Geochemical Regulation of Heavy Metal Speciation in Subtropical Peatlands: A Case Study in Dajiuhu Peatland
Abstract
1. Introduction
2. Materials and Method
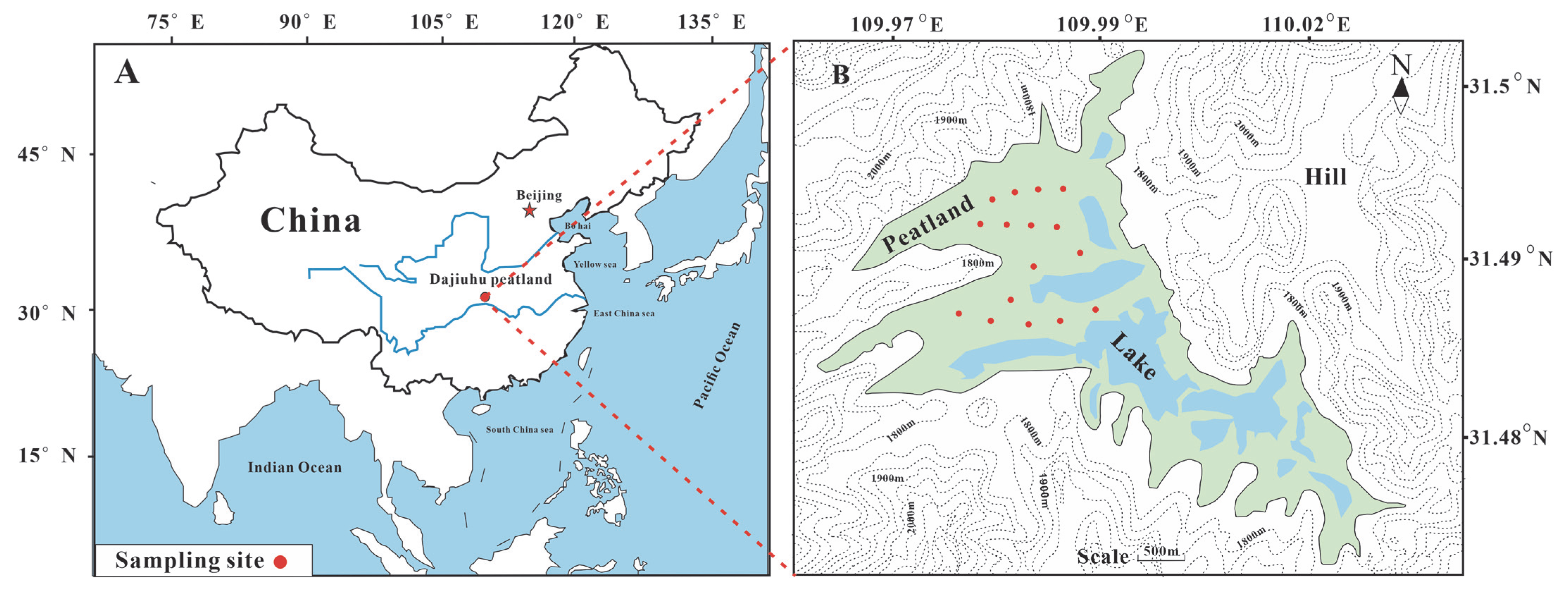
2.1. Study Area and Sample Preparation
2.2. Experimental Methods
2.3. Statistical Analyses
3. Results and Discussion
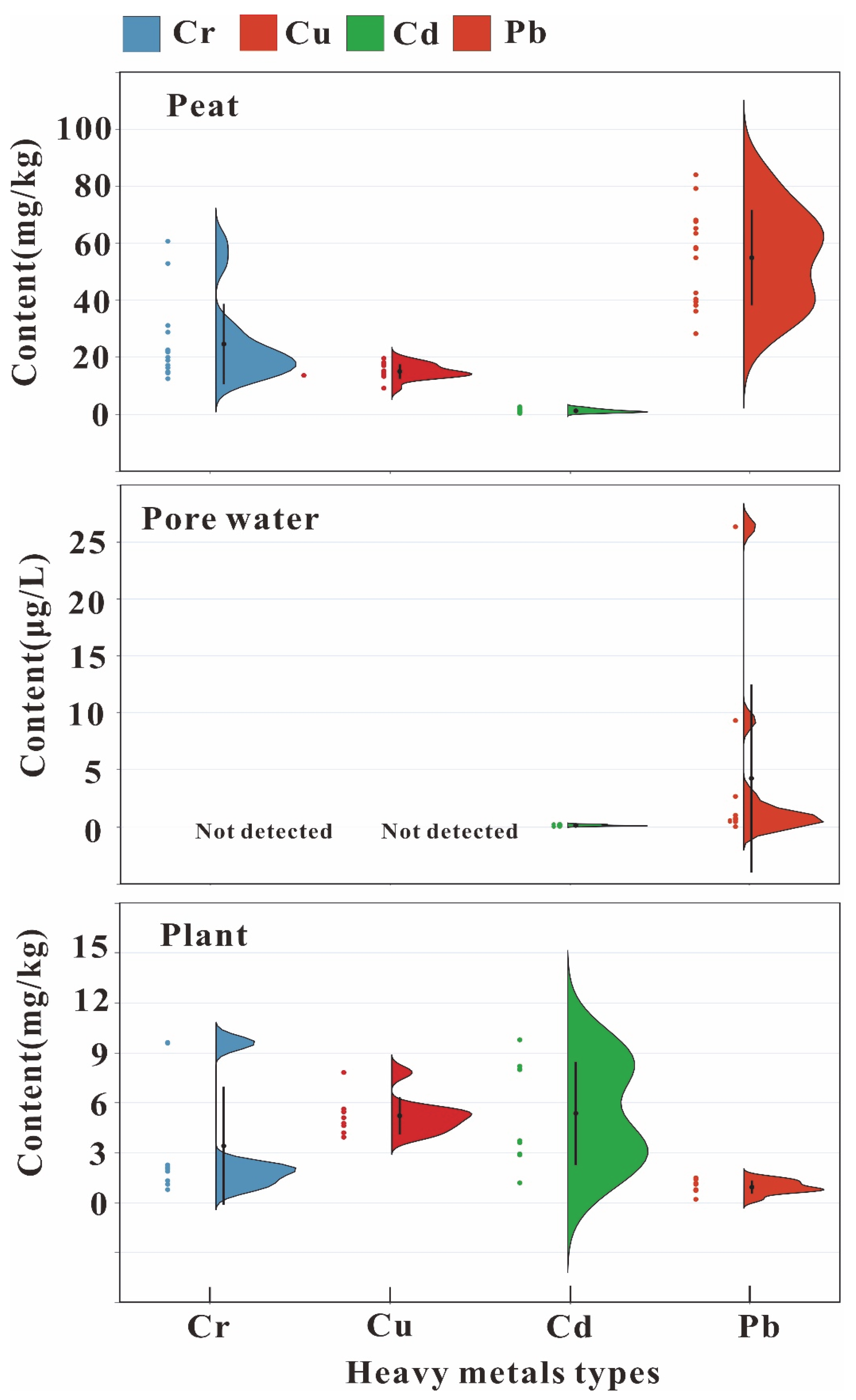
3.1. Descriptive Statistics of Heavy Metals and Chemical Parameters in Soil, Moss, and Pore Water
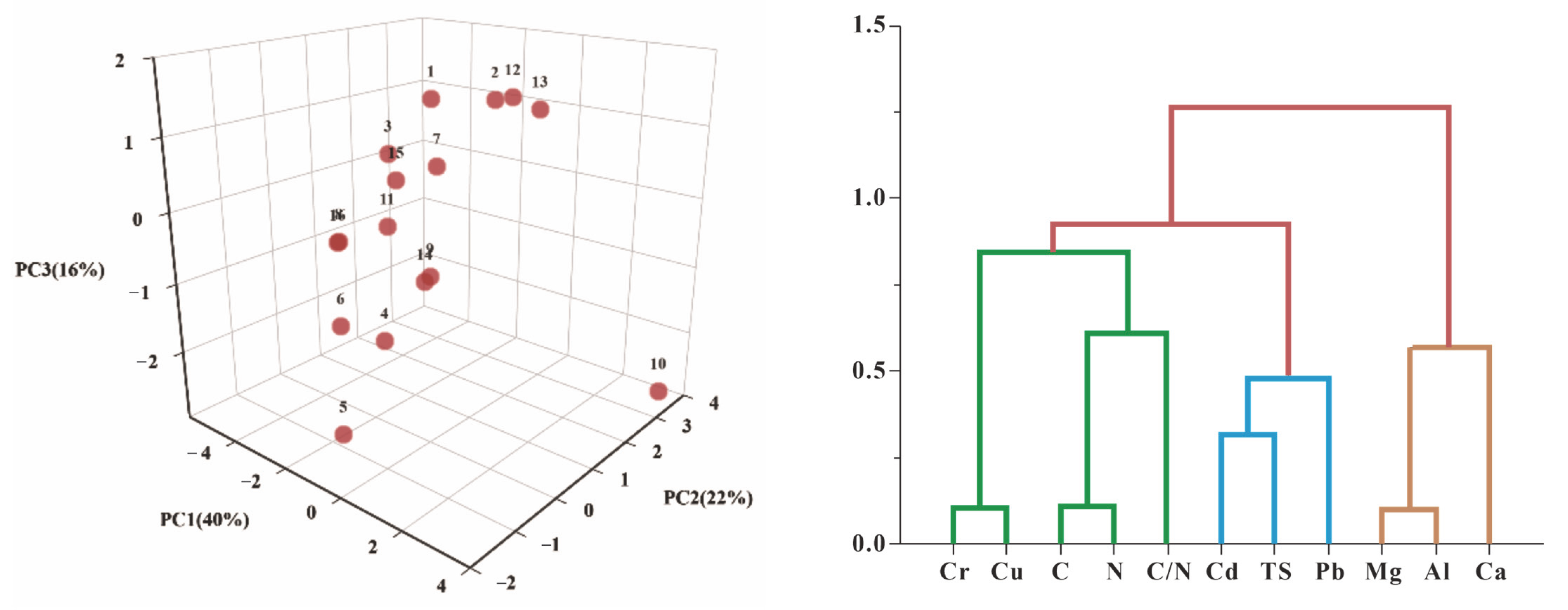
3.2. Sources of Heavy Metals in Peat
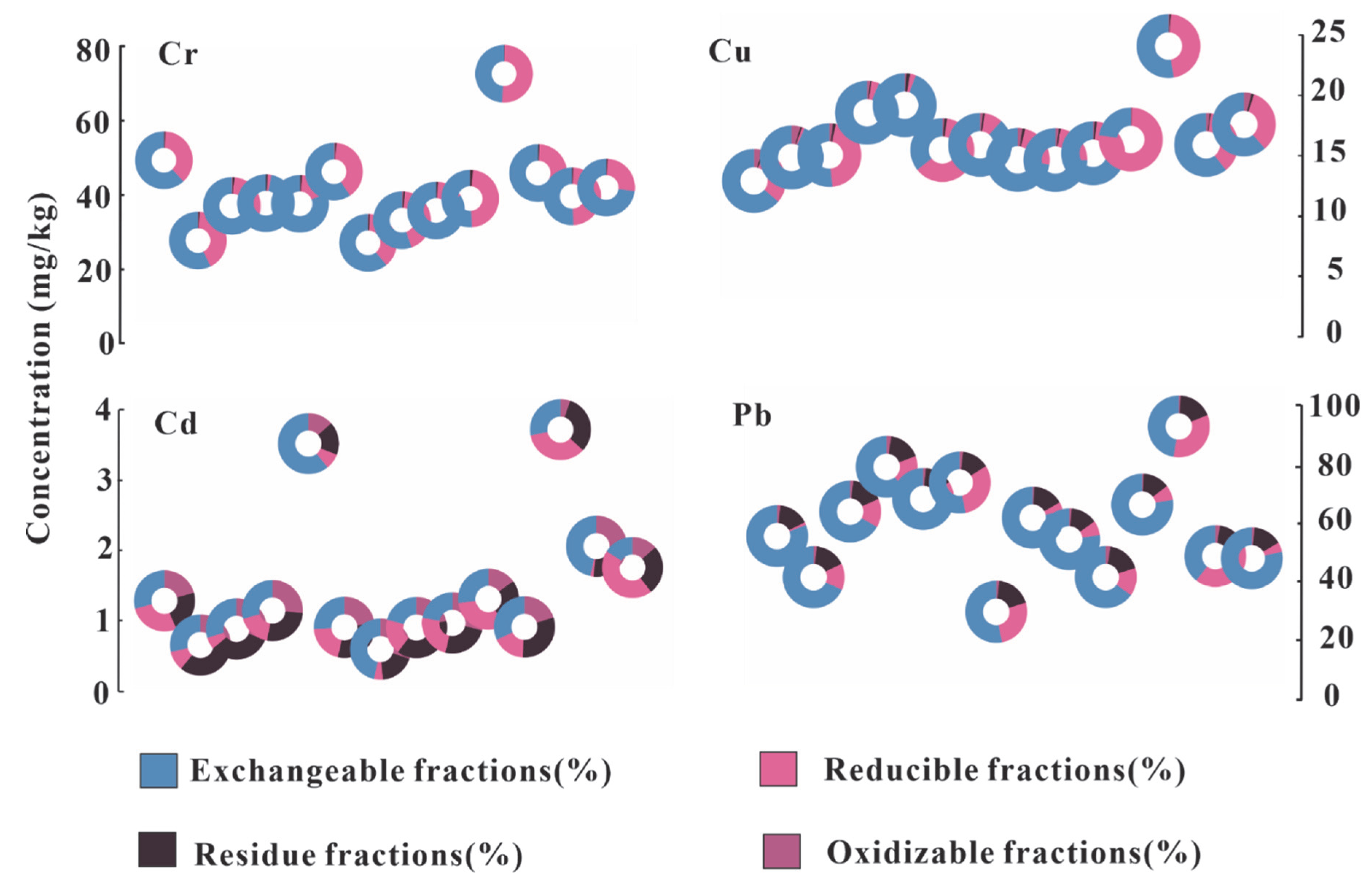
3.3. Heavy Metal Speciation in Sediments
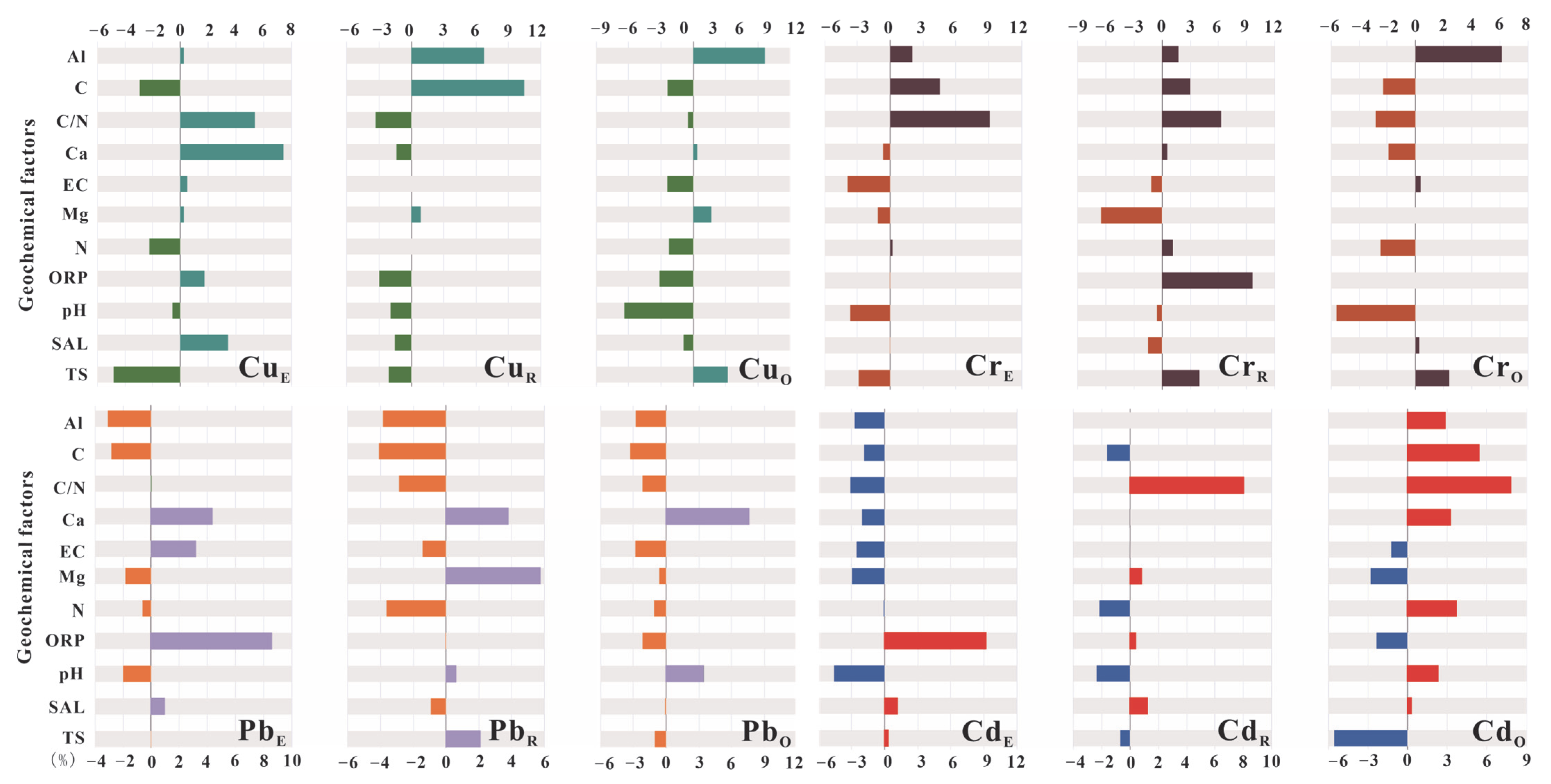
3.4. Geochemical Factors Affecting the Distribution of Heavy Metals in Peat
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, F.; Xu, Y.; Tan, Z.; Wu, Z.; Xue, H.; Shen, L.; Xu, X.; Han, Q.; Guo, H. Assessment of Pollution and Identification of Sources of Heavy Metals in Sediments from West Coast of Shenzhen, China. Environ. Sci. Pollut. Res. 2018, 25, 3647–3656. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhao, Q.; Lu, Q.; Wang, J.; Reddy, K.R. Effects of Freshwater Input on Trace Element Pollution in Salt Marsh Soils of a Typical Coastal Estuary, China. J. Hydrol. 2015, 520, 186–192. [Google Scholar] [CrossRef]
- Xiao, H.; Shahab, A.; Li, J. Distribution, Ecological Risk Assessment and Source Identification of Heavy Metals in Surface Sediments of Huixian Karst Wetland, China. Ecotoxicol. Environ. Saf. 2019, 185, 109700. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Y.; Li, L.; Tang, M.; Hu, W.; Chen, L.; Ai, S. Speciation of Heavy Metals in Soils and Their Immobilization at Micro-Scale Interfaces Among Diverse Soil Components. Sci. Total Environ. 2022, 825, 153862. [Google Scholar] [CrossRef]
- Robledo Ardila, P.A.; Álvarez-Alonso, R.; Árcega-Cabrera, F.; Valsero, J.J.D.; García, R.M.; Lamas-Cosío, E.; Oceguera-Vargas, I.; DelValls, A. Assessment and Review of Heavy Metals Pollution in Sediments of the Mediterranean Sea. Appl. Sci. 2024, 14, 1435. [Google Scholar] [CrossRef]
- Gandois, L.; Hoyt, A.M.; Mounier, S. From Canals to the Coast: Dissolved Organic Matter and Trace Metal Composition in Rivers Draining Degraded Tropical Peatlands in Indonesia. Biogeosciences 2020, 17, 1897–1909. [Google Scholar] [CrossRef]
- Hoang, H.G.; Hadi, M.; Nguyen, M.K. Assessing Heavy Metal Pollution Levels and Associated Ecological Risks in Peatland Areas in the Mekong Delta Region. Environ. Res. 2025, 274, 121319. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Y.; Ding, M. Level, Source Identification, and Risk Analysis of Heavy Metal in Surface Sediments from River-Lake Ecosystems in Poyang Lake, China. Environ. Sci. Pollut. Res. 2017, 24, 21902–21916. [Google Scholar] [CrossRef] [PubMed]
- Rudolf von Rohr, M.; Hering, J.G.; Kohler, H.P. Column Studies to Assess the Effects of Climate Variables on Redox Processes during Riverbank Filtration. Water Res. 2014, 61, 263–275. [Google Scholar] [CrossRef]
- Hudelson, K.E.; Muir, D.C.G.; Drevnick, P.E.; Kock, G.; Iqaluk, D.; Wang, X.; Kirk, J.L. Temporal Trends, Lake-to-Lake Variation, and Climate Effects on Arctic Char (Salvelinus alpinus) Mercury Concentrations from Six High Arctic Lakes in Nunavut, Canada. Sci. Total Environ. 2019, 678, 801–812. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, J.; Huang, X. Geochemical Cycle of Mercury Associated with Wet Deposition and Inflows in a Subalpine Wetland. Ecotoxicol. Environ. Saf. 2021, 208, 111507. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, P.; Drivel, T.C.; Bushey, T.J. Wetland Influence on Mercury Fate and Transport in a Temperate Forested Watershed. Environ. Pollut. 2008, 154, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huo, S.; Yeager, K.M. A Historical Sedimentary Record of Mercury in a Shallow Eutrophic Lake: Impacts of Human Activities and Climate Change. Engineering 2019, 5, 296–304. [Google Scholar] [CrossRef]
- Muller, F.L.L.; Chang, K.C.; Lee, C.L. Effects of Temperature, Rainfall and Conifer Felling Practices on the Surface Water Chemistry of Northern Peatlands. Biogeochemistry 2015, 126, 343–362. [Google Scholar] [CrossRef]
- Punia, A. Role of Temperature, Wind, and Precipitation in Heavy Metal Contamination at Copper Mines: A Review. Environ. Sci. Pollut. Res. 2021, 28, 4056–4072. [Google Scholar] [CrossRef]
- Chen, K.Y.; Liu, Y.T.; Hsieh, Y.C. Organic Fragments Newly Released from Heat-Treated Peat Soils Create Synergies with Dissolved Organic Carbon to Enhance Cr(VI) Removal. Ecotoxicol. Environ. Saf. 2020, 201, 110800. [Google Scholar] [CrossRef]
- Lim, A.G.; Krickov, I.V.; Pokrovsky, O.S. Organic Carbon, Major and Trace Element Release from and Adsorption onto Particulate Suspended Matter of the Ob River, Western Siberia. Sci. Total Environ. 2024, 948, 174735. [Google Scholar] [CrossRef] [PubMed]
- Mastný, J.; Kaštovská, E.; Bárta, J. Quality of DOC Produced during Litter Decomposition of Peatland Plant Dominants. Soil Biol. Biochem. 2018, 121, 221–230. [Google Scholar] [CrossRef]
- Szajdak, L.W.; Jezierski, A.; Wegner, K. Influence of Drainage on Peat Organic Matter: Implications for Development, Stability, and Transformation. Molecules 2020, 25, 2587. [Google Scholar] [CrossRef]
- Ratcliffe, J.L.; Lowe, D.J.; Schipper, L.A. Rapid Carbon Accumulation in a Peatland Following Late Holocene Tephra Deposition, New Zealand. Quat. Sci. Rev. 2020, 246, 106505. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.; Bisson, I.M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Davidson, C.M.; Thomas, R.P.; Mcvey, S.E. Evaluation of a Sequential Extraction Procedure for the Speciation of Heavy Metals in Sediments. Anal. Chim. Acta 1994, 291, 277–286. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Chemical Sequential Extraction for Metal Partitioning in Environmental Solid Samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef]
- Coetzee, P.P. Determination and Speciation of Heavy Metals in Sediments of the Hartbeespoort Dam by Sequential Chemical Extraction. Water SA 1993, 19, 291–300. [Google Scholar]
- Chowdhury, A.; Naz, A.; Maiti, S.K. Distribution, Speciation, and Bioaccumulation of Potentially Toxic Elements in the Grey Mangroves at Indian Sundarbans, in Relation to Vessel Movements. Mar. Environ. Res. 2023, 189, 106042. [Google Scholar] [CrossRef] [PubMed]
- Kozyatnyk, I.; Bouchet, S.; Bjorn, E.; Haglund, P. Fractionation and Size-Distribution of Metal and Metalloid Contaminants in a Polluted Groundwater Rich in Dissolved Organic Matter. J. Hazard. Mater. 2016, 318, 194–202. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, M.; Cui, Z. Assessment of Bioavailability of Selenium in Different Plant-Soil Systems by Diffusive Gradients in Thin-Films (DGT). Environ. Pollut. 2017, 225, 637–643. [Google Scholar] [CrossRef]
- Omanović, D.; Pizeta, I.; Vukosav, P. Assessing Element Distribution and Speciation in a Stream at Abandoned Pb-Zn Mining Site by Combining Classical, In-Situ DGT and Modelling Approaches. Sci. Total Environ. 2015, 511, 423–434. [Google Scholar] [CrossRef]
- Lu, H.; Mei, D.; Pavao-Zuckerman, M. Combination of DGT and Fluorescence Spectroscopy for Improved Understanding of Metal Behaviour in Mangrove Wetland. Chemosphere 2019, 229, 303–313. [Google Scholar] [CrossRef]
- Song, X.; Ning, Y.; Yang, S. Spatial Distribution, Pollution, and Ecological Risk Assessment of Metal(Loid)s in Multiple Spheres of the Shennongjia Alpine Critical Zone, Central China. Int. J. Environ. Res. Public Health 2023, 20, 1126. [Google Scholar] [CrossRef]
- Luo, K.; Liu, H.; Yu, E. Distribution and Release Mechanism of Heavy Metals in Sediments of Yelang Lake by DGT. Stoch. Environ. Res. Risk Assess. 2020, 34, 793–805. [Google Scholar] [CrossRef]
- Wali, A.; Colinet, G.; Ksibi, M. Speciation of Heavy Metals by Modified BCR Sequential Extraction in Soils Contaminated by Phosphogypsum in Sfax, Tunisia. Environ. Res. Eng. Manag. 2014, 70, 14–25. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 475–490. [Google Scholar]
- Zhou, X.; Wu, H.; Pan, J.; Chen, H.; Jin, B.; Yan, Z.; Xie, L.; Rogers, K.M. Geographical traceability of south-east Asian durian: A chemometric study using stable isotopes and elemental compositions. J. Food Compos. Anal. 2021, 101, 103940. [Google Scholar] [CrossRef]
- Reimann, C.; Caritat, P.D. Distinguishing between natural and anthropogenic sources for elements in the environment: Regional geochemical surveys versus enrichment factors. Sci. Total Environ. 2005, 337, 91–107. [Google Scholar] [CrossRef]
- Yan, J.L.; Yu, Z.L.; Yu, H.L.; Xiang, M.D.; Wang, C.H. Heavy Metal Pollution Characteristics and Risk Assessment of Golden Snub-Nosed Monkey (Rhinopithecus roxellana) Habitat in Shennongjia Mountains. Huanjing Kexue 2022, 43, 3288–3298. [Google Scholar]
- GB 15618-2018; Soil Environment Quality Risk Control Standard for Soil Contamination of Agriculture Land. Chinese Ministry of Environmental Protection: Beijing, China, 2015.
- Liu, M.; Han, X.; Liu, C.Q. Differences in the Spectroscopic Characteristics of Wetland Dissolved Organic Matter Binding with Fe3+, Cu2+, Cd2+, Cr3+ and Zn2+. Sci. Total Environ. 2021, 800, 149476. [Google Scholar] [CrossRef]
- GB3838-2002; Environmental Quality Standards for Surface Water. China Environmental Science Press: Beijing, China, 2002.
- Hernandez-Soriano, M.C.; Jimenez-Lopez, J.C. Effects of Soil Water Content and Organic Matter Addition on the Speciation and Bioavailability of Heavy Metals. Sci. Total Environ. 2012, 423, 55–61. [Google Scholar] [CrossRef]
- Fan, Q.; Sun, J.; Quan, G. Insights into the Effects of Long-Term Biochar Loading on Water-Soluble Organic Matter in Soil: Implications for the Vertical Co-Migration of Heavy Metals. Environ. Int. 2020, 136, 105439. [Google Scholar] [CrossRef]
- Ge, Y.S.; Cao, Y.L.; Zeng, C.H.; Li, Z.Q.; Wang, L. Monitoring Heavy Metal Pollutions in Chengdu Atmosphere Using Terrestrial Bryophytes. Ecol. Environ. Sci. 2013, 22, 844–850. (In Chinese) [Google Scholar]
- Bing, H.J.; Wu, Y.H.; Li, J.; Xiang, Z.X.; Luo, X.S.; Zhou, J.; Sun, H.Y.; Zhang, G. Biomonitoring Trace Element Contamination Impacted by Atmospheric Deposition in China’s Remote Mountains. Atmos. Res. 2019, 224, 30–41. [Google Scholar] [CrossRef]
- Shotyk, W.; Bicalho, B.; Cuss, C.W. Dust is the Dominant Source of “Heavy Metals” to Peat Moss (Sphagnum fuscum) in the Bogs of the Athabasca Bituminous Sands Region of Northern Alberta. Environ. Int. 2016, 92, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, L.; Zhang, X. Changes in Organic Carbon and Nitrogen in Soil with Metal Pollution by Cd, Cu, Pb and Zn: A Meta-Analysis. Eur. J. Soil Sci. 2016, 67, 237–246. [Google Scholar] [CrossRef]
- Huang, X.Y.; Xue, J.T.; Wang, X.X.; Meyers, P.A.; Huang, J.H.; Xie, S.C. Paleoclimate Influence on Early Diagenesis of Plant Triterpenes in the Dajiuhu Peatland, Central China. Geochim. Cosmochim. Acta 2013, 23, 106–119. [Google Scholar] [CrossRef]
- Zakharova, E.A.; Pokrovsky, O.S.; Dupré, B. Chemical Weathering of Silicate Rocks in Karelia Region and Kola Peninsula, NW Russia: Assessing the Effect of Rock Composition, Wetlands and Vegetation. Chem. Geol. 2007, 242, 255–277. [Google Scholar] [CrossRef]
- Das, R.; Mohtar, A.T.B.M.; Rakshit, D. Sources of Atmospheric Lead (Pb) in and Around an Indian Megacity. Atmos. Environ. 2018, 193, 57–65. [Google Scholar] [CrossRef]
- Connan, O.; Maro, D.; Hébert, D. Wet and Dry Deposition of Particle-Associated Metals (Cd, Pb, Zn, Ni, Hg) in a Rural Wetland Site, Marais Vernier, France. Atmos. Environ. 2013, 67, 394–403. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Wang, P. Inputs and Sources of Pb and Other Metals in Urban Area in the Post Leaded Gasoline Era. Environ. Pollut. 2022, 306, 119389. [Google Scholar] [CrossRef]
- Liu, Z.H. Effects of Plastics Types and Sizes on Microplastic Aging in Composting Process and Its Cd Adsorption. Master’s Thesis, Guilin University of Technology, Guilin, China, 2024; pp. 64–68. [Google Scholar]
- Frierdich, A.J.; Scherer, M.M.; Bachman, J. Inhibition of Trace Element Release during Iron(II)-Activated Recrystallization of Aluminum-, Chromium-, and Tin-Substituted Iron Oxide Minerals. Trace Elem. Cycl. Iron Oxide Miner. 2012, 1001, 100. [Google Scholar]
- Fulda, B. Changes in Copper and Cadmium Solubility and Speciation Induced by Soil Redox Dynamics: Competitive Metal Sulfide Formation and Interactions with Natural Organic Matter. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2013. [Google Scholar]
- Li, M.; Kuang, S.; Kang, Y. Recent Advances in Application of Fe/Mn Oxide Nanomaterials for Removal of Heavy Metals in the Aquatic Environment. Sci. Total Environ. 2022, 819, 153157. [Google Scholar] [CrossRef]
- Kaur, N.; Gräfe, M.; Singh, B. Simultaneous Incorporation of Cr, Zn, Cd, and Pb in the Goethite Structure. Clays Clay Miner. 2009, 57, 234–250. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Chen, T. An Insight into the Removal of Pb(II), Cu(II), Co(II), Cd(II), Zn(II), Ag(I), Hg(I), Cr(VI) by Na(I)-Montmorillonite and Ca(II)-Montmorillonite. Appl. Clay Sci. 2015, 118, 239–247. [Google Scholar] [CrossRef]
- Chou, P.I.; Ng, D.Q.; Li, I.C. Effects of Dissolved Oxygen, pH, Salinity and Humic Acid on the Release of Metal Ions from PbS, CuS, and ZnS during a Simulated Storm Event. Sci. Total Environ. 2018, 624, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Utrilla, J.; Bautista-Toledo, I.; Ferro-Garcıa, M.A.; Moreno-Castilla, C. Bioadsorption of Pb (II), Cd (II), and Cr (VI) on activated carbon from aqueous solutions. Carbon 2003, 41, 323–330. [Google Scholar] [CrossRef]
- Biester, H.; Knorr, K.H.; Schellekens, J. Comparison of Different Methods to Determine the Degree of Peat Decomposition in Peat Bogs. Biogeosciences 2014, 11, 2691–2707. [Google Scholar] [CrossRef]
- Li, Y.; Gong, X. Effects of Dissolved Organic Matter on the Bioavailability of Heavy Metals during Microbial Dissimilatory Iron Reduction: A Review. Rev. Environ. Contam. Toxicol. 2021, 257, 69–92. [Google Scholar]
- Zhang, S.; Peiffer, S.; Liao, X. Sulfidation of Ferric (Hydr)Oxides and Its Implication on Contaminants Transformation: A Review. Sci. Total Environ. 2022, 816, 151574. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Dahlgren, R.A. Nature, Properties and Function of Aluminum–Humus Complexes in Volcanic Soils. Geoderma 2016, 263, 110–121. [Google Scholar] [CrossRef]
- Papadas, I.T.; Kosma, C.; Deligiannakis, Y. Ternary [Al2O3–Electrolyte–Cu2+] Species: EPR Spectroscopy and Surface Complexation Modeling. J. Colloid Interface Sci. 2009, 339, 19–30. [Google Scholar] [CrossRef]
- Grybos, M.; Davranche, M.; Gruau, G. Is Trace Metal Release in Wetland Soils Controlled by Organic Matter Mobility or Fe-Oxyhydroxides Reduction? J. Colloid. Interface Sci. 2007, 314, 490–501. [Google Scholar] [CrossRef]
- Grybos, M.; Davranche, M.; Gruau, G. Increasing pH Drives Organic Matter Solubilization from Wetland Soils under Reducing Conditions. Geoderma 2009, 154, 13–19. [Google Scholar] [CrossRef]
- Su, R.; Li, C.; He, M. Catalytic Oxidation of Mn(II) on Ferrihydrite and Goethite Surfaces and the Subsequent Oxidation and Immobilization of Coexisting Cr(III). Appl. Geochem. 2024, 175, 106195. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Huang, X. Adsorption of Pb2+ from Aqueous Solutions Using Fe–Mn Binary Oxides-Loaded Biochar: Kinetics, Isotherm and Thermodynamic Studies. Environ. Technol. 2019, 40, 1853–1861. [Google Scholar] [CrossRef]
- Magdziak, Z.; Mleczek, M.; Kaczmarek, Z. Influence of Ca/Mg Ratio and Cd2+ and Pb2+ Elements on Low Molecular Weight Organic Acid Secretion by Salix viminalis L. Roots into Rhizosphere. Trees 2013, 27, 663–673. [Google Scholar] [CrossRef][Green Version]
- Li, Z.; Zhang, Z.; Xue, Z. Molecular Fingerprints of Soil Organic Matter in a Typical Freshwater Wetland in Northeast China. Chin. Geogr. Sci. 2019, 29, 700–708. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, L.X.; Xu, J.T. Release of Dissolved Organic Matter from Wetland Plants and Its Interaction with Polycyclic Aromatic Hydrocarbons. Environ. Res. 2023, 237, 116913. [Google Scholar] [CrossRef] [PubMed]
- Basti, S.; Sahu, C.; Pati, S.S. Impact of Organic Carbon on Heavy Metals of River Sediments and Aquatic Ecosystems: A Review. Environ. Qual. Manag. 2024, 34, 22253. [Google Scholar] [CrossRef]
- Kaplan, D.I.; Xu, C.; Huang, S. Unique Organic Matter and Microbial Properties in the Rhizosphere of a Wetland Soil. Environ. Sci. Technol. 2016, 50, 4169–4177. [Google Scholar] [CrossRef]
- Sóvágó, I.; Várnagy, K. Cadmium(II) Complexes of Amino Acids and Peptides. In Cadmium: From Toxicity to Essentiality; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 275–302. [Google Scholar]
- Lyu, P.; Li, L.; Huang, X. Ternary Ca–Mg–Al Layered Double-Hydroxides for Synergistic Remediation of As, Cd, and Pb from Both Contaminated Soil and Groundwater: Characteristics, Effectiveness, and Immobilization Mechanisms. J. Hazard. Mater. 2023, 442, 130030. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Ning, Y.; Liu, C.; Song, X.; Pang, Y.; Li, Q.; Yang, M.; Zeng, L. Geochemical Regulation of Heavy Metal Speciation in Subtropical Peatlands: A Case Study in Dajiuhu Peatland. Land 2025, 14, 1256. https://doi.org/10.3390/land14061256
Lu Z, Ning Y, Liu C, Song X, Pang Y, Li Q, Yang M, Zeng L. Geochemical Regulation of Heavy Metal Speciation in Subtropical Peatlands: A Case Study in Dajiuhu Peatland. Land. 2025; 14(6):1256. https://doi.org/10.3390/land14061256
Chicago/Turabian StyleLu, Zhuo, Yongqiang Ning, Chutong Liu, Xiannong Song, Yong Pang, Quanheng Li, Minglong Yang, and Liang Zeng. 2025. "Geochemical Regulation of Heavy Metal Speciation in Subtropical Peatlands: A Case Study in Dajiuhu Peatland" Land 14, no. 6: 1256. https://doi.org/10.3390/land14061256
APA StyleLu, Z., Ning, Y., Liu, C., Song, X., Pang, Y., Li, Q., Yang, M., & Zeng, L. (2025). Geochemical Regulation of Heavy Metal Speciation in Subtropical Peatlands: A Case Study in Dajiuhu Peatland. Land, 14(6), 1256. https://doi.org/10.3390/land14061256





