Abstract
Aiming at the remediation of soil in mining areas caused by mining activities, pot experiments were conducted using water jet-loom sludge (WJLS) and biochar as soil amendments to evaluate their potential for enhancing soil fertility and microbial communities of degraded mining soils. Six treatments with varying WJLS (0%, 5%, 15%) and biochar (0%, 3%) application rates were evaluated. Results showed that WJLS can significantly improve soil organic carbon (OC), total nitrogen (TN), total phosphorus (TP), and microbial biomass, while reducing soil pH and enhancing ryegrass biomass by 1.6–4.1 times. However, a 3% biochar addition may increase the soil sodium absorption ratio (SAR). Moreover, the role of biochar was mainly reflected in the microbiological properties. The combining of WJLS and biochar increased the soil microbial biomass and obviously improved the diversity and abundance of bacteria and fungi in the soil (p < 0.05) after the amendment, especially in the biochar addition groups. At the phylum level, the relative abundance of Proteobacteria, Firmicutes, and Actinobacteriota accounted for 72.4%~84.2% of soil bacteria in all treatments, while the fungi were dominated by Ascomycota (58.30%~95.36%) and Fungi_unclassified (1.26%~38.97%), all of which were significantly related to enhanced soil properties especially OC, TN, TP, and cation exchange capacity (CEC). Overall, WJLS and biochar demonstrate strong potential as sustainable amendments for improving soil fertility and biological quality in the reclamation of mining-affected lands.
1. Introduction
Lands in open-pit mining areas of China, particularly in the western and northwestern regions, have been severely affected by intensive mining activities and arid climatic conditions. These disturbances often result in the degradation of native soils, which are commonly transformed into sandy or sandy loam textures with diminished fertility [1]. Such soils are characterized by poor structural integrity, low organic matter content, limited microbial activity, and inadequate water-holding capacity. Furthermore, soluble salts from the subsoil or groundwater can migrate upward through capillary action and evaporative processes, leading to their accumulation in the surface layer and subsequent soil salinization [2,3]. These adverse conditions—including low moisture retention and salt-alkali stress—substantially reduce seed germination rates and significantly impede plant establishment and growth [4,5]. Soil quality is intrinsically linked to soil functionality, with both soil organic matter and biological components playing pivotal roles in enhancing overall soil performance, which is essential for maintaining soil functions and supporting sustainable plant growth [6]. Moreover, soil biodiversity is closely associated with functional diversity, and greater biodiversity is fundamental to the stability and resilience of ecosystem functions [7]. Although efforts to restore soils in open-pit mining areas within arid and semi-arid regions have increased, these restoration practices often depend heavily on artificial irrigation, leading to elevated costs [8] and limited long-term ecological sustainability [9]. Therefore, improving the quality of sandy loam soils through efficient organic amendment-based remediation is of great significance. This strategy not only increases soil organic matter content but also enhances soil structure and stimulates microbial abundance and diversity, thereby fostering a healthier and more functional soil ecosystem conducive to plant establishment and growth.
Biochar and sludge are two organic amendments rich in OC and essential nutrients such as nitrogen and phosphorus and are widely employed in soil remediation and quality enhancement studies [10,11,12]. Sludge, whether applied in its fresh or composted form, has been proposed as a cost-effective strategy for nutrient recovery from organic waste and for improving soil fertility [13]. WJLS, a type of textile sludge generated after the treatment of water-jet loom wastewater through membrane biotechnologies, is characterized by high clay and organic matter content, making it a promising amendment for enhancing soil OC levels and water-holding capacity. A recent study has demonstrated that WJLS modified with microbial inoculants can significantly improve the organic matter, nitrogen, and phosphorus content of degraded mining soils, thereby promoting plant growth [8]. Similarly, both fresh and composted textile sludge have been shown to enhance soil microbial biomass and reduce heavy metal concentrations in surface soils [10]. These findings highlight the potential of WJLS in remediating severely degraded mining lands, which are typically marked by disrupted surface structure, poor vegetation cover, low microbial abundance, and limited moisture retention. However, the profound disturbance of soil structure and vegetation in mining areas severely hampers microbial diversity and activity [14]. As such, the sole application of sludge may be insufficient to support microbial persistence and the effective recovery of soil functions. Moreover, existing research on sludge-based soil remediation has predominantly focused on municipal sources, such as sewage sludge [15,16,17], with relatively little attention given to industrial sludge. In particular, studies evaluating the effects of WJLS on soil physicochemical properties and microbial community structure remain scarce. Further investigation is therefore needed to elucidate the potential of WJLS as a sustainable amendment for soil restoration in mining-impacted environments.
Biochar is a carbon-rich material produced from the pyrolysis of organic biomass, such as crop residues and wood, under limited oxygen conditions [11]. Owing to its chemical stability and long environmental residence time, biochar has been widely recognized for its potential to enhance soil quality and increase soil organic carbon content, thereby contributing to long-term carbon sequestration and mitigating excessive CO2 emissions [18]. Moreover, biochar has garnered considerable attention for its ability to improve soil water retention, enhance enzymatic activity, and ultimately support plant growth [3,19,20]. The large specific surface area of biochar, which can provide a habitat for microorganisms, improves the CEC in saline-alkali soil, and this could ameliorate the adverse effects of salinity on soil and crops [3,21]. However, biochar may also negatively affect saline-alkali soil since the minerals, soluble alkali ions (such as K+, Na+, Mg2+, Ca2+), and oxygen-containing functional groups could increase soil salinity and alkalinity. While after incorporation into the soil, WJLS undergoes both anaerobic and aerobic microbial decomposition, which can potentially alter soil pH. This pH modulation, when combined with the buffering and ion exchange capacities of biochar, contributes to the regulation of salinity and alkalinity in soils. Therefore, the synergistic application of biochar with WJLS, is regarded as an effective strategy for soil remediation.
In this study, different application ratios of biochar and WJLS were employed to systematically investigate their effects on the remediation of sandy loam soil in mining areas. The primary objective was to evaluate the potential of these two soil amendments for enhancing the quality and functionality of degraded mining soils. Six treatments were designed in an experiment to explore (i) the effects of WJLS and biochar on the fertility of sandy loam soil; (ii) the beneficial effects of WJLS and biochar for reducing the salinization of sandy loam soil; (iii) the impact of WJLS and biochar on soil microbial biomass, diversity, and relative abundance. This study is expected to provide theoretical guidance for soil amelioration projects in degraded mining areas involving WJLS applications.
2. Materials and Methods
2.1. Preparation of Soil and Restorative Materials
The experiment was conducted in Wujiang District, Suzhou City, Jiangsu Province, China. The soil samples were sourced from coal mining areas in the Ningdong region of the Ningxia Hui Autonomous Region, specifically from residual coal-based solid waste dumpsites. The soil was classified as sandy loam, with a particle size distribution of 14% clay (<0.002 mm), 28% silt (0.002~0.02 mm), and 58% sand (>0.02 mm). Prior to use in the pot experiment, the collected soil was naturally air-dried, manually ground, and passed through a 5 mm sieve. The WJLS was obtained following biotechnological treatment of textile wastewater; it was also air-dried and pulverized prior to application. The biochar was produced from rice husks via pyrolysis at 500 °C. The basic physicochemical properties of the soil, WJLS, and biochar are presented in Table 1.

Table 1.
Basic characteristics of soil, WJLS, and biochar.
2.2. Pot Experiment
A total of 1.2 kg soil and WJLS were mixed together using different proportions as a matrix in a plastic pot (height: 14.5 cm, surface diameter: 16.5 cm, and bottom diameter: 12 cm). Next, biochar was added and mixed with that matrix. Then, a microbial inoculant (30 g·pot−1 according to the instruction) consisting mainly of Bacillus subtilis and B. licheniformis (effective number of viable bacteria ≥ 200 million per gram) was applied as a base biofertilizer to help improve soil structure. After 2 weeks of incubation at room temperature, 0.25 g of ryegrass seed was sown into each pot. A total of 6 treatments were set up, consisting of 3 replicates (pots) per treatment, as shown in Table 2. At the 5% and 15% WJLS application rates, the soil organic matter accounted for approximately 2.7% and 7.5% of the total soil weight, respectively—corresponding to levels that are close to or higher than those reported in the literature for the organic matter content of soils in northwestern, central, and northern China [22]. In addition, distilled water was used for irrigation during the whole experiment period, in which ryegrass was grown for a total of 72 days, after which soil and plant analyses were conducted.

Table 2.
Treatments of pot experiment.
2.3. Samples Collection and Analysis
2.3.1. Soil Sample Analysis
Soil pH and electric conductivity (EC) were determined with a digital pH and EC meter in a soil-to-water ratio of 1:2.5 and a 1:5 aqueous suspension, respectively. The soil OC content, TN, TP, and available phosphorus (AP) were, respectively, measured by the potassium dichromate oxidation-external heating method [23], Kjeldahl method, alkali fusion-Mo-Sb Anti spectrophotometric method, and sodium hydrogen carbonate solution-Mo-Sb anti-spectrophotometric method [24]. The CEC of soil samples was determined using the hexamminecobalt trichloride solution-spectrophotometric method (according to Chinese Environmental Protection Standard [25]). Available nitrogen (AN) content was quantified by extraction with potassium chloride solution-spectrophotometric methods according to Chinese Environmental Protection Standard [26], while the Na+, Ca2+, and Mg2+ were analyzed using the methodology described in [24]. The determination of the soil microbial biomass carbon (MBC), nitrogen (MBN), and phosphorus (MBP) was made according to the fumigation-extraction method.
Soil exchangeable sodium percentage (ESP, %) and SAR (%), were calculated as follows:
where CEC is cation exchange capacity, in cmol·kg−1; Na+, Ca2+, and Mg2+ are exchangeable sodium, magnesium, and calcium ion content, respectively, in cmol·kg−1.
2.3.2. Plant Sample Analysis
The ryegrass of all six treatment groups was washed with distilled water. Part of the aboveground plant was oven-dried at 105 °C for 30 min and then dried at 60 °C to a constant weight. An electronic balance was used to measure the dry weight of ryegrass samples.
2.4. Microbial Community (16S rDNA and ITS)
Soil samples were taken from the pots after 72 days to analyze their microbial community. Hangzhou Lianchuan Biotechnology Co., Ltd., Hangzhou, China. was responsible for testing these samples. Microbial community genomic DNA was extracted from sandy loam soil samples with the E.Z.N.A®Soil DNA Kit (D4015, Omega, Inc., Auburn, WA, USA). The hypervariable region V3–V4 of the bacterial 16S rDNA gene was amplified using the primer pair of 341F (5′-CCTACGGGNGGCWGCAG-3′) and 805R (5′-GACTACHVGGGTATCTAATCC-3′); for the fungi ITS2 region, the ITS1FI2 (5′-GTGARTCATCGAATCTTTG-3′) and ITS2 (5′-TCCTCCGCTTATTGATATGC-3′) primer pair was used. The ensuing PCR products were confirmed with 2% agarose gel electrophoresis, then purified by AMPure XT beads (Beckman Coulter Genomics, Danvers, MA, USA) and quantified by Qubit (Invitrogen, Carlsbad, CA, USA). The amplicon pools were prepared for sequencing and assessed, by, respectively, using the Agilent 2100 Bioanalyzer (Agilent, Lexington, MA, USA) and the Library Quantification Kit for Illumina (Kapa Biosciences, Woburn, MA, USA). The libraries were sequenced on the NovaSeq PE250 platform (Illumina, San Diego, CA, USA). Samples were sequenced on an Illumina NovaSeq platform; paired-end reads were assigned to samples based on their unique barcode and truncated by cutting off the barcode and primer sequence. The paired-end reads were then merged using Pear. Quality filtering of the raw reads was performed under specific filtering conditions to obtain high-quality clean tags. After filtering and dereplicating the chimeric sequences, the feature table and feature sequences were obtained. Alpha and beta diversity were calculated by QIIME2, for which the same number of sequences were extracted randomly by reducing the number of sequences to the minimum of certain samples and the relative abundancies used for fungi taxonomy. The sequence alignment of species annotation was performed by QIIME2’s plugin feature-classifier, using the alignment databases SILVA and UNITE.
2.5. SEM Analysis
The morphological structure and surface of biochar, WJLS, and origin soil were tested by scanning electron microscopy (SEM, Quanta 250, FEI Company, Hillsboro, OR, USA) after drying.
2.6. Statistical Analysis
A two-way ANOVA was conducted to assess the interactive effects of WJLS and biochar on soil properties. Additionally, the differences in means among the six treatments were analyzed using a one-way ANOVA at a significance level of p < 0.05, followed by the least significant difference (LSD) test, using SPSS 25.0 software. Redundancy analysis (RDA) was performed using the Canoco 5 program to explore the relationships between microbial communities and soil properties in the samples.
3. Results
3.1. Improvement of Soil Properties and Nutrients
3.1.1. Soil pH, OC, and N, P
The pH, OC, TN, TP, AN, and AP of the sandy loam soil under six different treatments are presented in Figure 1. The impact of WJLS, biochar, and their interaction on the above parameters was firstly assessed with a two-way ANOVA tool (Table 3). The results demonstrated that WJLS exerted a highly significant effect on soil properties (p < 0.01). In the control groups (S0 and S0B), the addition of biochar alone did not significantly alter soil pH, which remained relatively stable at approximately 9.1 (Figure 1a). In contrast, the incorporation of WJLS significantly reduced soil pH in the treated groups, the most pronounced reduction was observed in the S2B treatment, where the pH declined to 8.3—substantially lower than that in S1B (8.6) and S2 (8.61). As shown in Table 3, although the pH of biochar was relatively higher compared to that of soil and WJLS, it did not exert a significant effect on soil pH, whereas WJLS appeared to contribute to the observed acidification effect. As illustrated in Figure 1b, OC levels increased significantly across all amended groups. Notably, the OC concentrations in the S2 and S2B treatments reached 29.0 and 28.9 g·kg−1, respectively, representing an approximately 9.4-fold increase compared to the control group (S0), which recorded an OC content of only 3.1 g·kg−1.
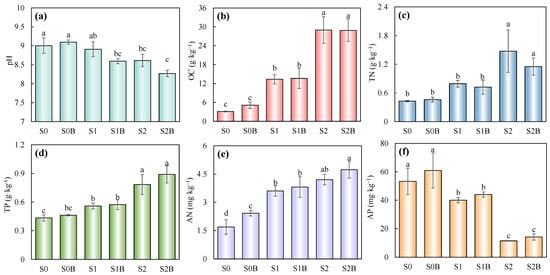
Figure 1.
Changes in soil pH (a), OC (b), TN (c), TP (d), AN (e) and AP (f) after reclamation. (Columns with different letters in the same figure show significant difference from each other (p < 0.05)).

Table 3.
Two-way ANOVA analysis of WJLS and biochar on soil properties.
The trends observed for TN, TP, and AN (Figure 1c–e) closely paralleled that of OC. In particular, the TN, TP, and AN in the WJLS and biochar-amended treatments (S1, S1B, S2, and S2B) were significantly higher than those in the control groups (S0 and S0B). Specifically, TN concentrations across the six treatments were 0.43, 0.46, 0.79, 0.72, 1.48, and 1.15 g·kg−1, respectively. A similar increasing trend was observed for TP and AN, with the highest values recorded in the S2B group (0.89 g·kg−1 for TP and 4.73 mg·kg−1 for AN). In contrast, AP content showed a decreasing trend with increasing WJLS application rate (Figure 1f), suggesting a potential immobilization or transformation of phosphorus under higher ratio of sludge additions.
3.1.2. Changes in EC, CEC, Soil Salinity, and Alkalinity
Soil salinization and alkalinization are critical environmental challenges contributing to land degradation in arid and semi-arid regions worldwide [27]. Given that the original soil already exhibits a high pH, it is necessary to closely monitor changes in salinity-related parameters such as soil EC throughout the improvement process. As shown in Figure 2a, the EC decreased to some extent in the S0B treatment group after the addition of biochar, with a value of 162 mS·m−1. However, with the increasing proportion of WJLS in the treated soil, EC gradually increased to 237 mS·m−1 in S2B, exhibiting a significant difference compared to S0B and S1, while no significant differences were observed between S2B and the other treatments.
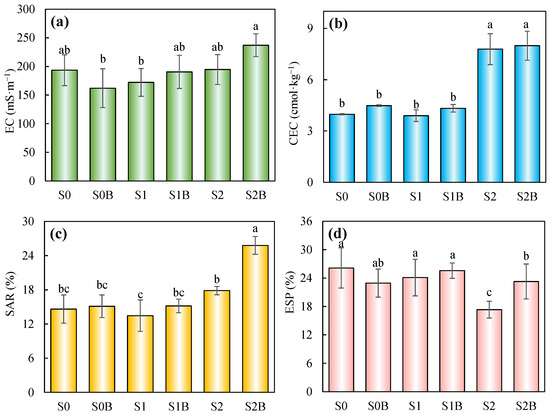
Figure 2.
Changes in EC (a), CEC (b), SAR (c), and ESP (d) in sandy loam soil under different treatments. (Columns with different letters in the same figure show significant difference from each other (p < 0.05)).
CEC is a key indicator of soil fertility, as it measures the soil’s ability to retain and supply essential plant nutrients, including calcium, magnesium, and potassium. As shown in Figure 2b, the group of S2 and S2B exhibited a significantly higher CEC of 7.79 and 7.99 cmol·kg−1, nearly twice that of its corresponding control group, whose value was 3.97 cmol·kg−1, this result is consistent with the findings of the interaction analysis (Table 3), indicating that WJLS plays the dominant role in soil CEC. Analyzing the concentrations of Na+, Ca2+, and Mg2+ in the treated soil samples (Table 4) revealed that Ca2+ was the dominant cation. However, the concentration of Ca2+ remained relatively stable across most treatment groups, with the exception of a noticeable increase in the S2 group, which may be attributed to the higher baseline calcium content in the soil. In contrast, Na+ and Mg2+ concentrations increased with higher WJLS application rates and the inclusion of biochar. The SAR, a key indicator for assessing changes in the physicochemical properties of saline-alkali soils [28], exhibited a marked rise in the S2B treatment group, reaching a maximum of 25.8%. In comparison, SAR values in the S0 to S2 groups ranged from 14.6% to 17.8%, the ESP decreased across all treatment groups following the application of WJLS and biochar, with the most substantial reduction observed in the S2 group, where ESP dropped to 17.3%. However, two-way ANOVA revealed that biochar, WJLS, and their interaction did not have a significant effect on ESP.

Table 4.
Concentrations of Na+, Ca2+and Mg2+ ions in the treated soil samples.
3.2. Microbial Biomass and Microbial Diversity in Soil
3.2.1. Microbial Biomass
The results of the MBC, MBN, and MBP analyses in soil are presented in Table 5. The two-way ANOVA results (Table 3) indicated that WJLS, biochar, and their interaction had significant effects on MBC, MBN, and MBP. For the same amount of WJLS added, treatments that included biochar exhibited relatively higher MBC, MBN, and MBP compared to those without biochar. When the WJLS proportion in the soil matrix reached 15% (S2, S2B), the resulting MBC and MBN were significantly higher than in the other four treatments. Specifically, the MBC in S2 and S2B was 1.8 and 3.3 times that of S0, respectively, while the MBN was 3.1 and 4.6 times higher than in S0. Additionally, the MBP levels in the five treatment groups were all significantly higher than in S0. The S2 and S2B groups, which exhibited the highest MBP, recorded values 4.1 and 8.0 times greater than S0, respectively, potentially explaining the lower AP content observed in these treatment groups. Moreover, treatments with biochar addition consistently showed higher MBC, MBN, and MBP, aligning with findings from previous studies [29]. The large surface area of biochar not only serves as an attachment site for soil nutrients but also provides a favorable substrate for microbial colonization [30], thereby enhancing microbial biomass.

Table 5.
Soil microbial biomass in different treatments.
3.2.2. Microbial Diversity and Communities
The microbial diversity results were shown in Figure 3, For bacterial diversity, the application of biochar and WJLS increased the Shannon and Chao1 richness, with a particularly noticeable increase in the Chao1 index in the S1B and S2B groups, while the impact on the Simpson index was not significant. Regarding fungal diversity, the effect of biochar was more pronounced, especially on the Chao1 index, where the Chao1 index of S2B was 2.7 times that of S0. The above results collectively indicated that microbial diversity and richness increased in response to WJLS and biochar application compared to the control. Additionally, the coverage of both bacteria and fungi were higher than 0.999.
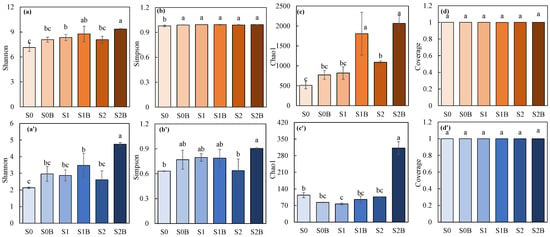
Figure 3.
Alpha-diversity index of soil bacteria (a–d) and fungi (a’–d’) community. (Columns with different letters in the same figure show significant difference from each other (p < 0.05)).
The structure and function of biotic communities in soils are inherently complex, with their distribution within a given soil type playing a crucial role in influencing overall soil function and health [31]. As shown in Figure 4a, the bacterial community at the phylum level was predominantly composed of Proteobacteria, Firmicutes, and Actinobacteriota, collectively accounting for 72.4% to 84.2% of the total relative abundance across all treatments. In the control group S0, the abundance of Proteobacteria was 34.2%; however, after the addition of WJLS and biochar, its abundance increased to 41.1% (S1), 43.3% (S1B), 70.4% (S2), and 51.5% (S2B). Moreover, Firmicutes is also a phylum whose members are metabolically versatile and can degrade a variety of complex organic materials [32,33]. Following soil remediation with WJLS and biochar, the relative abundance of Firmicutes significantly declined from 38.2% (S0) to 24.5% (S1), 18.7% (S1B), 5.5% (S2), and 6.4% (S2B), a similar trend was also observed at the genus level (Figure 4c). Fungi community composition at the phylum level is illustrated in Figure 4b, where Ascomycota clearly dominated with a relative abundance ranging from 67.6% to 95.4%. Additionally, the relative abundance of an unclassified fungal phylum increased in the treatment groups, and it emerged as a dominant group at the genus level (Figure 4d).
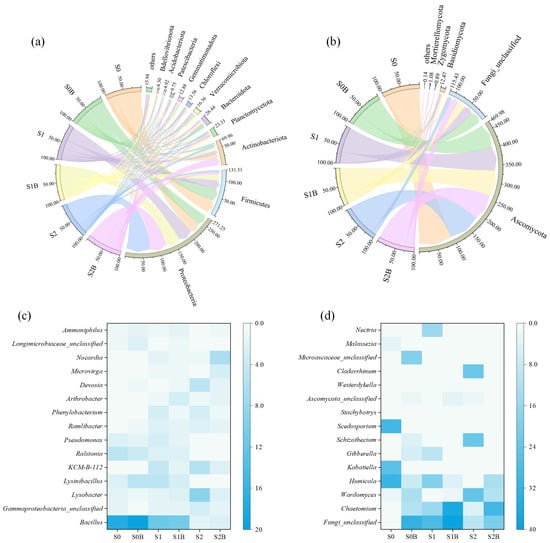
Figure 4.
Relative abundance of soil bacteria (a) and fungi (b) at the phylum level, and bacteria (c), fungi (d) at genus level (others mean the relative abundance of bacteria or fungi less than 1%).
To further elucidate the relationship between microbial diversity and soil physicochemical properties, RDA was conducted at the phylum level (Figure 5). The results indicated that a higher proportion of WJLS application significantly increased soil OC, TN, TP, and CEC, all of which showed strong positive correlations. These effects were further amplified in the S2B treatment group with the addition of biochar. Notably, variations in soil properties exhibited a stronger correlation with bacterial community composition than with fungal communities. For instance, Proteobacteria, Actinobacteriota, and Planctomycetota displayed a positive correlation with soil OC, TN, TP, and CEC, but a negative correlation with pH. In contrast, the increased abundance of Fungi_unclassified may be attributed to the combined input of WJLS and biochar, reflecting potential shifts in fungal community structure in response to organic amendments.
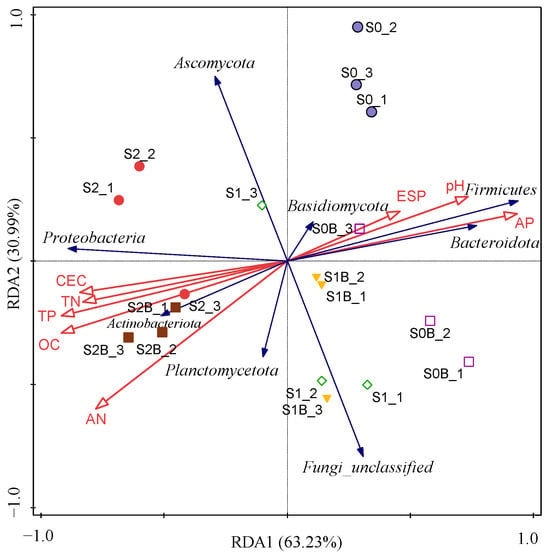
Figure 5.
RDA between microbial phylum relative abundance and soil properties of different samples.
3.3. Enhancement of Ryegrass Growth
The final growth status of ryegrass at the end of the experiment is presented in Figure 6. In the control group (S0), ryegrass showed limited growth, characterized by extensive leaf yellowing and a low biomass of 0.32 g·pot−1. In contrast, the S0B and S1 treatment groups exhibited visibly improved plant vigor, with denser growth and markedly fewer senescent or chlorotic leaves. Correspondingly, biomass increased to 0.51 and 0.59 g·pot−1, respectively, indicating a positive response to the soil amendments. Notably, the biomass of the S1B, S2, and S2B groups was significantly higher, at 3.2, 3.4, and 4.3 times that of S0. To elucidate the factors contributing to this enhanced growth, the correlations between plant biomass and soil physicochemical properties were analyzed across all six treatments. As shown in Figure 7, biomass exhibited significant correlations with soil pH, OC, TP, AN, AP, MBN, and MBP. Particularly, biomass was negatively correlated with soil pH, suggesting that the amendments effectively reduced soil alkalinity. The observed improvement in plant growth can be attributed to the increased availability of essential nutrients (OC, N, and P) and elevated microbial biomass following soil amendment.
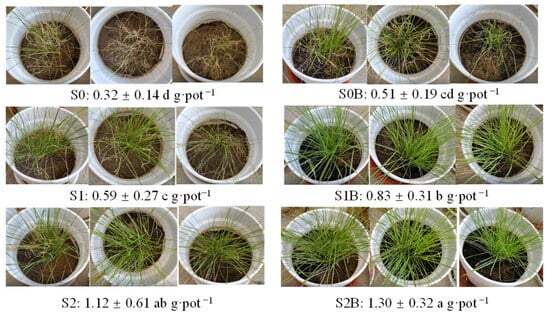
Figure 6.
Effect of WJLS and biochar on ryegrass growth.
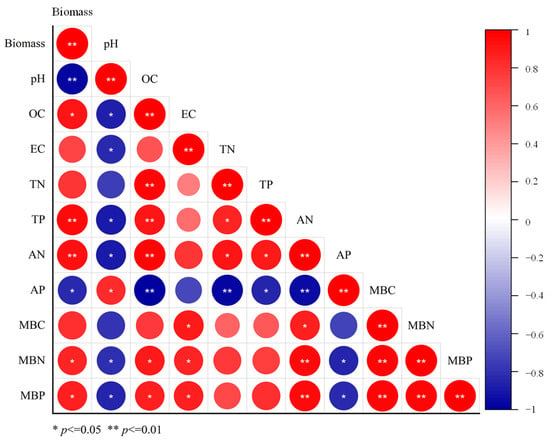
Figure 7.
Correlation between plant biomass and soil properties.
4. Discussion
4.1. Feasibility of the Application of Biochar and WJLS on Sandy-Loam Soil
Results shown that biochar, being inherently alkaline, does not significantly decrease soil pH and may even increase it in neutral or acidic soils [15]. However, the combined application of WJLS and biochar can moderately reduce soil alkalinity, offering a potential advantage in the remediation of high-pH mine soils. The notable increase in soil OC observed in the WJLS treated groups can be primarily attributed to the high organic matter content of the WJLS [17]. Although biochar is also rich in carbon, its addition did not lead to a significant increase in soil OC, suggesting that the OC in WJLS is more readily decomposable and more easily incorporated into the soil matrix than that in biochar. This may also indicate that biochar plays a more pronounced role in influencing soil microbial communities rather than directly contributing to soil OC levels. These findings are consistent with previous studies showing that sewage sludge application can substantially improve soil fertility and structure [10]. In contrast to the trends observed for TN and TP, the AP levels in the S1 through S2B treatments were comparatively lower, with the most pronounced reductions occurring in the treatments lacking biochar. Two possible explanations for this decline are (1) AP may have been rapidly taken up by microorganisms or assimilated by plants to support growth; and (2) phosphates and loosely bound phosphorus present in WJLS and biochar may have reacted with hydroxyl ions in the alkaline sandy loam soil [3,34], resulting in simultaneous reductions in both soil pH and AP availability.
Furthermore, potentially toxic elements (PTE) introduced by WJLS and biochar also need additional consideration (Table 1). The increasing of soil OC can enhance the soil’s capacity to retain PTE, which is another critical consideration in the context of soil remediation [15]. This effect is particularly pronounced when biochar is co-applied, as its surface properties facilitate the adsorption and immobilization of PTE, thereby reducing their leaching from the soil. However, it is worth noting that PTE enrichment may occur during the production of biochar and concentrated sludge [35,36]. Therefore, the application rates of both biochar and sludge in soil should be optimized based on comprehensive risk assessments of PTE contamination.
EC serves as an indicator of the dissolved salts content in the soil, the increase in EC in biochar and WJLS may be attributed to the high absorption of Na+ from the soil and the release of nutrients from the biochar [37]. Previous studies have suggested that Ca2+ and Mg2+ play a crucial role in stabilizing soil clay particles and enhancing the formation of a well-structured soil aggregate system [38]. The high organic matter content in WJLS and biochar, combined with biochar’s adsorption capacity for metal cations, likely contributes to the enhanced CEC [3]. While considering the concurrent changes in soil pH, SAR and ESP, the incorporation of WJLS and biochar in sandy loam soil appears to mitigate soil alkalization to some extent. However, the potential impact on soil salinity requires careful monitoring.
4.2. Effect of Biochar and WJLS on Microorganisms
Previous studies on the effects of sludge or biochar additions on soil microbial communities have primarily focused on agricultural applications [16,39], with little research addressing the combined use of WJLS and biochar for improving sandy loam soil. In this study, for the same WJLS addition ratio, treatments that included biochar (i.e., S0B, S1B, and S2B) exhibited significantly higher microbial biomass and diversity. This aligns with previous research showing that the addition of corn straw-derived biochar can markedly enhance soil bacterial diversity in farmland soils [39]. It is likely attributed to the high porosity and diverse chemical functional groups of biochar [3], which enhance compositional heterogeneity and create diverse ecological niches for microorganisms. Consequently, biochar provides a conducive habitat for microbial colonization and supports the proliferation of highly diverse microbial communities [40].
The dominated phyla of Proteobacteria, Firmicutes, and Actinobacteriota in remediated soil play a crucial role in enhancing soil nutrient cycling and accelerating the decomposition of organic matter [41,42]. Proteobacteria are actively involved in soil carbon and nitrogen cycling, which thrive in nutrient-rich soils [21,33] and have frequently been reported in studies on biochar-amended saline-alkali soils [43]. In this study, the microbial strains Bacillus subtilis and Bacillus licheniformis, both belonging to Firmicutes, were introduced at the experiment’s outset. The reduction in Firmicutes may be attributed to shifts in soil conditions, leading to Firmicutes being gradually replaced by other dominant bacterial groups, such as Proteobacteria. Additionally, Actinobacteriota has been reported to play a key role in breaking down recalcitrant polymers, contributing to the turnover of soil organic matter [44]. Other bacterial phyla, including Planctomycetota, Bacteroidota, and Chloroflexi, are ecologically significant for maintaining soil functionality [45]. For instance, these groups help enhance soil fertility [46] and actively participate in the carbon and nitrogen cycles [47]. The fungi phylum of Ascomycota is widely recognized as a prevalent fungal group in soils, known for its ability to secrete laccase, an enzyme that plays a crucial role in environmental protection by degrading organic pollutants [12,48]. The dominated phyla in the treatment group helps improve soil fertility and ecosystem function and plays a positive role in the development of soil remediation in a strong direction.
4.3. The Potential of Biochar and Sludge in Promoting Plant Growth
The finding of ryegrass growth indicated that the combined application of WJLS and biochar effectively enhanced the aboveground biomass of ryegrass. This aligns with previous studies demonstrating that the use of sludge and biochar in soil remediation significantly improves plant growth in degraded mining soils [15]. The biomass of ryegrass served as the most direct and compelling evidence that the combined application of biochar and WJLS enriched the nutrient content of sandy loam soil and significantly improved its overall quality.
Due to the short duration of the study and the constraints of indoor experiments, long-term continuous research is still required to investigate the sustained effects of biochar and WJLS on soil nutrient dynamics and the potential risk of salinity accumulation. In addition, it is necessary to integrate plant uptake assessments to clarify the potential risks of PTE contamination under long-term application of biochar and WJLS, thereby ensuring the environmental safety of their combined use as soil amendments.
5. Conclusions
In this study, biochar and WJLS were applied to assess their effectiveness in improving sandy loam soil properties. The results demonstrated that WJLS had significant effects on soil OC, N, and P, while biochar primarily influenced soil SAR and AN. The interaction of biochar and WJLS showed no significant impact on soil physicochemical properties. Furthermore, MBC, MBN, and MBP increased following soil improvement, especially in treatments with biochar application and higher sludge ratios. That is, interaction effects were observed mainly in microbial properties and in the biochar-induced enhancement of ryegrass biomass. Based on SAR values, soil alkalinity remains an issue requiring further attention. Overall, the combined application of WJLS and biochar constitutes an effective strategy for improving the quality of sandy loam soil.
Author Contributions
Conceptualization, M.J. and X.Z.; data curation, M.J., X.Z. and X.R.; formal analysis, M.J.; funding acquisition, M.J. and J.H.; investigation, M.J. and X.R.; methodology, X.R.; project administration, J.H.; software, M.J.; supervision, J.H.; validation, X.R.; visualization, X.Z. and X.R.; writing—original draft, M.J.; writing—review and editing, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Central Universities, Mechanisms of soil quality enhancement and microbial regulation in saline soils amended with biochar, grant number 2023QN1039; and Jiangsu Funding Program for Excellent Postdoctoral Talent, grant number 2023ZB622.
Data Availability Statement
The data presented in this study are available on request from the corresponding author; the data are not publicly available due to privacy.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| WJLS | Water jet-loom sludge |
| OC | Organic carbon |
| TN | Total nitrogen |
| TP | Total phosphorus |
| AN | Available nitrogen |
| AP | Available phosphorus |
| SAR | Sodium absorption ratio |
| CEC | Cation exchange capacity |
| EC | Electric conductivity |
| MBC | Microbial biomass carbon |
| MBN | Microbial biomass nitrogen |
| MBP | Microbial biomass phosphorus |
| ESP | Exchangeable sodium percentage |
| LSD | Least significant difference |
| RDA | Redundancy analysis |
| PTE | Potentially toxic elements |
References
- Burezq, H. Combating wind erosion through soil stabilization under simulated wind flow condition-Case of Kuwait. Int. Soil Water Conserv. Res. 2020, 8, 154–163. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, W.; Wen, Z.; Yang, Y.; Chang, X.; Yang, Q.; Meng, Y.; Liu, C. Mechanisms and feedbacks for evapotranspiration-induced salt accumulation and precipitation in an arid wetland of China. J. Hydrol. 2019, 568, 403–415. [Google Scholar] [CrossRef]
- Zhang, P.; Bing, X.; Jiao, L.; Xiao, H.; Li, B.; Sun, H. Amelioration effects of coastal saline-alkali soil by ball-milled red phosphorus-loaded biochar. Chem. Eng. J. 2022, 431, 133904. [Google Scholar] [CrossRef]
- Luo, S.; Wang, S.; Tian, L.; Shi, S.; Xu, S.; Yang, F.; Li, X.; Wang, Z.; Tian, C. Aggregate-related changes in soil microbial communities under different ameliorant applications in saline-sodic soils. Geoderma 2018, 329, 108–117. [Google Scholar] [CrossRef]
- Fu, J.; Xiao, Y.; Wang, Y.-F.; Liu, Z.-H.; Yang, K. Saline–alkaline stress in growing maize seedlings is alleviated by Trichoderma asperellum through regulation of the soil environment. Sci. Rep. 2021, 11, 11152. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.J.; Simpson, A.J. Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Yin, R.; Kardol, P.; Thakur, M.P.; Gruss, I.; Wu, G.-L.; Eisenhauer, N.; Schädler, M. Soil functional biodiversity and biological quality under threat: Intensive land use outweighs climate change. Soil Biol. Biochem. 2020, 147, 107847. [Google Scholar] [CrossRef]
- Ji, C.; Huang, J.; Tian, Y.; Liu, Y.; Barvor, J.B.; Shao, X.; Li, Z.A. Feasibility Study on the Application of Microbial Agent Modified Water-Jet Loom Sludge for the Restoration of Degraded Soil in Mining Areas. Int. J. Environ. Res. Public Health 2021, 18, 6797. [Google Scholar] [CrossRef]
- Bi, Y.; Guo, Y.; Sun, H. Arbuscular mycorrhizal fungal diversity in soils underlying moss biocrusts in coal mining subsidence areas. Environ. Sci. Pollut. Res. 2021, 28, 3484–3493. [Google Scholar] [CrossRef]
- Arif, M.S.; Riaz, M.; Shahzad, S.M.; Yasmeen, T.; Ashraf, M.; Siddique, M.; Mubarik, M.S.; Bragazza, L.; Buttler, A. Fresh and composted industrial sludge restore soil functions in surface soil of degraded agricultural land. Sci. Total Environ. 2018, 619, 517–527. [Google Scholar] [CrossRef]
- Baiamonte, G.; Crescimanno, G.; Parrino, F.; De Pasquale, C. Effect of biochar on the physical and structural properties of a sandy soil. Catena 2019, 175, 294–303. [Google Scholar] [CrossRef]
- Gao, W.; Gao, K.; Guo, Z.; Liu, Y.; Jiang, L.; Liu, C.; Liu, X.; Wang, G. Different responses of soil bacterial and fungal communities to 3 years of biochar amendment in an alkaline soybean soil. Front. Microbiol. 2021, 12, 630418. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, S.; Hamdi, H.; Aoyama, I.; Jedidi, N.; Hassen, A.; Dahmane, A. Assessment of the effect of repetitive municipal solid waste compost application on soil using physico-chemical analyses, solid-phase bioassays and microbial activity characterization. Jpn. J. Environ. Toxicol. 2007, 10, 19–30. [Google Scholar]
- Hu, Z.; Zhu, Q.; Liu, X.; Li, Y. Preparation of topsoil alternatives for open-pit coal mines in the Hulunbuir grassland area, China. Appl. Soil Ecol. 2020, 147, 103431. [Google Scholar] [CrossRef]
- Penido, E.S.; Martins, G.C.; Mendes, T.B.M.; Melo, L.C.A.; do Rosário Guimarães, I.; Guilherme, L.R.G. Combining biochar and sewage sludge for immobilization of heavy metals in mining soils. Ecotoxicol. Environ. Saf. 2019, 172, 326–333. [Google Scholar] [CrossRef]
- Markowicz, A.; Bondarczuk, K.; Cycoń, M.; Sułowicz, S. Land application of sewage sludge: Response of soil microbial communities and potential spread of antibiotic resistance. Environ. Pollut. 2021, 271, 116317. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lv, M.; Zuo, W.; Tang, Z.; Ding, C.; Yu, Z.; Shen, Z.; Gu, C.; Bai, Y. Sewage sludge application enhances soil properties and rice growth in a salt-affected mudflat soil. Sci. Rep. 2021, 11, 1402. [Google Scholar] [CrossRef]
- McHenry, M.P. Agricultural biochar production, renewable energy generation and farm carbon sequestration in Western Australia: Certainty, uncertainty and risk. Agric. Ecosyst. Environ. 2009, 129, 1–7. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, J.; Xu, R.; Li, Z. Adsorption of Pb (II) on variable charge soils amended with rice-straw derived biochar. Chemosphere 2012, 89, 249–256. [Google Scholar] [CrossRef]
- Yadav, V.; Jain, S.; Mishra, P.; Khare, P.; Shukla, A.K.; Karak, T.; Singh, A.K. Amelioration in nutrient mineralization and microbial activities of sandy loam soil by short term field aged biochar. Appl. Soil Ecol. 2019, 138, 144–155. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, C.; Kong, Y.; Cao, X.; Zhu, L.; Zhang, Y.; Ning, Y.; Tian, W.; Zhang, H.; Yu, Y. Biochar application alleviated rice salt stress via modifying soil properties and regulating soil bacterial abundance and community structure. Agronomy 2022, 12, 409. [Google Scholar] [CrossRef]
- Sheng, M.; Han, X.; Long, J.; Li, N. Characterization of soil organic matter in different regions of China. Soils Crops 2019, 8, 320–330. [Google Scholar]
- Kalembasa, S.J.; Jenkinson, D.S. A comparative study of titrimetric and gravimetric methods for the determination of organic carbon in soil. J. Sci. Food Agric. 1973, 24, 1085–1090. [Google Scholar] [CrossRef]
- Lu, R. Analytical Methods for Soil Agrochemistry; Chinese Agriculture Science and Technology Press: Beijing, China, 2000; pp. 34–36+147–175. [Google Scholar]
- HJ 889-2017; Soil Quality-Determination of Cation Exchange Capacity (CEC)-Hexamminecobalt Trichloride Solution-Spectrophotometric Method. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2017.
- HJ 634-2012; Soil-Determination of Ammonium, Nitrite and Nitrate by Extraction with Potassium Chloride Solution—Spectrophotometric Methods. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2012.
- Mahmoodabadi, M.; Yazdanpanah, N.; Sinobas, L.R.; Pazira, E.; Neshat, A. Reclamation of calcareous saline sodic soil with different amendments (I): Redistribution of soluble cations within the soil profile. Agric. Water Manag. 2013, 120, 30–38. [Google Scholar] [CrossRef]
- Qadir, M.; Sposito, G.; Smith, C.; Oster, J.D. Reassessing irrigation water quality guidelines for sodicity hazard. Agric. Water Manag. 2021, 255, 107054. [Google Scholar] [CrossRef]
- Ali, I.; Yuan, P.; Ullah, S.; Iqbal, A.; Zhao, Q.; Liang, H.; Khan, A.; Zhang, H.; Wu, X.; Wei, S. Biochar amendment and nitrogen fertilizer contribute to the changes in soil properties and microbial communities in a paddy field. Front. Microbiol. 2022, 13, 834751. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, B.; Zhu, L.; Xing, B. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Shanmugam, S.; Jenkins, S.N.; Mickan, B.S.; Jaafar, N.M.; Mathes, F.; Solaiman, Z.M.; Abbott, L.K. Co-application of a biosolids product and biochar to two coarse-textured pasture soils influenced microbial N cycling genes and potential for N leaching. Sci. Rep. 2021, 11, 955. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, J.; Zhang, S.; Zhang, X.; Chen, H. Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J. Hazard. Mater. 2020, 390, 121349. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Wang, M.; Li, Y.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Naidu, R.; Liu, Y. Land application of sewage sludge biochar: Assessments of soil-plant-human health risks from potentially toxic metals. Sci. Total Environ. 2021, 756, 144137. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a potential strategy for remediation of contaminated mining soils: Mechanisms, applications, and future perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef]
- Mehdizadeh, L.; Moghaddam, M.; Lakzian, A. Amelioration of soil properties, growth and leaf mineral elements of summer savory under salt stress and biochar application in alkaline soil. Sci. Hortic. 2020, 267, 109319. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, K.; Jiang, J.; Zhang, L.; Gao, C.; Qi, X.; Fan, J.; Li, Y.; Sun, S.; Fan, X. Soil aggregate construction: Contribution from functional soil amendment fertilizer derived from dolomite. Sustainability 2022, 14, 12287. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Hanzel, J.; Myrold, D.; Sessitsch, A.; Smalla, K.; Tebbe, C.C.; Totsche, K.U. Microbial Ecology of Biogeochemical Interfaces–Diversity, Structure, and Function of Microhabitats in Soil; Blackwell Publishing Ltd.: Oxford, UK, 2013; Volume 86, pp. 1–2. [Google Scholar]
- Takaichi, S.; Maoka, T.; Takasaki, K.; Hanada, S. Carotenoids of Gemmatimonas aurantiaca (Gemmatimonadetes): Identification of a novel carotenoid, deoxyoscillol 2-rhamnoside, and proposed biosynthetic pathway of oscillol 2, 2’-dirhamnoside. Microbiology 2010, 156, 757–763. [Google Scholar] [CrossRef]
- Kodama, Y.; Watanabe, K. Rhizomicrobium electricum sp. nov., a facultatively anaerobic, fermentative, prosthecate bacterium isolated from a cellulose-fed microbial fuel cell. Int. J. Syst. Evol. Microbiol. 2011, 61, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-y.; Liang, X.-y.; Zhang, H.-y.; Fu, R.; Li, M.; Chen, C.-j. Effect of biochar and bioorganic fertilizer on the microbial diversity in the rhizosphere soil of Sesbania cannabina in saline-alkaline soil. Front. Microbiol. 2023, 14, 1190716. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.; Qiu, X.; Zhang, J. Biochar influences the succession of microbial communities and the metabolic functions during rice straw composting with pig manure. Bioresour. Technol. 2019, 272, 10–18. [Google Scholar] [CrossRef]
- Kruczyńska, A.; Kuźniar, A.; Podlewski, J.; Słomczewski, A.; Grządziel, J.; Marzec-Grządziel, A.; Gałązka, A.; Wolińska, A. Bacteroidota structure in the face of varying agricultural practices as an important indicator of soil quality—A culture independent approach. Agric. Ecosyst. Environ. 2023, 342, 108252. [Google Scholar] [CrossRef]
- Wang, L.; Ye, X.; Hu, H.; Du, J.; Xi, Y.; Shen, Z.; Lin, J.; Chen, D. Soil bacterial community triggered by organic matter inputs supports a high-yielding pear production. SOIL 2021, 2021, 1–25. [Google Scholar]
- Zhou, X.; Xiao, C.; Zhang, B.; Chen, T.; Yang, X. Effects of Microplastics on Carbon Release and Microbial Community in Mangrove Soil Systems. J. Hazard. Mater. 2023, 465, 133152. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.; Song, S.; Mu, J.; Hu, W.; Xiao, P. Unearthing microbial diversity of Taxus rhizosphere via MiSeq high-throughput amplicon sequencing and isolate characterization. Sci. Rep. 2016, 6, 22006. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).