Pastoral Intensification and Peatland Drying in the Northern Tianshan Since 1560: Evidence from Fungal Spore Indicators
Abstract
1. Introduction
2. Study Area
3. Material and Methods
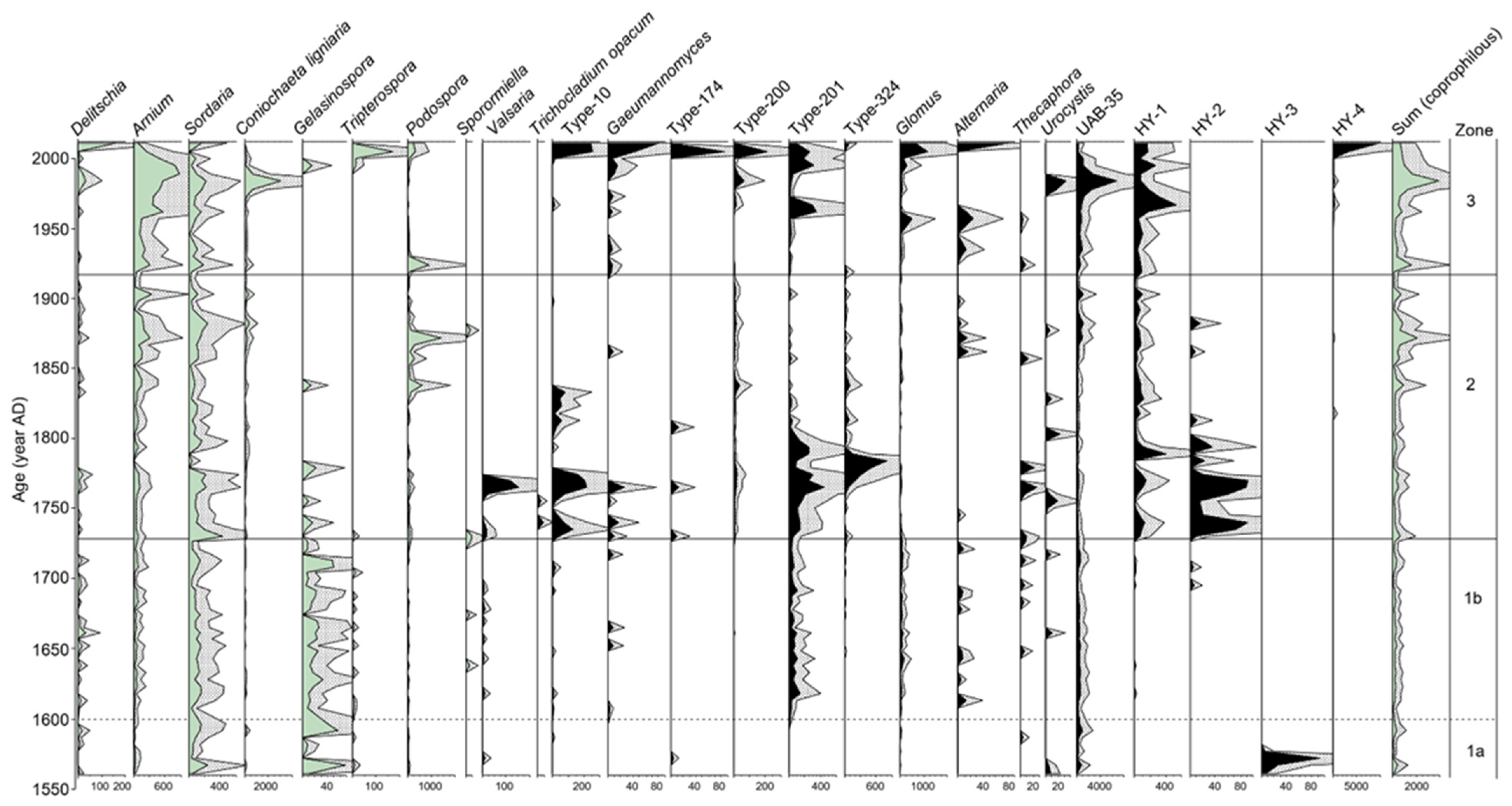
4. Results
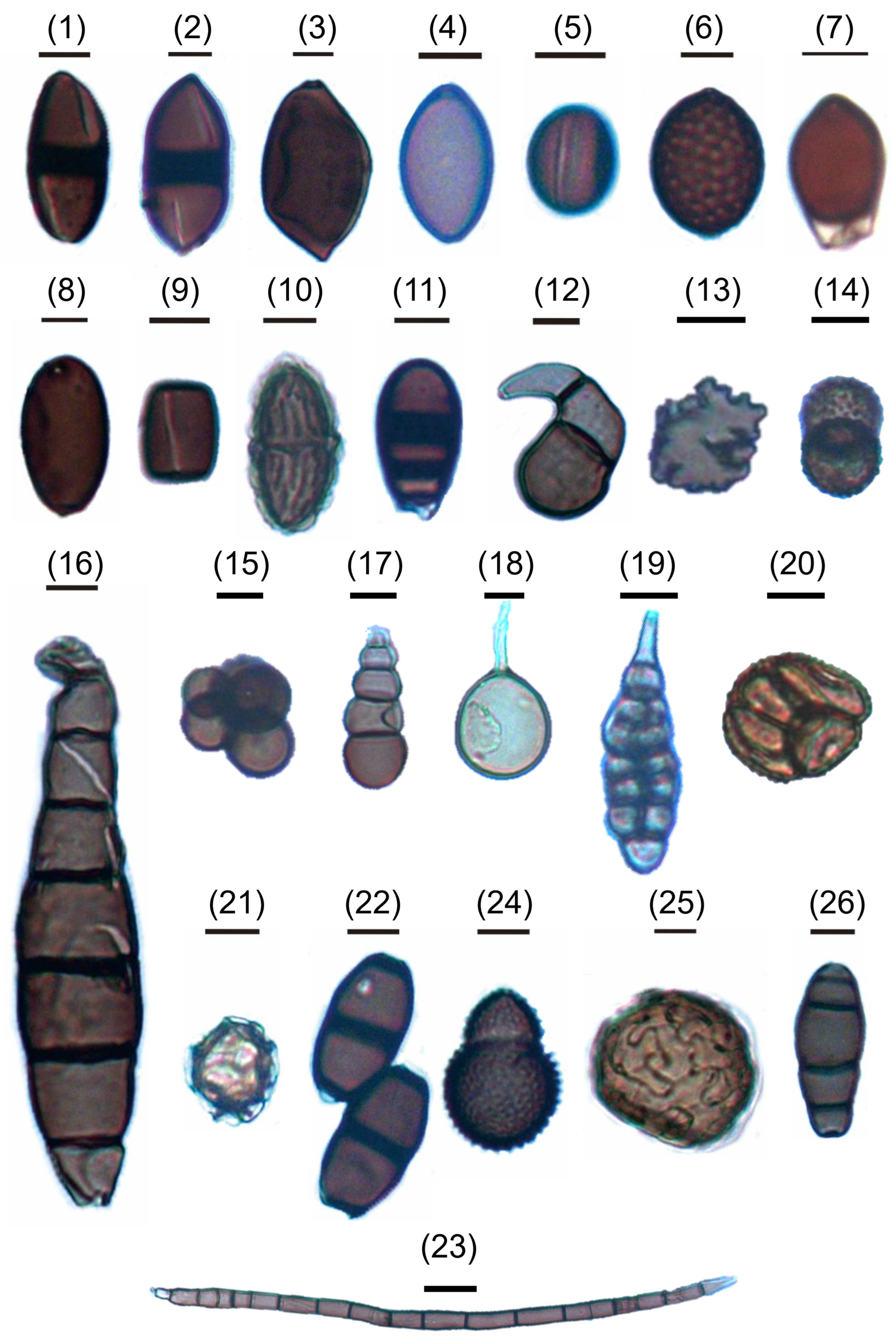
4.1. Fungal Assemblages
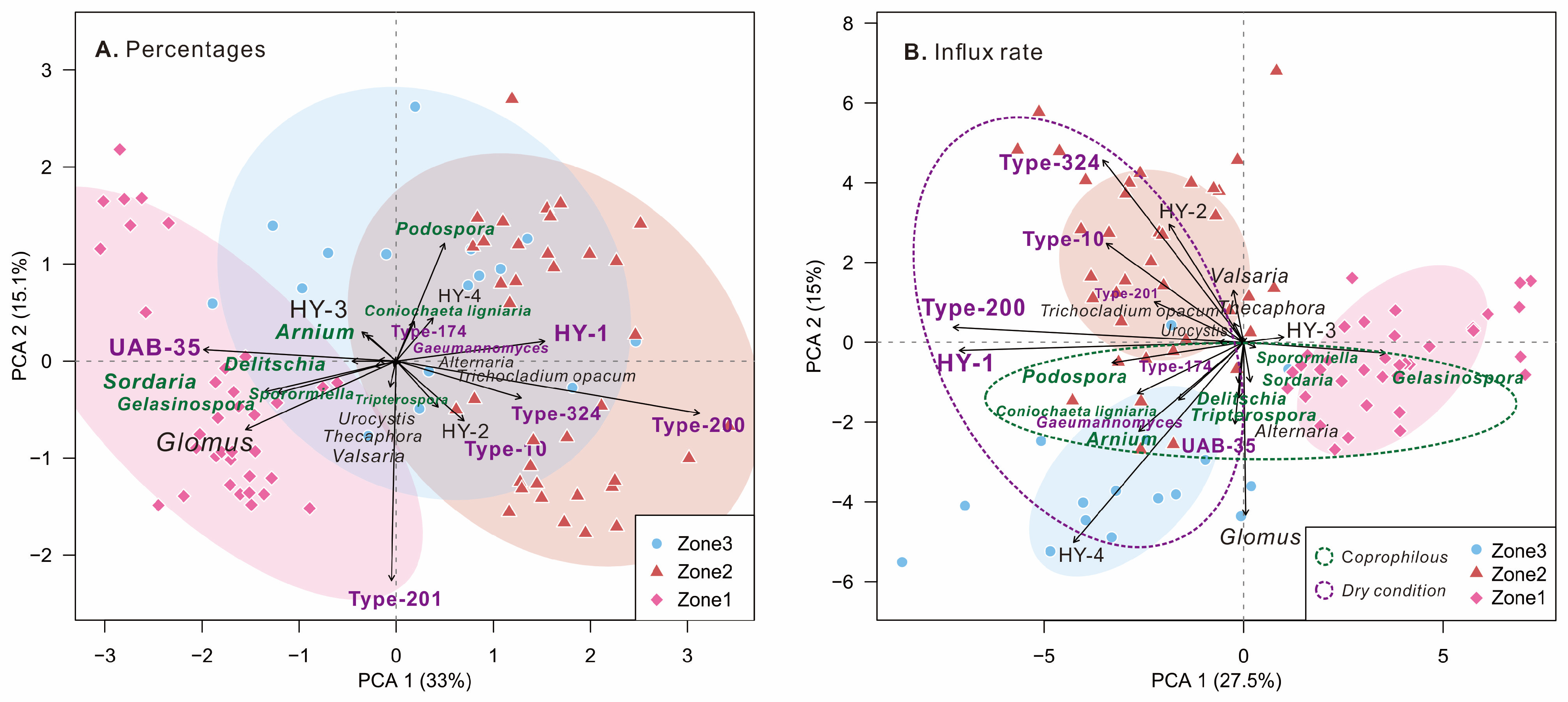
4.2. Results of PCA
5. Discussion
5.1. Ecological and Environmental Significance of Fungal Spores
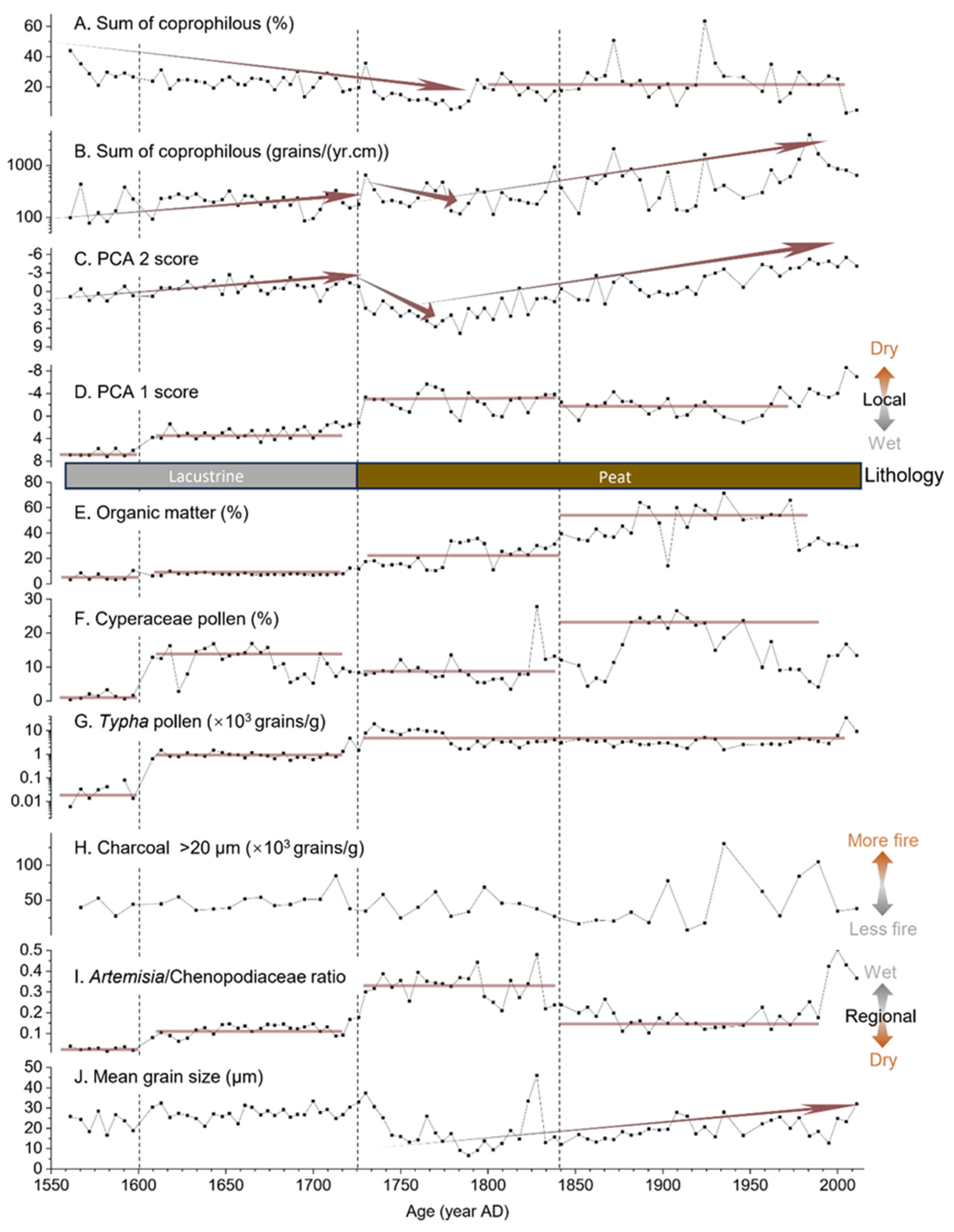
5.2. Pastoral Activities at Sichanghu Peatland Since 1560 AD
5.3. Divergence and Implications of Local and Regional Environmental Evolution at Sichanghu Peatland Since 1560 AD
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.P.; Ge, Q.S.; Wang, H.J.; Tao, Z.X. Climate change, migration, and regional administrative reform: A case study of Xinjiang in the middle Qing Dynasty (1760–1884). Sci. China Earth Sci. 2017, 60, 1328–1337. [Google Scholar] [CrossRef]
- Yan, S.; Kan, Y.P. Human activities and environmental changes during the history periods in Jimsar region. Geogr. Symp. Arid Zone 1993, 3, 162–175. (In Chinese) [Google Scholar]
- Chen, Y.Q. A Preliminary Study on the Development and Changes of Xinjiang’s Population and Their Causes from 1840 to 1949. Res. West. Reg. 1992, 1, 1–8. (In Chinese) [Google Scholar]
- Wu, Y.Q. A Study on Xinjiang Population History in the Qing Dynasty. Master’s Thesis, Xinjaing University, Xinjiang, China, 2001. (In Chinese). [Google Scholar]
- Chen, A.F. World Cultural Heritage: Gaochang Ancient City. Xinjiang Art Chin. Characters 2020, 1, 121–135. (In Chinese) [Google Scholar]
- Lu, Q.H. The historical changes of Gaochang and the Ruins of the Gaochang Ancient City. Local. Chron. Xinjiang 2021, 3, 58–61. (In Chinese) [Google Scholar]
- Ma, Z.K.; Liu, S.; Ren, M.; Ma, J.; Xi, T.Y.; Wang, J.X.; Wan, Z.W.; Tian, D.; Ren, W.H. Pastoralist and agriculturalist activities at the Shirenzigou Site in Xinjiang, China: Evidence from carbonized seed, phytolith and pollen. Acta Micropalaeontol. Sin. 2023, 40, 181–193. (In Chinese) [Google Scholar]
- Tian, D.; Ma, J.; Ren, M.; Xi, T.Y.; Wang, J.X.; Zhao, Z.J. Early barley production in Xinjiang: Evidence of plant remains from the Shirenzigou Site at the northern foot of the Tianshan Mountains. Agric. Hist. China 2021, 40, 44–55. (In Chinese) [Google Scholar]
- You, Y.; Lv, P.; Wang, J.X.; Ma, J.; Ren, M. The Appearance and Early Exploitation of Domestic Sheep in Xinjiang. Archaeology 2016, 12, 104–114+102. (In Chinese) [Google Scholar]
- Xia, X.C. A Summary of the Scientific Investigation in the Lop Nur Lake Region, Scientific Investigation and Research in the Lop Nur Lake Region; Science Press: Beijing, China, 1987. (In Chinese) [Google Scholar]
- Yu, Z.Y. Overview and Preliminary Study of 95MNIM8 at the Niya Site in Xinjiang. West. Reg. Stud. 1997, 1, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Sun, B.G.; Chen, G.; Feng, C.Z. Investigation of Beiting Ancient Town in Jimsar, Xinjiang. Archaeology 1982, 2, 165–178. (In Chinese) [Google Scholar]
- Jing, Q. The Karen Ruins of the Qing Dynasty. Res. Sun Tzu 2017, 6, 127–128. (In Chinese) [Google Scholar]
- Gao, Y.S.; Sang, Y.Y.; Xu, G.; Li, Y.L. The Rise and Decline of Loulan and the Environmental Changes, Catastrophe. J. Fuyang Norm. Univ. (Nat. Sci. Ed.) 2004, 3, 59–61. (In Chinese) [Google Scholar]
- Zhang, L. A Study on the Environmental Changes of the Ancient Lou-lan Oasis from the Han to the Jin Dynasty. Master’s Thesis, Shanxi Normal University, Xi’an, China, 2001. (In Chinese). [Google Scholar]
- Chen, J.H.; Chen, F.H.; Feng, S.; Huang, W.; Liu, J.B.; Zhou, A.F. Hydroclimatic changes in China and surroundings during the Medieval Climate Anomaly and Little Ice Age: Spatial patterns and possible mechanisms. Quat. Sci. Rev. 2015, 107, 98–111. [Google Scholar] [CrossRef]
- Ren, W.H.; Zhao, Y.; Li, Q.; Chen, J.H. Changes in vegetation and moisture in the northern Tianshan of China over the past 450 years. Front. Earth Sci. 2020, 14, 479–491. [Google Scholar] [CrossRef]
- Mansur, S.; Rahman, Y. Analysis on the spatial and temporial changes of population and its influencing factors in Xinjaing in the last 50 years. Hum. Geogr. 2007, 6, 114–119+146. (In Chinese) [Google Scholar]
- Gelorini, V.; Verbeken, A.; Van Geel, B.; Cocquyt, C.; Verschuren, D. Modern non-pollen palynomorphs from East African lake sediments. Rev. Palaeobot. Palynol. 2011, 164, 143–173. [Google Scholar] [CrossRef]
- Van Geel, B. Non-Pollen Palynomorphs; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 99–119. [Google Scholar]
- Van Geel, B.; Bohncke, S.J.P.; Dee, H. A palaeoecological study of an upper lateglacial and holocene sequence from ‘de borchert’. Rev. Palaeobot. Palynol. 1980, 31, 367–448. Available online: https://www.sciencedirect.com/science/article/abs/pii/0034666780900354 (accessed on 10 May 2025). [CrossRef]
- Van Geel, B.; Buurman, J.; Brinkkemper, O.; Schelvis, J.; Aptroot, A.; Van Reenen, G.; Hakbijl, T. Environmental reconstruction of a Roman Period settlement site in Uitgeest (The Netherlands), with special reference to coprophilous fungi. J. Archaeol. Sci. 2003, 30, 873–883. [Google Scholar] [CrossRef]
- Van Geel, B.; Guthrie, R.D.; Altmann, J.G.; Broekens, P.; Bull, I.D.; Gill, F.L.; Jansen, B.; Nieman, A.M.; Gravendeel, B. Mycological evidence of coprophagy from the feces of an Alaskan Late Glacial mammoth. Quat. Sci. Rev. 2011, 30, 2289–2303. [Google Scholar] [CrossRef]
- Huang, X.Z.; Zhang, J.; Storozum, M.; Liu, S.; Gill, J.L.; Xiang, L.X.; Ren, X.X.; Wang, J.L.; Qiang, M.R.; Chen, F.H.; et al. Long-term herbivore population dynamics in the northeastern Qinghai-Tibetan Plateau and its implications for early human impacts. Rev. Palaeobot. Palynol. 2020, 275, 104171. [Google Scholar] [CrossRef]
- Wei, H.C.; E, C.Y.; Duan, R.L.; Zhang, J.; Sun, Y.J.; Hou, G.L.; Gao, J.Y. Fungal spore record of pastoralism on the NE Qinghai-Tibetan Plateau since the middle Holocene. Sci. China Earth Sci. 2021, 64, 1318–1331. [Google Scholar] [CrossRef]
- Van Geel, B. A palaeoecological study of holocene peat bog sections in Germany and The Netherlands, based on the analysis of pollen, spores and macro- and microscopic remains of fungi, algae, cormophytes and animals. Rev. Palaeobot. Palynol. 1978, 25, 1–120. [Google Scholar] [CrossRef]
- Van Geel, B.; Hallewas, D.P.; Pals, J.P. A late holocene deposit under the Westfriese Zeedijk near Enkhuizen (Prov. of Noord-Holland, The Netherlands): Palaeoecological and archaeological aspects. Rev. Palaeobot. Palynol. 1983, 38, 269–335. [Google Scholar] [CrossRef]
- Yan, S.; Kong, Z.C.; Yang, Z.J. Pollen analysis and its significance of the Sichanghu section in Jimusaer county, Xijiang. Acta Bot. Boreali-Occident. Sin. 2003, 23, 531–536. (In Chinese) [Google Scholar]
- Chen, F.H.; Yu, Z.C.; Yang, M.L.; Ito, E.; Wang, S.M.; Madsen, D.B.; Huang, X.Z.; Zhao, Y.; Sato, T.; Birks, H.J.B.; et al. Holocene moisture evolution in arid central Asia and its out-of-phase relationship with Asian monsoon history. Quat. Sci. Rev. 2008, 27, 351–364. [Google Scholar] [CrossRef]
- Luo, C.X.; Zheng, Z.; Tarasov, P.; Pan, A.D.; Huang, K.Y.; Beaudouin, C.; An, F.Z. Characteristics of the modern pollen distribution and their relationship to vegetation in the Xinjiang region, northwestern China. Rev. Palaeobot. Palynol. 2009, 153, 282–295. [Google Scholar] [CrossRef]
- Yang, Z.J.; Zhang, Y.; Ren, H.B.; Yan, S.; Kong, Z.C.; Ma, K.P.; Ni, J. Altitudinal changes of surface pollen and vegetation on the north slope of the Middle Tianshan Mountains, China. J. Arid. Land. 2016, 8, 799–810. [Google Scholar] [CrossRef]
- Tan, B.; Wang, H.W.; Wang, X.Q.; Yi, S.Y.; Zhou, J.; Ma, C.; Dai, X.Y. Spatial distribution data of cultural sites from the Paleolithic to Bronze Age in Xinjiang, China. Sci. Data 2022, 9, 205. [Google Scholar] [CrossRef]
- Faegri, K.; Kaland, P.E.; Krzywinski, K. Textbook of Pollen Analysis; John Wiley & Sons Ltd.: New York, NY, USA, 1989. [Google Scholar]
- Van Geel, B. Palynology of a section from the raised peat bog ‘wietmarscher moor‘, with special reference to fungal remains. Acta Bot. Neerl. 1972, 21, 261–284. [Google Scholar] [CrossRef]
- Pals, J.P.; Vangeel, B.; Delfos, A. Paleoecological studies in the klokkeweel bog near hoogkarspel (prov of noord-holland). Rev. Palaeobot. Palynol. 1980, 30, 371–418. [Google Scholar] [CrossRef]
- Davis, O.K. Spores of the dung fungus Sporormiella: Increased abundance in historic sediments and before Pleistocene megafaunal extinction. Quat. Res. 1987, 28, 290–294. [Google Scholar] [CrossRef]
- Van Geel, B.; Coope, G.R.; Van Der Hammen, T. Palaeoecology and stratigraphy of the lateglacial type section at Usselo (the Netherlands). Rev. Palaeobot. Palynol. 1989, 60, 25–129. [Google Scholar] [CrossRef]
- Hyde, K.D.; Steinke, T.S. Two new species ofDelitschia from submerged wood. Mycoscience 1996, 37, 99–102. [Google Scholar] [CrossRef]
- Bell, A. An Illustrated Guide to the Coprophilous Ascomycetes of Australia; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2005. [Google Scholar]
- Cugny, C.; Mazier, F.; Galop, D. Modern and fossil non-pollen palynomorphs from the Basque mountains (western Pyrenees, France): The use of coprophilous fungi to reconstruct pastoral activity. Veg. Hist. Archaeobot 2010, 19, 391–408. [Google Scholar] [CrossRef]
- Quamar, M.F. Non-pollen palynomorphs from the late Quaternary sediments of southwestern Madhya Pradesh (India) and their palaeoenvironmental implications. Hist. Biol. 2014, 27, 1070–1078. [Google Scholar] [CrossRef]
- Miao, Y.F.; Jin, H.L.; Liu, B.; Herrmann, M.; Sun, Z.; Wang, Y.P. Holocene climate change on the northeastern Tibetan Plateau inferred from mountain-slope pollen and non-pollen palynomorphs. Rev. Palaeobot. Palynol. 2015, 221, 22–31. [Google Scholar] [CrossRef]
- Van Asperen, E.N.; Kirby, J.R.; Hunt, C.O. The effect of preparation methods on dung fungal spores: Implications for recognition of megafaunal populations. Rev. Palaeobot. Palynol. 2016, 229, 1–8. [Google Scholar] [CrossRef]
- Schlütz, F.; Shumilovskikh, L.S. Non-pollen palynomorphs notes: 1. Type HdV-368 (Podospora-type), descriptions of associated species, and the first key to related spore types. Rev. Palaeobot. Palynol. 2017, 239, 47–54. [Google Scholar] [CrossRef]
- Revelles, J.; Burjachs, F.; van Geel, B. Pollen and non-pollen palynomorphs from the Early Neolithic settlement of La Draga (Girona, Spain). Rev. Palaeobot. Palynol. 2016, 225, 1–20. [Google Scholar] [CrossRef]
- Grimm, E.C. CONISS—A FORTRAN 77 program for stratigraphically constrained cluster-analysis by the method of incremental sum of squares. Comput. Geosci. 1987, 13, 13–35. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Hao, X.D.; Weng, C.Y. The indicative significance of spores of coprophilous fungi in palaeoecological research. Mar. Geol. Quat. Geol. 2015, 35, 1–10. (In Chinese) [Google Scholar]
- Wood, J.R.; Wilmshurst, J.M. Accumulation rates or percentages? How to quantify Sporormiella and other coprophilous fungal spores to detect late Quaternary megafaunal extinction events. Quat. Sci. Rev. 2013, 77, 1–3. [Google Scholar] [CrossRef]
- Johnson, C.N.; Rule, S.; Haberle, S.G.; Turney, C.S.M.; Kershaw, A.P.; Brook, B.W. Using dung fungi to interpret decline and extinction of megaherbivores: Problems and solutions. Quat. Sci. Rev. 2015, 110, 107–113. [Google Scholar] [CrossRef]
- Chen, F.H.; Chen, J.H.; Holmes, J.; Boomer, I.; Austin, P.; Gates, J.B.; Wang, N.L.; Brooks, S.J.; Zhang, J.W. Moisture changes over the last millennium in arid central Asia: A review, synthesis and comparison with monsoon region. Quat. Sci. Rev. 2010, 29, 1055–1068. [Google Scholar] [CrossRef]
- Yan, S.; Li, S.F.; Kong, Z.C.; Yang, Z.J.; Ni, J. The pollen analyses and environment changes of the Dongdaohaizi area in Urumqi, Xinjiang. Quat. Sci. 2004, 24, 463–468. (In Chinese) [Google Scholar]
- Qiang, M.R.; Chen, F.H.; Zhou, A.F.; Xiao, S.; Zhang, J.W.; Jin, M. Preliminary study on dust storm events documented by grain size component of sugan lake sediments, north qaidam basin. Quat. Sci. 2006, 26, 915–922. (In Chinese) [Google Scholar]
- Eichler, A.; Tinner, W.; Brütsch, S.; Olivier, S.; Papina, T.; Schwikowski, M. An ice-core based history of Siberian forest fires since AD 1250. Quat. Sci. Rev. 2011, 30, 1027–1034. [Google Scholar] [CrossRef]
- Chen, F.H.; Huang, X.Z.; Zhang, J.W.; Holmes, J.A.; Chen, J.H. Humid Little Ice Age in and central Asia documented by Bosten Lake, Xinjiang, China. Sci. China Earth Sci. 2006, 49, 1280–1290. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, F.H.; Zhang, E.L.; Brooks, S.J.; Zhou, A.F.; Zhang, J.W. A 1000-year chironomid-based salinity reconstruction from varved sediments of Sugan Lake, Qaidam Basin, arid Northwest China, and its palaeoclimatic significance. Chin. Sci. Bull. 2009, 54, 3749–3759. [Google Scholar] [CrossRef]
- Putnam, A.E.; Putnam, D.E.; Andreu-Hayles, L.; Cook, E.R.; Palmer, J.G.; Clark, E.H.; Wang, C.Z.; Chen, F.; Denton, G.H.; Boyle, D.P.; et al. Little Ice Age wetting of interior Asian deserts and the rise of the Mongol Empire. Quat. Sci. Rev. 2016, 131, 33–50. [Google Scholar] [CrossRef]
- Florenzano, A. The History of Pastoral Activities in S Italy Inferred from Palynology: A Long-Term Perspective to Support Biodiversity Awareness. Sustainability 2019, 11, 404. [Google Scholar] [CrossRef]


| Depth (cm) | Stratigraphic Unit | 14C Age (cal yr AD) | Pollen Zones |
|---|---|---|---|
| 25–0 | Peat | 1880–2011 | SCH-4: high contents of Chenopodiaceae, Cyperaceae, and Artemisia |
| 56–25 | 1730–1880 | SCH-3: more Typha and Poaceae | |
| 84–56 | Silt clay | 1600–1730 | SCH-2: abundant Chenopodiaceae, Cyperaceae, and Artemisia |
| 92–84 | 1561–1600 | SCH-1: high percentages of Chenopodiaceae |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, W.; Liu, C.; Qin, F.; Li, Q.; Yi, G.; Chen, J.; Zhao, Y. Pastoral Intensification and Peatland Drying in the Northern Tianshan Since 1560: Evidence from Fungal Spore Indicators. Land 2025, 14, 1362. https://doi.org/10.3390/land14071362
Ren W, Liu C, Qin F, Li Q, Yi G, Chen J, Zhao Y. Pastoral Intensification and Peatland Drying in the Northern Tianshan Since 1560: Evidence from Fungal Spore Indicators. Land. 2025; 14(7):1362. https://doi.org/10.3390/land14071362
Chicago/Turabian StyleRen, Weihe, Cai Liu, Feng Qin, Quan Li, Guitian Yi, Jianhui Chen, and Yan Zhao. 2025. "Pastoral Intensification and Peatland Drying in the Northern Tianshan Since 1560: Evidence from Fungal Spore Indicators" Land 14, no. 7: 1362. https://doi.org/10.3390/land14071362
APA StyleRen, W., Liu, C., Qin, F., Li, Q., Yi, G., Chen, J., & Zhao, Y. (2025). Pastoral Intensification and Peatland Drying in the Northern Tianshan Since 1560: Evidence from Fungal Spore Indicators. Land, 14(7), 1362. https://doi.org/10.3390/land14071362





