Heavy Metal(oid)s in Soil–Tea System: Sources, Bioaccumulation, and Risks in Eastern Dabie Mountain
Abstract
1. Introduction
2. Materials and Methods
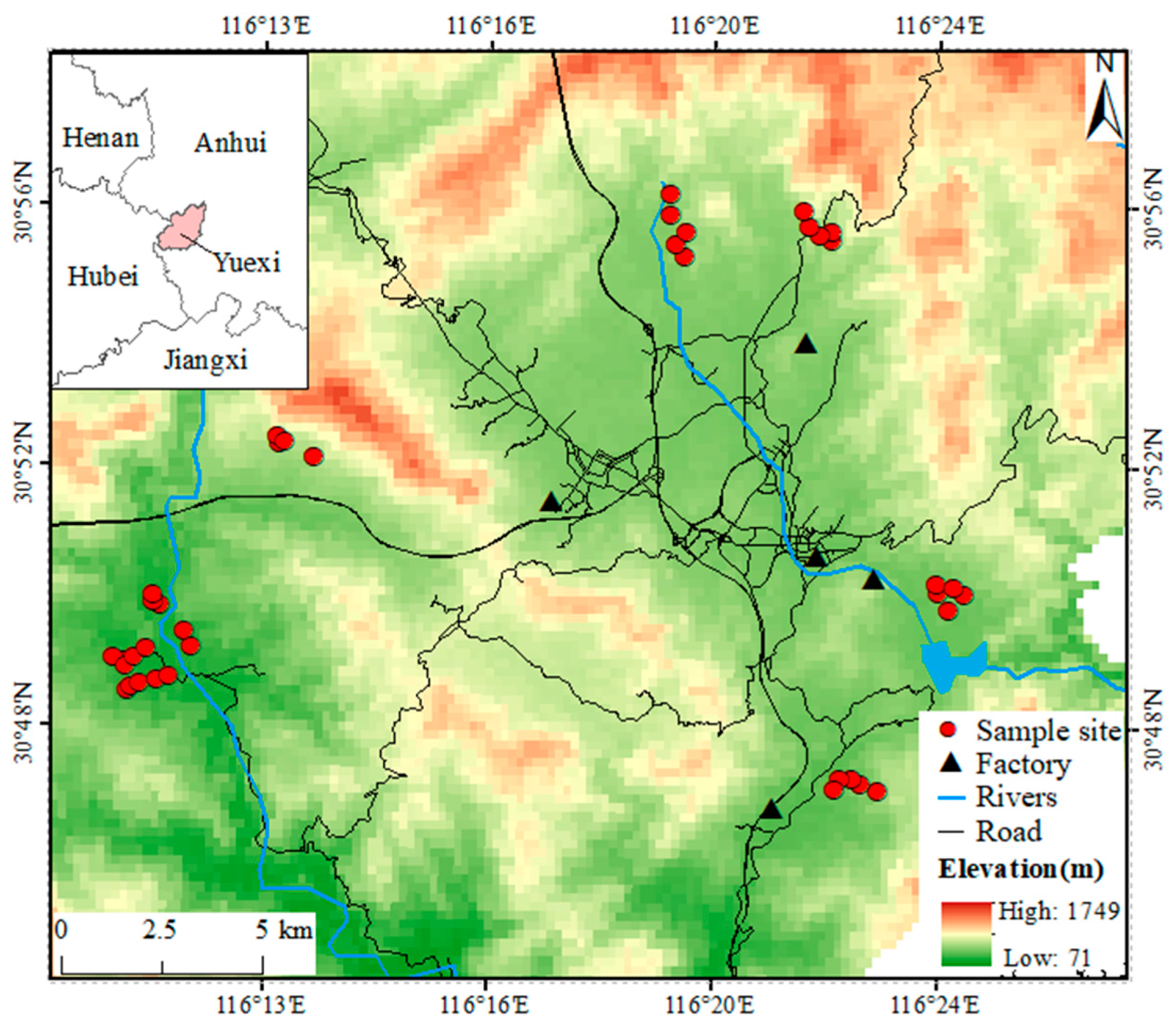
2.1. Study Area
2.2. Sample Collection and Preparation
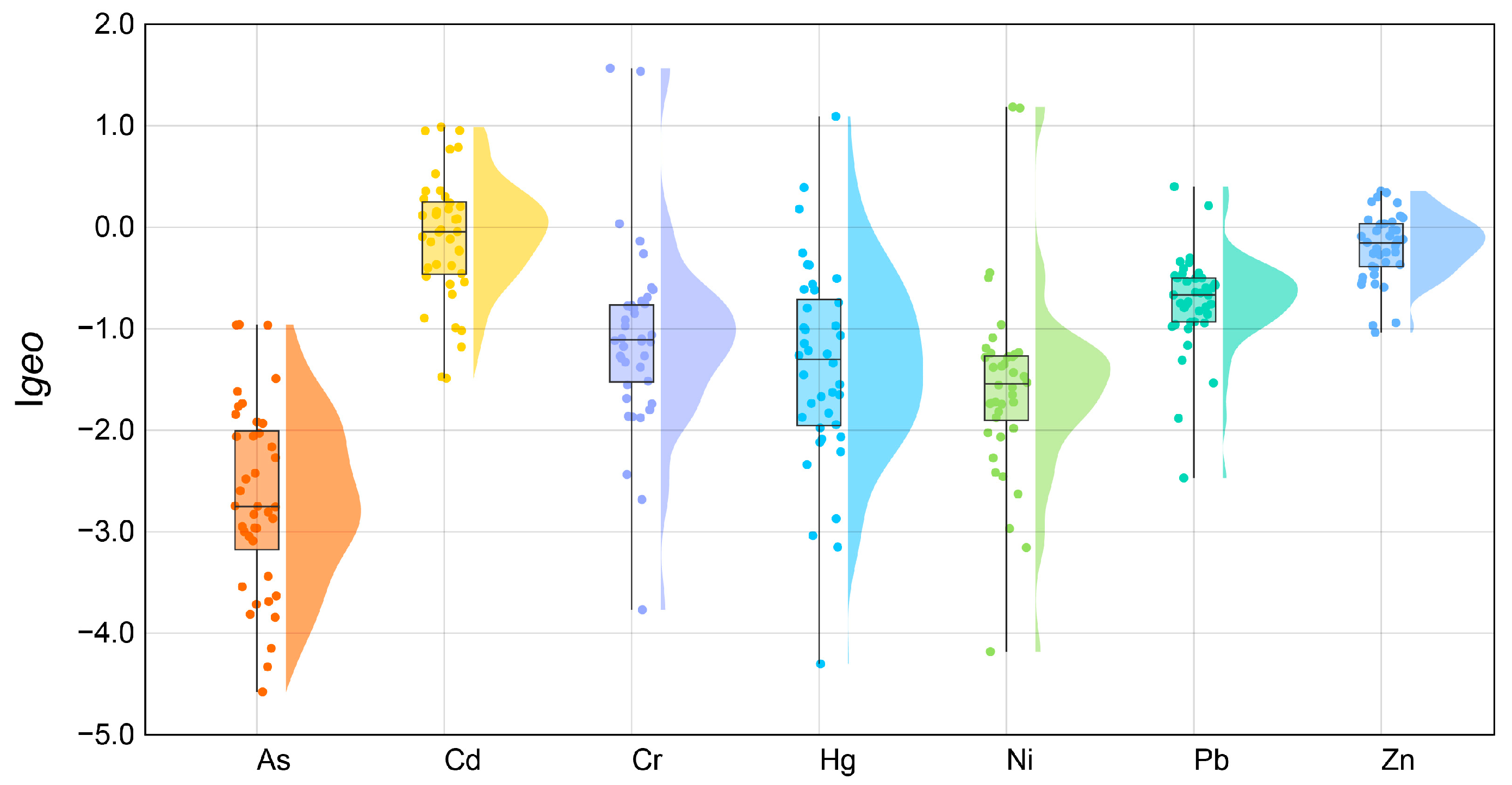
2.3. Pollution Assessment
2.4. Health Risk Assessment
2.5. Statistical Analysis
3. Results
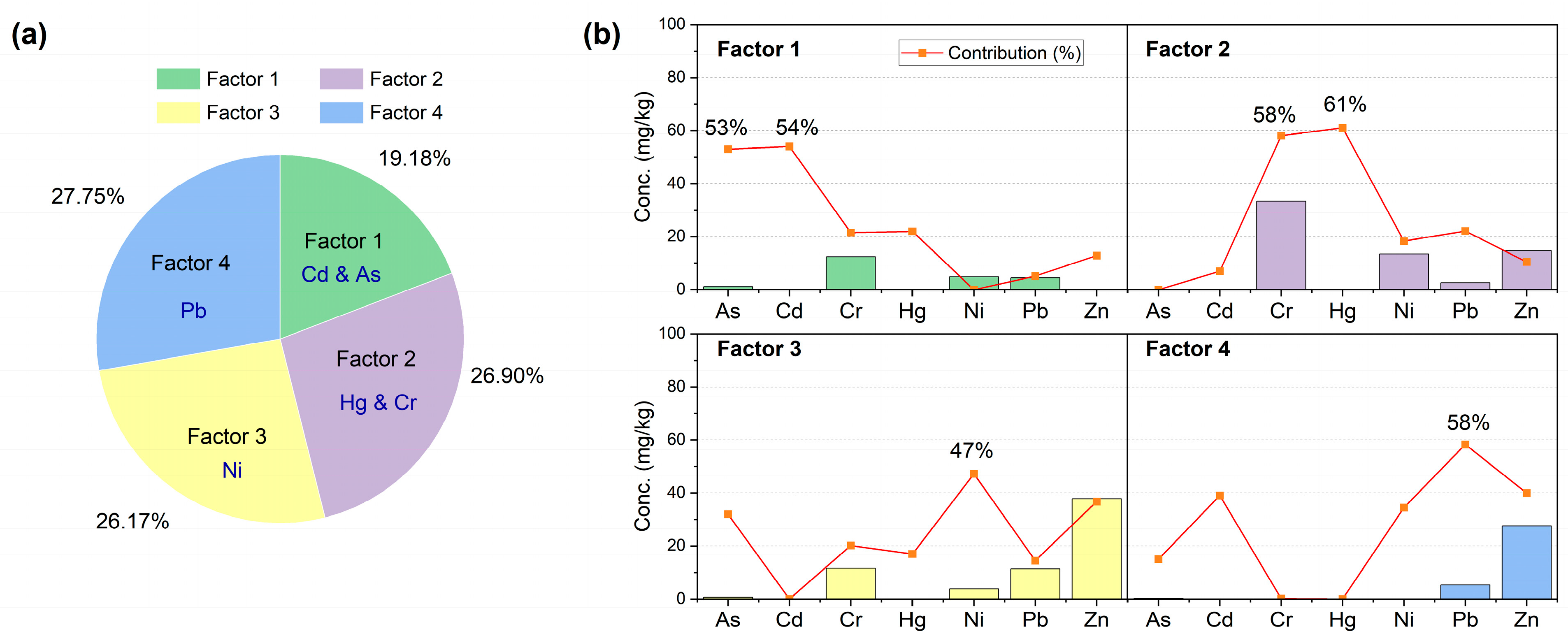
3.1. Heavy Metal(oid) Distribution and Source Apportionment in Soil
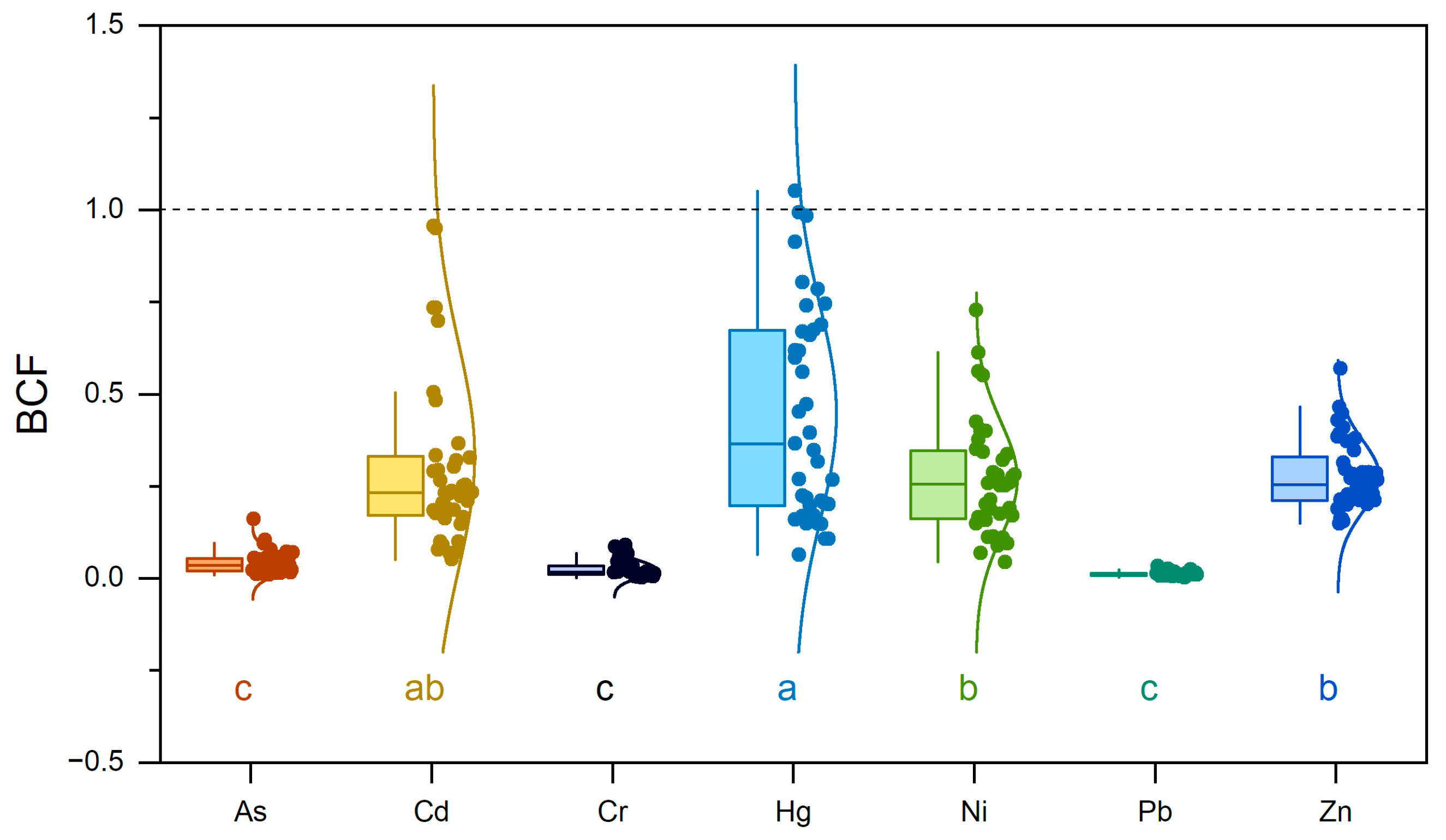
3.2. Heavy Metal(oid) Accumulation in Tea Leaves
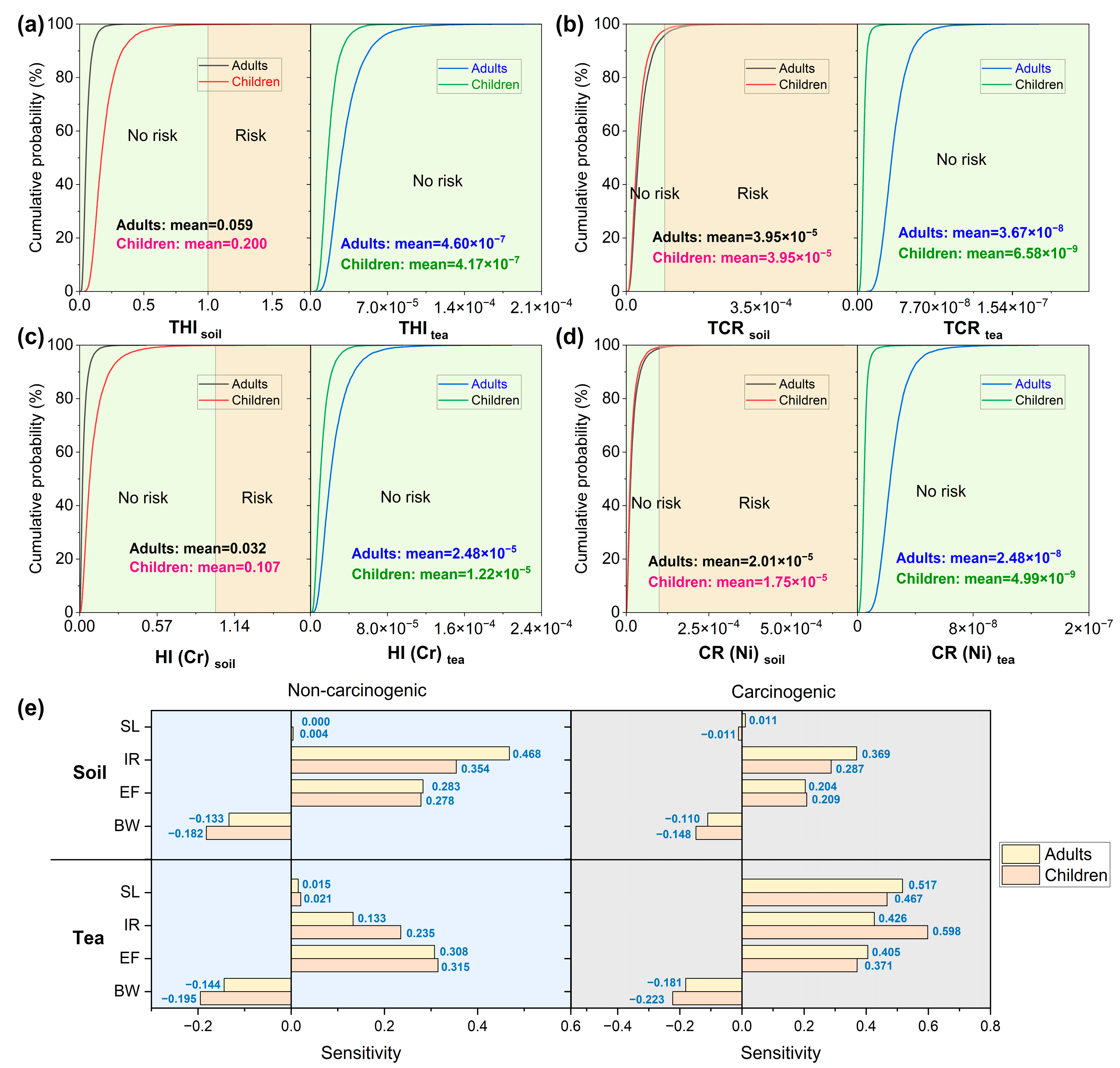
3.3. Health Risk Assessment
3.4. Soil–Tea System Interactions
4. Discussion
4.1. Regional-Specific Mechanisms of Heavy Metal(oid) Accumulation in Tea Gardens
4.2. Source Apportionment and Contribution Analysis of Heavy Metal(oid)s
4.3. Element-Specific Bioaccumulation Patterns in Tea Leaves
4.4. Implications for Risk Management and Quality Control
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| EF | Pollution Status | Igeo | Pollution Status | Ei | RI | Risk Level |
|---|---|---|---|---|---|---|
| ≤1 | No | ≤0 | No | <40 | <150 | Low |
| 1~2 | No to moderate | 0–1 | Slight | 40–80 | 150–300 | Moderate |
| 2~5 | Moderate | 1–2 | Slight to moderate | 80–160 | 300–600 | Moderate to high |
| 5~20 | Moderate to strong | 2–3 | Moderate | 160–320 | 600–1200 | High |
| 20~40 | Strong | 3–4 | Strong | >320 | >1200 | Very high |
| >40 | Extremely strong | 4–5 | Strong to very strong | |||
| ≥5 | Extremely strong |
| Parameter | Definition | Unit | Reference Values | |
|---|---|---|---|---|
| Adult | Children | |||
| Ring | ingestion rate | mg/day | 200 | 100 |
| Rinh | inhalation rate | m3/day | 15 | 7.5 |
| PEF | particle emission factor | m3/kg | 1.36 × 109 | 1.36 × 109 |
| SA | surface area of exposed skin | cm2 | 4350 | 1600 |
| AF | adhesiveness degree of skin | mg/(cm2 day) | 0.2 | 0.2 |
| BW | body weight | kg | 53.1 | 15 |
| ED | exposure duration | year | non-carcinogenic 24 carcinogenic 24 | non-carcinogenic 6 carcinogenic 30 |
| ABS | absorption factor of skin | — | 0.001 | 0.001 |
| EF | exposure frequency | day/year | 350 | 350 |
| AT | average exposure time | day | non-carcinogenic ED × 365 = 8760; carcinogenic 365 × 70 = 25,550 | non-carcinogenic ED × 365 = 2190; carcinogenic 365 × 70 = 25,550 |
| Heavy Metal(oid) | RfDing | RfDinh | RfDder | SFing | SFinh | SFder |
|---|---|---|---|---|---|---|
| As | 3.00 × 10−4 | 3.00 × 10−4 | 1.23 × 10−4 | 1.50 × 100 | 1.51 × 101 | 3.66 × 100 |
| Cd | 1.00 × 10−3 | 1.00 × 10−5 | 1.00 × 10−5 | |||
| Cr | 3.00 × 10−3 | 2.86 × 10−5 | 6.00 × 10−5 | 4.20 × 101 | ||
| Hg | 3.00 × 10−4 | 3.00 × 10−4 | 2.40 × 10−5 | |||
| Ni | 2.00 × 10−2 | 2.06 × 10−2 | 5.40 × 10−3 | 8.40E-01 | ||
| Pb | 3.50 × 10−3 | 3.52 × 10−3 | 5.25 × 10−4 | 8.50 × 10−3 | ||
| Zn | 3.00 × 10−1 | 3.00 × 10−1 | 6.00 × 10−2 |
References
- Xiang, M.; Li, Y.; Yang, J.; Lei, K.; Li, Y.; Li, F.; Zheng, D.; Fang, X.; Cao, Y. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 2021, 278, 116911. [Google Scholar] [CrossRef]
- Chen, L.; Luo, X.; He, H.; Duan, T.; Zhou, Y.; Yang, L.; Zeng, Y.; Chen, H.; Fang, L. Hg-mining-induced soil pollution by potentially toxic metal(loid)s presents a potential environmental risk and threat to human health: A global meta-analysis. Soil Ecol. Lett. 2024, 6, 240233. [Google Scholar] [CrossRef]
- Hakanson, L. An Ecological Risk Index for Aquatic Pollution Control—A Sedimentological Approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Meng, F.; Liu, D.; Bu, T.; Zhang, M.; Peng, J.; Ma, J. Assessment of pollution and health risks from exposure to heavy metals in soil, wheat grains, drinking water, and atmospheric particulate matter. J. Environ. Manag. 2025, 376, 124448. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Gupta, B.S.; Patidar, S.; Hernández-Martínez, J.L.; Martín-Romero, F.; Meza-Figueroa, D.; Martínez-Villegas, N. A comprehensive study of source apportionment, spatial distribution, and health risks assessment of heavy metal(loid)s in the surface soils of a semi-arid mining region in Matehuala, Mexico. Environ. Res. 2024, 260, 119619. [Google Scholar] [CrossRef]
- Goncharov, G.; Soktoev, B.; Farkhutdinov, I.; Matveenko, I. Heavy metals in urban soil: Contamination levels, spatial distribution and human health risk assessment (the case of Ufa city, Russia). Environ. Res. 2024, 257, 119216. [Google Scholar] [CrossRef]
- Singh, S.; Maiti, S.K.; Raj, D. An approach to quantify heavy metals and their source apportionment in coal mine soil: A study through PMF model. Environ. Monit. Assess. 2023, 195, 306. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Y.; Wang, J.; Liu, Y.; Guo, M.; Hu, C.; Shui, B. Analysis of heavy metal sources and potential ecological risk assessment of mangroves in Aojiang Estuary. Ecol. Indic. 2025, 173, 113343. [Google Scholar] [CrossRef]
- Liu, N.; Li, X.; Chen, P.; Yuan, W.; Wang, D.; Wang, X. Climate and vegetation controlling accumulation and translocation of heavy metals in water tower regions of Qinghai-Tibet Plateau. J. Hazard. Mater. 2025, 484, 136752. [Google Scholar] [CrossRef]
- Dai, H.; Xiao, J.; Wu, C.; Yu, L. The Accumulation and Physiological Responses of Camellia sinensis to Heavy Metals. Horticulturae 2024, 10, 680. [Google Scholar] [CrossRef]
- Lin, S.; Liu, X.; Yan, Q.; Liang, G.; Wang, D. Research on heavy metal enrichment and transportation in tea plant-soil systems of different varieties. Environ. Geochem. Health 2024, 46, 514. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Luo, Z.; Bi, J.; Liu, C.; Liu, X. Transfer of heavy metals from soil to tea and the potential human health risk in a regional high geochemical background area in southwest China. Sci. Total Environ. 2024, 908, 168122. [Google Scholar] [CrossRef] [PubMed]
- Angon, P.B.; Islam, M.S.; Kc, S.; Das, A.; Anjum, N.; Poudel, A.; Suchi, S.A. Sources, effects and present perspectives of heavy metals contamination: Soil, plants and human food chain. Heliyon 2024, 10, e28357. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil environmental quality—Risk control standard for soil contamination of agricultural land. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2018.
- GB 31608-2023; National food safety standards for tea. National Health Commission of the People’s Republic of China: Beijing, China, 2023.
- Muller, G. Index of Geoaccumulation in Sediments of the Rhine River. Geo J. 1969, 2, 109–118. [Google Scholar]
- Safadoust, A.; Khaleghi, S.; Kolahchi, Z. Environmental risks of heavy metals in railway soils: Challenges to ecosystem management. Sci. Total Environ. 2025, 974, 179217. [Google Scholar] [CrossRef] [PubMed]
- Gopal, V.; Krishnamurthy, R.R.; Indhumathi, A.; Sharon, B.T.X.; Priya, T.S.D.; Rathinavel, K.; Bharath, K.M.; Magesh, N.S.; Ayyamperumal, R. Geochemical evaluation, ecological and human health risk assessment of potentially toxic elements in urban soil, Southern India. Environ. Res. 2024, 248, 118413. [Google Scholar] [CrossRef]
- Korkanç, S.Y.; Korkanç, M.; Amiri, A.F. Effects of land use/cover change on heavy metal distribution of soils in wetlands and ecological risk assessment. Sci. Total Environ. 2024, 923, 171603. [Google Scholar] [CrossRef]
- China National Environmental Monitoring Centre. Background Values of Chinese Soil Elements; China Environmental Science Press: Beijing, China, 1990. [Google Scholar]
- De Nicola, F.; Maisto, G.; Alfani, A. Assessment of nutritional status and trace element contamination of holm oak woodlands through analyses of leaves and surrounding soils. Sci. Total Environ. 2003, 311, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ni, S.; Tuo, X.; Zhang, C. Calculation of heavy metals’ toxicity coefficient in the evaluation of potential ecological risk index. Environ. Sci. Technol. 2008, 31, 112–115. [Google Scholar]
- GB 2762-2022; National food safety standard—Limits of contaminants in food. National Health Commission of the People’s Republic of China: Beijing, China, 2018.
- NY 659-2003; Residue imits for chromium, cadmium, mercury, arsenic and fluoride in tea. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2003.
- Paatero, P. Least squares formulation of robust non-negative factor analysis. Chemom. Intell. Lab. Syst. 1997, 37, 23–35. [Google Scholar] [CrossRef]
- US EPA; Group, R.C.; Moya, J. Exposure Factors Handbook; BiblioGov: Washington, DC, USA, 1997.
- Zhou, L.; Zhao, X.; Teng, M.; Wu, F.; Meng, Y.; Wu, Y.; Byrne, P.; Abbaspour, K.C. Model-based evaluation of reduction strategies for point and nonpoint source Cd pollution in a large river system. J. Hydrol. 2023, 622, 129701. [Google Scholar] [CrossRef]
- Lwin, C.S.; Kim, Y.-N.; Lee, M.; Jung, H.-i.; Kim, K.-R. In Situ Immobilization of Potentially Toxic Elements in Arable Soil by Adding Soil Amendments and the Best Ways to Maximize Their Use Efficiency. J. Soil Sci. Plant Nutr. 2024, 24, 115–134. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 2010, 10, 40. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Mao, L.; Liu, S.; Mao, Y.; Ye, H.; Huang, T.; Li, F.; Chen, L. Enrichment and sources of trace metals in roadside soils in Shanghai, China: A case study of two urban/rural roads. Sci. Total Environ. 2018, 631–632, 942–950. [Google Scholar] [CrossRef]
- Chen, T.; Chang, Q.; Liu, J.; Clevers, J.G.P.W.; Kooistra, L. Identification of soil heavy metal sources and improvement in spatial mapping based on soil spectral information: A case study in northwest China. Sci. Total Environ. 2016, 565, 155–164. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Sun, X.; Wu, S.; Xia, L.; Sun, J.; Zha, Y.; Yang, P. Retrieval of chromium and mercury concentrations in agricultural soils: Using spectral information, environmental covariates, or a fusion of both? Ecol. Indic. 2024, 167, 112594. [Google Scholar] [CrossRef]
- Liu, S.; Pu, S.; Deng, D.; Huang, H.; Yan, C.; Ma, H.; Razavi, B.S. Comparable effects of manure and its biochar on reducing soil Cr bioavailability and narrowing the rhizosphere extent of enzyme activities. Environ. Int. 2020, 134, 105277. [Google Scholar] [CrossRef]
- Kung, H.C.; Wu, C.H.; Huang, B.W.; Chang-Chien, G.P.; Mutuku, J.K.; Lin, W.C. Mercury abatement in the environment: Insights from industrial emissions and fates in the environment. Heliyon 2024, 10, e28253. [Google Scholar] [CrossRef]
- Spivak-Birndorf, L.J.; Wang, S.-J.; Bish, D.L.; Wasylenki, L.E. Nickel isotope fractionation during continental weathering. Chem. Geol. 2018, 476, 316–326. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Soni, R.; Gupta, S.K.; Shukla, D.P. Mercury, arsenic, lead and cadmium in waters of the Singrauli coal mining and power plants industrial zone, Central East India. Environ. Monit. Assess. 2020, 192, 251. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, Q.; Wang, Y.; Wang, J.; Zhang, Y.; Pan, W.-P. Arsenic release and transformation in co-combustion of biomass and coal: Effect of mineral elements and volatile matter in biomass. Bioresour. Technol. 2020, 297, 122388. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Meng, Q.; Feng, Q.; Zhou, L.; Cui, Y.; Li, W. Occurrence and release of cadmium, chromium, and lead from stone coal combustion. Int. J. Coal Sci. Technol. 2019, 6, 586–594. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Wang, Z.; Guo, Y.; Yin, Y.; Zhang, X.; Cai, Y.; Jiang, G. Understanding foliar accumulation of atmospheric Hg in terrestrial vegetation: Progress and challenges. Crit. Rev. Environ. Sci. Technol. 2021, 52, 4331–4352. [Google Scholar] [CrossRef]
- Liu, C.; Wen, L.; Cui, Y.; Ahammed, G.J.; Cheng, Y. Metal transport proteins and transcription factor networks in plant responses to cadmium stress. Plant Cell Rep. 2024, 43, 218. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef]


| As | Cd | Cr | Hg | Ni | Pb | Zn | pH | |
|---|---|---|---|---|---|---|---|---|
| Min | 0.56 | 0.05 | 7.28 | 0.00 | 2.82 | 7.16 | 45.10 | 3.78 |
| Max | 6.91 | 0.29 | 294.00 | 0.11 | 116.00 | 52.40 | 118.00 | 7.80 |
| Mean | 2.56 | 0.15 | 58.96 | 0.02 | 22.18 | 25.01 | 82.65 | 5.58 |
| SE | 0.26 | 0.01 | 9.04 | 0.00 | 3.60 | 1.23 | 2.89 | 0.15 |
| CV (%) | 64.80 | 41.93 | 96.93 | 79.27 | 102.63 | 31.20 | 22.14 | 16.50 |
| Background value | 9.00 | 0.10 | 66.50 | 0.03 | 34.20 | 26.60 | 62.00 | — |
| t-value | −24.545 | 5.097 | −0.835 | −2.924 | −3.342 | −1.287 | 7.137 | — |
| p-value | 0.000 | 0.000 | 0.409 | 0.006 | 0.002 | 0.206 | 0.000 | — |
| As | Cd | Cr | Hg | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|
| Min | 0.05 | 0.01 | 0.24 | 0.00 | 0.94 | 0.12 | 12.10 |
| Max | 0.18 | 0.13 | 3.06 | 0.01 | 15.40 | 0.54 | 32.50 |
| Mean | 0.07 | 0.04 | 0.95 | 0.01 | 4.62 | 0.27 | 21.58 |
| SE | 0.00 | 0.00 | 0.10 | 0.00 | 0.46 | 0.01 | 0.63 |
| CV (%) | 31.99 | 61.83 | 64.01 | 35.63 | 62.66 | 35.38 | 18.34 |
| Criteria 1 | 2.00 | 5.00 | 5.00 | 0.30 | — | 5.00 | 50.00 |
| Criteria 2 | 0.10 | 0.30 | — | 0.10 | — | 0.50 | — |
| Pi | 0.007 ± 0.001 | 0.003 ± 0.000 | 0.061 ± 0.009 | 0.002 ± 0.001 | — | 0.015 ± 0.002 | 0.322 ± 0.015 |
| Over-standard rate | 2.5% | 0% | — | 0% | — | 2.5% | — |
| Source | HMs | Min | Max | Mean | SE | Contribution (%) | Min | Max | Mean | SE | Contribution (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-carcinogenic risks (Adults) | Non-carcinogenic risks (Children) | ||||||||||
| Soil | As | 8.8 × 10−4 | 1.7 × 10−1 | 1.3 × 10−2 | 9.9 × 10−3 | 22.78 | 3.2 × 10−3 | 4.1 × 10−1 | 4.6 × 10−2 | 3.2 × 10−2 | 22.90 |
| Cd | 1.1 × 10−5 | 3.1 × 10−3 | 2.6 × 10−4 | 2.1 × 10−4 | 0.43 | 5.5 × 10−5 | 1.7 × 10−2 | 8.7 × 10−4 | 6.9 × 10−4 | 0.44 | |
| Cr | 7.6 × 10−4 | 4.9 × 10−1 | 3.2 × 10−2 | 3.2 × 10−2 | 53.93 | 4.2 × 10−3 | 1.8 × 100 | 1.1 × 10−1 | 1.1 × 10−1 | 53.41 | |
| Hg | 5.5 × 10−6 | 1.8 × 10−3 | 1.3 × 10−4 | 1.1 × 10−4 | 0.22 | 2.1 × 10−5 | 5.2 × 10−3 | 4.4 × 10−4 | 3.6 × 10−4 | 0.22 | |
| Ni | 3.6 × 10−5 | 5.6 × 10−2 | 1.7 × 10−3 | 2.0 × 10−3 | 2.91 | 1.2 × 10−4 | 1.3 × 10−1 | 5.9 × 10−3 | 6.5 × 10−3 | 2.96 | |
| Pb | 1.7 × 10−3 | 4.7 × 10−2 | 1.1 × 10−2 | 5.0 × 10−3 | 19.00 | 5.9 × 10−3 | 1.6 × 10−1 | 3.9 × 10−2 | 1.5 × 10−2 | 19.33 | |
| Zn | 8.2 × 10−5 | 1.2 × 10−3 | 4.3 × 10−4 | 1.6 × 10−4 | 0.73 | 3.7 × 10−4 | 4.7 × 10−3 | 1.5 × 10−3 | 4.9 × 10−4 | 0.74 | |
| THI | 8.2 × 10−3 | 5.1 × 10−1 | 5.9 × 10−2 | 3.7 × 10−2 | 100.00 | 3.3 × 10−2 | 1.9 × 100 | 2.0 × 10−1 | 1.2 × 10−1 | 100.00 | |
| Carcinogenic risks (Adults) | Carcinogenic risks (Children) | ||||||||||
| Soil | As | 1.3 × 10−7 | 1.9 × 10−5 | 2.1 × 10−6 | 1.5 × 10−6 | 5.23 | 1.6 × 10−7 | 1.7 × 10−5 | 1.8 × 10−6 | 1.2 × 10−6 | 5.27 |
| Cd | 2.3 × 10−8 | 6.4 × 10−6 | 5.2 × 10−7 | 4.2 × 10−7 | 1.33 | 2.2 × 10−8 | 6.2 × 10−6 | 4.5 × 10−7 | 3.5 × 10−7 | 1.33 | |
| Cr | 2.7 × 10−7 | 7.1 × 10−4 | 1.7 × 10−5 | 1.9 × 10−5 | 42.32 | 3.1 × 10−7 | 2.1 × 10−4 | 1.4 × 10−5 | 1.4 × 10−5 | 41.31 | |
| Ni | 3.0 × 10−7 | 3.5 × 10−4 | 2.0 × 10−5 | 2.2 × 10−5 | 50.83 | 4.9 × 10−7 | 6.6 × 10−4 | 1.7 × 10−5 | 2.0 × 10−5 | 51.81 | |
| Pb | 2.1 × 10−8 | 4.7 × 10−7 | 1.2 × 10−7 | 5.0 × 10−8 | 0.29 | 2.2 × 10−8 | 3.4 × 10−7 | 9.8 × 10−8 | 3.9 × 10−8 | 0.29 | |
| TCR | 3.2 × 10−6 | 7.5 × 10−4 | 3.9 × 10−5 | 3.1 × 10−5 | 100.00 | 2.1 × 10−6 | 7.1 × 10−4 | 3.4 × 10−5 | 2.5 × 10−5 | 100.00 | |
| Non-carcinogenic risks (Adults) | Non-carcinogenic risks (Children) | ||||||||||
| Tea | As | 1.8 × 10−7 | 7.0 × 10−6 | 1.8 × 10−6 | 7.9 × 10−7 | 5.56 | 1.7 × 10−7 | 7.0 × 10−6 | 1.5 × 10−6 | 7.1 × 10−7 | 8.65 |
| Cd | 3.3 × 10−7 | 2.3 × 10−5 | 2.9 × 10−6 | 1.9 × 10−6 | 8.99 | 1.4 × 10−7 | 1.1 × 10−5 | 1.4 × 10−6 | 9.2 × 10−7 | 8.13 | |
| Cr | 1.8 × 10−6 | 2.1 × 10−4 | 2.5 × 10−5 | 1.7 × 10−5 | 76.77 | 6.2 × 10−7 | 9.6 × 10−5 | 1.2 × 10−5 | 8.2 × 10−6 | 69.36 | |
| Hg | 9.1 × 10−8 | 1.3 × 10−6 | 3.9 × 10−7 | 1.6 × 10−7 | 1.20 | 4.8 × 10−8 | 9.4 × 10−7 | 2.5 × 10−7 | 1.1 × 10−7 | 1.42 | |
| Ni | 4.2 × 10−7 | 3.2 × 10−6 | 1.5 × 10−6 | 4.6 × 10−7 | 4.54 | 1.8 × 10−7 | 2.8 × 10−6 | 1.3 × 10−6 | 4.6 × 10−7 | 7.57 | |
| Pb | 7.0 × 10−8 | 2.5 × 10−6 | 4.9 × 10−7 | 2.4 × 10−7 | 1.52 | 3.8 × 10−8 | 2.0 × 10−6 | 4.4 × 10−7 | 2.3 × 10−7 | 2.51 | |
| Zn | 9.4 × 10−8 | 1.4 × 10−6 | 4.6 × 10−7 | 1.7 × 10−7 | 1.42 | 5.4 × 10−8 | 1.2 × 10−6 | 4.2 × 10−7 | 1.7 × 10−7 | 2.37 | |
| THI | 5.5 × 10−6 | 2.2 × 10−4 | 3.2 × 10−5 | 1.7 × 10−5 | 100.00 | 2.8 × 10−6 | 1.0 × 10−4 | 1.8 × 10−5 | 8.6 × 10−6 | 100.00 | |
| Carcinogenic risks (Adults) | Carcinogenic risks (Children) | ||||||||||
| Tea | As | 4.8 × 10−11 | 1.8 × 10−9 | 3.3 × 10−10 | 1.5 × 10−10 | 0.89 | 6.8 × 10−12 | 5.3 × 10−10 | 6.5 × 10−11 | 3.3 × 10−11 | 0.99 |
| Cd | 2.5 × 10−11 | 4.8 × 10−9 | 5.2 × 10−10 | 3.9 × 10−10 | 1.40 | 2.2 × 10−12 | 1.0 × 10−9 | 1.1 × 10−10 | 8.5 × 10−11 | 1.74 | |
| Cr | 8.8 × 10−10 | 1.1 × 10−7 | 1.1 × 10−8 | 7.4 × 10−9 | 30.25 | 1.2 × 10−10 | 1.8 × 10−8 | 1.4 × 10−9 | 9.9 × 10−10 | 21.43 | |
| Ni | 5.6 × 10−9 | 1.2 × 10−7 | 2.5 × 10−8 | 1.1 × 10−8 | 67.43 | 5.4 × 10−10 | 1.0 × 10−7 | 5.0 × 10−9 | 3.0 × 10−9 | 75.82 | |
| Pb | 9.9 × 10−13 | 3.9 × 10−11 | 7.1 × 10−12 | 3.2 × 10−12 | 0.02 | 1.6 × 10−13 | 5.4 × 10−12 | 1.4 × 10−12 | 6.6 × 10−13 | 0.02 | |
| TCR | 9.4 × 10−9 | 1.8 × 10−7 | 3.7 × 10−8 | 1.4 × 10−8 | 100.00 | 1.1 × 10−9 | 1.2 × 10−7 | 6.6 × 10−9 | 3.5 × 10−9 | 100.00 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, M.; Liu, T.; Huang, J.; Xu, H.; Jiang, T.; Xie, X.; Yang, Y. Heavy Metal(oid)s in Soil–Tea System: Sources, Bioaccumulation, and Risks in Eastern Dabie Mountain. Land 2025, 14, 1269. https://doi.org/10.3390/land14061269
Luo M, Liu T, Huang J, Xu H, Jiang T, Xie X, Yang Y. Heavy Metal(oid)s in Soil–Tea System: Sources, Bioaccumulation, and Risks in Eastern Dabie Mountain. Land. 2025; 14(6):1269. https://doi.org/10.3390/land14061269
Chicago/Turabian StyleLuo, Minxuan, Tian Liu, Jinyan Huang, Honggen Xu, Ting Jiang, Xiang Xie, and Yujing Yang. 2025. "Heavy Metal(oid)s in Soil–Tea System: Sources, Bioaccumulation, and Risks in Eastern Dabie Mountain" Land 14, no. 6: 1269. https://doi.org/10.3390/land14061269
APA StyleLuo, M., Liu, T., Huang, J., Xu, H., Jiang, T., Xie, X., & Yang, Y. (2025). Heavy Metal(oid)s in Soil–Tea System: Sources, Bioaccumulation, and Risks in Eastern Dabie Mountain. Land, 14(6), 1269. https://doi.org/10.3390/land14061269








