Biochar Affects Greenhouse Gas Emissions from Urban Forestry Waste
Abstract
1. Introduction
2. Materials and Methods
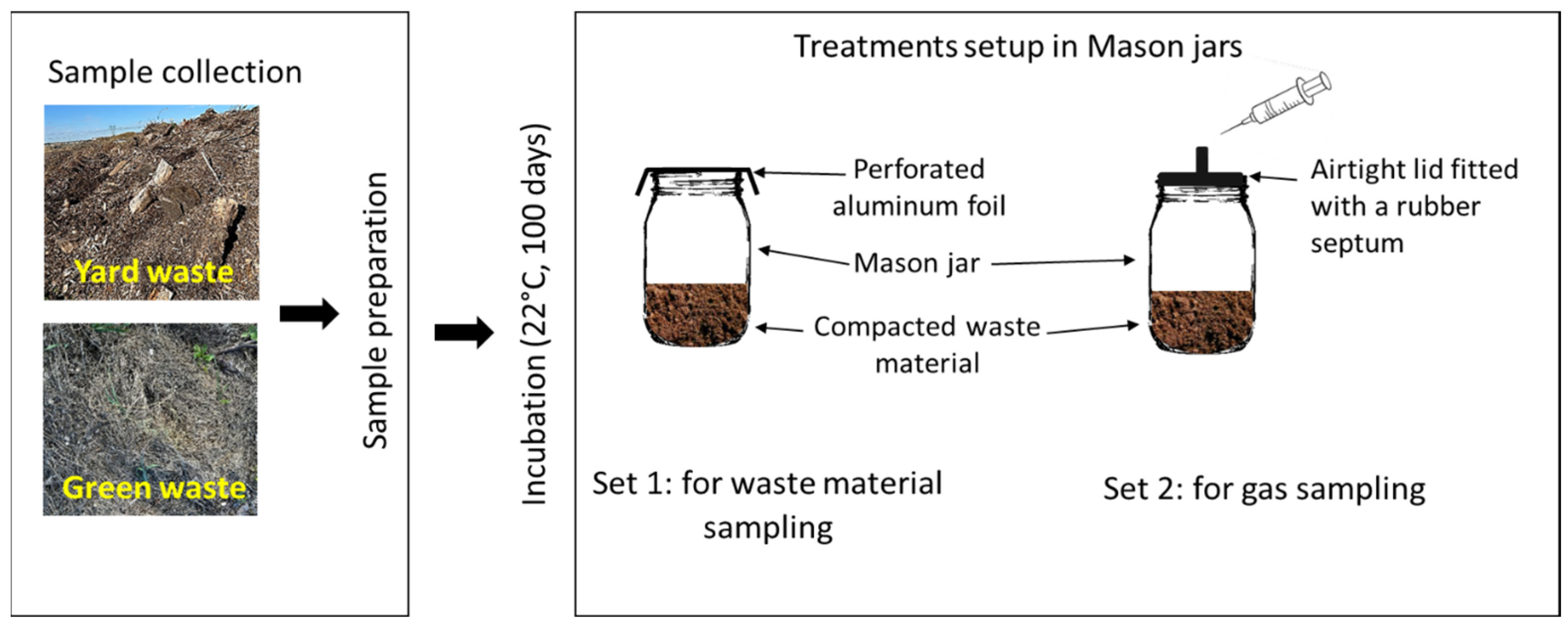
2.1. Study Materials
2.2. Experimental Design and Incubation Procedure
2.3. Gas and Incubation Material Sampling and Analysis
2.4. Analysis of Enzyme Activities and Physicochemical Properties of the Incubated Materials
2.5. Statistical Analyses
3. Results and Discussion
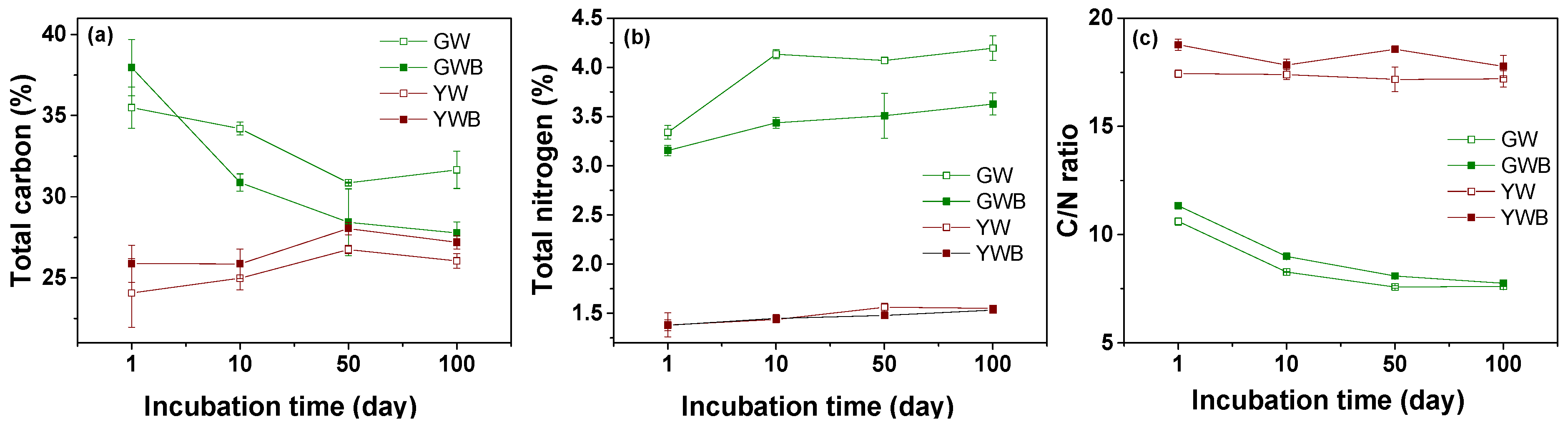
3.1. Biochar Effects on Physicochemical Properties
3.2. Biochar Effects on Enzyme Activities
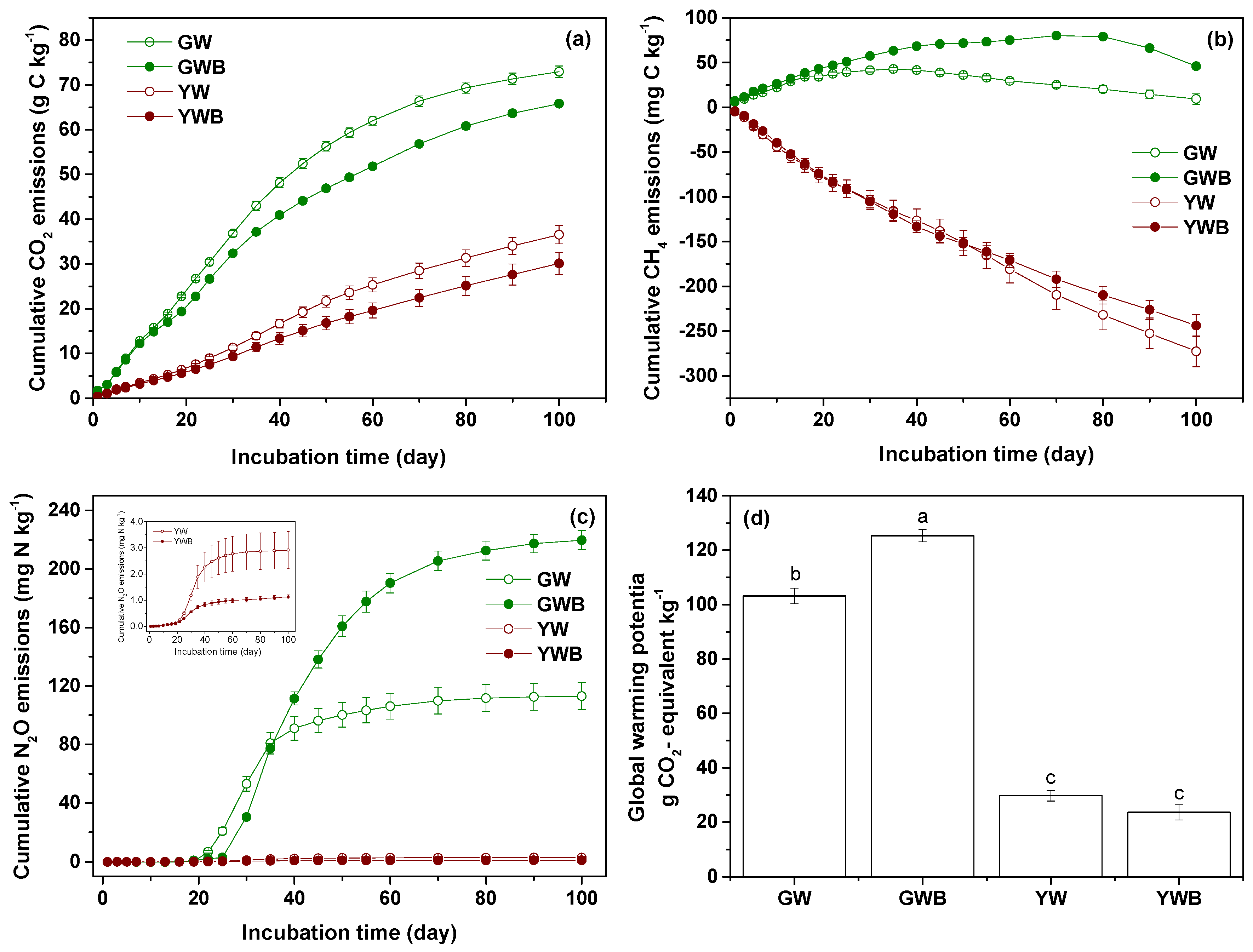
3.3. Biochar Effects on Greenhouse Gas Emissions and Global Warming Potential
3.4. Factors Affecting GHG Emissions from Green Waste Under Biochar Application
3.5. Factors Affecting GHG Emissions from Yard Waste Under Biochar Application
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ariluoma, M.; Ottelin, J.; Hautamäki, R.; Tuhkanen, E.M.; Mänttäri, M. Carbon sequestration and storage potential of urban green in residential yards: A case study from Helsinki. Urban For. Urban Green. 2021, 57, 126939. [Google Scholar] [CrossRef]
- Shafique, M.; Xue, X.; Luo, X. An overview of carbon sequestration of green roofs in urban areas. Urban For. Urban Green. 2020, 47, 126515. [Google Scholar] [CrossRef]
- Reyes-Riveros, R.; Altamirano, A.; De La Barrera, F.; Rozas-Vásquez, D.; Vieli, L.; Meli, P. Linking public urban green spaces and human well-being: A systematic review. Urban For. Urban Green. 2021, 61, 127105. [Google Scholar] [CrossRef]
- Lan, K.; Zhang, B.; Yao, Y. Circular utilization of urban tree waste contributes to the mitigation of climate change and eutrophication. One Earth 2022, 5, 944–957. [Google Scholar] [CrossRef]
- Araujo, Y.R.V.; Souza, B.I.; Carvalho, M. Greenhouse gas emissions associated with tree pruning residues of urban areas of Northeast Brazil. Resources 2024, 13, 127. [Google Scholar] [CrossRef]
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Gross, C.D.; Bork, E.W.; Carlyle, C.N.; Chang, S.X. Biochar and its manure-based feedstock have divergent effects on soil organic carbon and greenhouse gas emissions in croplands. Sci. Total Environ. 2022, 806, 151337. [Google Scholar] [CrossRef]
- Osra, F.A.; Elbisy, M.S.; Mosaıbah, H.A.; Osra, K.; Ciner, M.N.; Ozcan, H.K. Environmental impact assessment of a dumping site: A case study of Kakia dumping site. Sustainability 2024, 16, 3882. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef]
- Wang, B.; Chu, C.; Wei, H.; Zhang, L.; Ahmad, Z.; Wu, S.; Xie, B. Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals. Environ. Pollut. 2020, 267, 115411. [Google Scholar] [CrossRef]
- Sánchez, A.; Artola, A.; Font, X.; Gea, T.; Barrena, R.; Gabriel, D.; Sánchez-Monedero, M.Á.; Roig, A.; Cayuela, M.L.; Mondini, C. Greenhouse gas emissions from organic waste composting. Environ. Chem. Lett. 2015, 13, 223–238. [Google Scholar] [CrossRef]
- Chen, M.; Li, Q.; Liu, C.; Meng, E.; Zhang, B. Microbial degradation of lignocellulose for sustainable biomass utilization and future research perspectives. Sustainability 2025, 17, 4223. [Google Scholar] [CrossRef]
- Duan, M.; Wu, F.; Jia, Z.; Wang, S.; Cai, Y.; Chang, S.X. Wheat straw and its biochar differently affect soil properties and field-based greenhouse gas emission in a Chernozemic soil. Biol. Fertil. Soils 2020, 56, 1023–1036. [Google Scholar] [CrossRef]
- Liao, W.; Halim, M.A.; Kayes, I.; Drake, J.A.; Thomas, S.C. Biochar benefits green infrastructure: Global meta-analysis and synthesis. Environ. Sci. Technol. 2023, 57, 15475–15486. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, S.S.; Withana, P.A.; Lim, J.Y.; You, S.; Chang, S.X.; Wang, F.; Rhee, J.H.; Ok, Y.S. Carbon negative biochar systems contribute to sustainable urban green infrastructure: A critical review. Green Chem. 2024, 26, 10634–10660. [Google Scholar] [CrossRef]
- Kayes, I.; Halim, M.A.; Thomas, S.C. Biochar mitigates methane emissions from organic mulching in urban soils: Evidence from a long-term mesocosm experiment. J. Environ. Manag. 2025, 376, 124525. [Google Scholar] [CrossRef]
- Pokharel, P.; Ma, Z.; Chang, S.X. Biochar increases soil microbial biomass with changes in extra-and intracellular enzyme activities: A global meta-analysis. Biochar 2020, 2, 65–79. [Google Scholar] [CrossRef]
- Impraim, R.; Weatherley, A.; Chen, D.; Suter, H. Effect of lignite amendment on carbon and nitrogen mineralization from raw and composted manure during incubation with soil. Pedosphere 2022, 32, 785–795. [Google Scholar] [CrossRef]
- Li, J.; Kwak, J.-H.; Chang, S.X.; Gong, X.; An, Z.; Chen, J. Greenhouse gas emissions from forest soils reduced by straw biochar and nitrapyrin applications. Land 2021, 10, 189. [Google Scholar] [CrossRef]
- Li, X.; Yao, S.; Wang, Z.; Jiang, X.; Song, Y.; Chang, S.X. Polyethylene microplastic and biochar interactively affect the global warming potential of soil greenhouse gas emissions. Environ. Pollut. 2022, 315, 120433. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Saiya-Cork, K.; Long, T.; Osgood, M.; Neher, D.; Zak, D.; Norby, R. Soil microbial activity in a Liquidambar plantation unresponsive to CO2-driven increases in primary production. Appl. Soil Ecol. 2003, 24, 263–271. [Google Scholar] [CrossRef]
- Mori, T.; Aoyagi, R.; Kitayama, K.; Mo, J. Does the ratio of β-1,4-glucosidase to β-1,4-N-acetylglucosaminidase indicate the relative resource allocation of soil microbes to C and N acquisition? Soil Biol. Biochem. 2021, 160, 108363. [Google Scholar] [CrossRef]
- Mulvaney, R.L. Nitrogen—Inorganic forms. In Methods of Soil Analysis, Part 3, Chemical Methods; SSSA: Madison, WI, USA, 1996; pp. 1123–1184. [Google Scholar]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Wu, Q.; Kwak, J.-H.; Chang, S.X.; Han, G.; Gong, X. Cattle urine and dung additions differently affect nitrification pathways and greenhouse gas emission in a grassland soil. Biol. Fertil. Soils 2020, 56, 235–247. [Google Scholar] [CrossRef]
- Li, Y.; Hu, S.; Chen, J.; Müller, K.; Li, Y.; Fu, W.; Lin, Z.; Wang, H. Effects of biochar application in forest ecosystems on soil properties and greenhouse gas emissions: A review. J. Soils Sed. 2018, 18, 546–563. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Gholipour, S.; Mostafaii, G.; Yousefian, F. Biochar-amended food waste compost: A review of properties. Results Eng. 2024, 24, 103118. [Google Scholar] [CrossRef]
- Kauser, H.; Pal, S.; Haq, I.; Khwairakpam, M. Evaluation of rotary drum composting for the management of invasive weed Mikania micrantha Kunth and its toxicity assessment. Bioresour. Technol. 2020, 313, 123678. [Google Scholar] [CrossRef]
- Tian, X.; Gao, R.; Li, Y.; Liu, Y.; Zhang, X.; Pan, J.; Tang, K.H.D.; Scriber Ii, K.E.; Amoah, I.D.; Zhang, Z.; et al. Enhancing nitrogen conversion and microbial dynamics in swine manure composting process through inoculation with a microbial consortium. J. Clean. Prod. 2023, 423, 138819. [Google Scholar] [CrossRef]
- Alarefee, H.A.; Ishak, C.F.; Othman, R.; Karam, D.S. Effectiveness of mixing poultry litter compost with rice husk biochar in mitigating ammonia volatilization and carbon dioxide emission. J. Environ. Manag. 2023, 329, 117051. [Google Scholar] [CrossRef]
- Fetene, Y.; Addis, T.; Beyene, A.; Kloos, H. Valorisation of solid waste as key opportunity for green city development in the growing urban areas of the developing world. J. Environ. Chem. Eng. 2018, 6, 7144–7151. [Google Scholar] [CrossRef]
- Komilis, D.P. A kinetic analysis of solid waste composting at optimal conditions. Waste Manag. 2006, 26, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Wang, J.; Odinga, E.S.; Zhang, W.; Zhou, X.; Yang, B.; Waigi, M.G.; Gao, Y. Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: A synthesis study. Environ. Int. 2019, 130, 104899. [Google Scholar] [CrossRef]
- Belias, C.; Dassenakis, M.; Scoullos, M. Study of the N, P and Si fluxes between fish farm sediment and seawater. Results of simulation experiments employing a benthic chamber under various redox conditions. Mar. Chem. 2007, 103, 266–275. [Google Scholar] [CrossRef]
- El Barnossi, A.; Moussaid, F.; Iraqi Housseini, A. Decomposition of tangerine and pomegranate wastes in water and soil: Characterisation of physicochemical parameters and global microbial activities under laboratory conditions. Int. J. Environ. Stud. 2019, 76, 456–470. [Google Scholar] [CrossRef]
- Błońska, E.; Prażuch, W.; Lasota, J. Deadwood affects the soil organic matter fractions and enzyme activity of soils in altitude gradient of temperate forests. For. Ecosyst. 2023, 10, 100115. [Google Scholar] [CrossRef]
- Chen, P.; Ma, X.; Liang, J. Carbon conversion and microbial driving factors in the humification of green waste composts. Biomass Convers. Biorefin. 2025, 15, 14357–14367. [Google Scholar] [CrossRef]
- Sharma, D.; Pandey, A.K.; Yadav, K.D.; Kumar, S. Response surface methodology and artificial neural network modelling for enhancing maturity parameters during vermicomposting of floral waste. Bioresour. Technol. 2021, 324, 124672. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, G.; Yu, D.; Liu, R.; Chen, X.; Yang, Z.; Yao, T.; Gong, Y.; Shan, Y.; Wang, Y. Sludge composting with self-produced carbon source by phosphate buffer coupled hyperthermophilic pretreatment realizing nitrogen retention. Chem. Eng. J. 2023, 476, 146811. [Google Scholar] [CrossRef]
- Manga, M.; Camargo-Valero, M.A.; Anthonj, C.; Evans, B.E. Fate of faecal pathogen indicators during faecal sludge composting with different bulking agents in tropical climate. Int. J. Hyg. Environ. Health 2021, 232, 113670. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Zhou, X.; Moore, B. A simulation model linking crop growth and soil biogeochemistry for sustainable agriculture. Ecol. Model. 2002, 151, 75–108. [Google Scholar] [CrossRef]
- Cardoso, P.H.S.; Gonçalves, P.W.B.; Alves, G.d.O.; Pegoraro, R.F.; Fernandes, L.A.; Frazão, L.A.; Sampaio, R.A. Improving the quality of organic compost of sewage sludge using grass cultivation followed by composting. J. Environ. Manag. 2022, 314, 115076. [Google Scholar] [CrossRef] [PubMed]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, L.; Guo, J.; Guo, H.; Liu, M.; Guo, S.; Kuzyakov, Y.; Ling, N.; Shen, Q. Active microbial population dynamics and life strategies drive the enhanced carbon use efficiency in high-organic matter soils. mBio 2024, 15, e0017724. [Google Scholar] [CrossRef]
- Sun, B.; Kallenbach, C.M.; Boh, M.Y.; Clark, O.G.; Whalen, J.K. Enzyme activity after applying alkaline biosolids to agricultural soil. Can. J. Soil Sci. 2023, 103, 372–376. [Google Scholar] [CrossRef]
- Zaid, F.; Al-Awwal, N.; Yang, J.; Anderson, S.H.; Alsunuse, B.T.B. Effects of biochar-amended composts on selected enzyme activities in soils. Processes 2024, 12, 1678. [Google Scholar] [CrossRef]
- Hardy, B.; Sleutel, S.; Dufey, J.E.; Cornelis, J.-T. The long-term effect of biochar on soil microbial abundance, activity and community structure is overwritten by land management. Front. Environ. Sci. 2019, 7, 110. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, X. Effects of rhamnolipid and initial compost particle size on the two-stage composting of green waste. Bioresour. Technol. 2014, 163, 112–122. [Google Scholar] [CrossRef]
- Komilis, D.P.; Ham, R.K. The effect of lignin and sugars to the aerobic decomposition of solid wastes. Waste Manag. 2003, 23, 419–423. [Google Scholar] [CrossRef]
- Kalu, S.; Seppänen, A.; Mganga, K.Z.; Sietiö, O.-M.; Glaser, B.; Karhu, K. Biochar reduced the mineralization of native and added soil organic carbon: Evidence of negative priming and enhanced microbial carbon use efficiency. Biochar 2024, 6, 7. [Google Scholar] [CrossRef]
- Bekchanova, M.; Kuppens, T.; Cuypers, A.; Jozefczak, M.; Malina, R. Biochar’s effect on the soil carbon cycle: A rapid review and meta-analysis. Biochar 2024, 6, 88. [Google Scholar] [CrossRef]
- Gao, J.; Liu, L.; Shi, Z.; Lv, J. Biochar amendments facilitate methane production by regulating the abundances of methanogens and methanotrophs in flooded paddy soil. Front. Soil Sci. 2022, 2, 801227. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.; Gao, W.; Pan, W.; Jiang, C.; Lee, X.; Cheng, J. Response of soil N2O production pathways to biochar amendment and its isotope discrimination methods. Chemosphere 2024, 350, 141002. [Google Scholar] [CrossRef]
- Escuer-Gatius, J.; Shanskiy, M.; Soosaar, K.; Astover, A.; Raave, H. High-temperature hay biochar application into soil increases N2O fluxes. Agronomy 2020, 10, 109. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Hassan, N.S.; Jalil, A.A.; Izzuddin, N.M.; Bahari, M.B.; Hatta, A.H.; Kasmani, R.M.; Norazahar, N. Recent advances in lignocellulosic biomass-derived biochar-based photocatalyst for wastewater remediation. J. Taiwan Inst. Chem. Eng. 2024, 163, 105670. [Google Scholar] [CrossRef]
- Spokas, K.A.; Koskinen, W.C.; Baker, J.M.; Reicosky, D.C. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere 2009, 77, 574–581. [Google Scholar] [CrossRef]
- Liang, J.-F.; An, J.; Gao, J.-Q.; Zhang, X.-Y.; Song, M.-H.; Yu, F.-H. Interactive effects of biochar and AMF on plant growth and greenhouse gas emissions from wetland microcosms. Geoderma 2019, 346, 11–17. [Google Scholar] [CrossRef]
- Zhai, W.; Jia, L.; Zhao, R.; Chen, X.; Zhang, Y.; Wei, Z. Response characteristics of nitrous oxide related microorganisms to biochar addition during chicken manure composting. Process Saf. Environ. Prot. 2023, 169, 604–608. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Chen, C.; Xiang, Y.; Rezaei Rashti, M.; Li, Y.; Deng, Q.; Zhang, R. Effects of biochar application on soil nitrogen transformation, microbial functional genes, enzyme activity, and plant nitrogen uptake: A meta-analysis of field studies. GCB Bioenergy 2021, 13, 1859–1873. [Google Scholar] [CrossRef]
- Xiao, L.; Lichtfouse, E.; Kumar, P.S.; Wang, Q.; Liu, F. Biochar promotes methane production during anaerobic digestion of organic waste. Environ. Chem. Lett. 2021, 19, 3557–3564. [Google Scholar] [CrossRef]
- Pant, A.; Rai, J.P.N. Application of Biochar on methane production through organic solid waste and ammonia inhibition. Environ. Chall. 2021, 5, 100262. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, B.; Lal, R.; Lorenz, K.; Jacinthe, P.-A.; Shrestha, R.K.; Bai, X.; Singh, M.P.; Lindsey, L.E.; Ren, W. A global synthesis of biochar’s sustainability in climate-smart agriculture—Evidence from field and laboratory experiments. Renew. Sustain. Energy Rev. 2023, 172, 113042. [Google Scholar] [CrossRef]
- Nandipamu, T.M.K.; Nayak, P.; Chaturvedi, S.; Dhyani, V.C.; Sharma, R.; Tharayil, N. Chapter 17—Biochar-led methanogenic and methanotrophic microbial community shift: Mitigating methane emissions. In Biochar Production for Green Economy; Singh, S.V., Mandal, S., Meena, R.S., Chaturvedi, S., Govindaraju, K., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 335–358. [Google Scholar]
- Guo, X.; Xie, H.; Pan, W.; Li, P.; Du, L.; Zou, G.; Wei, D. Enhanced nitrogen removal via biochar-mediated nitrification, denitrification, and electron transfer in constructed wetland microcosms. Environ. Sci. Pollut. Res. 2023, 30, 72710–72720. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, J.; Ni, J. Influence of biochar on soil air permeability and greenhouse gas emissions in vegetated soil: A review. Biogeotechnics 2023, 1, 100040. [Google Scholar] [CrossRef]
- Edwards, J.D.; Pittelkow, C.M.; Kent, A.D.; Yang, W.H. Dynamic biochar effects on soil nitrous oxide emissions and underlying microbial processes during the maize growing season. Soil Biol. Biochem. 2018, 122, 81–90. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Y.; Liu, H.; Xie, S.; Abbas, F. Impact of different nitrogen source on the compost quality and greenhouse gas emissions during composting of garden waste. Process Saf. Environ. Prot. 2019, 124, 326–335. [Google Scholar] [CrossRef]
- Uwituze, Y.; Nyiraneza, J.; Fraser, T.D.; Dessureaut-Rompré, J.; Ziadi, N.; Lafond, J. Carbon, nitrogen, phosphorus, and extracellular soil enzyme responses to different land use. Front. Soil Sci. 2022, 2, 814554. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Li, Y.; Zeng, F.; Gufwan, L.A.; Yang, L.; Xia, L.; Song, S.; Montes, M.L.; Fernandez, M.A.; et al. The inclusion of clay minerals accelerates biocrust formation and potentially boosts carbon storage capabilities. Soil Tillage Res. 2025, 245, 106316. [Google Scholar] [CrossRef]
- Bodelier, P.L.E.; Laanbroek, H.J. Nitrogen as a regulatory factor of methane oxidation in soils and sediments. FEMS Microbiol. Ecol. 2004, 47, 265–277. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Zhang, J.; Dong, J.; Ren, S. The impact of global change factors on the functional genes of soil nitrogen and methane cycles in grassland ecosystems: A meta-analysis. Oecologia 2024, 207, 6. [Google Scholar] [CrossRef]
- Euler, S.; Jeffrey, L.C.; Maher, D.T.; Mackenzie, D.; Tait, D.R. Shifts in methanogenic archaea communities and methane dynamics along a subtropical estuarine land use gradient. PLoS ONE 2020, 15, e0242339. [Google Scholar] [CrossRef]
- Chen, S.; Sun, D.; Chung, J.-S. Simultaneous methanogenesis and denitrification of aniline wastewater by using anaerobic–aerobic biofilm system with recirculation. J. Hazard. Mater. 2009, 169, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Riyaz, Z.; Khan, S.T. Nitrogen fixation by methanogenic Archaea, literature review and DNA database-based analysis; significance in face of climate change. Arch. Microbiol. 2024, 207, 6. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, Z.; Li, M.; Clough, T.; Wrage-Mönnig, N.; Qin, S.; Ge, T.; Liao, H.; Zhou, S. Biochar’s role as an electron shuttle for mediating soil N2O emissions. Soil Biol. Biochem. 2019, 133, 94–96. [Google Scholar] [CrossRef]
- Liu, X.; Liu, X.; Gao, S. The electrochemical mechanism of biochar for mediating the product ratio of N2O/(N2O + N2) in the denitrification process. Sci. Total Environ. 2024, 951, 175566. [Google Scholar] [CrossRef]
- Liao, X.; Müller, C.; Jansen-Willems, A.; Luo, J.; Lindsey, S.; Liu, D.; Chen, Z.; Niu, Y.; Ding, W. Field-aged biochar decreased N2O emissions by reducing autotrophic nitrification in a sandy loam soil. Biol. Fertil. Soils 2021, 57, 471–483. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, S.; Hu, R.; Cheng, H.; Xiang, R.; Xu, H.; Zhao, J. Effects of biodegradable microplastics and straw addition on soil greenhouse gas emissions. Environ. Pollut. 2024, 356, 124315. [Google Scholar] [CrossRef]
- Yang, H.; Chen, X.; Tang, J.; Zhang, L.; Zhang, C.; Perry, D.C.; You, W. External carbon addition increases nitrate removal and decreases nitrous oxide emission in a restored wetland. Ecol. Eng. 2019, 138, 200–208. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, J.; Ye, C.; Zhu, P.; Ba, Q.; Pang, J.; Shu, L. Effects of biochar application on the abundance and community composition of denitrifying bacteria in a reclaimed soil from coal mining subsidence area. Sci. Total Environ. 2018, 625, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Yao, Y.; Gao, D.; Wang, H.; Song, L.; Sheng, H.; Cai, T.; Liang, H. Responses of N2O emissions to spring thaw period in a typical continuous permafrost region of the Daxing’an Mountains, northeast China. Atmos. Environ. 2019, 214, 116822. [Google Scholar] [CrossRef]
- Duan, T.; Zhao, J.; Zhu, L. Insights into CO2 and N2O emissions driven by applying biochar and nitrogen fertilizers in upland soil. Sci. Total Environ. 2024, 929, 172439. [Google Scholar] [CrossRef]
- Criscuoli, I.; Panzacchi, P.; Tognetti, R.; Petrillo, M.; Zanotelli, D.; Andreotti, C.; Loesch, M.; Raifer, B.; Tonon, G.; Ventura, M. Effects of woodchip biochar on temperature sensitivity of greenhouse gas emissions in amended soils within a mountain vineyard. Geoderma Reg. 2024, 38, e00847. [Google Scholar] [CrossRef]
- Saharan, B.S.; Dhanda, D.; Mandal, N.K.; Kumar, R.; Sharma, D.; Sadh, P.K.; Jabborova, D.; Duhan, J.S. Microbial contributions to sustainable paddy straw utilization for economic gain and environmental conservation. Curr. Res. Microb. Sci. 2024, 7, 100264. [Google Scholar] [CrossRef]


| Property | Treatment | Incubation Time | |||
|---|---|---|---|---|---|
| Day 1 | Day 10 | Day 50 | Day 100 | ||
| pH | GW | 7.69 (0.1) a | 7.8 (0.1) a | 7.3 (0.0) b | 7.73 (0.0) a |
| GWB | 7.65 (0.1) ab | 7.94 (0.1) a | 7.47 (0.0) b | 7.79 (0.0) ab | |
| EC (dS m−1) | GW | 2.38 (0.1) c | 3.36 (0.1) bc | 3.86 (0.1) ab | 4.04 (0.2) a |
| GWB | 2.24 (0.1) c | 3.6 (0.1) b | 4.08 (0.1) a | 3.94 (0.1) ab | |
| NH4+ (mg kg−1) | GW | 552.13 (43) a * | 534.95 (28) a * | 100.75 (2.2) b | 93.46 (3.8) b * |
| GWB | 732.53 (35) a * | 835.58 (53) a * | 205.34 (4.2) b | 263.47 (8.9) b * | |
| NO3− (mg kg−1) | GW | 237.83 (15) c | 298.91 (6.8) b | 282.74 (11) b | 696.52 (16) a |
| GWB | 231.86 (18) c | 235.67 (15) c | 372.63 (5.7) b | 819.62 (38) a | |
| TIN (mg kg−1) | GW | 789.96 (43) a * | 833.86 (26) a * | 383.49 (13) b * | 789.98 (15) a * |
| GWB | 964.39 (47) a * | 1071.25 (49) a * | 577.97 (8.9) b * | 1083.09 (33) a * | |
| Property | Treatment | Incubation Days | |||
|---|---|---|---|---|---|
| Day 1 | Day 10 | Day 50 | Day 100 | ||
| pH | YW | 7.62 (0.0) a | 7.55 (0.0) a | 7.38 (0.1) a | 7.5 (0.0) a |
| YWB | 7.69 (0.0) a | 7.73 (0.0) a | 7.43 (0.0) b | 7.61 (0.1) ab | |
| EC (dS m−1) | YW | 0.33 (0.0) b * | 0.36 (0.0) ab | 0.38 (0.0) a | 0.38 (0.0) a |
| YWB | 0.41 (0.0) b * | 0.41 (0.0) b | 0.42 (0.0) ab | 0.44 (0.0) a | |
| NH4+ (mg kg−1) | YW | 160.09 (2.3) a | 104.23 (6.1) b | 40.69 (1.4) c | 20.78 (0.2) d * |
| YWB | 168.15 (6.9) a | 131.33 (4.4) b | 71.75 (6.5) c | 38.73 (2.5) d * | |
| NO3− (mg kg−1) | YW | 24.65 (2.5) d | 38.86 (4.3) c | 102.82 (4.7) b | 143.01 (6.7) a |
| YWB | 31.95 (1) c | 42.81 (4.3) c | 114.1 (6.5) b | 183.43 (15.9) a | |
| TIN (mg kg−1) | YW | 184.74 (4.1) a | 143.08 (9.2) b | 143.51 (6) b * | 163.79 (6.8) ab * |
| YWB | 200.1 (6.5) ab | 174.14 (7) b | 185.86 (4.7) ab * | 222.16 (14.7) a * | |
| Green waste | ||||||||||
| TC | TN | NH4+ | NO3− | TIN | CO2 | CH4 | N2O | |||
| CO2 | 0.73 * | 0.76 * | −0.83 ** | −0.46 | −0.73 * | |||||
| CH4 | −0.79 ** | −0.78 ** | 0.65 | 0.77 ** | 0.89 ** | −0.69 * | ||||
| N2O | −0.69 * | −0.72 * | 0.705 * | 0.69 * | 0.88 ** | −0.80 ** | 0.91 ** | |||
| GWP | −0.63 | −0.65 * | 0.61 | 0.71 * | 0.86 ** | −0.66 * | 0.90 ** | 0.98 ** | ||
| Yard waste | ||||||||||
| pH | EC | NO3− | TIN | DON | BG | NG | CO2 | CH4 | N2O | |
| CO2 | −0.63 | −0.35 | −0.22 | −0.35 | 0.28 | 0.56 | 0.68 * | |||
| CH4 | −0.20 | 0.56 | 0.74 * | 0.71 * | −0.65 * | −0.57 | −0.30 | −0.05 | ||
| N2O | −0.41 | −0.77 * | −0.63 | −0.74 * | 0.53 | 0.68 * | 0.26 | 0.69 * | −0.33 | |
| GWP | −0.66 * | −0.29 | −0.13 | −0.26 | 0.20 | 0.48 | 0.63 | 0.99 ** | 0.10 | 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palansooriya, K.N.; Novera, T.M.; Qin, D.; An, Z.; Chang, S.X. Biochar Affects Greenhouse Gas Emissions from Urban Forestry Waste. Land 2025, 14, 1605. https://doi.org/10.3390/land14081605
Palansooriya KN, Novera TM, Qin D, An Z, Chang SX. Biochar Affects Greenhouse Gas Emissions from Urban Forestry Waste. Land. 2025; 14(8):1605. https://doi.org/10.3390/land14081605
Chicago/Turabian StylePalansooriya, Kumuduni Niroshika, Tamanna Mamun Novera, Dengge Qin, Zhengfeng An, and Scott X. Chang. 2025. "Biochar Affects Greenhouse Gas Emissions from Urban Forestry Waste" Land 14, no. 8: 1605. https://doi.org/10.3390/land14081605
APA StylePalansooriya, K. N., Novera, T. M., Qin, D., An, Z., & Chang, S. X. (2025). Biochar Affects Greenhouse Gas Emissions from Urban Forestry Waste. Land, 14(8), 1605. https://doi.org/10.3390/land14081605








