rock;
- C (parent rock): unaltered or slightly altered carbonate rock.
Despite their overall fertility, these soils tend to be shallow and rocky, which may limit their agricultural use [15]. At the Experimental Station for Cultivar Testing in Uhnin: Haplic Luvisols.
In Uhnin, experiments were conducted on Haplic Luvisols soils (Figure S11a in the Supplementary Materials). These soils are characterized by the presence of a diagnostic argic horizon (washed-in clay), formed as a result of loessification (movement of clay from the upper layers) [15]. The soil profile at the Uhnin Experimental Station for Cultivar Testing consisted of:
- −
- Cavity level (Level A): the surface humus layer from which clay is washed out;
- −
- Washing-out level (Level E): a light-colored eluvial horizon from which clay, iron, and aluminum have been washed out;
- −
- Immersion level (Level Bt/Argic): an illuvial horizon, with a more intense color and higher density, where washed-in clay accumulates;
- −
- Parent rock (Level C): unaltered or slightly altered parent rock. Haplic Luvisols are typical soils with a well-developed clay movement process, which often makes them fertile agricultural soils [WRB 2022].
Experimental Station for Cultivar Testing in Węgrzce: Eutric Cambisols (Brown Soil).
Eutric Cambisols (Brown Soils) occur in ESCT Węgrzce (Figure S11b in the Supplementary Materials). These are soils with a diagnostic cambic horizon (Bw), formed as a result of weathering and browning. The key characteristic is the qualifier “Eutric,” indicating
- −
- High content of base cations (e.g., Ca, Mg, K, Na) and high base saturation (>50%);
- −
- Fertility and alkaline/neutral pH, favorable for plant growth.
A typical profile for these soils is:
- −
- Humus (A): Dark, organic-rich surface layer;
- −
- Brownification level (Bw): cambic horizon, brown, with visible changes in structure;
- −
- Parent rock (C): the parent rock from which the soil was formed [15].
Primary minerals are transformed into secondary clay minerals (illite, montmorillonite). There is no downward movement of weathering products due to intensive biological circulation and the predominance of humic acids.
2.6.2. Physico-Chemical Properties of Soil
Before establishing the annual field experiments, soil samples were collected to determine mineral fertilization rates. This procedure was performed immediately after harvesting the preceding crop or after basic post-harvest tillage, always before applying mineral fertilization. Sampling was performed in accordance with the Polish standard PN-R-04-028:1997 [23]. The number of samples taken depended on the size and variability of the field, with one sample consisting of an average of 10 to 15 individual samples. Soil pH was determined in KCl according to PN-ISO 10390:1997 [24], phosphorus P2O5 according to PN-R-04023:1996 [25], potassium K2O according to PN-R-04022:1996/Az1:2002 [26], and magnesium Mg PN-R-04020:1994/Az1:2004 [27].
Analysis of the data in Table 2 reveals variation in soil chemical properties across locations and years.

Table 2.
Soil characteristics before establishing the experiment (2021–2023).
Macronutrient content (P, K, Mg): phosphorus (P): Phosphorus levels were variable, from the lowest in Uhnin (e.g., 14.6 mg·100 g−1 in 2023) to the highest in Słupia (37.0 mg·100 g−1 in 2021). The average for all locations and years was 25.3 mg·100 g−1.
Potassium (K): Potassium content also showed significant fluctuations, from 12.8 mg·100 g−1 in Uhnin (2021) to 36.0 mg·100 g−1 in Węgrzce (2022). The average was 21.1 mg·100 g−1.
Magnesium (Mg): The greatest variability was observed for magnesium, with values ranging from very low (2.6 mg·100 g−1 in Uhnin in 2022) to high (16.1 mg·100 g−1 in Przecław in 2021). The average magnesium content was 9.0 mg·100 g−1. Low magnesium levels in Uhnin require special attention when planning fertilization.
The pH (in KCl) ranged from slightly acidic (5.7 in Uhnin) to neutral (7.2 in Przecław). The average pH was 6.3, indicating optimal conditions for most crops, although in Uhnin, the trend toward a lower pH may require liming. This data is crucial for precise planning of mineral fertilization, providing plants with appropriate nutrients and optimal soil pH to maximize yields.
2.7. Meteorological Conditions
Table S1 presents monthly and summary data on air temperature, precipitation, and the Sielianinov hydrothermal coefficient (KHT) for four locations (Przecław, Słupia, Uhnin, Węgrzce) during the potato vegetation period (April–September) in 2021–2023. Analysis of this data allows for the assessment of humidity and thermal conditions in individual years and locations, which is crucial for plant development.
In 2021, meteorological conditions were generally favorable in terms of humidity:
Precipitation: 2021 was characterized by high precipitation totals during the growing season. Węgrzce (668.6 mm) and Przecław (599.5 mm) recorded the highest values. Particularly high rainfall occurred in July and August in Przecław (188.3 mm and 145.9 mm), and in August in Słupia (257.1 mm) and Węgrzce (225.8 mm). Temperatures: Average monthly temperatures remained at optimal levels, typical for potato vegetation, with increases from April to July/August (maximum temperatures in July/August ranging from 20.4 to 22.2 °C). Sielianionov’s hydrothermal coefficient (KHT): Most months, especially July and August (the period of intense tuber formation), had KHT values significantly above 1.5 and often above 2.0 (e.g., Przecław in July 2.8, August 2.7; Słupia in August 5.0; Węgrzce in August 3.9). This indicates very good or even excessively humid conditions during these months, which could favor tuber development, but in extreme cases (such as Słupia in August with KHT 5.0) these conditions could lead to problems with excessive humidity, fungal diseases, or harvest difficulties. June in Przecław (0.5) and Słupia (1.4), and July in Słupia (0.9), indicated periods bordering on drought or moderate conditions, respectively. Overall 2021 can be considered a wet year, with conditions ranging from sufficiently wet to excessively wet. This generally favored potato growth but locally could have led to adverse effects from excess water (Table S1).
Meteorological conditions in 2022, a dry year, particularly in Przecław: Precipitation: 2022 was significantly drier than 2021, with lower rainfall totals. Przecław recorded only 290.7 mm, a significant decrease compared to the previous year. Other locations also had lower rainfall totals (Słupia 425.7 mm, Uhnin 367.0 mm, Węgrzce 403.7 mm). Temperatures: July and August were often higher than in 2021 (e.g., Przecław in August 20.7 °C, Węgrzce in August 21.8 °C), which, combined with low rainfall, exacerbated the drought effect. Sielianionov’s Hydrothermal Coefficient (KHT): Many months in 2022 were characterized by low KHT values, often below 1.0, or even below 0.5, indicating drought. Przecław: May (0.5), June (0.4), August (0.6)—periods of drought or severe drought. Węgrzce: May (0.4), June (1.2), August (1.2)—also indicate periods of drought at the beginning of the growing season and moderate conditions in mid-summer. Even in September, despite the drop in temperatures, the KHT often did not indicate hydration. Overall, 2022 was a difficult year for potato cultivation due to water shortages, particularly noticeable in Przecław and Węgrzce, which likely negatively impacted yields (Table S1).
Conditions in 2023—this was an intermediate year, with local differences: Precipitation: Precipitation totals in 2023 fell between those of 2021 and 2022. Przecław (521.9 mm) and Węgrzce (487.0 mm) had moderate rainfall, while Uhnin (297.3 mm) was again very dry, and Słupia (405.6 mm) was also relatively dry. Temperatures: July and August remained high (often above 20 °C), similar to previous years. September was also relatively warm in some locations (e.g., Przecław 17.3 °C, Uhnin 18.8 °C, Węgrzce 19.1 °C). Sielianionov’s Hydrothermal Coefficient (KHT): There were both months with favorable KHT (e.g., May in Przecław 2.0, May in Uhnin 2.1, May in Węgrzce 2.5) and periods of drought. Uhnin: July (0.9), August (0.5), September (0.3)—indicating severe drought later in the growing season. Przecław and Słupia: These had more balanced conditions, with KHT often in the range of 1.0–2.5, although September in Przecław (0.9) indicated drought. The year 2023 was a year of variable weather conditions. While some locations (Przecław, Węgrzce) experienced moderately favorable conditions, Uhnin and Słupia faced severe water shortages during key stages of potato development, particularly at the end of the growing season. Long-Term General Summary: Inter-Summer Variability: The data shows significant variability in agroclimatic conditions between 2021 and 2023. The year 2021 was wet, 2022 was dry, and 2023 was intermediate, with local droughts. This variability in weather conditions is a key factor in potato-yield volatility. Drought Risk: In each year, regardless of the general trend, there were periods with a KHT below 1.0, indicating water shortages. This is particularly evident during the summer months (June-September), when temperatures are highest and potato water demand is highest. Impact on Yield: Extreme conditions (both excess and deficit water) can negatively impact potato development and yield. The high yield in 2021 (as suggested by the data in the previous table) may have been related to optimal or excessive rainfall, while the yield decline in 2022 and local declines in 2023 may have been directly related to drought. Significance of Location: Clear differences are evident between locations, suggesting that microclimate and local hydrological conditions play a significant role in plant-water availability. Uhnin appears to be the most vulnerable to drought during the study period (Table S1).
This analysis allows for a better understanding of the environmental factors that shape potato yield in individual years and regions, which is crucial for agronomic planning and assessing variety resilience.
2.8. Statistics Calculations
Statistical analysis of the results was mainly performed using ANOVA [27]. The significance of sources of variation was tested using the Fischer–Snedecor “F” test, and the significance of differences using the Tukey test.
To meet the assumptions of ANOVA, specifically normality of distribution and homogeneity of variances, a normalizing transformation was applied to the percentage-expressed outcome data. The arcsin (arcsine square root) transformation was used, as it is the classic and most recommended transformation for data expressed as proportions or percentages. This transformation effectively converts the data, so its distribution becomes more closely approximated to normal, and its variance becomes more homogeneous.
The correct formula for this transformation is
Letter symbols (e.g., “a,” “b,” “c”) in analysis of variance (ANOVA), placed next to mean values in the results tables, were used to visually represent the results of post-hoc tests. The main purpose of these symbols is to indicate which groups (e.g., cultivars, locations, years) differ statistically significantly from each other in terms of the trait being tested. Same letters: If two or more mean values have the same letter (e.g., “a” and “a,” or “ab” and “b”), this means there is no statistically significant difference between these means. Different letters: If two mean values have different letters (e.g., “a” and “b,” or “a” and “c”), this means there is a statistically significant difference between these means at the assumed significance level (usually p < 0.05).
In order to determine the share of individual sources of variation and their interaction in the total variability of the traits studied, an assessment of variance components was carried out according to a random model, using the following designations:
σ2e—assessment of environmental variability associated with repeated observations or measurements in time;
σ2G—assessment of genotypic (varietal) variability;
σ2Y—assessment of variability associated with years of study (years);
σ2p—assessment of phenotypic (total) variability.
The empirical values of mean squares obtained from the analysis of variance were compared with their expected values. By solving the systems of equations in this way, an estimate of variance components corresponding to individual sources of variability was obtained. The mutual relations of the determined variance component estimates and their percentage structure were the basis for assessing the influence of environmental, genotypic, and year factors on the variability of tuber-yield structure traits and starch and dry-matter content and yield.
Additionally, descriptive statistics were calculated using SPSS software version 28 [28]. The variability of the research results was assessed using the following measures: arithmetic mean, which provides a measure of central tendency; standard deviation for all assessed traits presented within the results alongside other variability indicators for a comprehensive comparison, which quantifies the spread or dispersion of the data around the mean; and the coefficient of variation (V), which is a dimensionless measure calculated as the ratio of the standard deviation to the mean. It allows for the comparison of variability between different datasets or different characteristics within the same dataset, irrespective of the units of measurement. Furthermore, Pearson’s simple correlation coefficients were calculated to determine the strength and direction of linear relationships between variables.
3. Results
3.1. Total and Marketable Tuber Yield
Table 3 presents the total and marketable yield for seven potato varieties (Irmina, Jurek, Laskara, Mazur, Otolia, Satina, Tajfun) grown in four locations (Przecław, Uhnin, Słupia, Węgrzce) over three years (2021–2023). The letters (e.g., “a,” “b,” “c”) attached to the numbers are crucial for statistical interpretation.

Table 3.
Total and commercial yield of tubers [t ha−1].
Table 3 presents the dynamics of the total and marketable yield of seven potato varieties.
Differences between years: Significant variability in yield is visible between years. The year 2021 was characterized by the highest average total yield (54.67 t ha−1) and marketable yield (50.32 t ha−1), which was statistically significantly higher than in 2022 (45.31 t ha−1 of marketable yield) and generally more favorable than in 2023. The year 2022 was the least favorable, with a significant decrease in marketable yield (average 45.31 t ha−1), suggesting that conditions this year were less favorable, especially for the marketable quality of tubers. This is consistent with the previous analysis of agroclimatic conditions, which indicated a drier year in 2022 (Table 3).
Differences between locations: Słupia and Węgrzce consistently achieved higher average total yields (62.47 t ha−1 and 60.29 t ha−1, respectively) and marketable yields (53.47 t ha−1 and 57.25 t ha−1, respectively) compared to Przecław (44.29 t ha−1 total yield, 38.30 t ha−1 marketable yield) and Uhnin (46.99 t ha−1 total yield, 44.72 t ha−1 marketable yield). This means that Słupia and Węgrzce had more favorable conditions for potato yields during the period under review. Przecław and Uhnin showed lower yields, which correlates with the unfavorable agroclimatic conditions identified earlier (e.g., droughts in Uhnin) (Table 3).
The Jurek variety has the highest average total yield (60.68 t ha−1) and marketable yield (56.15 t ha−1), making it the leader in terms of productivity. These values are statistically significantly higher than most other varieties. Satina consistently records the lowest total yield (45.03 t ha−1) and marketable yield (39.95 t ha−1), indicating its poorer adaptation to the growing conditions in these locations or lower yield potential. The Laskara and Tajfun varieties also demonstrate high yield potential, particularly in some locations and years. Interactions (L × V; Y × L; Y × V): High LSD values for interactions (especially L × V: 22.8 for total yield and 21.0 for marketable yield; Y × V: 17.1 and 15.8) indicate that the response of varieties to environmental conditions (location and year) is highly variable.
3.2. Yield Structure
Analysis of the effect of experimental factors on the mass fraction of potato tubers with different diameters (below 35 mm, 36–50 mm, 51–60 mm, and above 60 mm) revealed significant differences depending on location, tested varieties, and years of cultivation (Table 4).

Table 4.
Effect of location, varieties, and years on the mass fraction of tubers with diameters.
The location had a significant impact on the tuber fraction proportions. Przecław had the highest share of the smallest tubers (<35 mm), at 4.8%. At the same time, the share of the largest tubers (>60 mm) was relatively low (38.9%) compared to other locations. In Uhnin, the medium-large tuber fraction (36–50 mm in diameter) dominated, reaching a share of 52.2%, the highest in this category. The lowest share of the largest tubers (>60 mm) was also observed there, at just 8.2%. Słupia and Węgrzce had the highest share of tubers over 60 mm in diameter, at 48.8% and 51.5%, respectively, indicating favorable conditions for the development of large tubers in these locations. It is also worth noting that Węgrzce had a relatively low share of tubers with a diameter of 36–50 mm (14.7%). The lowest proportion of tubers < 35 mm was recorded in Uhnin (1.6%) and Słupia (2.1%) (Table 4).
The genetic characteristics of the potato varieties significantly differentiated the distribution of tuber size (Table 4). Mazur and Otolia showed the highest proportion of large tubers (>60 mm), at 48.2% and 47.0%, respectively, suggesting their predisposition to form large-sized tubers. These varieties also had the lowest proportion of the smallest tubers (<35 mm)—Otolia at 1.2% and Mazur at 2.2%. In contrast, varieties such as Irmina, Jurek, Laskara, and Tajfun had a high proportion of tubers in the 36–50 mm range (above 27%). Tajfun, in particular, achieved the highest result in this category—33.1%. This variety also had one of the highest proportions of the smallest tubers (<35 mm)—4.1%, while also having the lowest proportion of the largest tubers (>60 mm)—25.7%. The Laskara variety, on the other hand, had the highest proportion of tubers in the 51–60 mm fraction (34.5%) (Table 4).
The year significantly modified the proportions of individual fractions. In 2021, the highest proportion of medium-large tubers (36–50 mm) was recorded at 29.9%, and the highest proportion of tubers was recorded with a diameter of 51–60 mm (35.5%). At the same time, this year was characterized by the lowest proportion of the largest tubers (>60 mm)—26.3%. In 2022 and 2023, a significant increase in the share of the largest tubers (>60 mm) was observed, reaching 42.0% and 42.2%, respectively. These years favored the development of large tubers. In 2022, the lowest share of medium-large tubers (36–50 mm) was recorded at 19.4%, and in 2023, the lowest share of tubers 51–60 mm was recorded (27.5%) (Table 4).
On average, for all experimental factors, the largest mass share was accounted for by tubers with a diameter of 51–60 mm (31.9%) and >60 mm (36.9%). The smallest tubers (<35 mm) accounted for only 3.0% of the mass, and the 36–50 mm fraction accounted for 25.1% (Table 4).
Based on a comprehensive ANOVA analysis of tuber yield structure, the following locations and varieties were identified as having the largest tuber diameters:
Large Tuber Diameters: The analysis showed that Słupia and Węgrzce consistently provided the most favorable conditions for the development of large tubers. They recorded the highest proportion of tubers over 60 mm in diameter, reaching 48.8% and 51.5%, respectively. Uhnin, on the other hand, had the lowest proportion of tubers > 60 mm (8.2%), and Przecław also achieved a relatively lower proportion of large tubers (38.9%).
Varieties Predisposed to Forming Large Tuber: Among the varieties tested, the Mazur and Otolia varieties demonstrated a strong genetic predisposition to forming large tubers. These varieties had the highest proportion of tubers over 60 mm in diameter, reaching 48.2% for Mazur and 47.0% for Otolia. These varieties also had the lowest percentage of the smallest tubers (<35 mm) in the yield. Varieties such as Tajfun, on the other hand, had the lowest percentage of the largest tubers (>60 mm)—25.7%.
Annual trends: The years 2022 and 2023 significantly favored the development of larger tubers, with a significant increase in the share of tubers over 60 mm in diameter, which, compared to 2021 (26.3%), amounted to 42.0% and 42.2%, respectively.
A detailed analysis of the tuber-size distribution, presented in Table 4, is crucial for optimizing crop planning and selecting varieties based on the desired tuber size for specific market needs.
The interaction between year and variety also proved significant (Figure 2. In 2022 and 2023, a clear upward trend was observed in the share of the largest tubers (>60 mm) in the total yield, to 42.0% and 42.2%, respectively, compared to 26.3% in 2021. At the same time, these years were characterized by a lower share of the medium-sized tuber fraction (36–50 mm and 51–60 mm) compared to 2021. The share of the smallest tubers (<35 mm) was the highest in 2021 (3.4%) and decreased in the following years (Figure 2).
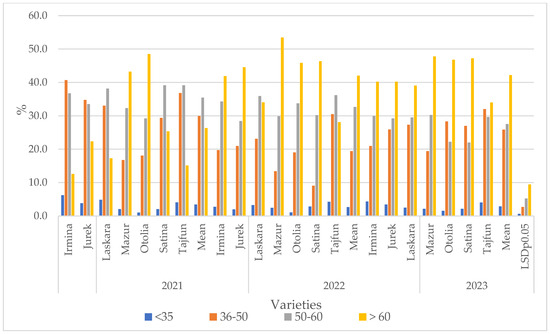
Figure 2.
Effect of cultivars and years on the mass share of tubers with a diameter of <35, 36–50, 51–60, and >60 mm.
The Otolia and Mazur varieties consistently showed a very low proportion of tubers below 35 mm in all years studied, indicating a lower tendency to form small tubers. The proportion of medium-sized tubers (36–50 mm) dominated in 2021 (average 29.9%). The Irmina (40.7%) and Tajfun (36.8%) varieties achieved particularly high values this year. In 2022 and 2023, the average proportion of this fraction dropped significantly (19.4% and 25.8%, respectively), which may suggest favorable conditions for further tuber growth. Despite the overall decline, the Tajfun variety maintained a high proportion of this fraction in 2023 (32.0%). The Mazur and Satina varieties showed a very low proportion of this fraction in 2022 (13.4% and 9.1%, respectively). Share of medium-sized tubers (36–50 mm): 2021 had the highest share of this fraction (average 29.9%). The Irmina (40.7%) and Tajfun (36.8%) varieties achieved particularly high values this year. In 2022 and 2023, the average share of this fraction decreased significantly (19.4% and 25.8%, respectively), which may suggest favorable conditions for further tuber growth. Despite the overall decline, the Tajfun variety maintained a high share of this fraction in 2023 (32.0%). The Mazur and Satina varieties showed a very low share of this fraction in 2022 (13.4% and 9.1%, respectively) (Figure 2).
Share of medium-large tubers (51–60 mm): The highest was in 2021 (35.5%). Satina (39.1%), Tajfun (39.2%), and Laskara (38.2%) were the leading varieties in this category. In 2022 and 2023, the share of this fraction was lower, especially in 2023 (average 27.5%). This year, the Otolia and Satina varieties showed a significant decline in this fraction, which may have contributed to the increase in the share of the largest tubers (Figure 2).
Proportion of largest tubers (>60 mm): An increase in their share was observed in 2022 and 2023. This is the most prominent fraction, with a dynamic increase in share in the last two years. In 2021, the average share was 26.3%, while in 2022 and 2023 it reached 42.0% and 42.2%, respectively. The Mazur variety consistently dominated this category in all years, achieving the highest values: 43.2% (2021), 53.5% (2022), and 47.8% (2023). Otolia also showed a very high share of the largest tubers, particularly in 2021 (48.5%) and 2022 (45.9%). Satina also had a high share of this fraction in 2022 and 2023 (46.4% and 47.2%). Varieties with a lower share: Irmina, Tajfun, and Laskara consistently showed a lower share of the largest tubers compared to Mazur, Otolia, and Satina, especially in 2021 (Figure 2). The results indicate an interaction between variety and growing year in shaping the size structure of tuber yield. The years 2022 and 2023 were clearly more favorable for the growth of large tubers, as reflected by a significantly higher proportion of the >60 mm fraction. Varieties such as Mazur, Otolia, and Satina demonstrated genetic predisposition to form large tubers, especially under favorable conditions. In contrast, Irmina and Tajfun were more likely to have a higher proportion of medium-sized tubers. This information is crucial for optimizing variety selection to meet specific market requirements regarding tuber sizing (Figure 2).
3.3. Starch Content and Yield
Table 5 provides information on tuber quality in terms of starch content and yield.

Table 5.
Starch content and yield of starch.
Starch content—differences between years: 2022 had the highest average starch content (14.5%), statistically significantly higher than in 2021 (13.0%) and 2023 (13.5%). This may be due to stress conditions (e.g., drought) in 2022, which often favor the accumulation of dry matter and starch in tubers. Differences between locations: Uhnin (14.4%) and Słupia (14.3%) had the highest average starch content, while Przecław (12.6%) and Węgrzce (13.5%) had the lowest. Differences between varieties: Tajfun (15.6%) and Laskara (14.9%) had the highest average starch content, while Satina (11.9%) and Irmina (12.2%) had the lowest values (Table 5).
Starch yield: Differences between years: 2022 recorded the highest average starch yield (7.79 t ha−1), which is statistically significantly higher than in 2021 (7.11 t ha−1) and 2023 (7.12 t ha−1). Despite lower overall yields in 2022, the higher starch content translated into higher starch yields. Differences between locations: Słupia (9.01 t ha−1) and Uhnin (6.78 t ha−1) achieved higher average starch yields, compared to Przecław (5.42 t ha−1) and Węgrzce (8.15 t ha−1). Starch yields were significantly higher in Słupia. Variety differences: Laskara (8.42 t ha−1) and Tajfun (8.44 t ha−1) had the highest average starch yields, consistent with their high starch content. Jurek (7.73 t ha−1) also had very good starch yields. Satina (5.39 t ha−1) again had the lowest values, confirming its overall poor productivity (Table 5).
Interactions (Y × L × V): The very high LSDs (17.2 for starch content and 9.4 for starch yield) for the Y × L × V interaction emphasize that starch content and yield are very strongly dependent on the specific combination of variety, location, and year (Table 5).
3.4. Dry-Matter Content and Yield
Table 6 provides information on dry-matter content and yield in tubers. Dry-matter content: Differences between years: 2022 (19.4%) showed the highest average dry-matter content, statistically significantly higher than 2021 (18.4%) and 2023 (18.7%). This is similar to the trend observed for starch, suggesting that conditions in 2022 (likely drought) favored dry-matter accumulation.

Table 6.
Dry-matter content and yield.
Differences between locations: Uhnin (19.2%) and Słupia (19.3%) had the highest average dry-matter content, while Przecław (18.2%) and Węgrzce (18.7%) had the lowest (Table 6). Differences between varieties: Tajfun (21.1%) and Laskara (20.3%) had the highest average dry-matter content. Satina (17.2%) and Irmina (16.9%) recorded the lowest values. Dry-matter yield: Differences between years: 2022 (10.41 t ha−1) showed the highest average dry-matter yield, statistically significantly higher than in 2021 (10.09 t ha−1) and 2023 (9.82 t ha−1). Again, despite the overall lower tuber yields in 2022, the higher dry-matter concentration translated into higher yields of this nutrient (Table 6).
Differences between locations: Słupia (12.13 t ha−1) had the highest average dry-matter yield, which was statistically significantly higher than in the other locations. Uhnin (9.03 t ha−1), Przecław (7.97 t ha−1), and Węgrzce (11.29 t ha−1) had lower values. Differences between varieties: Laskara (11.45 t ha−1) and Tajfun (11.41 t ha−1) achieved the highest average dry-matter yields. Jurek (10.91 t ha−1) also performed very well. Satina (7.76 t ha−1) had the lowest dry-matter yield (Table 6).
Interactions (Y × L × V): As with starch, the high LSD values (24.0 for dry-matter content and 13.4 for dry-matter yield) for the Y × L × V interaction indicate a strong dependence of these traits on the specific combination of variety, location, and year (Table 6).
3.5. Influence of Genotypic and Environmental Factors
Table 7 provides key information on the relative importance of genotype, environment, and their interactions in shaping the studied traits.

Table 7.
Influence of genotypic (varieties) and environmental (year) factors on yield, yield structure, and dry-matter and starch content and yield of potatoes.
Dominant influence of the year (environment): Total and marketable tuber yield: Year (as an environmental factor) is the dominant factor influencing yield, accounting for 68.2% of the total variability in tuber yield and 67.1% of marketable yield. This confirms previous observations of a strong influence of weather conditions (especially in 2022) on yield. The Variety × Year interaction (25.9% and 25.3%) also plays a significant role, emphasizing that varieties respond differently to changing weather conditions. Varieties alone have a relatively small direct effect (4.1% and 5.6%) on yield compared to years and interactions (Table 7).
Dry-Matter and Starch Yield: Year (47.7% and 45.2%) and the Variety × Year interaction (37.3% and 35.2%) also have a significant, though slightly smaller, effect on dry-matter and starch yield compared to overall yield (Table 7).
Influence of Variety (Genotype) on Quality: Dry-Matter and Starch Content: Unlike yield, varieties are the dominant factor influencing dry-matter (37.4%) and starch (49.8%) content in tubers. This indicates that the genetic potential of a variety is crucial for determining its quality. The effect of year (28.9% and 26.7%) and the interaction (32.1% and 16.4%) are also significant, but smaller than that of yield (Table 7).
Effects on Yield Structure: For tuber-weight fractions (e.g., <4 cm, 4–5 cm, >6 cm), both year and the Variety × Year interaction have a dominant effect, often exceeding 50%, meaning that the tuber-size distribution is strongly modulated by environmental conditions. Varieties alone have a smaller direct effect (Table 7).
Effects of All Factors on All Traits: All tested factors (Varieties, Years, Varieties × Years) had a statistically significant effect (marked **) on all tested traits, except for the share of 5–6 cm tubers, where the effect of varieties was insignificant (Ns) (Table 7).
Hierarchy of Influence: For quantitative traits (total and marketable yield, dry-matter and starch yield), the effect of year (environment) is dominant, followed by the Variety × Year interaction. The effect of varieties alone is relatively small. For quality traits (dry-matter and starch content), the influence of cultivars (genotype) dominates, although year and interactions also play a significant role. For yield structure (tuber fraction), the influence of year and interactions again dominates. These results provide a solid basis for further recommendations for farmers and breeders, emphasizing the importance of both cultivar selection and adapting cultivation practices to prevailing environmental conditions.
3.6. Descriptive Statistics of Yield Characteristics
Table 8 presents descriptive statistics for ten different potato-yield characteristics, designated by symbols y through x9. Analysis of these indices allows for an in-depth characterization of the distribution, typical values, variability, and shape of the distribution for each of the studied characteristics across the entire dataset.

Table 8.
Descriptive statistics of potato yield and its quality characteristics.
The data analyzed consisted primarily of continuous variables, such as yield (t ha−1), starch content (%), and other quantitative quality parameters. Regarding the sample size, for each variety at each location and year, the data was derived from three experimental repetitions (plots). Therefore, the total sample size for the analysis of yield and quality parameters across the entire study was 252 (7 varieties × 3 years × 4 locations × 3 repetitions).
Mean and Median: Traits y (total tuber yield) and x1 (marketable tuber yield) exhibit the highest mean values (53.51 and 48.43, respectively), with medians very close to the means (51.81 and 48.02). This convergence of the mean and media suggests that the distributions of these key yield traits are relatively symmetrical, indicating the absence of strong extreme values that could significantly distort the mean. Traits x2 to x9 have significantly lower mean and median values, indicating that they represent different, specific aspects of yield (e.g., nutrient content, tuber fraction) measured on a different scale (Table 8).
Standard Deviation and Coefficient of Variation. Variability of Yield Traits (y, x1): The standard deviation for y is 12.06, and for x1 it is 11.40. Coefficients of variation of 22.53% and 23.53%, respectively, indicate moderate yield variability. This means that yields differ between the conditions studied and cultivars, but this variability is typical of agricultural data, where the influence of environmental and genetic factors is significant (Table 9).

Table 9.
Pearson’s simple correlation coefficients of yield and its quality characteristics.
Traits with the highest variability: Traits x6 (coefficient of variation 84.06%) and x7 (78.01%) exhibit relatively high relative variability. Such high CV values (significantly above 50%) suggest that the data for these traits are highly scattered and irregular. This may indicate a high sensitivity of these traits to subtle changes in environmental conditions, measurement errors, or the strong influence of outliers. These traits require special attention in further analysis. Trait x9 (63.97%) is characterized by relatively high variability (Table 9). Traits with the lowest variability: Traits x4 (10.18% CV) and x2 (15.03% CV) have the lowest relative variability. This means their values are relatively stable and close to the mean, suggesting that these traits are less sensitive to changes in growing conditions over the study period (Table 8).
Skewness: Skewness measures the asymmetry of the data distribution. Positive Skewness: Traits y (0.55), x1 (0.37), x4 (0.43), x5 (0.72), x6 (1.44), and x7 (1.21) exhibit positive skewness. This means that their distributions have a longer “tail” on the right side, with most of the data clustered to the left of the mean. For x6 and x7, high skewness values (over 1.0) indicate significant asymmetry, with a clear predominance of lower values and the presence of several very high outliers. Negative skewness: Features x2 (−0.37), x8 (−0.16), and x9 (−0.06) exhibit negative skewness, meaning that their distributions have a longer “tail” on the left side. However, they are relatively close to symmetry. Feature x3 (0.66) has moderate positive skewness (Table 8).
Kurtosis: Kurtosis measures the “spiciness” or “flatness” of a distribution compared to the normal distribution (which has a kurtosis close to 0). Negative Kurtosis: The features y (−0.05), x1 (−0.02), x2 (−0.37), x4 (−0.60), x8 (−0.72), and x9 (−1.36) exhibit negative kurtosis. This means that their distributions are flatter at the peak and have lighter (less extreme) tails than the normal distribution. x9 (−1.36), in particular, is clearly platykurtic. Leptokurtic (Positive Kurtosis): The features x3 (0.26), x5 (0.25), x6 (1.79), and x7 (0.40) exhibit positive kurtosis. This means that their distributions have more pointed peaks and thicker (more extreme) tails than the normal distribution. The high kurtosis for x6 (1.79) suggests a greater concentration of data around the mean, but also a greater number of values far from the mean (which correlates with its high variability and skewness) (Table 8).
Range, Minimum, and Maximum: Analysis of the range (the difference between the maximum and minimum values) confirms the observations regarding variability. Traits x7 (range 78.10) and x9 (range 78.70) have the largest range of values, consistent with their high coefficients of variation and suggesting highly variable observations, from low to very high. It is worth noting that x7 reaches a maximum of 81.90, and x9 a maximum of 78.70. Traits y (total yield) and x1 (marketable yield) also have a wide range (53.86 and 53.52), indicating a wide range in observed yields. The minimum values for x6 (0.00) and x9 (0.00) may indicate cases of complete absence or very low values of these particular yield traits, which is important information in the context of their variability (Table 8).
3.7. Pearson Correlation Coefficients Between Yield and Potato-Quality Characteristics
Table 9 presents Pearson’s simple correlation coefficients (r) between ten selected potato tuber-yield and quality characteristics. These coefficients measure the strength and direction of the linear relationship between two variables, with values closer to 1 or −1 indicating a strong positive or negative correlation, respectively, and values closer to 0 indicating no linear correlation.
3.7.1. Strong Positive Correlations with Total and Marketable Yield
Total Yield (y) and Marketable Yield (x1): There is a very strong positive correlation (r = 0.82) between total yield and marketable yield. This means that varieties and environmental conditions that favor high total yield typically translate into high marketable yield, which is extremely beneficial from an economic perspective (Table 9).
Total Yield (y) and Dry-Matter Yield (x5): There is a very strong positive correlation (r = 0.91) between total yield and dry-matter yield. This is logical, as higher tuber yield naturally leads to higher dry-matter yield (Table 9).
Total Yield (y) and Starch Yield (x3): There is a strong positive correlation (r = 0.85) between total yield and starch yield. As with dry matter, high tuber yield largely determines the resulting starch yield. Marketable yield (x1) with starch yield (x3, r = 0.71) and dry-matter yield (x5, r = 0.75): These correlations are also strong and positive, indicating that varieties with high marketable yield are also effective in producing starch and dry matter (Table 9).
3.7.2. Correlations of Nutrient Content with Yield and Among Themselves
Starch content (x2) and dry-matter content (x4): A very strong positive correlation (r = 0.91) was found between starch content and dry-matter content. This is expected, as starch is the main component of dry matter in potato tubers. Starch content (x2) and starch yield (x3): There is a moderate positive correlation (r = 0.59) between starch content and yield. This means that while a higher starch concentration promotes higher starch yield, total tuber yield plays a significantly greater role in the final starch yield (as indicated by the correlation of y with x3, r = 0.85) (Table 9).
Dry-matter content (x4) and dry-matter yield (x5): Similarly, a moderate positive correlation is observed (r = 0.48). This is slightly weaker than for starch, further emphasizing the dominant effect of total tuber yield on dry-matter yield (Table 9).
No or very weak correlations between nutrient content and total/marketable yield: Starch content (x2) and dry-matter content (x4) show very weak or no correlation with total yield (y, r = 0.09 for both) and marketable yield (x1, r = 0.11 for x2, r = 0.09 for x4). This is an important observation because it means that high tuber yield does not necessarily correlate with high dry-matter or starch content, and vice versa. Varieties with high levels of these components may have lower overall yields, and vice versa. This is a common problem in breeding, where combining these two traits is difficult (Table 9).
3.7.3. Correlations with Yield Structure
Negative correlation of the small tuber fraction with yield: The fractions of tubers < 35 mm in diameter (x6) and 36–50 mm in diameter (x7) show weak to moderate negative correlations with overall yield (y, r = −0.22 and r = −0.33) and marketable yield (x1, r = −0.26 and r = −0.21). This means that a higher proportion of very small tubers is associated with lower yields, which is unfavorable. The higher absolute value for x7 of y (r = −0.33) suggests that a larger number of tubers in the medium range (36–50 mm) is more detrimental to overall yield than very small tubers. Positive correlation of the large tuber fraction with yield: The fraction of tubers > 60 mm in diameter (x9) shows a moderate positive correlation with overall yield (y, r = 0.41) and marketable yield (x1, r = 0.33). This indicates that a higher proportion of large tubers is beneficial and correlates with higher yield (Table 9).
A 51–60 mm fraction (x8): This fraction shows very weak correlations with both total and marketable yield (r = −0.09 and r = −0.04), suggesting that its share has little direct impact on overall yield (Table 9. Strong negative correlation between tuber fractions: There is a very strong negative correlation (r = −0.86) between the 36–50 mm fraction (x7) and the >60 mm fraction (x9). This is obvious and expected, as a higher proportion of small tubers naturally translates to a lower proportion of large tubers in the total yield. Similarly, the 51–60 mm fraction (x8) is moderately negatively correlated with the >60 mm fraction (x9, r = −0.55). The correlations between tuber fractions are logical and confirm that these traits are mutually exclusive within a given yield (Table 9).
3.7.4. Other Significant Correlations
Starch content (x2) and tuber fractions: Starch content shows very weak or no correlation with tuber fractions (r = −0.08 with x6, r = 0.15 with x7, r = 0.16 with x8, r = −0.12 with x9). This indicates that tuber size does not have a strong linear relationship with its starch content (Table 9).
Dry-matter content (x4) and tuber fractions: Similar to starch, dry-matter content shows weak correlations with tuber fractions (r = −0.05 with x6, r = 0.11 with x7, r = 0.20 with x8, r = −0.11 with x9) (Table 9). Weak or no correlation: Total yield (Y) showed a strong correlation with starch content (x2) and dry-matter content (x4). Here, low coefficients (r = 0.09) indicate a lack of a significant linear relationship between yield and quality. This is an important, key finding for breeding, confirming the difficulty of simultaneously increasing both traits.
3.8. Pearson Correlation Coefficients Between Yield and Potato-Quality Characteristics with Soils Parameters
The following results were found: strong positive (0.8–0.9): total yield (y) and starch yield (x3): 0.85; total yield (y) and commercial yield (x1): 0.82 moderate positive (0.5–0.8): pH (x6) and Mg (x9): 0.73; K (x8) and Mg (x9): 0.58 weak negative (negative but close to zero). Most correlations with P (x7) are weakly negative (Table 10).

Table 10.
Pearson’s simple correlation coefficients of yield and its soil-quality characteristics.
The strongest correlations visible in the heatmap are starch yield (x3) and yield of dry matter (x5): 0.97; total yield (y) and yield of dry matter (x5): 0.91; starch content (x2) and dry-matter content (x4): 0.91; total yield (y) and starch yield (x3): 0.85; and total yield (y) and commercial yield (x1): 0.82 (Table 10).
Based on Pearson’s simple correlation coefficients, a heat map was generated, which is an excellent visualization tool. It allows for quick identification of the strength and direction of relationships between variables. Colors indicate the strength of correlations: from blue for strongly negative correlations, through white for no correlation, to red for strongly positive relationships. Interpretation of key simple Pearson correlations are displayed in Figure 3.
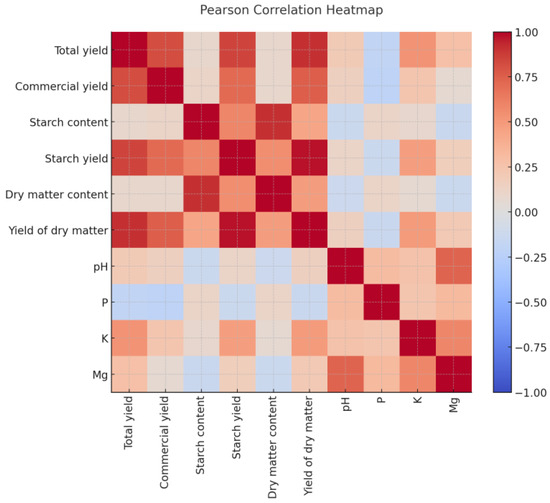
Figure 3.
Pearson correlation heatmap of yield, quality, and soil parameters.
Once again, strong positive correlations (red) were confirmed: total yield (Y) with starch yield (x3) and dry-matter yield (x5). The correlation coefficients of 0.85 and 0.91 indicate a very strong positive relationship, meaning that the higher the tuber yield, the higher the starch and dry-matter yield, which is a logical relationship. Also, x2 (starch content) and x4 (dry-matter content) were strongly positively related (r = 0.91), almost perfectly correlated, which is the expected result, confirmed by many authors [2,3].
Soil parameters were found to be associated with tuber yield and quality (Figure 3). Soil acidity (x6) was strongly positively related (red) to Mg content (x9). The correlation coefficient (r = 0.73) demonstrates this strong relationship between pH and magnesium content in the soil (Figure 3). Negative correlations (blue) were found between starch content (x2) and soil pH (x6). However, the simple correlation coefficient (r = −0.13) suggests a very weak, negative relationship between these variables (Figure 3).
Total yield (Y) was negatively associated with phosphorus content (x7). The coefficient (r = −0.18), however, indicates a weak negative correlation, which may be the result of complex interactions with other factors, such as climatic variability or the specificity of the studied locations (Figure 3).
Dry-matter content (x4) and Mg content (x9) were found to be weakly negatively associated. The coefficient (r = −0.13) indicates a weak negative relationship, which is inconsistent with the general assumptions (Figure 3). However, this requires further analysis and potential explanation.
This visualization helps identify relationships between different yield and quality parameters in agricultural data.
4. Discussion
4.1. Stability and Variability of Potato-Yield and -Quality Traits in the Face of Genotypic–Environmental Interactions
The primary goal of potato cultivation is to achieve high and high-quality tuber yields. This trait is influenced by complex interactions between genotypic (varietal) factors, environmental conditions (soil, climate, location, year), and agronomic factors (fertilization, irrigation, plant protection) [2,28,29,30]. Early studies [2,31,32,33,34,35,36,37,38] confirmed that most important potato-production traits are characterized by significant phenotypic variability, resulting from strong genotypic–environmental (G × E) interactions.
Modern agriculture, driven by ongoing climate change and growing market demands, has intensified the need to understand and manage this variability. Recent research consistently confirms the dominant influence of G × E interactions on potato yield and quality. For example, the work of authors such as Zhou et al. [39] and Yang et al. [40], using advanced statistical models (e.g., GGE biplot, AMMI), emphasizes that the environment often explains a larger portion of the total yield variability than genotype alone. This variability results from differences in precipitation, temperature, sunlight, and soil physicochemical and biological properties across years and locations.
The dispersion of the obtained results was characterized by the coefficient of variation (CV), which, being the ratio of the standard deviation to the mean, is a dimensionless measure allowing for comparison of trait variation both within the same community and between different communities. It was demonstrated that the lower its value, the more stable the given trait. In our study, the average coefficient of variation for total yield (y) was 22.53% and for marketable yield (x1) was 23.53%. This value is comparable to data from Aliche et al. [29] showed a similarly low stability of this trait (28.1%). These values highlight moderate but significant yield variability, necessitating the search for varieties with increased stability.
Of the studied traits, the highest stability was observed for dry-matter content (x4, CV = 10.18%) and starch content (x2, CV = 15.03%). This is consistent with the general trend that qualitative traits are often less sensitive to environmental fluctuations than quantitative traits such as yield. On the other hand, the highest variability (lowest stability) was observed for the fraction of tubers with a diameter of <35 mm (x6, CV = 84.06%) and the fraction of tubers 36–50 mm (x7, CV = 78.01%). The fraction >60 mm (x9) also exhibited high variability (CV = 63.97%). These data confirm studies [2,29,36], which also indicated significant variability in yield structure, especially for small and very large tubers. This high variability in fractions is particularly important because it directly affects the commercial value and intended use of the crop.
Seid and Tessema [41] long ago noted the enormous, even fourteen-fold, differences in yield within the same variety. They attributed this significant variability to plant variability within ridges (up to 50% of the variability in tuber mass) and between ridges. Contemporary studies, such as those by Song et al. [42], further deepen this perspective, pointing to the heterogeneity of soil conditions in small areas and the precise mapping of within-field variability as key to understanding and minimizing these differences.
From a physiological perspective, yield variability is also influenced by the processes of photosynthesis and assimilate allocation. According to Starck [43], each variety produces and distributes assimilates individually. As the leaf blade grows and then ages, the supply of nutrients to the tubers is limited, which can lead to a reduction in chlorophyll (Fo) fluorescence, which reflects the efficiency of photosynthetic processes. According to [44] and more recent studies, e.g., [45], Fo levels depend primarily on the genetic characteristics of the varieties, and maximum photosynthetic efficiency does not always correlate with maximum assimilation surface area. Optimal dry-matter accumulation in tubers often occurs when the leaf-blade area has already reached its minimum size and may then decrease with increasing aging of the assimilation apparatus [46]. These results provide a solid basis for further recommendations for farmers and breeders, emphasizing the importance of both varietal selection and adapting cultivation practices to prevailing environmental conditions. In light of the above, developing plant varieties appropriate for a given use is a fundamental task of breeding. To properly guide breeding efforts for a given species, including potatoes, a deeper understanding of the range of variability and trait interdependencies, both within and between years, is essential [8,43,47].
Genotype–environment interactions (G × E, G × L, G × Y) were confirmed, which significantly modified the phenotypic expression of potato-yield and tuber-quality traits, resulting in a variable level of stability of the tested varieties in different locations and years. “Deriving plant varieties appropriate for a given use is the task of breeding. To properly direct breeding efforts for a given species, it is essential to understand the range of variability and interdependencies of traits both within a given year and between years.” Potato traits that are economically important should be subjected to such research.
Confirming significant genotype–environment interactions (G × E, G × L—genotype × location, G × Y—genotype × year) in our study is crucial for understanding the phenotypic expression of potato-yield and tuber-quality traits. This implies that the behavior of a given variety is not constant but varies depending on environmental conditions, which influences its stability. This is discussed in detail and expanded upon in the discussion of the results [1,2,3].
How did this happen? Mechanisms of G × E Interactions
G × E interactions result from complex mechanisms in which a variety’s genotype responds differently to changing environmental factors. Here are some key mechanisms:
Phenotypic Plasticity: Mechanism: Each genotype has the ability to modify its phenotype (i.e., observable traits such as yield or chemical composition) in response to different environmental conditions. Different varieties possess varying degrees of this plasticity. A variety with high plasticity may adapt well to a wide range of conditions, while another, less plastic, may be more specialized and may achieve optimal results only under very specific conditions [9].
In our research we see this when one variety yields excellently in a dry year, while another, which is normally a leader, performs less well in the same dry conditions.
Differential Response to Abiotic and Biological Stress: Mechanism: Potato varieties differ genetically in their tolerance to abiotic stresses (e.g., drought, high temperature, nutrient deficiency, specific soil pH) and biotic stresses (e.g., diseases, pests). Genes responsible for resistance or tolerance are activated to varying degrees, or not at all, depending on the variety and the presence of the stressor [2].
In our own research different locations (L) and years (Y) were characterized by different weather conditions (e.g., rainfall distribution, temperature) and potentially different disease or pest intensities. The drought-resistant variety maintained a more stable yield in a dry year, while the late blight-susceptible variety experienced a drastic yield decline in a wet year favoring pathogen development.
- ○
- Resource Use Efficiency (RUE): Mechanism: Genotypes differ in their efficiency in uptake and utilization of available environmental resources, such as water, nitrogen, phosphorus, and potassium. This efficiency may vary depending on the availability of these resources in the soil at a given location. In our study a variety that utilizes nitrogen efficiently may yield better in soils deficient in this element, while another more demanding variety requires higher nutrient availability to reach its full potential;
- ○
- Physiological Interactions of Genes and the Environment: Mechanism: These interactions manifest at the level of plant physiological processes, such as photosynthesis, transpiration, and assimilate accumulation in tubers. The environment can influence the expression of genes responsible for these processes, and the genotype, in turn, determines how the plant responds to these environmental signals. In our research, optimal light and temperature conditions in one year can activate genes responsible for intense photosynthesis in one variety, leading to high yields, while another variety may not show such a strong response;
- ○
- Confirmation of the G × E (G × L, G × Y) interaction in our study clearly indicates that variety selection should be closely matched to specific location conditions and expected annual conditions. This is fundamental for maximizing yield and tuber quality, as well as for improving the stability of potato production in a variable environment [2,9].
Understanding Variety Performance: The Role of Genotype–Environment Interaction
The observed variability in yield performance across different years and locations underscores a crucial aspect of potato cultivation: there is not a single “universal” best variety for all conditions. Instead, the optimal variety often depends on the specific environmental context of a given year and location [2,9].
This phenomenon is a classic example of the Genotype × Environment (G × E) interaction. While a variety like ‘Jurek’ might exhibit superior overall performance when averaged across all tested conditions, other varieties may demonstrate exceptional localized adaptation. For instance, as highlighted in Table 3, ‘Otolia’ achieved an impressive total yield of 83.28 t ha−1 in Węgrzce in 2021. Such specific, high-performing location–year combinations indicate that certain varieties are particularly well-suited to distinct environmental niches.
Understanding these G × E interactions is paramount for agronomic planning. It suggests that selecting varieties based solely on their average performance might not always maximize yield potential. Instead, tailored recommendations, considering local climatic patterns and soil conditions, could lead to more efficient and productive potato cultivation. This also highlights the importance of multi-location and multi-year trials in identifying varieties with broad adaptability versus those with specific, high-potential niches.
4.2. Analysis of Pearson Correlation Coefficients—Interrelationships Between Potato-Yield and -Quality Traits
Pearson correlation coefficient analysis is a fundamental tool for understanding the interrelationships between various crop traits. In this study, examining the correlation between total and marketable yield and key quality traits (starch content and yield, dry matter) and yield structure (tuber fractions) allows for the identification of traits that can be simultaneously improved in breeding programs, as well as those that exhibit negative correlations, posing a challenge for breeders and agronomists. The Pearson correlation coefficient analysis serves as a fundamental tool for understanding the interrelationships between various crop traits, directly connecting to and building upon the findings of previous research in potato breeding and agronomy [2,9]. This analysis is crucial for two main reasons: identifying traits for simultaneous improvement and recognizing negative correlations that pose breeding challenges.
Identification of Traits for Simultaneous Improvement. How it relates to previous research: Previous breeding programs and agronomic studies have often focused on improving individual traits in isolation (e.g., maximizing yield or increasing starch content). However, in reality, traits are rarely independent; they are governed by shared genetic pathways and physiological processes. When a strong positive correlation is found between two desirable traits (e.g., total yield and marketable yield, or dry-matter content and starch content), it indicates that they tend to increase or decrease together. This knowledge is invaluable for breeders. If previous research identified a high-yielding variety, a positive correlation with a quality trait (like starch content) means that selection for higher yield might also inadvertently improve starch content, or vice-versa. This allows breeders to conduct indirect selection, making breeding programs more efficient. Instead of selecting for two traits separately, they can focus on one, knowing the other will likely improve concurrently. This aligns with the goal of developing multi-purpose varieties that meet diverse market and processing demands.
Recognizing Negative Correlations and Breeding Challenges. How it relates to previous research: Often, the pursuit of one desirable trait in breeding can unintentionally lead to a decline in another important trait. For instance, increasing total yield might sometimes come at the expense of lower dry-matter content or reduced disease resistance. When Pearson correlation analysis reveals a significant negative correlation between two traits (e.g., very high total yield and low tuber dry matter, or large tuber size and poor storage quality), it highlights a breeding challenge. Significance: Previous research and breeding efforts have frequently encountered such trade-offs. Identifying these negative correlations quantitatively, as performed through Pearson analysis, helps breeders and agronomists anticipate and strategize. Instead of being surprised by undesirable outcomes, they can proactively
Implement more complex breeding strategies: This might involve molecular breeding techniques, such as marker-assisted selection, to break undesirable genetic linkages;
Develop management practices: Agronomists can devise specific cultivation techniques (e.g., adjusted fertilization, irrigation) to mitigate the negative impact of one trait on another, aiming to optimize both [1,9].
Redefine breeding objectives: In some cases, acknowledging strong negative correlations might lead to a re-evaluation of breeding priorities, balancing the improvement of one trait against the acceptable levels of another. For example, if extremely high yield severely compromises processing quality, breeders might aim for an optimal yield rather than a maximal one [1,2,3].
4.2.1. Strong Positive Correlations—Synergy and Breeding Efficiency
The strong correlation between total and marketable yield was highly desirable. It means that the majority of tubers produced are of sufficient size and quality for sale. These very high correlations (dry-matter yield (x5 (r = 0.91) and starch yield (x3) (r = 0.85) are logical and fundamental from the perspective of production efficiency. A high tuber yield naturally translates into high yields of these key components, regardless of their percentage content. This confirms that the primary factor limiting dry-matter and starch production per hectare is tuber biomass.
Similar relationships are commonly reported in the literature [46,47], emphasizing that genotypes that efficiently produce tuber biomass are also effective in accumulating nutrients. Direct proportionality of total yield to total dry matter/starch: For example, if a potato plant produces a high total tuber mass per hectare (e.g., 50 t), even if the dry-matter content of these tubers is average (e.g., 20%), the total dry-matter yield per hectare will still be significant (50 t ha−1 × 0.20 = 10 t dry matter per hectare). In contrast, a variety with a very high dry-matter content (e.g., 25%) but a low total tuber yield (e.g., 30 t ha−1) will produce significantly less total dry matter (30 t ha−1 × 0.25 = 7.5 t dry matter per hectare). This shows that tuber biomass has a greater impact on total content than “concentration” (percentage content), especially when the differences in total yield are large. Numerous studies on potato productivity and breeding goals consistently indicate that achieving high total biomass is a key factor in increasing total stored carbon production. Researchers often focus first on breeding for higher yield potential, as this sets a ceiling for total solids accumulation.
Genotypes that efficiently produce high tuber biomass are those that efficiently partition (transfer) carbohydrates (sugars) produced during photosynthesis from leaves and stems to developing tubers. This process is known as the source–sink relationship, where leaves are the “source” of sugars and tubers are the “sink,” where these sugars are converted into starch and other dry-matter components for storage. Varieties with high tuber uptake tend to accumulate higher biomass [3]. Plant physiological studies have detailed the mechanisms of carbon allocation [2,3,9]. Efficient partitioning into tubers is a key feature of high-yielding potato varieties. This efficiency ensures that a greater proportion of the plant’s photosynthetic product reaches the harvestable parts, contributing directly to both tuber yield and overall dry-matter and starch accumulation.
While breeders also strive to improve the starch and dry-matter percentages for specific processing purposes, the primary goal of most potato breeding programs for yield-oriented segments is to maximize tuber yield per unit area. This is because high tuber yield inherently drives overall dry-matter and starch production per hectare. Achieving very high yields often comes at the expense of overall yield. A genotype may have a very high starch percentage but produce fewer, smaller tubers, leading to lower overall starch yield per hectare. Therefore, finding varieties that combine high yield with acceptable quality is crucial. Potato breeding reviews often emphasize that while quality traits are important, yield stability and yield potential remain priorities, as they determine the economic viability of the crop. Studies comparing different breeding lines often show that absolute dry-matter or starch yield is more strongly correlated with fresh tuber yield than with dry-matter or starch percentage. In essence, our findings confirm a well-established principle in potato breeding: while dry-matter percentage is important for specific end uses, the total mass of tubers produced is the most important factor in determining the total dry matter and starch harvested from the field. High-yielding varieties are, by definition, those that efficiently convert solar energy and nutrients from the soil into stored tuber biomass.
4.2.2. Weak or Absent Correlations—Breeding Challenges
One of the most critical findings from the correlation analysis is the lack of a strong positive relationship between yield (both total and marketable) and starch content (x2) and dry-matter content (x4). The correlation coefficients ranged from r = 0.09 to 0.11, indicating a virtually non-linear relationship. This result is consistent with numerous studies [48,49], which consistently demonstrate that breeding varieties combining high yield with high quality (high dry-matter and starch content) is challenging. A phenomenon known as “nutrient dilution” often occurs here—higher tuber biomass can lead to lower nutrient concentrations. For breeders, this means balancing these traits and selecting genotypes that, despite the lack of a strong correlation, achieve acceptable levels of both parameters.
4.2.3. Correlations Within Quality Traits—Physiological Basis
Starch is the main component of tuber dry matter (usually 60–80% of dry matter); hence their interrelationship is fundamental. Varieties with high dry-matter content are usually also characterized by high starch content. The trade-off between yield and quality, often embodied in the phenomenon of nutrient dilution, poses a fundamental challenge for breeders. The observation that combining high yield with high quality (specifically, high dry-matter and starch content) in potato varieties is difficult, leading to a phenomenon known as “nutrient dilution,” is a well-established principle in crop science. It highlights the inherent physiological and genetic trade-off within the plant [2,9,31]. This is the physiological basis: resource allocation and absorption power.
- Limited resources: A plant has a limited number of resources (carbon absorbed through photosynthesis, water, and nutrients absorbed from the soil) available for growth and development. These resources must be distributed among various “sinks”—vegetative growth (leaves, stems, roots), reproductive structures (flowers, seeds), and storage organs (tubers in potatoes) [9,31]. Yield-Dilution Trade-Off: When a potato plant is bred or maintained to achieve exceptionally high tuber biomass (yield), this means that a larger total volume of storage tissue is produced. However, the plant’s ability to synthesize and transport specific compounds, such as starch, or to accumulate minerals may not be proportional to the increase in biomass;
- Rapid Growth: High-yielding varieties often achieve high biomass due to rapid growth and efficient water uptake. This rapid biomass accumulation can “dilute” the concentration of certain nutrients and quality components into a larger volume of tissue. Metabolic Bottlenecks: Biochemical pathways responsible for starch synthesis or the transport of specific minerals (e.g., phosphorus, potassium, trace elements) to the tuber may not reach their maximum capacity. Even if the plant produces raw biomass, the enzymes or transporters involved in the synthesis or loading of these specific compounds may not be able to keep up with the rate of biomass accumulation. This leads to lower concentrations per unit of fresh or dry weight [9].
- Genetic Basics: Pleiotropy and Linkage Pleiotropy: This occurs when a single gene influences multiple, seemingly unrelated traits. It is possible that genes contributing to high yield (e.g., genes promoting rapid cell division or large cell size) also have a pleiotropic effect that inadvertently reduces the concentration of certain quality components. Linkage Disequilibrium: Even if the genes for yield and quality are distinct, they may be located very close to each other on the chromosome. This “linkage” makes it difficult for breeders to separate desirable high-yielding alleles from undesirable low-quality alleles through conventional crossbreeding. When selecting for high yield, breeders may inadvertently transfer alleles that lead to lower concentrations of quality traits. Breaking these linkages often requires extensive breeding efforts and large populations;
- Environmental Interactions: Nutrient Availability and Growth-Conditions Nutrient Availability: Although innate physiological and genetic mechanisms are fundamental, environmental factors can exacerbate or mitigate nutrient dilution. If soil nutrient resources are insufficient to meet the needs of a fast-growing, high-yielding crop, the dilution effect will be more pronounced. Growth Conditions: Factors such as water availability and temperature can influence the rate of biomass accumulation compared to the rate of nutrient uptake and assimilation, further influencing final concentrations. Consistency with Other Studies: “Yield-Quality Trade-Off” This phenomenon is not unique to potatoes and is a well-documented “yield-quality trade-off” in many crop species. Numerous studies confirm this inverse relationship, making it a key challenge in crop improvement. Potato Research: Aliche et al. [29]: Studies on genetically modified or conventionally bred potatoes often demonstrate the difficulty of simultaneously increasing yield and the content of specific nutrients (e.g., iron, zinc, and even starch) without compromise [46,47,48]. They discuss that while increasing total tuber biomass can increase the total amount of nutrients harvested, their concentration in the tuber may decrease. Nitrogen Fertilization and Quality Studies: Many studies examine the effects of nitrogen fertilization on potato yield and quality. While moderate nitrogen application can increase both, excessive nitrogen application can lead to higher yields (biomass) but lower dry-matter and starch content due to increased vegetative growth.
Genetic Predisposition and Environmental Influence: While the physiological relationship is universal, the extent of starch and dry-matter accumulation is influenced by both the genetics of the potato variety and environmental factors (such as temperature, light, water availability, and nutrient supply) during tuberization and bulking. Varieties bred for processing (e.g., for French fries, crisps, or industrial starch production) are specifically selected for their genetic ability to prioritize starch synthesis, leading to inherently higher dry-matter and starch levels. Optimal environmental conditions that favor sustained photosynthesis and assimilate partitioning to tubers will further enhance both parameters [2,3]. Implications for Breeding and Processing: This robust correlation between starch content and dry-matter content is immensely beneficial for potato breeding and the processing industry. Breeders can effectively select varieties suitable for processing by primarily focusing on increasing dry-matter content, knowing that starch content will almost invariably increase concomitantly. This simplifies screening processes, as specific gravity (which is highly correlated with dry-matter content) can be easily measured as an indirect indicator [9,31].
4.2.4. The Impact of Yield Structure on Yield Value—Commercial Aspects
The assessment of the commercial value of a crop is closely related to its fractional composition. A weak-to-moderate negative correlation (r = −0.21 to −0.33) was observed between the small and medium-sized tuber fractions (<35 mm and 36–50 mm) and the total and marketable yield. A high proportion of small tubers reduces the commercial value of the crop. This is expected, as a larger proportion of tubers outside the preferred marketable size reduces the overall marketable yield. Positive correlations with large tuber fraction (x9): The >60 mm (x9) fraction shows a moderate positive correlation with total yield (y, r = 0.41) and marketable yield (x1, r = 0.33). This indicates that varieties producing larger tubers typically achieve higher marketable yields, which is crucial for their market attractiveness, particularly in the fresh market and processing sectors. Strong negative correlation between tuber fractions (x7 and x9) (r = −0.86) (Table 9): A strong negative correlation between two different fractions (e.g., “small” vs. “large” tubers) is a natural and widely recognized phenomenon in potato-yield structure. The explanation is simple: if a plant allocates more assimilates (nutrients) to growing tubers in one fraction (e.g., large tubers), it necessarily has fewer resources to produce tubers in another fraction (e.g., small tubers). This relationship is a key element in breeding programs where the goal is to create varieties with a specific tuber size-distribution profile. For example, breeders working on potato-chip varieties strive to obtain very uniform, large-sized tubers, while breeders working on “new potatoes” focus on smaller fractions. Confirmation of these relationships can be found in numerous scientific works that use correlation analysis and path analysis to examine the relationships between yield components (such as the number of tubers, their average weight, percentage of different fractions) and the final yield [3,36,37,46] (Table 9).
Analysis of Pearson correlation coefficients provides a comprehensive picture of the interrelationships between the most important yield and quality traits in potato tubers. These results are crucial for potato breeding programs, enabling effective selection identifying traits that can be simultaneously improved (e.g., total yield and marketable yield; starch and dry-matter content). The relationship between potato-tuber yield and quality: In potato breeding, there is often a trade-off between quantity (high yield) and quality (high starch and dry-matter content). The plant’s biomass accumulation mechanism prioritizes either rapid tuber growth, which leads to “dilution” (higher water content), or slower, “denser” growth, resulting in higher starch concentration. Breeders must find the optimal balance depending on the variety’s intended use [3,37]. For example, varieties for the fresh market may have a lower starch concentration to achieve high yield, while varieties for processing (French fries, chips) require a high dry-matter content, which is crucial for their commercial value [36,47].
Soil parameters showed weaker, although significant, relationships with yield and quality. Soil pH strongly correlated with magnesium content (r ≈ 0.73), which is consistent with the observations of Kołodziejczyk [36] and Pszczółkowski and Sawicka [37], indicating the key role of soil pH in the availability of base cations such as Mg and K. Phosphorus content, on the other hand, was not significantly related to yield, which may be due to its adequate saturation in the soil or low variability under experimental conditions, as previously suggested by Scavo [5]. These results indicate that under the studied conditions, it is primarily the relationships between yield-forming traits that determine productivity and quality, while the influence of soil factors is limited to selected relationships.
Informed trade-off management: Understanding that high tuber yield does not always correlate with high starch or dry-matter content, requiring a careful balance between quantity and quality depending on the intended use of the variety.
Agronomic optimization: Adjusting cultural practices (e.g., spacing, fertilization, irrigation) to promote the desired yield structure (tuber fraction) directly translates into commercial value. In the context of future research, it would be worthwhile to deepen correlation analysis, considering not only simple Pearson coefficients but also more advanced methods, such as path analysis or correlation networks, which allow for the distinction between direct and indirect correlations and the identification of “key” traits that have a dominant influence on others. Furthermore, analysis of correlation stability across environments (correlation × environment interaction) could provide further insights into the stability of genotypes in terms of their ability to maintain desired relationships between traits, regardless of conditions [1,2,3].
4.3. Limitations of Research
Every scientific study, regardless of its scope and thoroughness, has certain limitations that must be clearly presented to ensure transparency and reliability of the results. In the case of the potato research described, several key areas of potential limitations can be identified:
Number of years (2021–2023): a three-year period may be insufficient to capture the full range of weather variability, including extreme climate events that can drastically impact potato yields. Studies lasting 5–10 years better reflect the stability of varieties in a changing climate. Number of locations: four locations may not fully capture the diversity of soil and agroclimatic conditions across the country, omitting the specific characteristics of microregions, heavy soil, or areas with increased pest and disease pressure.
Methodological limitations and study representativeness: The three-year study period (2021–2023) may be insufficient to capture the full range of weather variability, including extreme climatic events that can drastically impact potato yield. Studies lasting 5–10 years better reflect the stability of varieties in a changing climate. The four study locations may not fully reflect the diversity of soil and agroclimatic conditions in Poland, omitting the specific characteristics of microregions, heavy soils, or areas with increased pest and disease pressure. The COBORU methodology, although standardized, assumes uniform agronomic practices, which limits the assessment of variety stability in changing growing conditions. Furthermore, the lack of detailed analysis of intra-field variability (e.g., using yield maps) and the impact of diseases and pests constitutes significant gaps in the evaluation of the results.
Scope of analysis and conclusions: The study tested seven mid-early varieties, which limits the extrapolation of results to the full spectrum of potato varieties, including early, late, and industrially specific varieties. The analysis focused on basic quality traits (starch, dry matter), ignoring other important parameters such as vitamins and antioxidants. The use of the Pearson correlation coefficient limits conclusions to linear relationships. In agriculture, many relationships are nonlinear, and correlation does not always imply causality [47]. Failure to employ more advanced statistical methods (e.g., path analysis, linear mixed models, GGE/AMMI biplots for G × E) limits the depth of conclusions [49,50,51,52]. According to Islam [31], Song et al. [42], Uzair & Sharma [52] and An et al. [53], phenotyping has become a bottleneck in plant breeding, and overcoming this bottleneck will require phenomics.
The study provides valuable information on potato-yield stability and quality in diverse Polish conditions. However, its results should be interpreted with the above limitations in mind, as is standard scientific practice. Identifying these limitations enhances the credibility and scientific value of the work.
4.4. Practical Agronomic Recommendations: A Decision Matrix for Variety Selection
Our research clearly demonstrates that optimal potato variety selection requires a flexible approach that considers specific growing conditions and production goals. Based on the strong genotype–environment (G × E) interactions, we have identified key factors that farmers should consider. The following decision matrix, developed based on our findings, aims to simplify the variety selection process and minimize production risk (Table 11).

Table 11.
Practical agronomic recommendations.
A decision matrix should be considered a tool to support practical agronomic decisions, in this case, regarding potato-variety selection. Its role is to simplify the decision-making process and reduce production risk by identifying which variety traits are most important under specific farm conditions.
To use the matrix effectively, you should:
- -
- Define your production goals—e.g., high marketable yield, high starch content, resistance to water stress or disease;
- -
- Determine growing conditions—soil (fertility, pH, nutrient content), irrigation availability, site type, and potential constraints (e.g., diseases, shorter growing season);
- -
- Find the criteria in the table that best suit your situation—the matrix will indicate which varieties (or variety types) are best suited to your conditions and priorities;
- -
- Use the table as a preliminary filter—select a few varieties that meet the most key criteria and only then compare them in terms of seed availability, costs, and local farmers’ experience. In other words, the table does not provide one universal answer but makes it easier to match the variety to the farm, considering both environmental conditions and market and technological expectations.
5. Conclusions and Prospects for Further Research
The main finding of the study is that climatic variability was the dominant factor influencing potato yield, accounting for 67–68% of the total yield variability. This phenomenon highlights the need for flexible agronomic strategies, such as water management based on weather conditions. Equally important is the understanding that there is no one-size-fits-all potato variety; optimal genotype selection requires consideration of specific location conditions and annual variability, which results from the strong genotype–environment interaction (G × E). This finding provides crucial information for breeders and farmers, confirming that variety selection must be tailored to the specific region.
It was noted that tuber-quality traits, such as starch and dry-matter content, are largely determined by cultivar genetics, as evidenced by the high contribution of these parameters in cultivars such as ‘Laskara’ and ‘Tajfun’. The study also demonstrated strong synergies between total and marketable yield, as well as between tuber yield and starch and dry-matter yield. At the same time, total yield was weakly correlated with starch content, posing a significant breeding challenge, requiring optimal agronomic solutions. A high proportion of large tubers (>60 mm) was also found to positively correlate with yield, while a high proportion of small tubers (<50 mm) was negatively correlated.
Future research should further explore the factors contributing to higher yields in locations such as Słupia and Węgrzce, as well as examining the impact of stress on starch content during dry years. It is also essential to expand this research to include detailed analysis of the impact of extreme weather events, precision farming, and further exploration of the G × E interaction, which will allow for the development of more stable and resilient potato varieties.
A key conclusion from our research is that there is no single, universal potato variety. Optimal genotype selection must always be based on informed farmer decision-making, considering specific soil types, anticipated climatic conditions, and the intended use of the crop. Our results clearly demonstrate that while there is a strong synergy between total yield and marketable yield, the correlation between high total yield and high starch content is weak. This phenomenon highlights the fact that yield does not always correlate with quality, which poses a key challenge in both breeding and agronomic management. Farmers should strive to find the optimal compromise, selecting varieties that best suit their individual conditions and production goals.
The use of GGE biplot analysis will be a key element of our future research, which will be a continuation of this work.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/land14091777/s1, Figure S1. Map of the Przecław commune in Mielec County, Przecław commune, Podkarpackie Voivodeship, Poland; Figure S2. Field map of the Experimental Variety Testing Station in Przecław; Figure S3. Map of the Słupia commune, Jędrzejów County, Świętokrzyskie Voivodeship; Figure S4. Field map of the Experimental Variety Testing Station in Słupia; Figure S5. Map of the Dębowa Kłoda commune, Uhnin village, Parczew district, Lublin Voivodeship, Poland; Figure S6. Field map of the Experimental Variety Testing Station in Uhnin; Figure S7. Map of the Zielonki commune, Węgrzce village, Kraków county, Lesser Poland Voivodeship; Figure S8. Field map of the Experimental Variety Testing Station in Węgrzce; Figure S9. Hydrostatic balance for determining starch in potatoes. Table S1. Air temperatures, precipitation, and Sielianinov hydrothermal coefficient during the potato vegetation period in 2021–2023. link to the Experimental Variety Testing Station in Słupia. Figure S10. Soil profile (a) Brown alluvial, (b) Calcaric Cambisols; Source: own. Figure S11. Soil profile (a) Haplic Luviosils, (b) Eutric Cambisols; Source: own.
Author Contributions
Conceptualization, P.P., B.S., P.B. and B.K.-M.; methodology, P.P., B.S., P.B. and B.K.-M.; software, P.N.; validation, P.N., B.K.-M. and P.B.; formal analysis, P.N.; investigation, P.P. and P.B.; resources, B.K.-M. and P.N.; data curation, P.N. and P.B.; writing—original draft preparation, P.P., B.S., P.B., B.K.-M. and P.N.; writing—review and editing, P.P., B.S., P.B. and B.K.-M.; visualization, P.P., P.B. and B.S.; supervision, B.S. and B.K.-M.; project administration, P.P.; funding acquisition, B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to thank the Management of the Central Research Centre for Cultivated Plants in Słupia Wielka for technical and administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AMMI | Additive Main Effects and Multiplicative Interaction |
| BBCH | Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie |
| COBORU | Research Centre for Cultivar Testing |
| MET | Multi-Environment Trials |
| PN | Polish Standard |
| ESCT | Experimental Station for Cultivar Testing |
| WRB | World Reference Base for Soil Resources. |
References
- Bitew, M.W.; Bogale, A.T.; Tegegne, E.M.; Fentie, M.E.; Mebratie, B.G.; Emrie, G.A.; Wasie, T.A.; Kolech, S.A.; Mekonen, D.A.; Abereha, A.M.; et al. Yield performance and Stability of Potato (Solanum tuberosum L.) Genotypes Derived from Crossing for Variety Development in Ethiopia. Am. J. Potato Res. 2024, 101, 450–467. [Google Scholar] [CrossRef]
- Sawicka, B. Studies on the Variability of Selected Traits and Degeneration of Various Potato Varieties in the Biała Podlaska Region; Habilitation thesis; Series 141; Agricultural Academy Publishing House: Lublin, Poland, 1991; ss. 76, PL; ISSN 0860-4355. (In Polish) [Google Scholar]
- Pszczółkowski, P.; Sawicka, B.; Jariene, E.; Kiełtyka-Dadasiewicz, A. Phenotypic yield and its structure variability of moderately late and late potato cultivars. Agron. Sci. 2020, 75, 21–38. [Google Scholar] [CrossRef]
- Tadege, M.B.; Utta, H.Z.; Aga, A.A. Association of statistical methods used to explore genotype × environment interaction (GEI) and varietal stability. Afr. J. Agric. Res. 2014, 29, 2231–2237. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G.; Ierna, A. Dissecting the Genotype × Environment Interaction for Potato Tuber Yield and Components. Agronomy 2023, 13, 101. [Google Scholar] [CrossRef]
- Hassanpanah, D.; Azimi, J. Yield Stability Analysis of Potato Cultivars in Spring Cultivation and after Barley Harvest Cultivation. Am. Eurasian J. Agric. Environ. Sci. 2010, 9, 140–144. [Google Scholar]
- Gastelo, M.; Perez, W.; Quispe, K.; Pozo, V. Phenotypic Stability and Correlation for Late Blight Resistance in Advanced Potato Clones Under Field and Controlled Conditions. Am. J. Potato Res. 2022, 99, 150–159. [Google Scholar] [CrossRef]
- Shakshi; Srivastava, R.P.; Yadav, S.; Singh, M.K. Integrating Genomics and Phenomics in Agricultural Breeding: A Comprehensive Review. Asian Res. J. Agric. 2024, 17, 116–125. [Google Scholar] [CrossRef]
- Sawicka, B.; Michałek, W.; Pszczółkowski, P. The relationship of potato tubers chemical composition with selected physiological indicators. Zemdirb. Agric. 2015, 102, 41–50. [Google Scholar] [CrossRef][Green Version]
- Woś, A. The Polish Climate; PWN: Warsaw, Poland, 1999; pp. 79–224. ISBN 978-83-01-12780-0. (In Polish) [Google Scholar]
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1971−2010). Fragm. Agron. 2014, 31, 74–87. [Google Scholar][Green Version]
- Kondracki, J. Regional Geography of Poland; State Scientific Publishing House: Warsaw, Poland, 2002. (In Polish) [Google Scholar]
- Chełmnicki, W. Sources of the Kraków-Wieluń and Miechów Uplands; IGIGP UJ: Kraków, Poland, 2001. (In Polish) [Google Scholar]
- Lenartowicz, T.Z. Methodology of Examining the Economic Value of Varieties; COBORU Publishing House: Słupia Wielka, Poland, 2020; p. 41. (In Polish) [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Mocek, A. Soil Science; State Scientific Publisher: Warsaw, Poland, 2015; p. 571. (In Polish) [Google Scholar]
- Kabała, C.; Świtoniak, M.; Charzyński, P. Correlation between the Polish Soil Classification (2011) and international soil classification system World Reference Base for Soil Resources (2015). Soil Sci. Annu. 2016, 67, 88. [Google Scholar] [CrossRef]
- Duer, I.; Fotyma, M.; Madej, A. Code of Good Agricultural Practice; Ministry of Agriculture and Rural Development: Warsaw, Poland, 2004; p. 93. (In Polish) [Google Scholar]
- Strażyński, P. (Ed.) Recommendations for the Protection of Agricultural Plants; Volume I. Oilseeds, Roots, Legumes, and Herbs; IOR-PIB: Poznań, Poland, 2017; ISBN 978-83-64655-55-5. (In Polish) [Google Scholar]
- Piecuch, K.; Lenartowicz, T. Descriptive List of Agricultural Cultivars; COBORU Publishing House: Słupia Wielka, Poland, 2023; p. 39. (In Polish) [Google Scholar]
- Bleinholder, H.L.; Weber, E.; Buhr, L.; Feller, C.; Hess, M.; Wicke, H.; Meier, U.; Van Den Boom, T.; Lancashire, P.; Buhr, D.L.; et al. Compendium of Stage Identification Keys for Mono-and Dicotyledonous Plants, BBCH Monograph, 2nd ed.; Meier, U., Ed.; Federal Center for Agricultural and Forest Biological Research: Braunschweig, Germany, 2001. [Google Scholar]
- Baryłko-Pikielna, N.; Matuszewska, I. Sensory Food Research. Basics—Methods—Applications, 2nd ed.; Scientific Publishers PTTZ: Krakow, Poland, 2014; p. 375. ISBN 978-83-935421-3-0. [Google Scholar]
- Polish Standard PN-R-04031:1997. Agrochemical Soil Analyses—Sampling; Polish Committee for Standardization: Warsaw, Poland, 1997. [Google Scholar]
- Polish Standard PN-ISO 10390:1997P; Soil Quality—Determination of pH. Polish Committee for Standardization: Warsaw, Poland, 1997.
- Polish Standard PN-R-04023:1996P; Chemical and Agricultural Analysis of Soil. Determination of Available Phosphorus Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 1996.
- Polish Standard PN-R-04022:1996 +Az1: 2002P; Chemical and Agricultural Analysis of Soil. Determination of Available Potassium Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Polish Standard PN-R-04020 1994 + Az1:2004; Chemical and Agricultural Analysis of Soil. Polish Committee for Standardization: Warsaw, Poland, 2004.
- IBM Statistics SPSS 28. Available online: https://www.ibm.com/docs/en/SSLVMB_28.0.0/pdf/pl/IBM_SPSS_Statistics_Core_System_User_Guide.pdf (accessed on 10 July 2025).
- Aliche, E.B.; Oortwijn, M.; Theeuwen, T.P.J.M.; Bachem, C.W.B.; van Eck, H.J.; Visser, R.G.F.; van der Linden, C.G. Genetic mapping of tuber size distribution and marketable tuber yield under drought stress in potatoes. Euphytica 2019, 215, 186. [Google Scholar] [CrossRef]
- Leonel, M.; do Carmo, E.L.; Fernandes, A.M.; Soratto, R.P.; Ebúrneo, J.A.M.; Garcia, E.L.; Dos Santos, T.P.R. Chemical composition of potato tubers: The effect of cultivars and growth conditions. J. Food Sci. Technol. 2017, 54, 2372–2378. [Google Scholar] [CrossRef]
- Islam, M.M.; Naznin, S.; Naznin, A.; Uddin, M.N.; Amin, M.N.; Rahman, M.M.; Tipu, M.M.H.; Alsuhaibani, A.M.; Gaber, A.; Ahmed, S. Dry Matter, Starch Content, Reducing Sugar, Color and Crispiness Are Key Parameters of Potatoes Required for Chip Processing. Horticulturae 2022, 8, 362. [Google Scholar] [CrossRef]
- Bodor, K.; Tamási, B.; Len, A.; Keresztesi, Á.; Szép, R.; Bodor, Z. Investigation of the properties of different potato varieties and analysis of the starch structure. Appl. Food Res. 2025, 5, 100998. [Google Scholar] [CrossRef]
- Tatarowska, B.E.; Plich, J.; Milczarek, D.; Boguszewska-Mańkowska, D.; Zarzyńska, K. Genotype by Environment Interaction (GEI) Effect for Potato Tuber Yield and Their Quality Traits in Organic Multi-Environment Domains in Poland. Agriculture 2024, 14, 1591. [Google Scholar] [CrossRef]
- Hanász, A.; Zsombik, L.; Magyar-Tábori, K.; Mendler-Drienyovszki, N. Effect of Drought and Seed Tuber Size on Agronomical Traits of Potato (Solanum tuberosum L.) under In Vivo Conditions. Agronomy 2024, 14, 1131. [Google Scholar] [CrossRef]
- Meise, P.; Seddig, S.; Uptmoor, R.; Ordon, F.; Schum, A. Assessment of Yield and Yield Components of Starch Potato Cultivars (Solanum tuberosum L.) Under Nitrogen Deficiency and Drought Stress Conditions. Potato Res. 2019, 62, 193–220. [Google Scholar] [CrossRef]
- Kołodziejczyk, M. Phenotypic variation of yielding of medium-early cultivars of table potato. Bull. Inst. Plant Breed. Acclim. 2021, 294, 27–33. (In Polish) [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. Phenotypic variability of the yield and structure of mid-early potato cultivars. Acta Sci. Pol. Agric. 2017, 16, 147–161. [Google Scholar]
- Slater, A.T.; Cogan, N.O.I.; Rodoni, B.C.; Daetwyler, H.D.; Hayes, B.J.; Caruana, B.; Badenhorst, P.E.; Spangenberg, G.C.; Forster, J.W. Breeding Differently—The Digital Revolution: High-Throughput Phenotyping and Genotyping. Potato Res. 2017, 60, 337–352. [Google Scholar] [CrossRef]
- Zhou, B.; Yuan, J.; Liang, L.; Zhang, F.; Wang, Y. Genotype × environment interactions for potato yield and quality traits: Identification of ideotypes adapted to different ecological regions of northwest China. BMC Plant Biol. 2025, 25, 737. [Google Scholar] [CrossRef]
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop Phenomics and High-Throughput Phenotyping: Past Decades, Current Challenges, and Future Perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef]
- Seid, E.; Tessema, L. Evaluation of tuber quality, yield and yield-related traits of potato (Solanum tuberosum L.) genotypes in Holetta, central Ethiopia. CABI Agric. Biosci. 2024, 5, 99. [Google Scholar] [CrossRef]
- Song, P.; Wang, J.; Guo, X.; Yang, W.; Zhao, C. High-throughput phenotyping: Breaking through the bottleneck in future crop breeding. Crop. J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- Starck, Z. Plant physiology: Yesterday, today and what will bring tomorrow? Kosmos. Probl. Biol. Sci. 2014, 6, 569–589. (In Polish) [Google Scholar]
- Puła, J.; Skrzypek, E.; Łabza, T.; Durert, F. Chlorophyll fluorescence as one of the potato yield indicators. Scientific Conference Materials: Edible potatoes for food pro-cessing—Agrotechnical and storage factors determining quality. Radzików 1999, 23–25.02, 110–122. (In Polish) [Google Scholar]
- Rahman, M.H.; Islam, M.J.; Mumu, U.H.; Ryu, B.-R.; Lim, J.-D.; Azad, M.O.K.; Cheong, E.J.; Lim, Y.-S. Growth and yield of potato tubers (Solanum tuberosum L.) under variable LED light spectrum in controlled greenhouse conditions. Horticulturae 2024, 10, 254. [Google Scholar] [CrossRef]
- Tong, C.; Ma, Z.; Chen, H.; Gao, H. Toward an understanding of potato starch structure, function, biosynthesis, and applications. Food Front. 2023, 4, 980–1000. [Google Scholar] [CrossRef]
- Monneveux, P.; Rekika, D.; Acevedo, E.; Merah, O. Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes. Plant Sci. 2006, 170, 867–872. [Google Scholar] [CrossRef]
- Haase, N.U. Estimation of dry matter and starch concentration in potatoes by determination of under-water weight and near infrared spectroscopy. Potato Res. 2003, 46, 117–127. [Google Scholar] [CrossRef]
- Pęksa, A.; Tajner-Czopek, A.; Gryszkin, A.; Miedzianka, J.; Rytel, E.; Wolny, S. Assessment of the Content of Glycoalkaloids in Potato Snacks Made from Colored Potatoes, Resulting from the Action of Organic Acids and Thermal Processing. Foods 2024, 13, 1712. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kalaji, M.H.; Carpentier, R.; Allakverdiev, S.I. Photosystem II thermostability in situ: Environmentally induced acclimation and genotype specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Barbaś, P.; Skiba, D.; Pszczółkowski, P.; Sawicka, B. Mechanisms of Plant Natural Immunity and the Role of Selected Oxylipins as Molecular Mediators in Plant Protection. Agronomy 2022, 12, 2619. [Google Scholar] [CrossRef]
- Uzair, A.; Sharma, L. A review of Best Management Practices for potato crop using Precision Agricultural Technologies. Smart Agric. Tech. 2023, 4, 100220. [Google Scholar] [CrossRef]
- An, L.; Fang, H.; Zhang, X.; Tang, J.; Gong, J.; Yi, Y.; Tang, M. Genome-Wide Identification and Characterization of the CDPK Family of Genes and Their Response to High-Calcium Stress in Yinshania henryi. Genes 2025, 16, 109. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).