Improving Soil Health Using Date Palm Residues in Southern Tunisian Olive Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental and Sampling Design
2.3. Analytical Methods and Incubation Under Different Moisture Conditions
2.3.1. Soil Chemical and Physico-Chemical Properties
2.3.2. Soil Basal Respiration and Soil N Mineralisation Capacity
2.3.3. Microbial Biomass
2.3.4. Soil Enzyme Activities
2.3.5. Community-Level Physiological Profiling (CLPP)
2.3.6. Statistical Analysis
3. Results
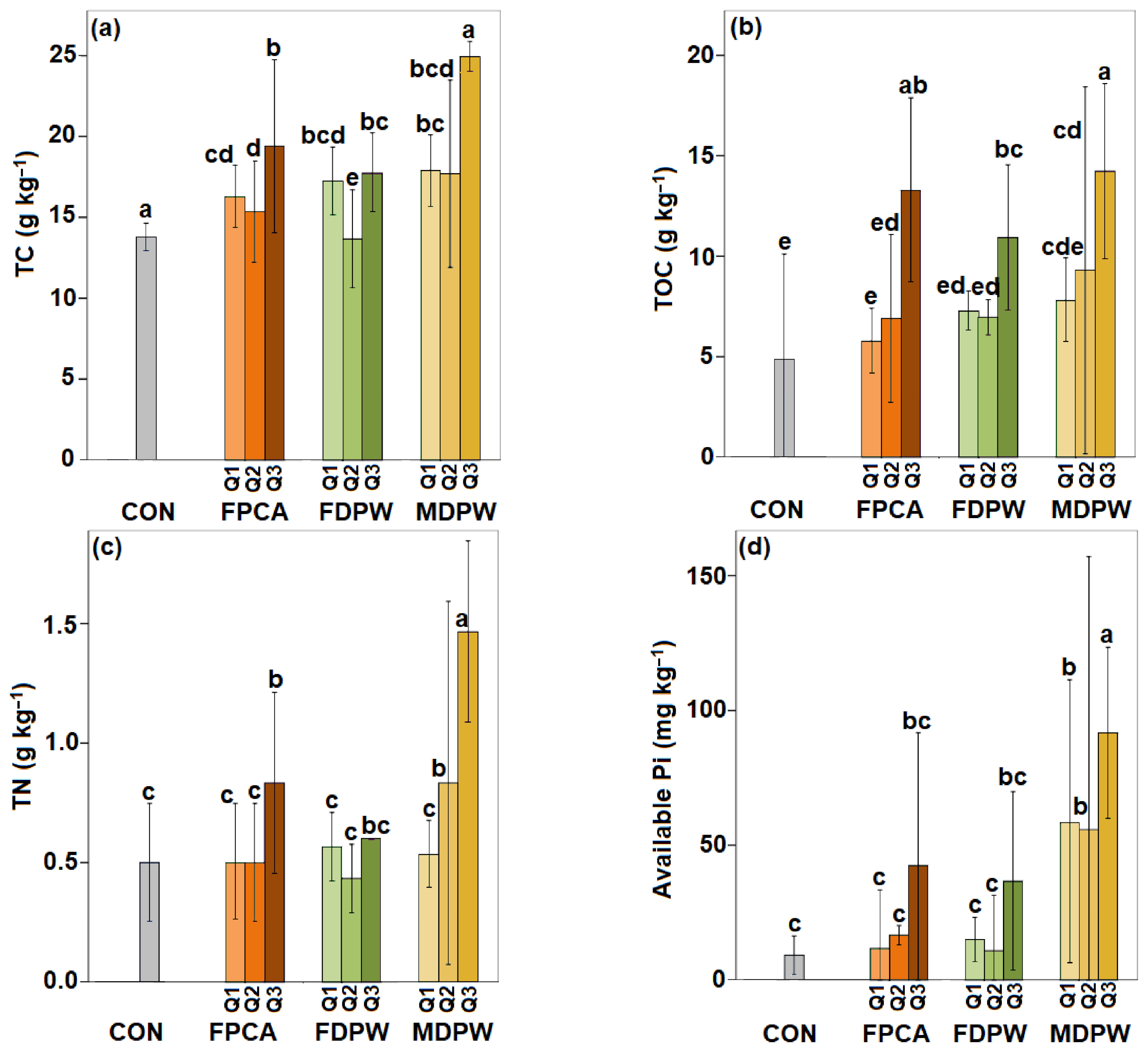
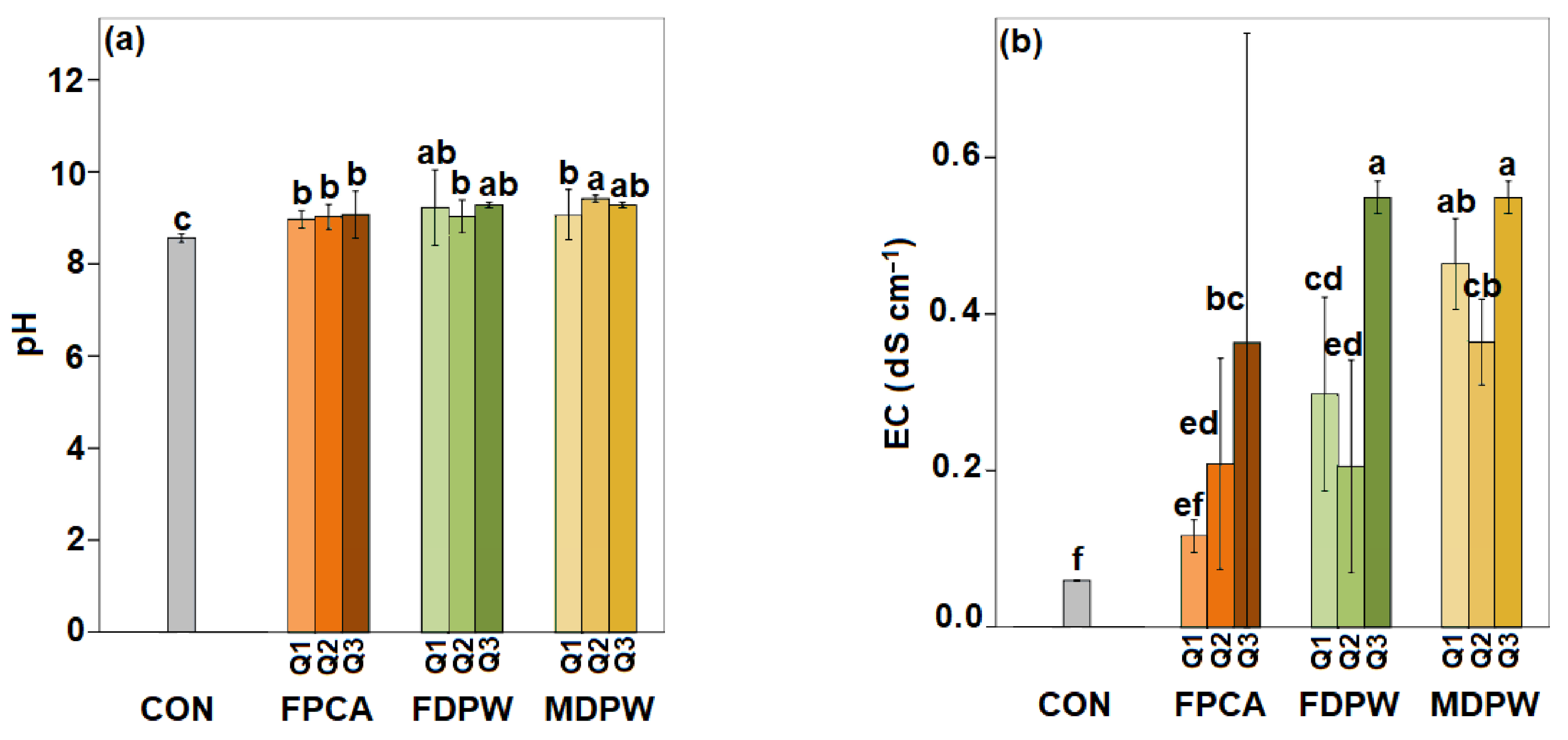
3.1. Effects of Organic Amendments on the Main Physico-Chemical Properties of the Soil
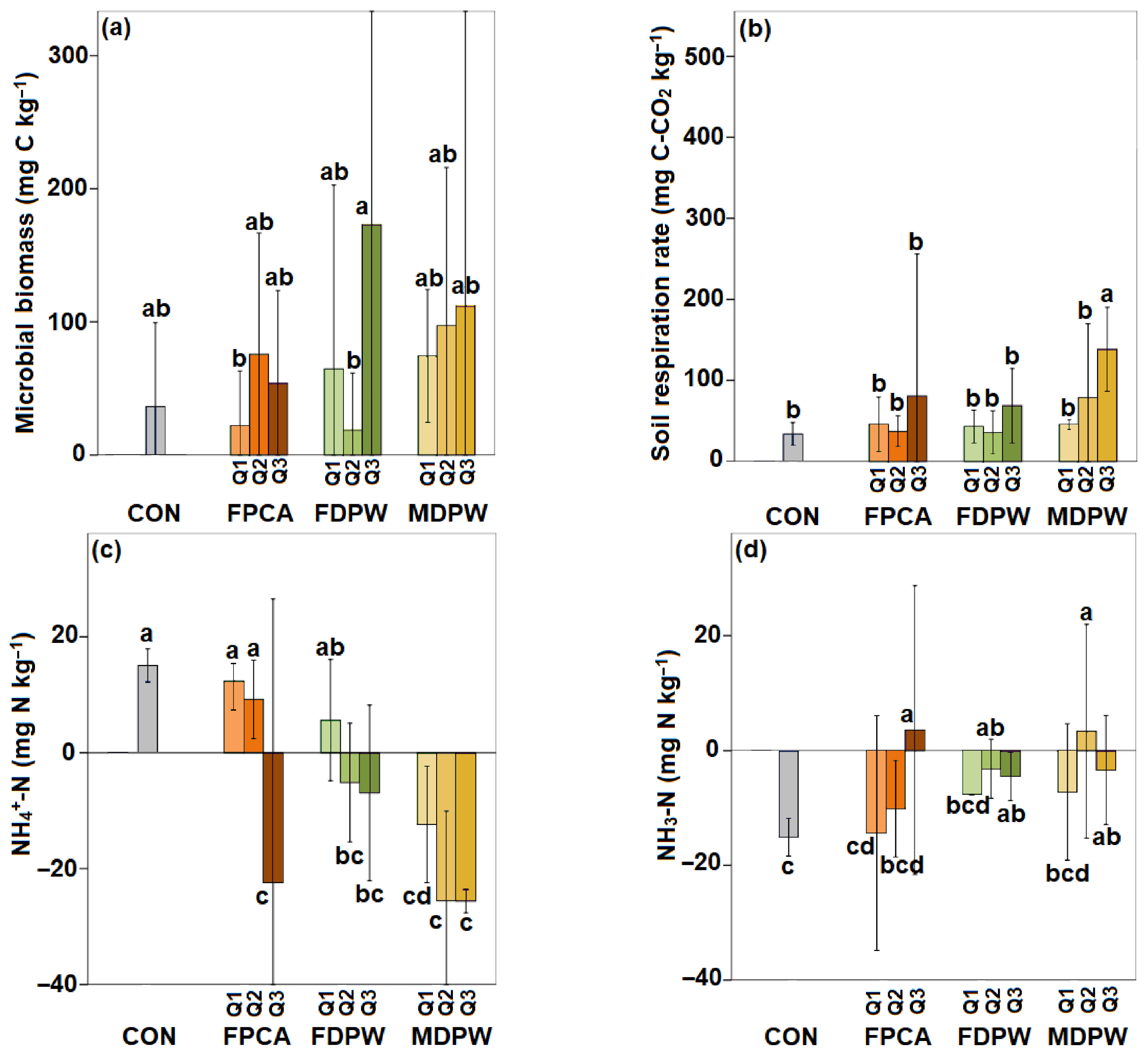
3.2. Effect of the Organic Amendments on Microbial Biomass C
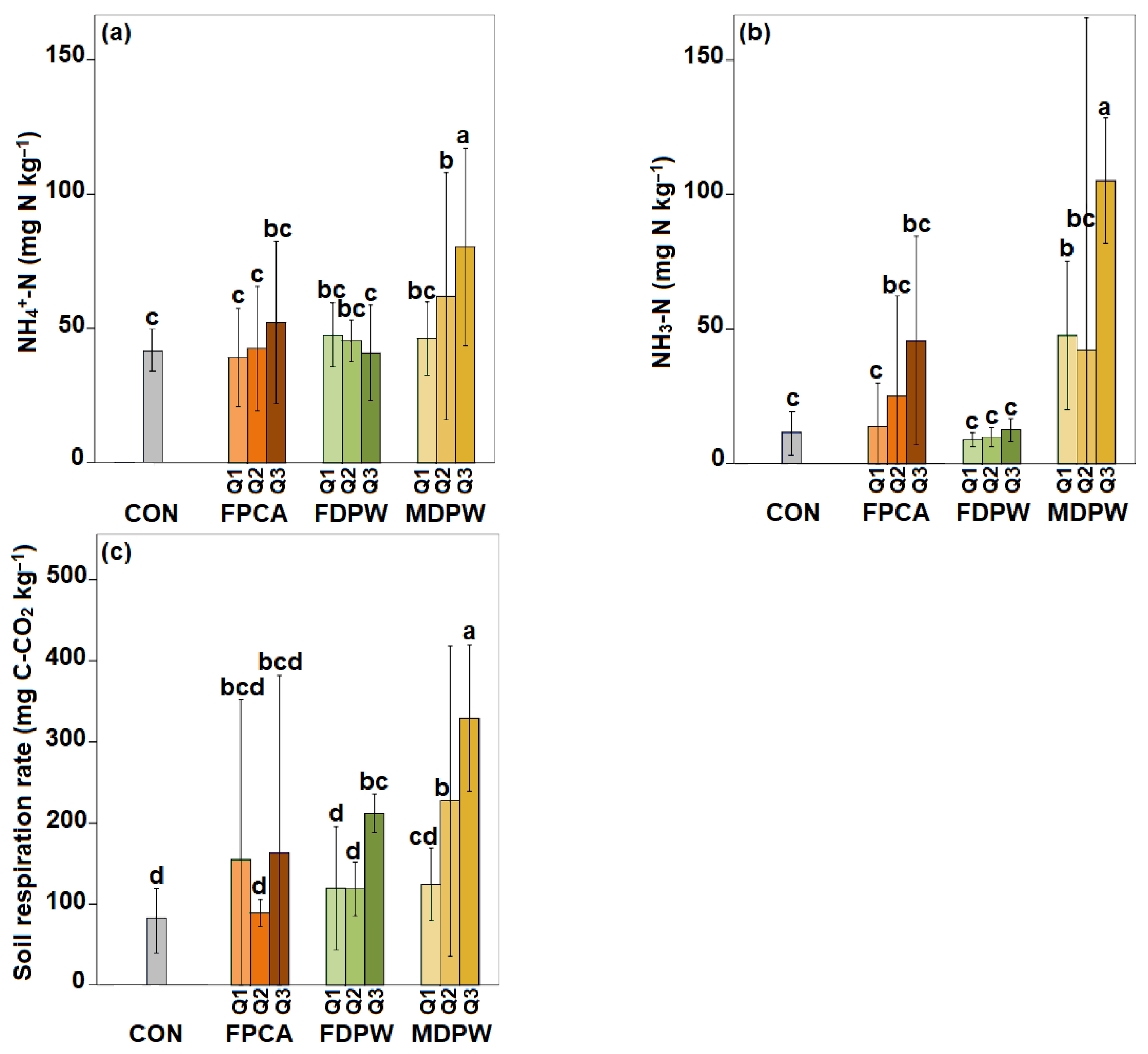
3.3. Effect of the Organic Amendments on Soil Basal Respiration and Nitrogen Mineralisation Capacity
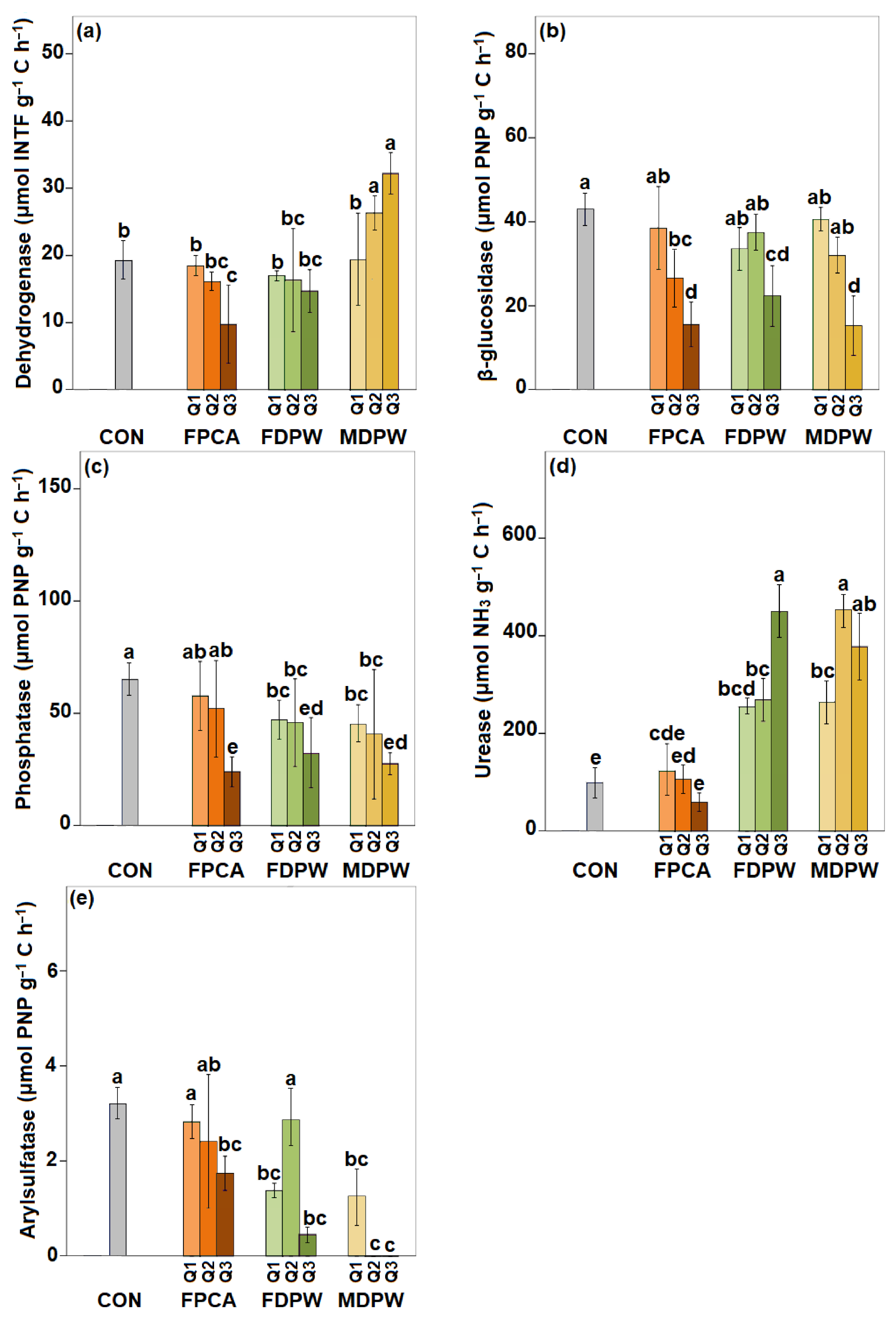
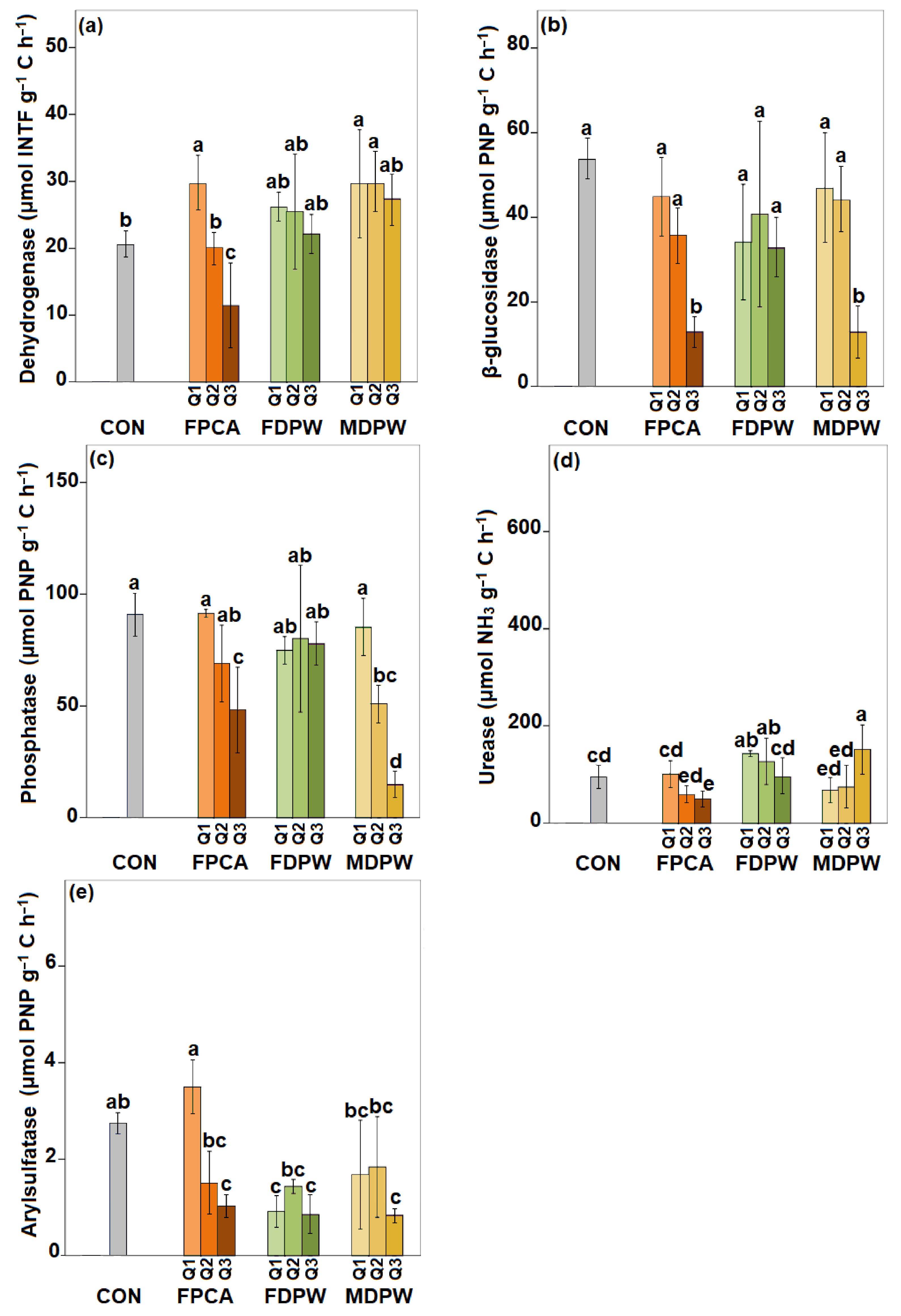
3.4. Effect of the Organic Amendments on Soil Enzyme Activities
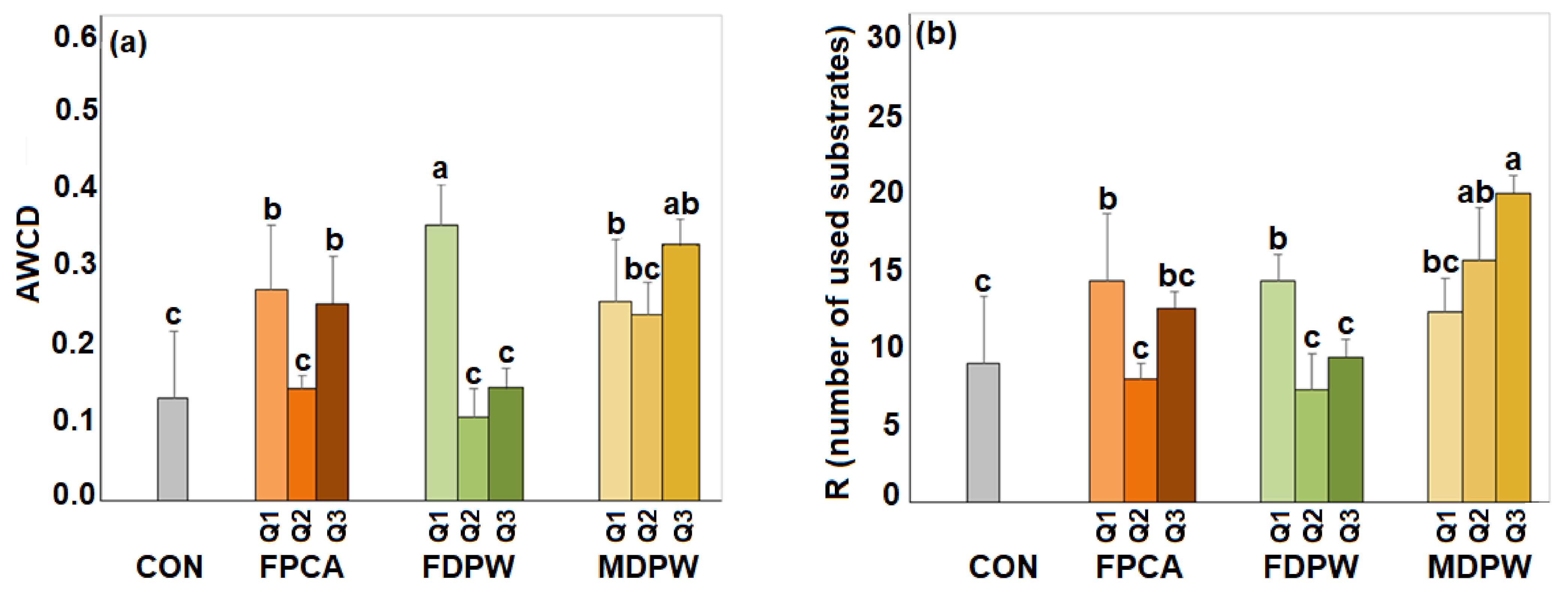
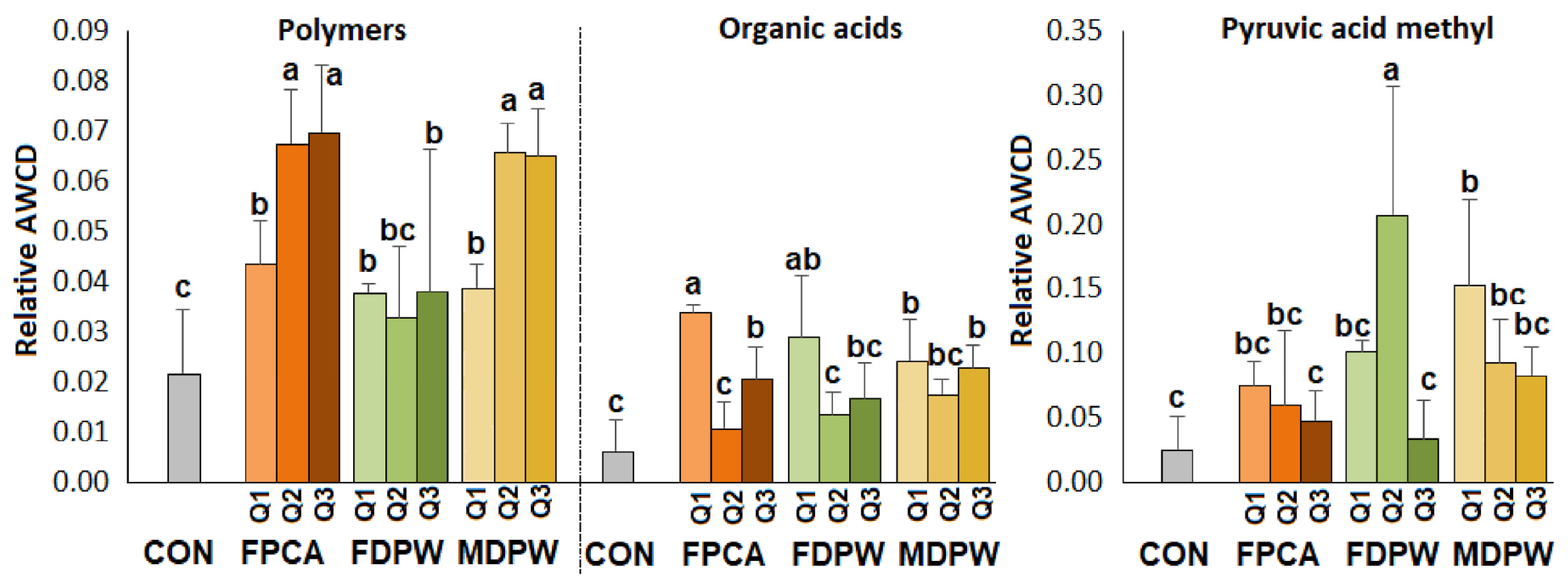
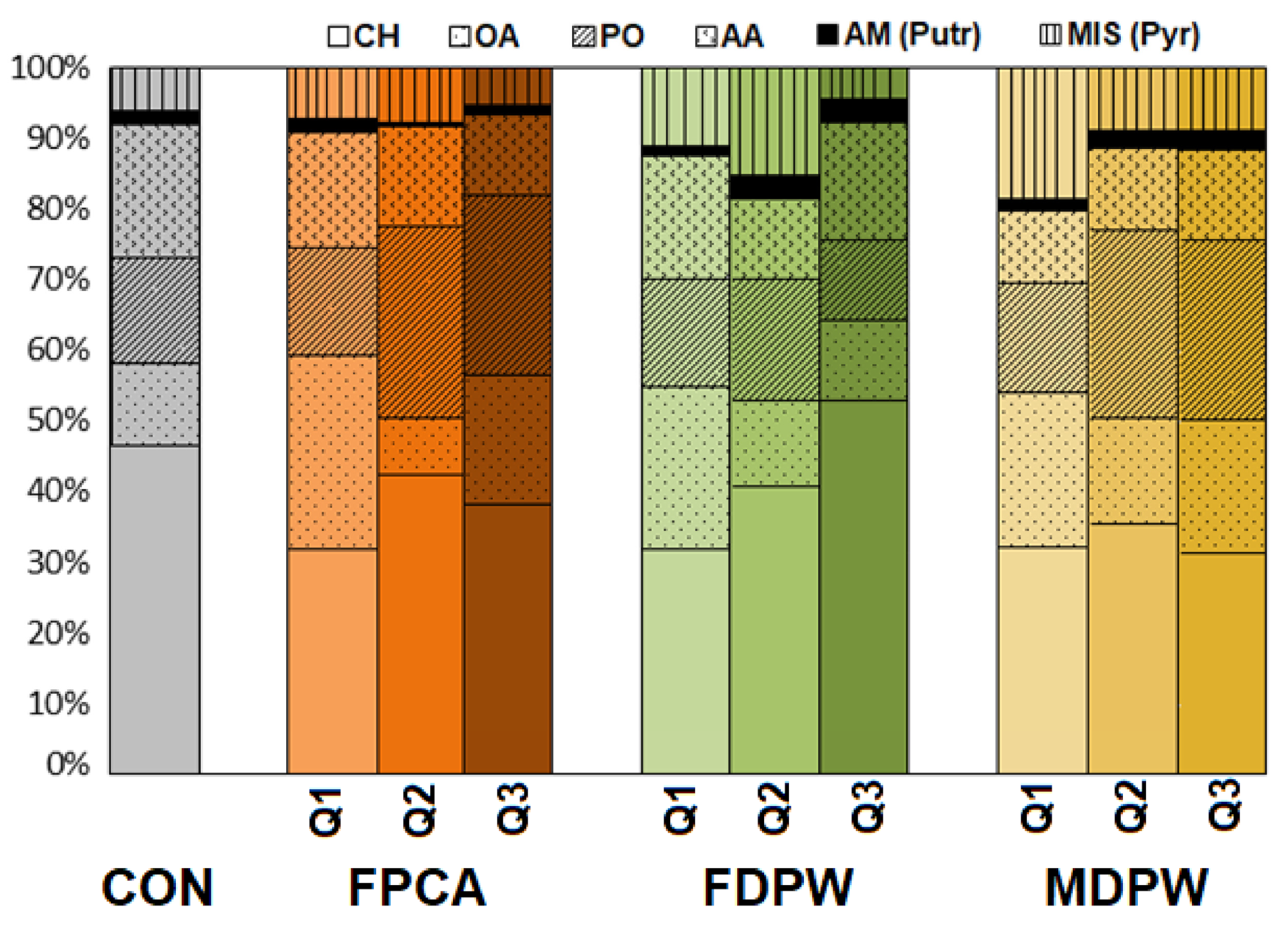
3.5. Effect of the Organic Amendments on Community Level Physiological Profile (CLPP)
3.6. Effect of the Organic Amendments on Soil Basal Respiration and Nitrogen Mineralisation Capacity in Moist Soils
3.7. Effect of the Organic Amendments on Soil Enzyme Activities in Moist Soils
4. Discussion
4.1. Effects of Organic Amendments on the Main Physico-Chemical Properties of the Soil
4.2. Effects of Organic Amendments on the Biological and Biochemical Properties of Dry Soil
4.3. Effects of Organic Amendments on the Biological and Biochemical Properties of Re-Wetted Soil
4.4. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Yu, H.; Dai, A.; Wei, Y.; Kang, L. Drylands Face Potential Threat under 2 °C Global Warming Target. Nat. Clim. Change 2017, 7, 417–422. [Google Scholar] [CrossRef]
- El Belghiti, F.-Z.; Benidire, L.; M’Barki, K.; Dounas, H.; Boularbah, A. Soil System Status, Threats, Utilization Pattern, and Sustainable Management Strategies in the Global South: Overview of the Agriculture Soils in Maghreb Countries. In Sustainable Soil Systems in Global South; Ogwu, M.C., Izah, S.C., Dessureault-Rompré, J., Gasparatos, D., Eds.; Springer: Singapore, 2024; pp. 689–718. [Google Scholar] [CrossRef]
- Brahim, N.; Ibrahim, H.; Hatira, A. Tunisian Soil Organic Carbon Stock–Spatial and Vertical Variation. Procedia Eng. 2014, 69, 1549–1555. [Google Scholar] [CrossRef]
- Yao, H.; Bowman, D.; Shi, W. Seasonal Variations of Soil Microbial Biomass and Activity in Warm-and Cool-Season Turfgrass Systems. Soil Biol. Biochem. 2011, 43, 1536–1543. [Google Scholar] [CrossRef]
- Bastida, F.; Moreno, J.L.; Hernandez, T.; García, C. Microbiological Activity in a Soil 15 Years after Its Devegetation. Soil Biol. Biochem. 2006, 38, 2503–2507. [Google Scholar] [CrossRef]
- Fterich, A.; Mahdhi, M.; Mars, M. Seasonal Changes of Microbiological Properties in Steppe Soils from Degraded Arid Area in Tunisia. Arid Land Res. Manag. 2014, 28, 49–58. [Google Scholar] [CrossRef]
- Madline, A.; Benidire, L.; Pereira, S.I.; Khalil, H.E.; Michalski, A.; Castro, P.M.; Charzyński, P.; Boularbah, A. Optimizing Biological and Physicochemical Properties of Acidic Mine Tailings through Combined Organo-Mineral Amendments and Topsoil Application. J. Soils Sediments 2025, 25, 609–624. [Google Scholar] [CrossRef]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological Practices for Sustainable Agriculture. A Review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef]
- Brahim, N.; Karbout, N.; Dhaouadi, L.; Bouajila, A. Global Landscape of Organic Carbon and Total Nitrogen in the Soils of Oasis Ecosystems in Southern Tunisia. Agronomy 2021, 11, 1903. [Google Scholar] [CrossRef]
- Dittrich, F.; Iserloh, T.; Treseler, C.-H.; Hüppi, R.; Ogan, S.; Seeger, M.; Thiele-Bruhn, S. Crop Diversification in Viticulture with Aromatic Plants: Effects of Intercropping on Grapevine Productivity in a Steep-Slope Vineyard in the Mosel Area, Germany. Agriculture 2021, 11, 95. [Google Scholar] [CrossRef]
- Sobeh, M.; Boularbah, A.; Yasri, A. Chemically Degraded Soil Rehabilitation Process Using Medicinal and Aromatic Plants. Environ. Sci. Pollut. Res. 2021, 28, 73–93. [Google Scholar] [CrossRef]
- Steenwerth, K.; Belina, K.M. Cover Crops Enhance Soil Organic Matter, Carbon Dynamics and Microbiological Function in a Vineyard Agroecosystem. Appl. Soil Ecol. 2008, 40, 359–369. [Google Scholar] [CrossRef]
- Liang, Z.; He, Z.; Zhou, X.; Powell, C.A.; Yang, Y.; He, L.M.; Stoffella, P.J. Impact of Mixed Land-Use Practices on the Microbial Water Quality in a Subtropical Coastal Watershed. Sci. Total Environ. 2013, 449, 426–433. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Tejada, M.; Moreno, J.L.; Hernández, M.T.; García, C. Soil Amendments with Organic Wastes Reduce the Toxicity of Nickel to Soil Enzyme Activities. Eur. J. Soil Biol. 2008, 44, 129–140. [Google Scholar] [CrossRef]
- Bastida, F.; Kandeler, E.; Hernández, T.; García, C. Long-Term Effect of Municipal Solid Waste Amendment on Microbial Abundance and Humus-Associated Enzyme Activities under Semiarid Conditions. Microb. Ecol. 2008, 55, 651–661. [Google Scholar] [CrossRef]
- Hammad, H.M.; Khaliq, A.; Abbas, F.; Farhad, W.; Fahad, S.; Aslam, M.; Shah, G.M.; Nasim, W.; Mubeen, M.; Bakhat, H.F. Comparative Effects of Organic and Inorganic Fertilizers on Soil Organic Carbon and Wheat Productivity under Arid Region. Commun. Soil Sci. Plant Anal. 2020, 51, 1406–1422. [Google Scholar] [CrossRef]
- Karbout, N.; Brahim, N.; Mlih, R.; Moussa, M.; Bousnina, H.; Weihermuller, L.; Bol, R. Bentonite Clay Combined with Organic Amendments to Enhance Soil Fertility in Oasis Agrosystem. Arab. J. Geosci. 2021, 14, 428. [Google Scholar] [CrossRef]
- Liu, M.; Hu, F.; Chen, X.; Huang, Q.; Jiao, J.; Zhang, B.; Li, H. Organic Amendments with Reduced Chemical Fertilizer Promote Soil Microbial Development and Nutrient Availability in a Subtropical Paddy Field: The Influence of Quantity, Type and Application Time of Organic Amendments. Appl. Soil Ecol. 2009, 42, 166–175. [Google Scholar] [CrossRef]
- Elfstrand, S. Impact of Green Manure on Soil Organisms: With Emphasis on Microbial Community Composition and Function. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2007. Available online: https://pub.epsilon.slu.se/1349/1/SE_thesis.pdf (accessed on 2 May 2025).
- Eid, E.M.; Shaltout, K.H.; Abdallah, S.M.; Galal, T.M.; El-Bebany, A.F.; Sewelam, N.A. Uptake Prediction of Ten Heavy Metals by Eruca Sativa Mill. Cultivated in Soils Amended with Sewage Sludge. Bull. Environ. Contam. Toxicol. 2020, 104, 134–143. [Google Scholar] [CrossRef]
- Rasoulzadeh, A.; Yaghoubi, A. Effect of Cattle Manure on Soil Physical Properties on a Sandy Clay Loam Soil in North-West Iran. J. Food Agric. Environ. 2010, 8, 976–979. [Google Scholar]
- Bell, M.J.; Worrall, F. Charcoal Addition to Soils in NE England: A Carbon Sink with Environmental Co-Benefits? Sci. Total Environ. 2011, 409, 1704–1714. [Google Scholar] [CrossRef]
- Roy, R.N.; Finck, A.; Blair, G.J.; Tandon, H.L.S. Plant Nutrition for Food Security. Guide Integr. Nutr. Manag. FAO Fertil. Plant Nutr. Bull. 2006, 16, 201–214. [Google Scholar]
- Zrelli, A.; Elfalleh, W.; Ghorbal, A.; Chaouachi, B. Valorization of Date Palm Wastes by Lignin Extraction to Be Used for the Improvement of Polymeric Membrane Characteristics. Period. Polytech. Chem. Eng. 2022, 66, 70–81. [Google Scholar] [CrossRef]
- Oueriemmi, H.; Kidd, P.S.; Trasar-Cepeda, C.; Rodríguez-Garrido, B.; Zoghlami, R.I.; Ardhaoui, K.; Prieto-Fernández, Á.; Moussa, M. Evaluation of Composted Organic Wastes and Farmyard Manure for Improving Fertility of Poor Sandy Soils in Arid Regions. Agriculture 2021, 11, 415. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Rao, K.J.; Reza, S.K.; Padua, S.; Dinesh, D.; Dharumarajan, S. Influence of Inorganic Fertilizers and Organic Amendments on Plant Nutrients and Soil Enzyme Activities under Incubation. Int. J. Bio-Resour. Stress Manag. 2016, 7, 924–932. [Google Scholar] [CrossRef]
- Arshad, M.A.; Coen, G.M. Characterization of Soil Quality: Physical and Chemical Criteria. Am. J. Altern. Agric. 1992, 7, 25–31. [Google Scholar] [CrossRef]
- Parr, J.F.; Papendick, R.I.; Hornick, S.B.; Meyer, R.E. Soil Quality: Attributes and Relationship to Alternative and Sustainable Agriculture. Am. J. Altern. Agric. 1992, 7, 5–11. [Google Scholar] [CrossRef]
- Ros, M.; Rodríguez, I.; García, C.; Hernández, M.T. Bacterial Community in Semiarid Hydrocarbon Contaminated Soils Treated by Aeration and Organic Amendments. Int. Biodeterior. Biodegrad. 2014, 94, 200–206. [Google Scholar] [CrossRef]
- Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S. Different Approaches to Evaluating Soil Quality Using Biochemical Properties. Soil Biol. Biochem. 2005, 37, 877–887. [Google Scholar] [CrossRef]
- Nannipieri, P.; Greco, S.; Ceccanti, B. Ecological Significance of the Biological Activity in Soil. In Soil Biochemistry; Bollag, J.-M., Stotzky, G., Eds.; CRC Press: New York, NY, USA, 1990; Volume 6, Chapter 6; pp. 293–356. [Google Scholar]
- Visser, S.; Parkinson, D. Soil Biological Criteria as Indicators of Soil Quality: Soil Microorganisms. Am. J. Altern. Agric. 1992, 7, 33–37. [Google Scholar] [CrossRef]
- Jordán, A.; Zavala, L.M.; Gil, J. Effects of Mulching on Soil Physical Properties and Runoff under Semi-Arid Conditions in Southern Spain. Catena 2010, 81, 77–85. [Google Scholar] [CrossRef]
- Brahim, N.; Ibrahim, H.; Mlih, R.; Bouajila, A.; Karbout, N.; Bol, R. Soil OC and N Stocks in the Saline Soil of Tunisian Gataaya Oasis Eight Years after Application of Manure and Compost. Land 2022, 11, 442. [Google Scholar] [CrossRef]
- Armas, C.M.; Santana, B.; Mora, J.L.; Notario, J.S.; Arbelo, C.D.; Rodríguez-Rodríguez, A. A Biological Quality Index for Volcanic Andisols and Aridisols (Canary Islands, Spain): Variations Related to the Ecosystem Degradation. Sci. Total Environ. 2007, 378, 238–244. [Google Scholar] [CrossRef]
- Hannachi, N.; Cocco, S.; Fornasier, F.; Agnelli, A.; Brecciaroli, G.; Massaccesi, L.; Weindorf, D.; Corti, G. Effects of Cultivation on Chemical and Biochemical Properties of Dryland Soils from Southern Tunisia. Agric. Ecosyst. Environ. 2015, 199, 249–260. [Google Scholar] [CrossRef]
- Miralles, I.; Trasar-Cepeda, C.; Leirós, M.C.; Barbosa-Pereira, L.; Gil-Sotres, F. Capacity of Biological Soil Crusts Colonized by the Lichen Diploschistes to Metabolize Simple Phenols. Plant Soil 2014, 385, 229–240. [Google Scholar] [CrossRef]
- Benidire, L.; Madline, A.; Pereira, S.I.A.; Castro, P.M.L.; Boularbah, A. Synergistic Effect of Organo-Mineral Amendments and Plant Growth-Promoting Rhizobacteria (PGPR) on the Establishment of Vegetation Cover and Amelioration of Mine Tailings. Chemosphere 2021, 262, 127803. [Google Scholar] [CrossRef]
- Mohamed, M.A.E.-H.; Moursy, F.I.; Darrag, M.H.; El-Mahdy, M.E.-S. Assessment of Long-Term Trends and Mapping of Drought Events in Tunisia. Sci. Afr. 2023, 21, e01766. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. World Soil Resources Reports No. 106. Rome, Italy, FAO. 2015. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/bcdecec7-f45f-4dc5-beb1-97022d29fab4/content (accessed on 15 April 2025).
- Dick, R.P. Methods of Soil Enzymology; Wiley Online Library: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mylavarapu, R.; Bergeron, J.; Wilkinson, N. Soil pH and Electrical Conductivity: A Country Extension Soil Laboratory Manual. IFAS Extension. University of Florida, USA. Available online: https://edis.ifas.ufl.edu/ss118 (accessed on 15 April 2025).
- Guitián-Ojea, F.; Carballas-Fernández, T. Técnicas de Análisis de Suelos, 2nd ed.; Eitorial PS: Santiago de Compostela, España, 1976. [Google Scholar]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Trasar-Cepeda, M.C.; Gil-Sotres, F.; Guitian-Ojea, F. Relation between Phosphorus Fractions and Development of Soils from Galicia (NW Spain). Geoderma 1990, 47, 139–150. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Bremner, J.M. Inorganic Forms of Nitrogen. In Methods of Soil Analysis. Part 2 Agronomy; Black, C.A., Evans, D.D., Clark, F.E., Eds.; American Society of Agronomy Inc.: Madison, WI, USA, 1965; pp. 1179–1237. [Google Scholar]
- Jenkinson, D.S.; Powlson, D.S. The Effects of Biocidal Treatments on Metabolism in Soil—V: A Method for Measuring Soil Biomass. Soil Biol. Biochem. 1976, 8, 209–213. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. Microbial Biomass Measurements in Forest Soils: Determination of Kc Values and Tests of Hypotheses to Explain the Failure of the Chloroform Fumigation-Incubation Method in Acid Soils. Soil Biol. Biochem. 1987, 19, 689–696. [Google Scholar] [CrossRef]
- Camiña, F.; Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, C. Measurement of Dehydrogenase Activity in Acid Soils Rich in Organic Matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar] [CrossRef]
- Nannipieri, P.; Ceccanti, B.; Cervelli, S.; Matarese, E. Extraction of Phosphatase, Urease, Proteases, Organic Carbon, and Nitrogen from Soil. Soil Sci. Soc. Am. J. 1980, 44, 1011–1016. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of P-Nitrophenyl Phosphate for Assay of Soil Phosphatase Activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Trasar-Cepeda, M.C.; Gil-Sotres, F. Phosphatase Activity in Acid High Organic Matter Soils in Galicia (NW Spain). Soil Biol. Biochem. 1987, 19, 281–287. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase Activity of Soils. Soil Sci. Soc. Am. J. 1970, 34, 225–229. [Google Scholar] [CrossRef]
- Trasar-Cepeda, C.; Leirós, M.C.; Gil-Sotres, F. Hydrolytic Enzyme Activities in Agricultural and Forest Soils. Some Implications for Their Use as Indicators of Soil Quality. Soil Biol. Biochem. 2008, 40, 2146–2155. [Google Scholar] [CrossRef]
- Pardo, T.; Rodríguez-Garrido, B.; Saad, R.F.; Soto-Vázquez, J.L.; Loureiro-Viñas, M.; Prieto-Fernández, Á.; Echevarria, G.; Benizri, E.; Kidd, P.S. Assessing the Agromining Potential of Mediterranean Nickel-Hyperaccumulating Plant Species at Field-Scale in Ultramafic Soils under Humid-Temperate Climate. Sci. Total Environ. 2018, 630, 275–286. [Google Scholar] [CrossRef]
- Garland, J.L. Analysis and Interpretation of Community-Level Physiological Profiles in Microbial Ecology. FEMS Microbiol. Ecol. 1997, 24, 289–300. [Google Scholar] [CrossRef]
- Muniz, S.; Lacarta, J.; Pata, M.P.; Jimenez, J.J.; Navarro, E. Analysis of the Diversity of Substrate Utilisation of Soil Bacteria Exposed to Cd and Earthworm Activity Using Generalised Additive Models. PLoS ONE 2014, 9, e85057. [Google Scholar] [CrossRef]
- Garau, G.; Castaldi, P.; Santona, L.; Deiana, P.; Melis, P. Influence of Red Mud, Zeolite and Lime on Heavy Metal Immobilization, Culturable Heterotrophic Microbial Populations and Enzyme Activities in a Contaminated Soil. Geoderma 2007, 142, 47–57. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Plaza, C.; Saiz, H.; Manzano, R.; Flagmeier, M.; Maestre, F.T. Aridity and Reduced Soil Micronutrient Availability in Global Drylands. Nat. Sustain. 2019, 2, 371–377. [Google Scholar] [CrossRef]
- Gargouri, K.; Bentaher, H.; Rhouma, A. A Novel Method to Assess Drought Stress of Olive Tree. Agron. Sustain. Dev. 2012, 32, 735–745. [Google Scholar] [CrossRef]
- Kefi, M.; Yoshino, K.; Setiawan, Y.; Zayani, K.; Boufaroua, M. Assessment of the Effects of Vegetation on Soil Erosion Risk by Water: A Case of Study of the Batta Watershed in Tunisia. Environ. Earth Sci. 2011, 64, 707–719. [Google Scholar] [CrossRef]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant Nutrition under Climate Change and Soil Carbon Sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Cherif, H.; Ayari, F.; Ouzari, H.; Marzorati, M.; Brusetti, L.; Jedidi, N.; Hassen, A.; Daffonchio, D. Effects of Municipal Solid Waste Compost, Farmyard Manure and Chemical Fertilizers on Wheat Growth, Soil Composition and Soil Bacterial Characteristics under Tunisian Arid Climate. Eur. J. Soil Biol. 2009, 45, 138–145. [Google Scholar] [CrossRef]
- Siles, J.A.; García-Sánchez, M.; Gómez-Brandón, M. Studying Microbial Communities through Co-Occurrence Network Analyses during Processes of Waste Treatment and in Organically Amended Soils: A Review. Microorganisms 2021, 9, 1165. [Google Scholar] [CrossRef]
- Zriba, Z.; Karbout, N.; Azaiez, F.E.B.; Lamourou, H.; Bousnina, H.; Moussa, M. Effect of Different Soil Amendments on Irrigation and Crop Yields in the Oases of Southern Tunisia. Emir. J. Food Agric. 2023, 35, 297–304. [Google Scholar] [CrossRef]
- Zanuzzi, A.; Arocena, J.M.; Van Mourik, J.M.; Cano, A.F. Amendments with Organic and Industrial Wastes Stimulate Soil Formation in Mine Tailings as Revealed by Micromorphology. Geoderma 2009, 154, 69–75. [Google Scholar] [CrossRef]
- Touceda-González, M.; Álvarez-López, V.; Prieto-Fernández, Á.; Rodríguez-Garrido, B.; Trasar-Cepeda, C.; Mench, M.; Puschenreiter, M.; Quintela-Sabarís, C.; Macías-García, F.; Kidd, P.S. Aided Phytostabilisation Reduces Metal Toxicity, Improves Soil Fertility and Enhances Microbial Activity in Cu-Rich Mine Tailings. J. Environ. Manag. 2017, 186, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Huang, J.; Fallah, N.; Lin, W.; Yuan, Z.; Hu, C. Combining N Fertilization with Biochar Affects Root-Shoot Growth, Rhizosphere Soil Properties and Bacterial Communities under Sugarcane Monocropping. Ind. Crop. Prod. 2022, 182, 114899. [Google Scholar] [CrossRef]
- Le Guyader, E.; Morvan, X.; Miconnet, V.; Marin, B.; Moussa, M.; Intrigliolo, D.S.; Delgado-Iniesta, M.J.; Girods, P.; Fontana, S.; Sbih, M. Influence of Date Palm-Based Biochar and Compost on Water Retention Properties of Soils with Different Sand Contents. Forests 2024, 15, 304. [Google Scholar] [CrossRef]
- Cantrell, I.C.; Linderman, R.G. Preinoculation of Lettuce and Onion with VA Mycorrhizal Fungi Reduces Deleterious Effects of Soil Salinity. Plant Soil 2001, 233, 269–281. [Google Scholar] [CrossRef]
- Ghimire, R.; Bista, P.; Machado, S. Crop Yield Limitation by Soil Organic Matter Decline: A Case Study from the US Pacific Northwest. In Advances in Understanding Soil Degradation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 609–621. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzálvez, J.; Tortosa, G.; Baddi, G.A.; Cegarra, J. Evaluation of “Alperujo” Composting Based on Organic Matter Degradation, Humification and Compost Quality. Biodegradation 2009, 20, 257–270. [Google Scholar] [CrossRef]
- Marinari, S.; Mancinelli, R.; Campiglia, E.; Grego, S. Chemical and Biological Indicators of Soil Quality in Organic and Conventional Farming Systems in Central Italy. Ecol. Indic. 2006, 6, 701–711. [Google Scholar] [CrossRef]
- Mhadhbi, T.; Pringault, O.; Nouri, H.; Spinelli, S.; Beyrem, H.; Gonzalez, C. Evaluating Polar Pesticide Pollution with a Combined Approach: A Survey of Agricultural Practices and POCIS Passive Samplers in a Tunisian Lagoon Watershed. Environ. Sci. Pollut. Res. 2019, 26, 342–361. [Google Scholar] [CrossRef]
- Azaiez, N.; Alleoua, A.; Baazaoui, N.; Qhtani, N. Assessment of Soil Loss in the Mirabah Basin: An Overview of the Potential of Agricultural Terraces as Ancestral Practices (Saudi Arabia). Open J. Soil Sci. 2020, 10, 159. [Google Scholar] [CrossRef]
- Abid, W.; Ammar, E. Date Palm (Phoenix dactylifera L.) Wastes Valorization: A Circular Economy Approach. In Mediterranean Fruits Bio-Wastes: Chemistry, Functionality and Technological Applications; Springer: Berlin/Heidelberg, Germany, 2022; pp. 403–430. [Google Scholar] [CrossRef]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and Defixation of Ammonium in Soils: A Review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef]
- Scherer, H.W.; Metker, D.J.; Welp, G. Effect of Long-Term Organic Amendments on Chemical and Microbial Properties of a Luvisol. Plant Soil Environ. 2011, 57, 513–518. [Google Scholar] [CrossRef]
- Hernández, T.; García, C. Estimación de La Respiración Microbiana Del Suelo. In Técnicas de Análisis de Parámetros Bioquímicos en Suelos; Mundi-Prensa: Madrid, Spain, 2003; pp. 311–346. [Google Scholar]
- Miguéns, T.; Leirós, M.C.; Gil-Sotres, F.; Trasar-Cepeda, C. Biochemical Properties of Vineyard Soils in Galicia, Spain. Sci. Total Environ. 2007, 378, 218–222. [Google Scholar] [CrossRef]
- Miralles, I.; Ortega, R.; Almendros, G.; Gil-Sotres, F.; Trasar-Cepeda, C.; Leirós, M.C.; Soriano, M. Modifications of Organic Matter and Enzymatic Activities in Response to Change in Soil Use in Semi-arid Mountain Ecosystems (Southern Spain). Eur. J. Soil Sci. 2012, 63, 272–283. [Google Scholar] [CrossRef]
- Bastida, F.; Torres, I.F.; Moreno, J.L.; Baldrian, P.; Ondoño, S.; Ruiz-Navarro, A.; Hernández, T.; Richnow, H.H.; Starke, R.; García, C. The Active Microbial Diversity Drives Ecosystem Multifunctionality and Is Physiologically Related to Carbon Availability in Mediterranean Semi-arid Soils. Mol. Ecol. 2016, 25, 4660–4673. [Google Scholar] [CrossRef]
- Zornoza, R.; Faz, A.; Carmona, D.M.; Martínez-Martínez, S.; Acosta, J.A. Plant Cover and Soil Biochemical Properties in a Mine Tailing Pond Five Years after Application of Marble Wastes and Organic Amendments. Pedosphere 2012, 22, 22–32. [Google Scholar] [CrossRef]
- Karbout, N.; Moussa, M.; Gasmi, I.; Bousnina, H. Effect of Clay Amendment on Physical and Chemical Characteristics of Sandy Soil in Arid Areas: The Case of Ground South-Eastern Tunisian. Appl. Sci. Rep. 2015, 11, 43–48. [Google Scholar] [CrossRef]
- Douh, B.; Mguidiche, A.; Zouari, I.; Gargouri, K.; Ayachi, M. Impact of Different Organic Amendments on: Hydrodynamic Soil Properties and Olive Tree Behavior Conducted under Deficit Irrigation. Commun. Soil Sci. Plant Anal. 2021, 52, 2256–2270. [Google Scholar] [CrossRef]
- Masmoudi, S.; Magdich, S.; Rigane, H.; Medhioub, K.; Rebai, A.; Ammar, E. Effects of Compost and Manure Application Rate on the Soil Physico-Chemical Layers Properties and Plant Productivity. Waste Biomass Valorization 2020, 11, 1883–1894. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Trasar-Cepeda, C.; Leirós, M.C.; Seoane, S.; Gil-Sotres, F. Biochemical Properties of Acid Soils under Native Grassland in a Temperate Humid Zone. N. Z. J. Agric. Res. 2007, 50, 537–548. [Google Scholar] [CrossRef]
- Zornoza, R.; Mataix-Solera, J.; Guerrero, C.; Arcenegui, V.; Mayoral, A.M.; Morales, J.; Mataix-Beneyto, J. Soil Properties under Natural Forest in the Alicante Province of Spain. Geoderma 2007, 142, 334–341. [Google Scholar] [CrossRef]
- Bastida, F.; Hernández, T.; Albaladejo, J.; García, C. Phylogenetic and Functional Changes in the Microbial Community of Long-Term Restored Soils under Semiarid Climate. Soil Biol. Biochem. 2013, 65, 12–21. [Google Scholar] [CrossRef]
- Siles, J.A.; José, M.; González-Pérez, J.A.; Fernández-Pérez, V.; García-Díaz, C.; Moreno, J.L.; García, C.; Bastida, F. Long-Term Restoration with Organic Amendments Is Clearer Evidenced by Soil Organic Matter Composition than by Changes in Microbial Taxonomy and Functionality. Appl. Soil Ecol. 2024, 198, 105383. [Google Scholar] [CrossRef]
- Kavvadias, V.; Le Guyader, E.; El Mazlouzi, M.; Gommeaux, M.; Boumaraf, B.; Moussa, M.; Lamine, H.; Sbih, M.; Zoghlami, I.R.; Guimeur, K. Using Date Palm Residues to Improve Soil Properties: The Case of Compost and Biochar. Soil Syst. 2024, 8, 69. [Google Scholar] [CrossRef]

| Treatment | pH H2O | EC (dS cm−1) | % P | % K | % Ca | % Mg | % TC | % TN | C/N |
|---|---|---|---|---|---|---|---|---|---|
| FPCA | 6.6 | 1.21 | 4 | 2 | 3.5 | 0.9 | 14.6 | 1.3 | 10.9 |
| FDPW | 7.6 | 0.66 | 4.5 | 3.6 | 2.1 | 0.5 | 16.1 | 0.8 | 20.1 |
| MDPW | 6.4 | 0.99 | 7 | 7 | 2.3 | 0.6 | 6.4 | 0.6 | 10.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chniguir, N.; Bouajila, A.; Prieto-Fernández, Á.; Omar, Z.; Mahmoudi, S.; Trasar-Cepeda, C. Improving Soil Health Using Date Palm Residues in Southern Tunisian Olive Orchards. Land 2025, 14, 1414. https://doi.org/10.3390/land14071414
Chniguir N, Bouajila A, Prieto-Fernández Á, Omar Z, Mahmoudi S, Trasar-Cepeda C. Improving Soil Health Using Date Palm Residues in Southern Tunisian Olive Orchards. Land. 2025; 14(7):1414. https://doi.org/10.3390/land14071414
Chicago/Turabian StyleChniguir, Najoua, Abdelhakim Bouajila, Ángeles Prieto-Fernández, Zohra Omar, Salah Mahmoudi, and Carmen Trasar-Cepeda. 2025. "Improving Soil Health Using Date Palm Residues in Southern Tunisian Olive Orchards" Land 14, no. 7: 1414. https://doi.org/10.3390/land14071414
APA StyleChniguir, N., Bouajila, A., Prieto-Fernández, Á., Omar, Z., Mahmoudi, S., & Trasar-Cepeda, C. (2025). Improving Soil Health Using Date Palm Residues in Southern Tunisian Olive Orchards. Land, 14(7), 1414. https://doi.org/10.3390/land14071414







