Vegetation Dynamics, Productivity, and Carbon Stock in Plant Matter in the Drained Berkazan-Kamysh Peatland (Bashkir Cis-Urals) After Rewetting
Abstract
1. Introduction
2. Materials and Methods
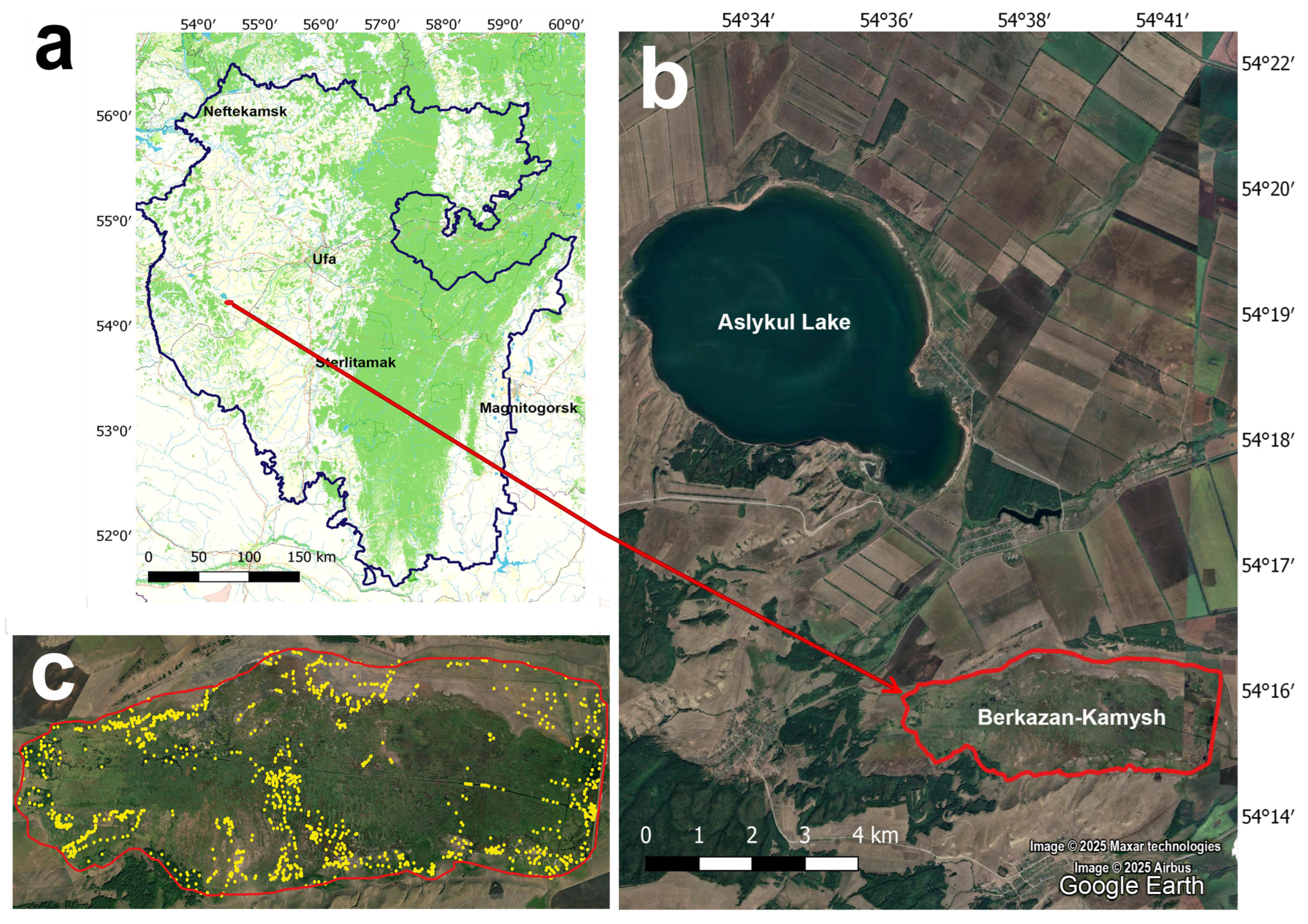
2.1. Site Description
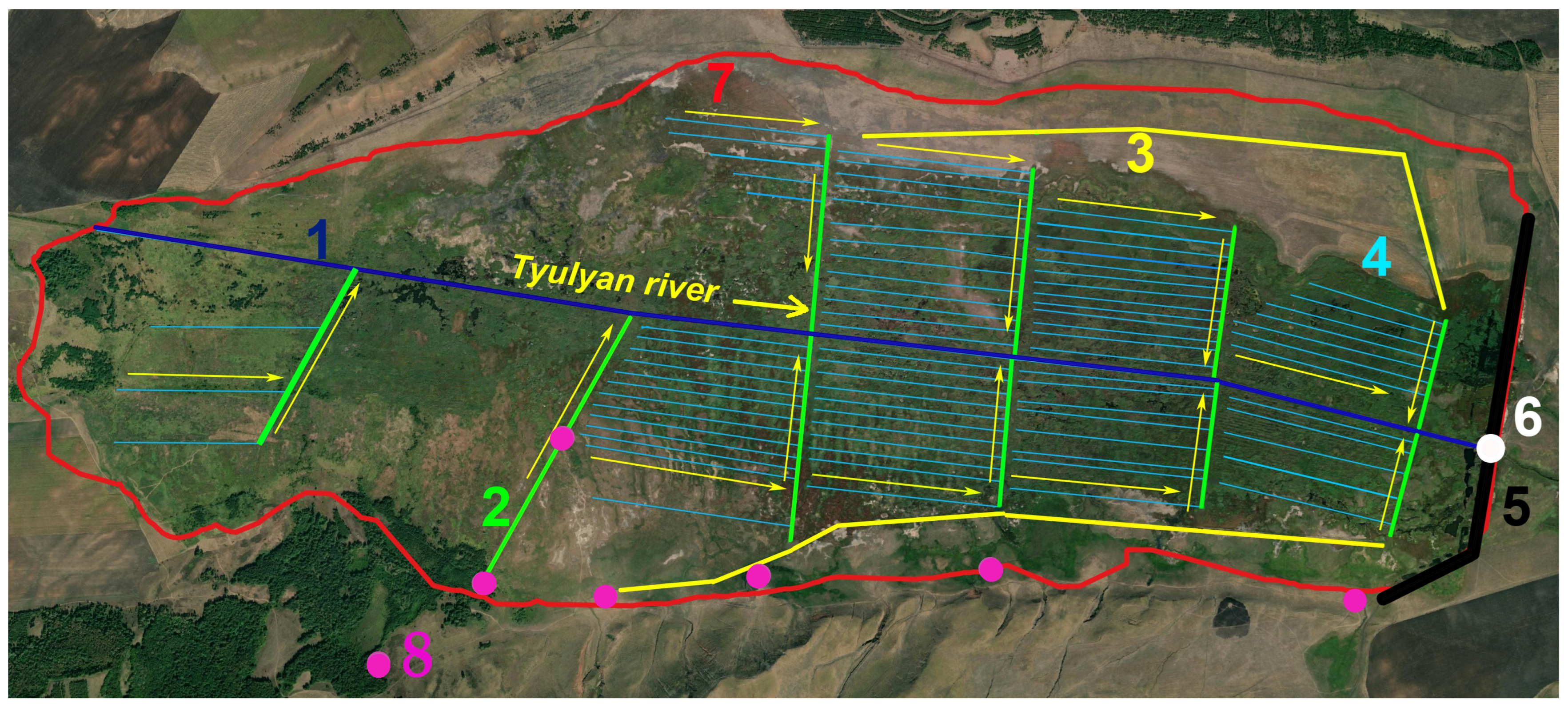
2.2. Study and Mapping of Vegetation Cover on the Berkazan-Kamysh Peatland
2.3. Estimation of the Live Phytomass and Mortmass of Herbaceous Plants
2.4. Analysis of the Samples’ Carbon Contents
2.5. Vegetation of the Berkazan-Kamysh Peatland
3. Results
3.1. Analysis of Productivity and Stocks of Aboveground and Root Phytomass in Different Types of Plant Communities on the Berkazan-Kamysh Peatland
3.2. Total Carbon Stock in the Vegetation of the Berkazan-Kamysh Peatland
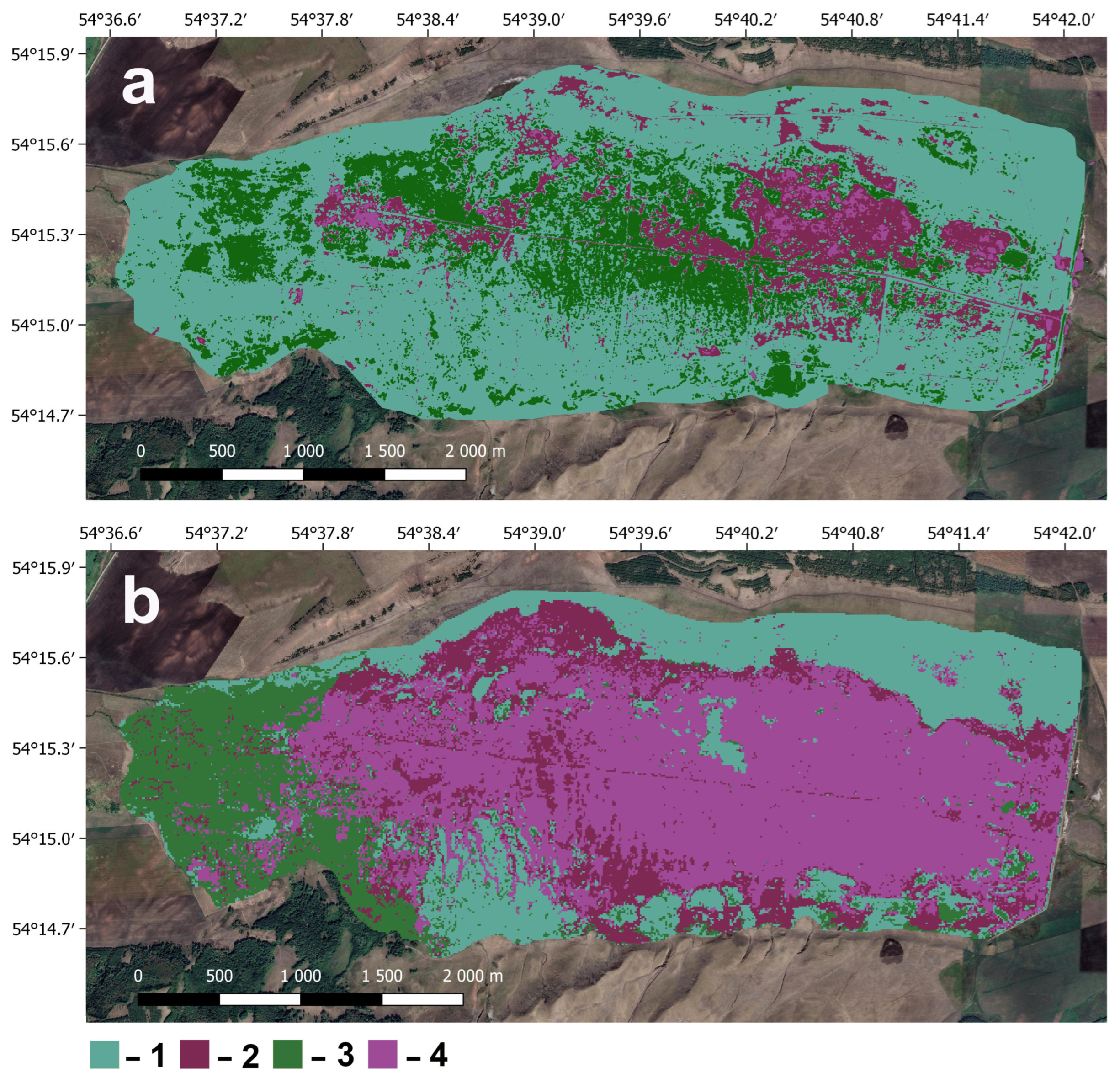
3.3. Dynamics of Vegetation on the Berkazan-Kamysh Peatland After Rewetting
4. Discussion
4.1. Vegetation Dynamics After Rewetting
4.2. Comparison of Productivity and Stocks of Aboveground and Root Phytomass of Plant Communities on the Berkazan-Kamysh Peatland with Peatlands of Other Regions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Assessment on Peatlands, Biodiversity and Climate Change: Main Report; Parish, F., Ed.; Kuala Lumpur, Malaysia; Global Environment Centre: Wageningen, The Netherlands; Wetlands International: Ede, The Netherlands, 2008; 179p, Available online: https://hazeportal.asean.org/publications/assessment-on-peatlands-biodiversity-and-climate-change-main-report/ (accessed on 21 August 2025).
- Yu, Z.C. Northern peatland carbon stocks and dynamics: A review. Biogeosciences 2012, 9, 4071–4085. [Google Scholar] [CrossRef]
- Loisel, J.; Yu, Z.; Beilman, D.W.; Camill, P.; Alm, J.; Amesbury, M.J.; Anderson, D.; Andersson, S.; Bochicchio, C.; Barber, K.; et al. A database and synthesis of northern peatland soil properties and Holocene carbon and nitrogen accumulation. Holocene 2014, 24, 1028–1042. [Google Scholar] [CrossRef]
- Joosten, H.; Tanneberger, F.; Moen, A. Mires and Peatlands of Europe: Status, Distribution and Conservation; Schweizerbart Science Publishers: Stuttgart, Germany, 2017; 780p. [Google Scholar]
- Moore, T.R.; Bubier, J.L.; Bledzki, L. Litter decomposition in temperate peatland ecosystems. The effect of substrate and site. Ecosystems 2007, 10, 949–963. [Google Scholar] [CrossRef]
- Vompersky, S.E. Biogeocenotic Features of Swamps and Their Rational Use; Nauka: Moscow, Russia, 1994; pp. 5–37. [Google Scholar]
- Kosykh, N.P.; Koronatova, N.G.; Naumova, N.B.; Titlyanova, A.A. Above- and below-ground phytomass and net primary production in boreal mire ecosystems Western Sibiria. Wetl. Ecol. Manag. 2008, 16, 139–153. [Google Scholar] [CrossRef]
- Kosykh, N.P.; Mironycheva-Tokareva, N.P.; Peregon, A.M.; Parshina, E.K. Biological productivity of bogs in the middle taiga subzone of Western Siberia. Russ. J. Ecol. 2008, 39, 8–16. [Google Scholar] [CrossRef]
- Zhong, Y.; Jiang, M.; Middleton, B.A. Effects of water level alteration on carbon cycling in peatlands. Ecosyst. Health Sustain. 2020, 6, 1806113. [Google Scholar] [CrossRef]
- Serk, H.; Nilsson, M.B.; Figueira, J.; Wieloch, T.; Schleucher, J. CO2 fertilization of Sphagnum peat mosses is modulated by water table level and other environmental factors. Plant Cell Environ. 2021, 44, 1756–1768. [Google Scholar] [CrossRef]
- Titlyanova, A.A.; Bazilevich, N.I.; Snytko, V.A.; Dubynina, S.S.; Kopoteva, T.A.; Magomedova, L.N.; Mironycheva-Tokareva, N.P.; Nefedyeva, L.G.; Titlyanova, A.A.; Vishnyakova, E.K. Changes in the productivity of wetland and grass ecosystems along the latitudinal gradient. Soils Environ. 2022, 5, 32–50. [Google Scholar]
- Joosten, H. The Global Peatland CO2 Picture: Peatland Status and Drainage Related Emissions in All Countries of the World; Wetland International: Ede, The Netherlands, 2010. [Google Scholar]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Joosten, H. Peatlands across the globe. In Peatland Restoration and Ecosystem Services. Science, Policy and Practice; Joosten, H., Ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 19–43. [Google Scholar]
- Frolking, S.; Talbot, J.; Jones, M.C.; Treat, C.C.; Kauffman, J.B.; Tuittila, E.S.; Roulet, N. Peatlands in the Earth’s 21st century climate system. Environ. Rev. 2011, 19, 371–396. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Leifeld, J. Prologue paper: Soil carbon losses from land-use change and the global agricultural greenhouse gas budget. Sci. Total Environ. 2013, 465, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Zak, D.; Gelbrecht, J. The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry 2007, 85, 141–151. [Google Scholar] [CrossRef]
- Tiemeyer, B.; Kahle, P. Nitrogen and dissolved organic carbon (DOC) losses from an artificially drained grassland on organic soils. Biogeosciences 2014, 11, 4123–4137. [Google Scholar] [CrossRef]
- Lamers, L.P.M.; Vile, M.A.; Grootjans, A.P.; Acreman, M.C.; van Diggelen, R.; Evans, M.G.; Richardson, C.J.; Rochefort, L.; Kooijman, A.M.; Roelofs, J.G.M.; et al. Ecological restoration of rich fens in Europe and North America: From trial and error to an evidence-based approach. Biol. Rev. Camb. Philos. Soc. 2015, 90, 182–203. [Google Scholar] [CrossRef]
- Bonn, A. (Ed.) Peatland Restoration and Ecosystem Services: Science, Policy, and Practice; Cambridge University Press: Cambridge, UK, 2016; 510p. [Google Scholar] [CrossRef]
- Günther, A.; Huth, V.; Jurasinski, G.; Glatzel, S. The effect of biomass harvesting on greenhouse gas emissions from a rewetted temperate fen. GCB Bioenergy 2015, 7, 1092–1106. [Google Scholar] [CrossRef]
- Tuittila, E.S.; Vasander, H.; Laine, J. Impact of rewetting on the vegetation of a cut-away peatland. Appl. Veg. Sci. 2000, 3, 205–212. [Google Scholar] [CrossRef]
- Liu, N.; Li, Y.; Wang, Q.C.; Zhou, R.; Gaffney, P.P.; Liu, M.; Shi, R.; Gao, Z.; Chu, H.; Niu, S.; et al. Restoration recovers plant diversity but changes species composition and biomass allocation in an alpine peatland. Ecol. Process. 2025, 14, 24. [Google Scholar] [CrossRef]
- Malloy, S.; Price, J.S. Fen restoration on a bog harvested down to sedge peat: A hydrological assessment. Ecol. Eng. 2014, 64, 151–160. [Google Scholar] [CrossRef]
- Evans, C.D.; Peacock, M.; Baird, A.J.; Evans, C.D.; Peacock, M.; Baird, A.J.; Artz, R.R.E.; Burden, A.; Callaghan, N.; Chapman, P.J.; et al. Morrison5Overriding water table control on managed peatland greenhouse gas emissions. Nature 2021, 593, 548–552. [Google Scholar] [CrossRef]
- Günther, A.; Barthelmes, A.; Huth, V.; Joosten, H.; Jurasinski, G.; Koebsch, F.; Couwenberg, J. Prompt rewetting of drained peatlands reduces climate warming despite methane emissions. Nat. Commun. 2020, 11, 1644. [Google Scholar] [CrossRef]
- Järveoja, J.; Nilsson, M.B.; Crill, P.M.; Peichl, M. Bimodal diel pattern in peatland ecosystem respiration rebuts uniform temperature response. Nat. Commun. 2020, 11, 4255. [Google Scholar] [CrossRef]
- Peichl, M.; Gažovič, M.; Vermeij, I.; de Goede, E.; Sonnentag, O.; Limpens, J.; Nilsson, M.B. Peatland vegetation composition and phenology drive the seasonal trajectory of maximum gross primary production. Sci. Rep. 2018, 8, 8012. [Google Scholar] [CrossRef]
- Schwieger, S.; Kreyling, J.; Peters, B.; Gillert, A.; Freiherr von Lukas, U.; Jurasinski, G.; Köhn, D.; Blume-Werry, G. Rewetting prolongs root growing season in minerotrophic peatlands and mitigates negative drought effects. J. Appl. Ecol. 2022, 59, 2106–2116. [Google Scholar] [CrossRef]
- Mursyid, H.; Ramadhan, R.; Irawan, A.; Sadono, R.; Putra, E.T.S.; Suryanto, P. A global development and dynamics of peatland restoration: A bibliometric analysis. Ecol. Indic. 2025, 177, 113724. [Google Scholar] [CrossRef]
- Kotowski, W.; Ackreman, M.; Grootjans, A.; Klimkowska, A.; Rößling, H.; Wheeler, B. Restoration of Temperate Fens: Matching Strategies with Site Potential. In Peatland Restoration and Ecosystem Services: Science, Policy and Practice; Bonn, A., Allott, T., Evans, M., Joosten, H., Stoneman, R., Eds.; Cambridge University Press, British Ecological Society: Cambridge, MA, USA, 2016; pp. 170–191. [Google Scholar]
- Kreyling, J.; Tanneberger, F.; Jansen, F.; Van Der Linden, S.; Aggenbach, C.; Blüml, V.; Couwenberg, J.; Emsens, W.-J.; Joosten, H.; Klimkowska, A.; et al. Rewetting does not return drained fen peatlands to their old selves. Nat. Commun. 2021, 12, 5693. [Google Scholar] [CrossRef]
- Kotowski, W.; Thörig, W.; van Diggelen, R.; Wassen, M.J. Competition as a factor structuring species zonation in riparian fens—A transplantation experiment. Appl. Veg. Sci. 2006, 9, 231–240. [Google Scholar] [CrossRef]
- Minayeva, T.; Sirin, A.; Bragg, O. A Quick Scan of Peatlands in Central and Eastern Europe; Wetlands International: Wageningen, The Netherlands, 2009; p. 132. [Google Scholar]
- Sirin, A.A. Peatbogs and anthopogenically modified peatlands: Carbon, greenhouse gases and climate change. Uspekhi Sovrem. Biol. 2022, 142, 560–577. [Google Scholar] [CrossRef]
- Ilyasov, D.V.; Sirin, A.A.; Suvorov, G.G.; Meteleva, M.M.; Markina, A.V. Restoration of forest–steppe degrading peatlands and their ecosystem functions. Aktual. Napravleniya Nauchnykh Issled. XXI Veka Teor. I Prakt. 2017, 5, 400–406. [Google Scholar]
- Minayeva, T.; Sirin, A.; Stracher, G. The Peat Fires of Russia. In Coal and Peat Fires: A Global Perspective; Stracher, G., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2013; pp. 376–394. [Google Scholar]
- Sirin, A.A.; Medvedeva, M.A.; Itkin, V.Y. Rewetting of disused drained peatlands and reduction of greenhouse gas emissions. Bull. Russ. Acad. Sciences. Geogr. Ser. 2023, 87, 597–618. [Google Scholar] [CrossRef]
- Mirkin, B.M.; Martynenko, V.B. (Eds.) Natural Conditions and Biota of the Asly-Kul Nature Park; Bashkir encyclopedia: Ufa, Russia, 2018; 456p. [Google Scholar]
- Kadilnikov, I.P. (Ed.) Physical and Geographical Zoning of the Bashkir ASSR; Bashkir State University: Ufa, Russia, 1964; 210p. [Google Scholar]
- Gabbasova, I.M.; Suleymanov, R.R.; Garipov, T.T.; Komissarov, M.A.; Suleymanov, A.R. Changes in soil properties under drainage and secondary watering of the steppe zone, Southern Ural Region. Arid Ecosys. 2024, 30, 91–99. [Google Scholar]
- Yamalov, S.M.; Martynenko, V.B.; Abramova, L.M.; Golub, V.B.; Baisheva, E.Z.; Bayanov, A.V. Prodromus of Plant Communities of the Republic of Bashkortostan: Preprint; Gilem: Ufa, Russia, 2012; 83p. [Google Scholar]
- Hennekens, S.M.; Schaminée, J.H.J. TURBOVEG, a comprehensive data base management system for vegetation data. J. Veg. Sci. 2001, 12, 589–591. [Google Scholar] [CrossRef]
- Tichý, L.; Holt, J.; Nejezchlebová, M. Program for Management, Analysis and Classification of Ecological Data; Masaryk University: Brno, Czech Republic, 2011; 61p. [Google Scholar]
- Westhoff, V.; van der Maarel, E. The Braun-Blanquet approach. In Classification of Plant Communities; Whittaker, R.H., Ed.; Dr. W. Junk Publishers: Hague, The Netherlands, 1978. [Google Scholar]
- Mirkin, B.M.; Solomeshch, A.I.; Zhuravleva, S.E. Vegetation of Russia in the area of the syntaxonomy of Braun-Blanquet: Development and results. Zhurnal Obs. Biol. 2000, 1, 5–21. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde; Springer: Wien, Austria; New York, NY, USA, 1964; 631p. [Google Scholar]
- Conrad, O.; Bechtel, B.; Bock, M.; Dietrich, H.; Fischer, E.; Gerlitz, L.; Wehberg, J.; Wichmann, V.; Böhner, J. System for Automated Geoscientific Analyses (SAGA) v. 2.1.4. Geosci. Model Dev. 2015, 8, 1991–2007. [Google Scholar] [CrossRef]
- Golovatskaya, E.A. Biological productivity of oligotrophic and eutrophic mires in the southern taiga of Western Siberia. J. Sib. Fed. Univ. Ser. Biol. 2009, 2, 38–53. [Google Scholar]
- Khaziev, F.K.; Mukatanov, A.K. Rational Use of Drained Lands; Bashkirskoye Knizhnoye Izdatel’stvo: Ufa, Russia, 1985; 104p. [Google Scholar]
- Komissarov, A.V.; Kovshov, Y.A.; Ishbulatov, M.G. Monitoring of reclaimed lands in the Republic of Bashkortostan. Land Manag. Cadastre Land Monit. 2011, 10, 56–61. [Google Scholar]
- Baisheva, E.Z.; Shirokih, P.S.; Martynenko, V.B.; Bikbaev, I.G. On the results of inventarisation of anthropogenically modified peatlands in the Bashkir Cis-Urals. Bull. Ufa Sci. Cent. Russ. Acad. Sci. 2022, 3, 55–61. [Google Scholar] [CrossRef]
- Karpov, D.N.; Yuritsyna, N.A. Vegetation of Saline Soils of the Southern Urals and Adjacent Territories; Institute of Ecology of the Volga Basin of the RAS: Tolyatti, Russia, 2006; 124p. [Google Scholar]
- Ilyasov, D.V.; Sirin, A.A.; Suvorov, G.G.; Meteleva, M.M.; Maslov, A.A.; Muldashev, A.A.; Shirokikh, P.S.; Bikbaev, I.G.; Martynenko, V.B. Soils and vegetation of anthropogenic changed steppe peatland (Berkazan-Kamysh, Bashkiriya). Agrochemistry 2018, 12, 46–59. [Google Scholar] [CrossRef]
- Ilyasov, D.V.; Sirin, A.A.; Suvorov, G.G.; Martynenko, V.B. The summer carbon dioxide and methane fluxes on drained peatland in forest–steppe of Bashkortostan. Agrochemistry 2017, 1, 50–62. [Google Scholar]
- Suvorov, G.G.; Chistotin, M.V.; Sirin, A.A. The carbon losses from a drained peatland in Moscow oblast used for peat extraction and agriculture. Agrochemistry 2015, 11, 51–62. [Google Scholar]
- Tsvelev, N.N. (Ed.) Flora of Eastern Europe; Publishing House of SPHFA: St. Petersburg, Russia, 2001; 670p. [Google Scholar]
- van den Berg, M.; Gremmen, T.M.; Vroom, R.J.E.; van Huissteden, J.; Boonman, J.; van Huissteden, C.J.A.; van der Velde, Y.; Smolders, A.J.P.; van de Riet, B.P. A case study on topsoil removal and rewetting for paludiculture: Effect on biogeochemistry and greenhouse gas emissions from Typha latifolia, Typha angustifolia, and Azolla filiculoides. Biogeosciences 2024, 21, 2669–2690. [Google Scholar] [CrossRef]
- Baur, P.A.; Maier, A.; Buchsteiner, C.; Zechmeister, T.; Glatzel, S. Consequences of intense drought on CO2 and CH4 fluxes of the reed ecosystem at Lake Neusiedl. Environ. Res. 2024, 262, 119907. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, M.; Chen, L.; Zhang, S.; Luo, Y.; Cao, W.; Zhao, J.; Wang, L.; Jia, Z.; Bao, Z. Methanotrophs contribute to nitrogen fixation in emergent macrophytes. Front. Microbial. 2022, 13, 851424. [Google Scholar] [CrossRef] [PubMed]
- Fauβer, A.C.; Hoppert, M.; Walther, P. Roots of the wetland plants Typha latifolia and Phragmites australis are inhabited by methanotrophic bacteria in biofilms. Flora-Morphology, Distribution. Funct. Ecol. Plants 2012, 207, 775–782. [Google Scholar] [CrossRef]
- Laanbroek, H.J. Methane emission from natural wetlands: Interplay between emergent macrophytes and soil microbial processes. A mini-review. Ann. Bot. 2010, 105, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Bazilevich, N.I. Biological Productivity of the Ecosystems of Northern Eurasia; Nauka: Moskow, Russia, 1993; 293p. [Google Scholar]
- Titlyanova, A.A. Net primary production of grass and marsh ecosystems. Sib. Ecol. J. 2007, 5, 763–770. [Google Scholar]
- Kosykh, N.P. Biological productivity of bog in forest–steppe. Vestn. Tomsk. Gos. Pedagog. Univ. 2009, 3, 87–90. [Google Scholar]
- Titlyanova, A.A.; Bazilevich, N.I.; Shmakova, E.I.; Snytko, V.A.; Dubynina, S.S.; Magomedova, L.N.; Nefedeva, L.G.; Semenyuk, N.V.; Tishkov, A.A.; Tran, T.; et al. Biological Productivity of Grasslands. Geographical Regularities and Ecological Features, 2nd ed.; revised and supplemented; IPA SB RAS: Novosibirsk, Russia, 2018; 110p. [Google Scholar] [CrossRef]
- Borkenhagen, A.; Cooper, D.J.; House, M.; Vitt, D.H. Establishing peat-forming plant communities: A comparison of wetland reclamation methods in A lberta’s oil sands region. Ecol. Appl. 2024, 34, e2929. [Google Scholar] [CrossRef]
- Zubkova, K.A.; Gonina, E.S.; Shikhova, L.N.; Lisitsyn, E.M. Analysis of climatic stages of swamp formation based on the botanical composition of peat. Vestn. Orenb. Gos. Univ. 2016, 193, 57–64. [Google Scholar]


| Plant Matter Fractions | Types of Plant Community | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Average stock of plant biomass, g m−2 | ||||||||
| Live aboveground phytomass | 103.2 ± 10.6 | 415.9 ± 41.0 | 869.8 ± 90.0 | 278.6 ± 14.1 | 515.9 ± 31.9 | 1101.6 ± 81.0 | 734.6 ± 32.2 | 1238.9 ± 94.7 |
| Mortmass | 0 ± 0 | 324.2 ± 42.9 | 416.2 ± 43.6 | 167.5 ± 19.2 | 391.1 ± 43.8 | 265.9 ± 20.5 | 0 ± 0 | 0 ± 0 |
| Root phytomass | 346.9 ± 59.6 | 881.9 ± 113.0 | 530.0 ± 68.4 | 933.9 ± 88.7 | 1041.2 ± 174.4 | 1039.8 ± 83.4 | 1299.7 ± 239.8 | 0 ± 0 |
| Average stock of plant biomass | 450.1 ± 59.3 | 1621.9 ± 104.4 | 1816.0 ± 118.9 | 1380.1 ± 97.1 | 1948.2 ± 181.9 | 2407.2 ± 82.7 | 2034.3 ± 244.3 | 1238.9 ± 94.7 |
| Total stock of plant biomass calculated taking into account the areas occupied by different types of plant communities in the Berkazan-Kamysh peatland, t | ||||||||
| Live aboveground phytomass | 34.37 | 437.52 | 243.55 | 502.12 | 184.17 | 4611.12 | 958.64 | 287.42 |
| Mortmass | 0 ± 0 | 341.01 | 116.52 | 301.85 | 139.62 | 1113.18 | 0 ± 0 | 0 ± 0 |
| Root phytomass | 115.51 | 927.72 | 148.39 | 1682.89 | 371.72 | 4352.43 | 1696.15 | 0 ± 0 |
| Total stock of plant biomass | 149.88 | 1706.25 | 508.47 | 2486.86 | 695.51 | 10076.73 | 2654.79 | 287.42 |
| Plant Matter Fractions | Types of Plant Community | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Carbon content, % | ||||||||
| Live aboveground phytomass | 29.7 ± 1.2 | 41.6 ± 0.2 | 42.7 ± 0.4 | 40.8 ± 0.2 | 42.7 ± 0.2 | 42.7 ± 0.1 | 42.7 ± 0.2 | 42.3 ± 0.4 |
| Mortmass | – | 37.1 ± 1.0 | 42.5 ± 0.3 | 38.3 ± 0.5 | 35.0 ± 1.3 | 39.9 ± 0.5 | – | – |
| Root phytomass | 30.8 ± 1.0 | 40.9 ± 0.6 | 40.2 ± 0.7 | 35.0 ± 0.7 | 34.9 ± 1.3 | 39.6 ± 0.8 | 37.8 ± 0.8 | – |
| Carbon stock, g m−2 | ||||||||
| Live aboveground phytomass | 30.6 ± 3.2 | 172.9 ± 16.8 | 371.1 ± 37.1 | 114.4 ± 6.0 | 219.8 ± 13.3 | 469.6 ± 34.3 | 313.6 ± 13.9 | 525.3 ± 41.6 |
| Mortmass | – | 125.3 ± 18.6 | 176.1 ± 17.8 | 63.3 ± 6.9 | 136.8 ± 16.7 | 106.5 ± 8.8 | – | – |
| Root phytomass | 108.6 ± 20.3 | 355.0 ± 44.2 | 211.6 ± 27.5 | 325.8 ± 31.6 | 368.3 ± 63.2 | 413.6 ± 34.3 | 491.7 ± 93.3 | – |
| Total stock of plant biomass | 139.2 ± 20.6 | 653.2 ± 42.1 | 758.8 ± 47.9 | 503.5 ± 35.6 | 724.9 ± 64.5 | 989.6 ± 35.4 | 805.2 ± 95.0 | 525.3 ± 41.6 |
| Total carbon stock in plant matter calculated taking into account the areas occupied by different plant communities in the Berkazan-Kamysh peatland, t | ||||||||
| Live aboveground phytomass | 10.18 | 181.87 | 103.90 | 206.12 | 78.46 | 1965.76 | 409.19 | 121.87 |
| Mortmass | – | 131.86 | 49.31 | 114.05 | 48.85 | 445.74 | – | – |
| Root phytomass | 36.17 | 373.47 | 59.26 | 587.08 | 131.48 | 1731.17 | 641.65 | – |
| Total stock of plant biomass | 46.35 | 687.21 | 212.47 | 907.26 | 258.80 | 4142.66 | 1050.84 | 121.87 |
| Types of Plant Community | |
|---|---|
| Before Rewetting | After Rewetting |
| saline meadows | Festuca regeliana + Hordeum nevskianum wet meadows (var. typicum, var. Artemisia austriaca), salt-marsh communities |
| reed–sedge communities with tuber-reeds | tuber-reed communities with Bolboschoenus maritimus, sedge communities with Carex acuta |
| slightly saline wet meadows | wet meadows with Calamagrostis epigeios, Festuca regeliana-forb meadows |
| reed–cattail communities | reed communities with Phragmites australis and cattail communities with Typha angustifolia |
| Types of Plant Community | Before Rewetting (2017) | After Rewetting (2024) | ||
|---|---|---|---|---|
| Area, ha | Share of Total Peatland Area, % | Area, ha | Share of Total Peatland Area, % | |
| Mesophytic plant communities | ||||
| saline meadows + salt-marsh communities | 682 | 66 | 242.9 | 24.1 |
| slightly saline wet meadows | 126 | 12 | 156.0 | 15.5 |
| Hygrophytic and hydrophytic plant communities | ||||
| reed–sedge communities with tuber-reeds | 180 | 18 | 166.2 | 16.5 |
| reed–cattail communities | 38 | 4 | 441.8 | 43.9 |
| Total area | 1026 | 100 | 1006.9 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedorov, N.; Shirokikh, P.; Baisheva, E.; Zhigunova, S.; Muldashev, A.; Tuktamyshev, I.; Bikbaev, I.; Martynenko, V.; Naumova, L. Vegetation Dynamics, Productivity, and Carbon Stock in Plant Matter in the Drained Berkazan-Kamysh Peatland (Bashkir Cis-Urals) After Rewetting. Land 2025, 14, 1729. https://doi.org/10.3390/land14091729
Fedorov N, Shirokikh P, Baisheva E, Zhigunova S, Muldashev A, Tuktamyshev I, Bikbaev I, Martynenko V, Naumova L. Vegetation Dynamics, Productivity, and Carbon Stock in Plant Matter in the Drained Berkazan-Kamysh Peatland (Bashkir Cis-Urals) After Rewetting. Land. 2025; 14(9):1729. https://doi.org/10.3390/land14091729
Chicago/Turabian StyleFedorov, Nikolay, Pavel Shirokikh, Elvira Baisheva, Svetlana Zhigunova, Albert Muldashev, Ilshat Tuktamyshev, Ilnur Bikbaev, Vasiliy Martynenko, and Leniza Naumova. 2025. "Vegetation Dynamics, Productivity, and Carbon Stock in Plant Matter in the Drained Berkazan-Kamysh Peatland (Bashkir Cis-Urals) After Rewetting" Land 14, no. 9: 1729. https://doi.org/10.3390/land14091729
APA StyleFedorov, N., Shirokikh, P., Baisheva, E., Zhigunova, S., Muldashev, A., Tuktamyshev, I., Bikbaev, I., Martynenko, V., & Naumova, L. (2025). Vegetation Dynamics, Productivity, and Carbon Stock in Plant Matter in the Drained Berkazan-Kamysh Peatland (Bashkir Cis-Urals) After Rewetting. Land, 14(9), 1729. https://doi.org/10.3390/land14091729





