Modeling Plant Diversity Responses to Fire Recurrence in Disjunct Amazonian Savannas
Abstract
1. Introduction
2. Materials and Methods
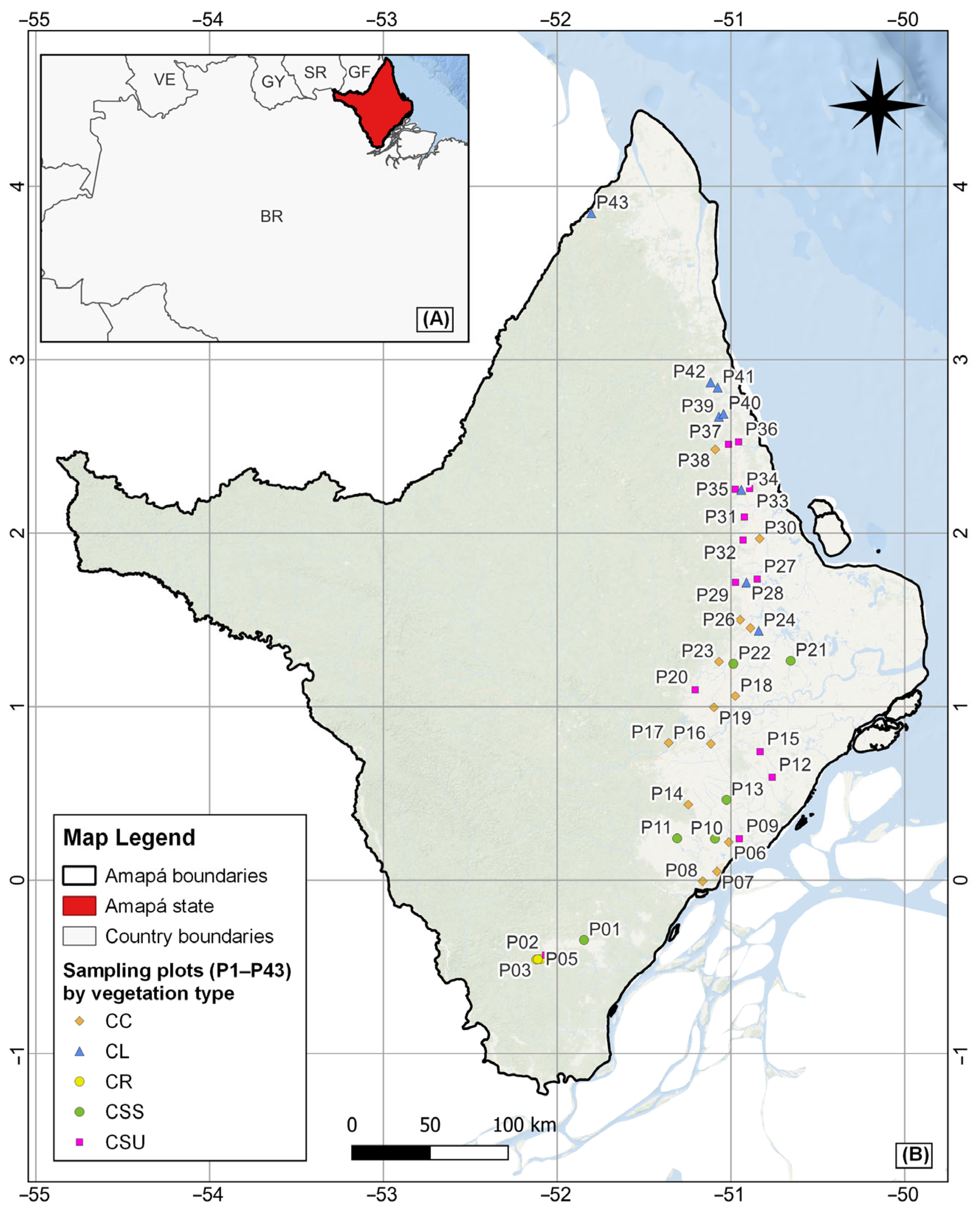
2.1. Vegetation Sampling and Functional Trait Data
2.2. Metrics of Taxonomic and Functional Diversity
2.3. Reconstruction of Fire History and Multiscale Landscape Analysis
2.4. Statistical Analysis
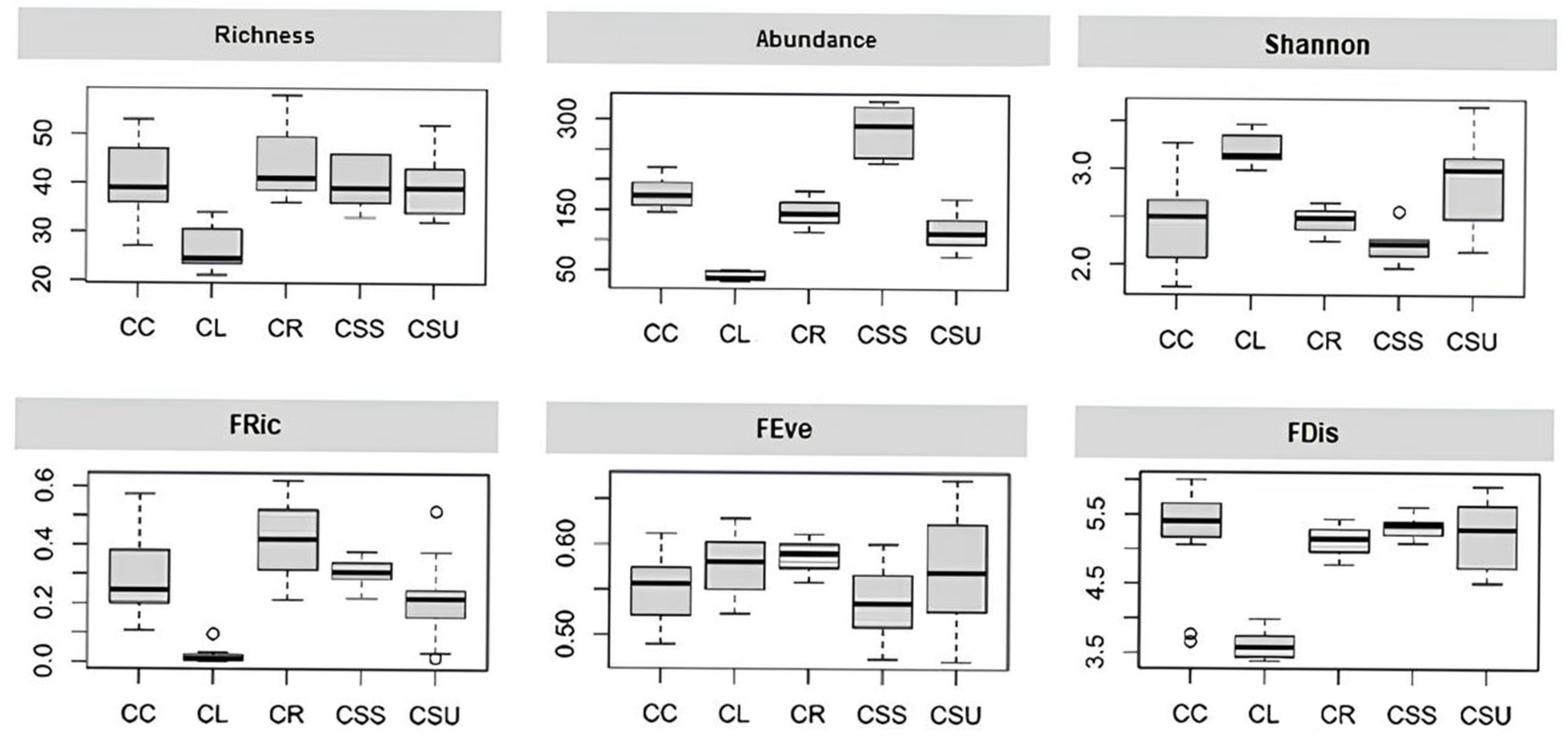
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLauchlan, K.K.; Higuera, P.E.; Miesel, J.; Rogers, B.M.; Schweitzer, J.; Shuman, J.K.; Tepley, A.J.; Varner, J.M.; Veblen, T.T.; Adalsteinsson, S.A.; et al. Fire as a fundamental ecological process: Research advances and frontiers. J. Ecol. 2020, 108, 2047–2069. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B.; Pausas, J.G. Fire as a key driver of Earth’s biodiversity. Biol. Rev. 2019, 94, 1983–2010. [Google Scholar] [CrossRef]
- Santos, F.; Bailey, J.K.; Schweitzer, J.A. The eco-evolutionary role of fire in shaping terrestrial ecosystems. Funct. Ecol. 2023, 37, 2090–2095. [Google Scholar] [CrossRef]
- Topp, E.N.; Tscharntke, T.; Loos, J. Fire and landscape context shape plant and butterfly diversity in a South African shrubland. Divers. Distrib. 2022, 28, 357–371. [Google Scholar] [CrossRef]
- Uys, R.G.; Bond, W.J.; Everson, T.M. The effect of different fire regimes on plant diversity in southern African grasslands. Biol. Conserv. 2004, 118, 489–499. [Google Scholar] [CrossRef]
- Ferreira, P.M.A.; Ely, C.V.; Beal-Neves, M. Different post-fire stages encompass different plant community compositions in fire-prone grasslands from Southern Brazil. Flora Morphol. Distrib. Funct. Ecol. Plants 2021, 285, 151937. [Google Scholar] [CrossRef]
- Brewer, J.S. Mechanisms of fire-maintained plant species diversity in species-rich wet pine savannas. Ecosphere 2023, 14, e4387. [Google Scholar] [CrossRef]
- Machida, W.S.; Gomes, L.; Moser, P.; Castro, I.B.; Miranda, S.C.; da Silva-Júnior, M.C.; Bustamante, M.M.C. Long term post-fire recovery of woody plants in savannas of central Brazil. For. Ecol. Manag. 2021, 493, 119255. [Google Scholar] [CrossRef]
- Das Chagas, D.B.; Pelicice, F.M. Response of vegetation to fire disturbance: Short-term dynamics in two savanna physiognomies. Community Ecol. 2018, 19, 211–222. [Google Scholar] [CrossRef]
- Joubert, D.F.; Stolter, C.; Krewenka, K.M.; Amputu, N.U.; Nghalipo, E.; Thompson, S.; Schütte, K.; Kruspe, M.; Throop, H.; du Preez, P.; et al. Impacts of fire history in a semi-arid woodland savanna. Biodivers. Ecol. 2018, 6, 207–218. [Google Scholar] [CrossRef]
- Lebbink, G.; Fensham, R.; Cowley, R. Vegetation responses to fire history and soil properties in grazed semi-arid tropical savanna. Rangel. J. 2018, 40, 271–285. [Google Scholar] [CrossRef]
- Furley, P. Tropical savannas: Biomass, plant ecology, and the role of fire and soil on vegetation. Prog. Phys. Geogr. 2010, 34, 563–585. [Google Scholar] [CrossRef]
- Teixeira, J.; Souza, L.; Le Stradic, S.; Fidelis, A. Fire promotes functional plant diversity and modifies soil carbon dynamics in tropical savanna. Sci. Total Environ. 2022, 812, 152317. [Google Scholar] [CrossRef] [PubMed]
- Giroldo, A.B.; Scariot, A.; Ferreira, J.B.; Moser, P.; Lima, I.L.P.; Hoffmann, W.A. An enemy’s enemy is an ally: Competitive indirect interactions mediate coexistence of trees, grasses, and subshrubs in neotropical savanna. Biotropica 2025, 57, e13399. [Google Scholar] [CrossRef]
- Abreu, R.C.R.; Durigan, G.; Melo, A.C.G.; Pilon, N.A.L.; Hoffmann, W.A. Facilitation by isolated trees triggers woody encroachment and a biome shift at the savanna–forest transition. J. Appl. Ecol. 2021, 58, 2650–2660. [Google Scholar] [CrossRef]
- Cruz Júnior, P.F.D.; Pinheiro, L.F.S.; Rossatto, D.R.; Kolb, R.M. Woody encroachment and leaf functional traits of ground-layer savanna species. Flora Morphol. Distrib. Funct. Ecol. Plants 2025, 326, 152709. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Zirondi, H.L.; Fidelis, A. Fire frequency affects fire behavior in open savannas of the Cerrado. For. Ecol. Manag. 2021, 482, 118850. [Google Scholar] [CrossRef]
- Zizka, A.; Govender, N.; Higgins, S.I. How to tell a shrub from a tree: A life-history perspective from a South African savanna. Austral Ecol. 2014, 39, 767–778. [Google Scholar] [CrossRef]
- Bond, W.J. Ancient grasslands at risk. Science 2016, 351, 120–122. [Google Scholar] [CrossRef]
- Stevens, N.; Lehmann, C.E.R.; Murphy, B.P.; Durigan, G. Savanna woody encroachment is widespread across three continents. Glob. Change Biol. 2017, 23, 235–244. [Google Scholar] [CrossRef]
- Passos, F.B.; Marimon, B.S.; Phillips, O.L.; Morandi, P.S.; das Neves, E.C.; Elias, F.; Reis, S.M.; de Oliveira, B.; Feldpausch, T.R.; Júnior, B.H.M. Savanna turning into forest: Concerted vegetation change at the ecotone between the Amazon and “Cerrado” biomes. Rev. Bras. Bot. 2018, 41, 611–619. [Google Scholar] [CrossRef]
- Cianciaruso, M.V.; Auŕlio Da Silva, I.; Batalha, M.A. Aboveground biomass of functional groups in the ground layer of savannas under different fire frequencies. Aust. J. Bot. 2010, 58, 169–174. [Google Scholar] [CrossRef]
- Chiminazzo, M.A.; Bombo, A.B.; Charles-Dominique, T.; Fidelis, A. To protect or to hide: Why not both? An investigation of fire-related strategies in Cerrado woody species. Flora Morphol. Distrib. Funct. Ecol. Plants 2023, 306, 152350. [Google Scholar] [CrossRef]
- Pilon, N.A.L.; Cava, M.G.B.; Hoffmann, W.A.; Abreu, R.C.R.; Fidelis, A.; Durigan, G. The diversity of post-fire regeneration strategies in the cerrado ground layer. J. Ecol. 2021, 109, 154–166. [Google Scholar] [CrossRef]
- Bombo, A.B.; Appezzato-da-Glória, B.; Martins, R.; Fidelis, A. Belowground organs and bud bank: Insights on morphoanatomical functional traits related to fire. Folia Geobot. 2024, 58, 259–273. [Google Scholar] [CrossRef]
- Le Stradic, S.; Roumet, C.; Durigan, G.; Cancian, L.; Fidelis, A. Variation in biomass allocation and root functional parameters in response to fire history in Brazilian savannas. J. Ecol. 2021, 109, 4143–4157. [Google Scholar] [CrossRef]
- Zupo, T.; Daibes, L.F.; Pausas, J.G.; Fidelis, A. Post-fire regeneration strategies in a frequently burned Cerrado community. J. Veg. Sci. 2021, 32, e12968. [Google Scholar] [CrossRef]
- Fidelis, A.; Zirondi, H.L.; Rossatto, D.R.; Zanzarini, V. Fire stimulates grass flowering in the Cerrado independent of season. J. Veg. Sci. 2022, 33, e13125. [Google Scholar] [CrossRef]
- Fidelis, A.; Zirondi, H.L. And after fire, the Cerrado flowers: A review of post-fire flowering in a tropical savanna. Flora Morphol. Distrib. Funct. Ecol. Plants 2021, 280, 151849. [Google Scholar] [CrossRef]
- Dairel, M.; Fidelis, A. Fire stimulates seedling recruitment from the seed bank in the Cerrado. J. Veg. Sci. 2024, 35, e13268. [Google Scholar] [CrossRef]
- Barbosa, L.C.; Viana, P.L.; Teodoro, G.S.; Caldeira, C.F.; Ramos, S.J.; Gastauer, M. A wildfire in an Amazonian canga community maintained important ecosystem properties. Int. J. Wildland Fire 2020, 29, 943–949. [Google Scholar] [CrossRef]
- de Oliveira Buzatti, R.S.; Pfeilsticker, T.R.; de Magalhães, R.F.; Bueno, M.L.; Lemos-Filho, J.P.; Lovato, M.B. Genetic and historical colonization analyses of an endemic savanna tree, qualea grandiflora, reveal ancient connections between amazonian savannas and cerrado core. Front. Plant Sci. 2018, 9, 981. [Google Scholar] [CrossRef]
- Beltran, J.; de Toledo, M.B.; Palace, M.; Dibb, J.; Bush, M.B. Holocene histories of biome stability in northern Amazonian savannas. Holocene 2024, 34, 283–296. [Google Scholar] [CrossRef]
- de Fátima Rossetti, D.; Gribel, R.; Cohen, M.C.L.; de Morisson Valeriano, M.; Tatumi, S.H.; Yee, M. The role of Late Pleistocene-Holocene tectono-sedimentary history on the origin of patches of savanna vegetation in the middle Madeira River, southwest of the Amazonian lowlands. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 526, 136–156. [Google Scholar] [CrossRef]
- Hoorn, C.; Wesselingh, F.P.; Ter Steege, H.; Bermudez, M.A.; Mora, A.; Sevink, J.; Sanmartín, I.; Sanchez-Meseguer, A.; Anderson, C.L.; Figueiredo, J.P.; et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 2010, 330, 927–931. [Google Scholar] [CrossRef]
- Bueno, M.L.; Pennington, R.T.; Dexter, K.G.; Kamino, L.H.Y.; Pontara, V.; Neves, D.M.; Ratter, J.A.; de Oliveira-Filho, A.T. Effects of Quaternary climatic fluctuations on the distribution of Neotropical savanna tree species. Ecography 2017, 40, 403–414. [Google Scholar] [CrossRef]
- de Moura Araújo, M.A.; da Rocha, A.E.S.; de Souza Miranda, I.; Barbosa, R.I. Hydro-edaphic conditions defining richness and species composition in savanna areas of the northern Brazilian Amazonia. Biodivers. Data J. 2017, 5, e13829. [Google Scholar] [CrossRef]
- Devecchi, M.F.; Lovo, J.; Moro, M.F.; Andrino, C.O.; Barbosa-Silva, R.G.; Viana, P.L.; Giulietti, A.M.; Antar, G.; Watanabe, M.T.C.; Zappi, D.C. Beyond forests in the Amazon: Biogeography and floristic relationships of the Amazonian savannas. Bot. J. Linn. Soc. 2021, 193, 478–503. [Google Scholar] [CrossRef]
- da Costa-Coutinho, J.M.; da Costa-Neto, S.V.; Jardim, M.A.G. Floristic and structure of the woody species in five savannas in the state of Pará, Brazil. Rev. Bras. Geogr. Fis. 2021, 14, 215–228. [Google Scholar] [CrossRef]
- Rull, V.; Montoya, E.; Vegas-Vilarrúbia, T.; Ballesteros, T. New insights on palaeofires and savannisation in northern SouthAmerica. Quat. Sci. Rev. 2015, 122, 158–165. [Google Scholar] [CrossRef]
- Iriarte, J.; Power, M.J.; Rostain, S.; Mayle, F.E.; Jones, H.; Watling, J.; Whitney, B.S.; McKey, D.B. Fire-free land use in pre-1492 Amazonian savannas. Proc. Natl. Acad. Sci. USA 2012, 109, 6473–6478. [Google Scholar] [CrossRef]
- Ribeiro, J.F.; Walter, B.M.T. As principais fitofisionomias do bioma Cerrado. In Cerrado: Ecologia e Flora; Embrapa Cerrado. In Cerrado: Ecologia e Flora; Embrapa Cerrados: Flantina, Brazil, 2008. [Google Scholar]
- Costa Neto, S.V. Fitofisionomias e Florística de Savanas no Amapá. 2014. Available online: https://oasisbr.ibict.br/vufind/Record/BRCRIS_bdee249654b272c8becc23d29e0b423f (accessed on 25 May 2020).
- Nogueira, E.M.; Fearnside, P.M.; Nelson, B.W.; França, M.B. Wood density in forests of Brazil’s “arc of deforestation”: Implications for biomass and flux of carbon from land-use change in Amazonia. For. Ecol. Manag. 2007, 248, 119–135. [Google Scholar] [CrossRef]
- Nogueira, E.M.; Nelson, B.W.; Fearnside, P.M. Wood density in dense forest in central Amazonia, Brazil. For. Ecol. Manag. 2005, 208, 261–286. [Google Scholar] [CrossRef]
- Ruschel, A.R.; Coelho, F.d.A.; Carneiro, F.D.S.; dos Santos, J.C.; Pinheiro, K.A.O.; D’arace, L.M.B.; de Freitas, L.J.M. Densidade Da Madeira de Espécies Florestais de Quatro Áreas Experimentais Da Amazônia Oriental Brasileira; Atena: São Paulo, Brazil, 2020. [Google Scholar] [CrossRef]
- Amaral, A.G.; Munhoz, C.B.R.; Eugênio, C.U.O.; Felfili, J.M. Vascular flora in dry-shrub and wet grassland Cerrado seven years after a fire, Federal District, Brazil. Check List 2013, 9, 487–503. [Google Scholar] [CrossRef]
- Fidelis, A.; Rosalem, P.; Zanzarini, V.; Camargos, L.S.; Martins, A.R. From ashes to flowers: A savanna sedge initiates flowers 24 h after fire. Ecology 2019, 100, e02648. [Google Scholar] [CrossRef] [PubMed]
- Dutra, V.F.; Vieira, M.F.; Garcia, F.C.P.; Lima, H.C.d. Fenologia reprodutiva, síndromes de polinização e dispersão em espécies de Leguminosae dos campos rupestres do Parque Estadual do Itacolomi, Minas Gerais, Brasil. Rodriguésia 2009, 60, 371–387. [Google Scholar] [CrossRef]
- da Silva Júnior, M.C. 100 Árvores Do Cerrado: Cerrado Sentido Restrito. Guia de Campo; Seed Network of the Cerrado: Brasilia, Brazil, 2012. [Google Scholar]
- Kinoshita, L.S.; Torres, R.B.; Forni-Martins, E.R.; Spinelli, T.; Yu, J.A.; Constâncio, S.S. Composição florística e síndromes de polinização e de dispersão da mata do Sítio São Francisco, Campinas, SP, Brasil. Acta Bot. Bras. 2006, 20, 313–327. [Google Scholar] [CrossRef]
- Tannus, J.L.S.; Assis, M.A. Composição de espécies vasculares de campo sujo e campo úmido em área de cerrado, Itirapina—SP, Brasil. Rev. Bras. Botânica 2004, 27, 489–506. [Google Scholar] [CrossRef]
- Barbosa, R.I.; Fearnside, P.M. Wood density of trees in open savannas of the Brazilian Amazon. For. Ecol. Manag. 2004, 199, 115–123. [Google Scholar] [CrossRef]
- Cheek, M.; da Ribeiro, J.E.L.; Hopkins, M.J.G.; Vicentini, A.; Sothers, C.A.; da, S. Costa, M.A.; de Brito, J.M.; de Souza, M.A.D.; Martins, L.H.P.; Lohmann, L.G.; et al. Flora da Reserva Ducke. Guia de identificacao das plantas vasculares de uma floresta de terra-firme na Amazonia Central. Kew Bull. 2002, 57, 238. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas Do Brasil; The Plantarum Institute: São Paulo, Brazil, 1992. [Google Scholar]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef] [PubMed]
- Laliberte, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Oksanen, J.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M. Vegan: Community Ecology Package; R Package Version 2.5-7; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=vegan (accessed on 19 November 2021).
- Arruda, V.L.S.; Piontekowski, V.J.; Alencar, A.; Pereira, R.S.; Matricardi, E.A.T. An alternative approach for mapping burn scars using Landsat imagery, Google Earth Engine, and Deep Learning in the Brazilian Savanna. Remote Sens. Appl. Soc. Environ. 2021, 22, 100472. [Google Scholar] [CrossRef]
- de Santana, M.M.M.; de Vasconcelos, R.N.; Mariano-Neto, E. Fire propensity in Amazon savannas and rainforest and effects under future climate change. Int. J. Wildland Fire 2022, 32, 149–163. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Taylor & Francis: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2019; Volume 53. [Google Scholar]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. R Core Team. nlme: Linear and Nonlinear Mixed Effects Models, Version 3.1-149; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://CRAN.R-project.org/package=nlme (accessed on 19 November 2021).
- Burnham, K.; Anderson, D. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Connell, J.H. Diversity in tropical rain forests and coral reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- He, T.; Lamont, B.B. Baptism by fire: The pivotal role of ancient conflagrations in evolution of the Earth’s flora. Natl. Sci. Rev. 2018, 5, 237–254. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Geiger, E.L.; Gotsch, S.G.; Rossatto, D.R.; Silva, L.C.R.; Lau, O.L.; Haridasan, M.; Franco, A.C.; Lloret, F. Ecological thresholds at the savanna-forest boundary: How plant traits, resources and fire govern the distribution of tropical biomes. Ecol. Lett. 2012, 15, 759–768. [Google Scholar] [CrossRef]
- Cianciaruso, M.V.; Silva, I.A.; Batalha, M.A.; Gaston, K.J.; Petchey, O.L. The influence of fire on phylogenetic and functional structure of woody savannas: Moving from species to individuals. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 205–216. [Google Scholar] [CrossRef]
- Guidoni-Martins, K.G.; Maracahipes, L.; Melo, A.S.; Cianciaruso, M.V. Annual fires reduce local species richness but do not homogenize the composition of savanna woody species. Flora Morphol. Distrib. Funct. Ecol. Plants 2021, 281, 151868. [Google Scholar] [CrossRef]
- Sarmiento, G. Biodiversity and Water Relations in Tropical Savannas. In Biodiversity and Savanna Ecosystem Processes; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar] [CrossRef]
- de Paula Loiola, P.; Cianciaruso, M.V.; Silva, I.A.; Batalha, M.A. Functional diversity of herbaceous species under different fire frequencies in Brazilian savannas. Flora Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 674–681. [Google Scholar] [CrossRef]
- Osborne, C.P.; Charles-Dominique, T.; Stevens, N.; Bond, W.J.; Midgley, G.; Lehmann, C.E.R. Human impacts in African savannas are mediated by plant functional traits. New Phytol. 2018, 220, 10–24. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- de Souza, G.F.; Ferreira, M.C.; Munhoz, C.B.R. Decrease in species richness and diversity, and shrub encroachment in Cerrado grasslands: A 20 years study. Appl. Veg. Sci. 2022, 25, e12668. [Google Scholar] [CrossRef]
- De Carvalho, W.D.; Mustin, K. The highly threatened and little known Amazonian savannahs. Nat. Ecol. Evol. 2017, 1, 100. [Google Scholar] [CrossRef]
- de Santana, M.M.M.; de Vasconcelos, R.N.; Mariano Neto, E.; da Franca Rocha, W.d.J.S. Machine Learning Model Reveals Land Use and Climate’s Role in Amazon Wildfires: Present and Future Scenarios. Fire 2024, 7, 338. [Google Scholar] [CrossRef]
| Feature | Category | Functional Importance |
|---|---|---|
| Raunkiaer’s Life Form | Phanerophytes; Chamaephytes; Hemicryptophytes; Geophyte; or Therophyte | Competitive strength |
| Height 1 | Low, Medium, or High | Tolerance or avoidance of disturbances and competitive vigor |
| Stem circumference 2 | Low, Medium, or High | Competitive vigor, survivability after fire |
| Herbaceous cover 3 | Low, Medium, or High | Competitive strength |
| Wood density 4 | Light, Medium, or Heavy | Structural strength, resistance against physical damage |
| Presence of exudates | Presence or Absence of volatile oils, waxes, and resins | Flammability |
| Bark characteristics | Presence or Absence of suberose bark | Mechanical protection |
| Deciduousness | Deciduous, Semideciduous, or Evergreen | Resistance to environmental disturbance |
| Pollination | Anemophily or Zoophily | Traits of regeneration linked to the ability to (re)colonize |
| Dispersal | Anemochory, Zoochory, or Autochory | Traits of regeneration linked to the ability to (re)colonize |
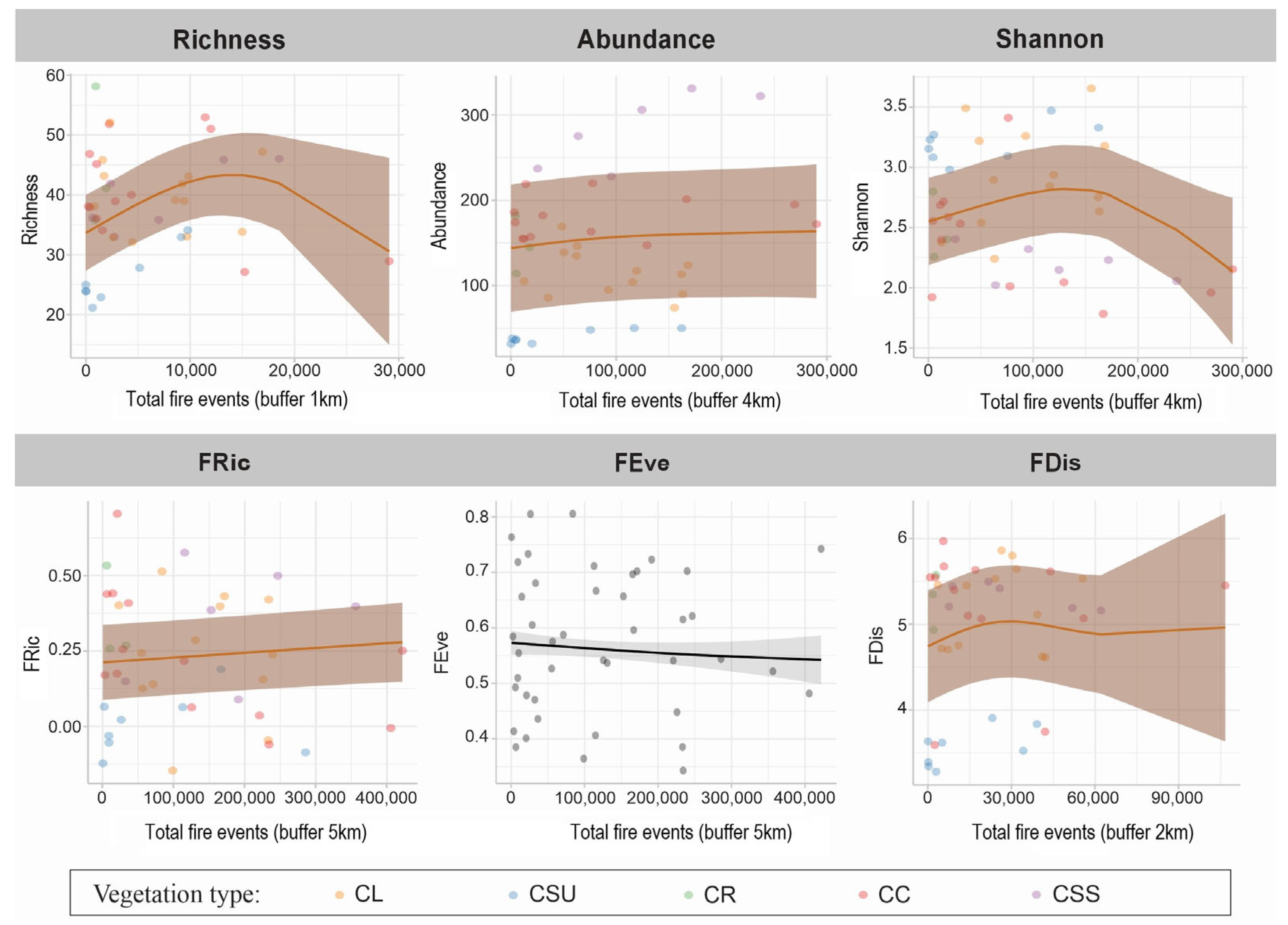
| Response Variable | Scale (Km) | Best Model | D (%) | edf | R2 adj | p-Smoother (Fire) | Pr(>|t|) Intercept |
|---|---|---|---|---|---|---|---|
| Richness | 1 | Fixed effect, combined with a random term for phytophysiognomy, and modeled variance for phytophysiognomy | 3.93 | 2.4 | −0.707 | 1.04 × 10−5 | 1.13 × 10−14 |
| Abundance | 4 | Fixed effect, combined with a random term for phytophysiognomy, and modeled variance for phytophysiognomy | 7.27 | 1.7 | −17.6 | 3.55 × 10−5 | 0.00033 |
| Shannon | 4 | Fixed effect, combined with a random term for phytophysiognomy, and modeled variance for phytophysiognomy | 14.7 | 2.5 | −0.91 | 0.00389 | <2 × 10−16 |
| FRic | 5 | Fixed effect, combined with a random term for phytophysiognomy, and modeled variance for phytophysiognomy | 0.67 | 1 | −2.52 | 0.0346 | 0.00068 |
| FEve | 5 | Fixed effect, combined with a random term for phytophysiognomy, and modeled variance for phytophysiognomy | 4.18 | 1.1 | 0.01 | 0.288 | <2 × 10−16 |
| FDis | 2 | Fixed effect, combined with a random term for phytophysiognomy, and modeled variance for phytophysiognomy, with a spatial correlation structure term | 3.00 | 2.3 | −0.56 | <2 × 10−16 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, M.M.M.d.; Vasconcelos, R.N.d.; Neto, S.V.d.C.; Neto, E.M.; Rocha, W.d.J.S.d.F. Modeling Plant Diversity Responses to Fire Recurrence in Disjunct Amazonian Savannas. Land 2025, 14, 1455. https://doi.org/10.3390/land14071455
Santana MMMd, Vasconcelos RNd, Neto SVdC, Neto EM, Rocha WdJSdF. Modeling Plant Diversity Responses to Fire Recurrence in Disjunct Amazonian Savannas. Land. 2025; 14(7):1455. https://doi.org/10.3390/land14071455
Chicago/Turabian StyleSantana, Mariana Martins Medeiros de, Rodrigo Nogueira de Vasconcelos, Salustiano Vilar da Costa Neto, Eduardo Mariano Neto, and Washington de Jesus Sant’Anna da Franca Rocha. 2025. "Modeling Plant Diversity Responses to Fire Recurrence in Disjunct Amazonian Savannas" Land 14, no. 7: 1455. https://doi.org/10.3390/land14071455
APA StyleSantana, M. M. M. d., Vasconcelos, R. N. d., Neto, S. V. d. C., Neto, E. M., & Rocha, W. d. J. S. d. F. (2025). Modeling Plant Diversity Responses to Fire Recurrence in Disjunct Amazonian Savannas. Land, 14(7), 1455. https://doi.org/10.3390/land14071455










