Soil Fungal Communities in the Rhizosphere of Sauvignon Blanc Grapes Subjected to Various Agricultural Management Practices
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Sampling
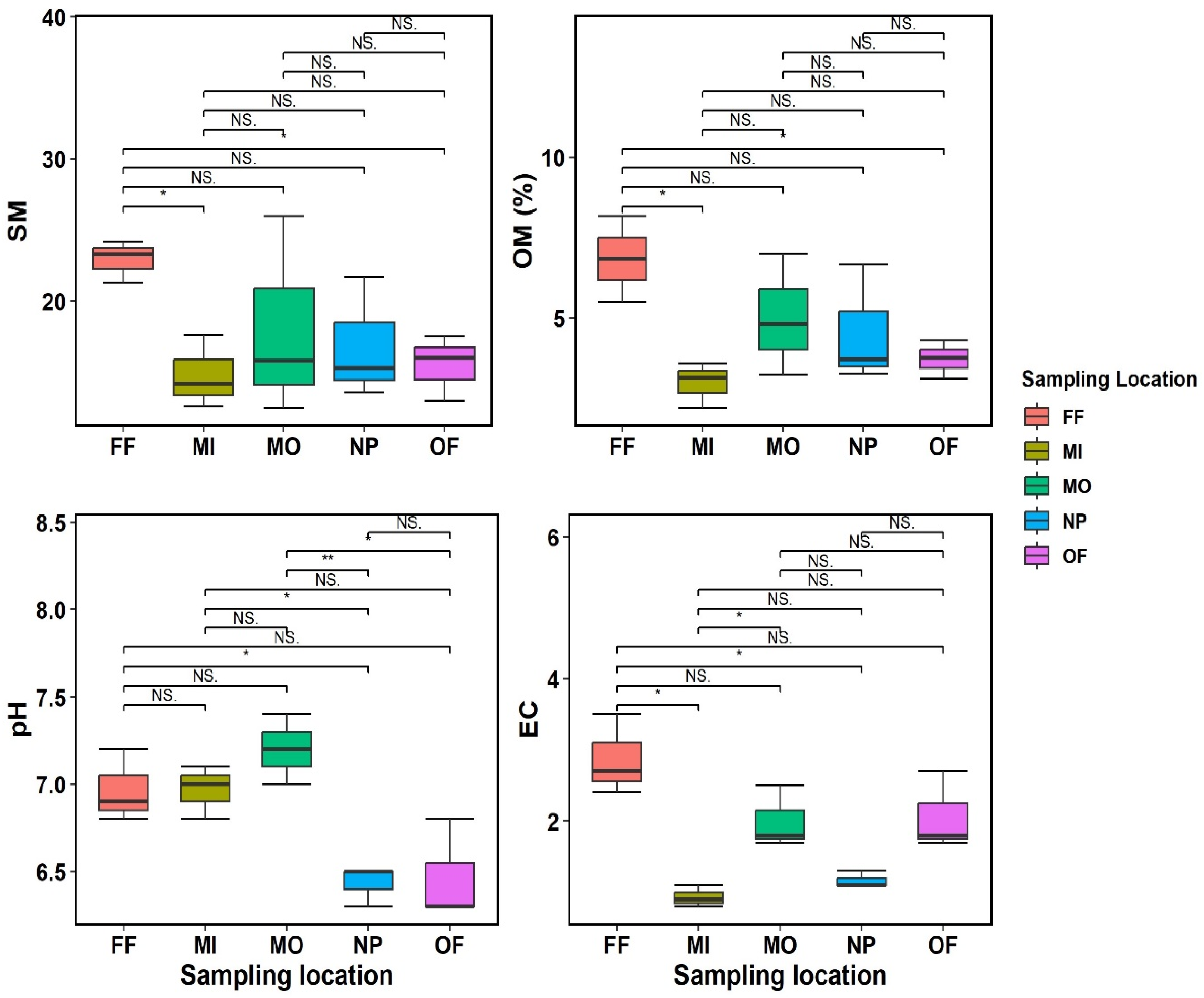
2.2. Soil Abiotic Variable Determination
2.3. Soil Biotic Components Analysis
3. Data Analysis
Phylogenetic Marker and Diversity
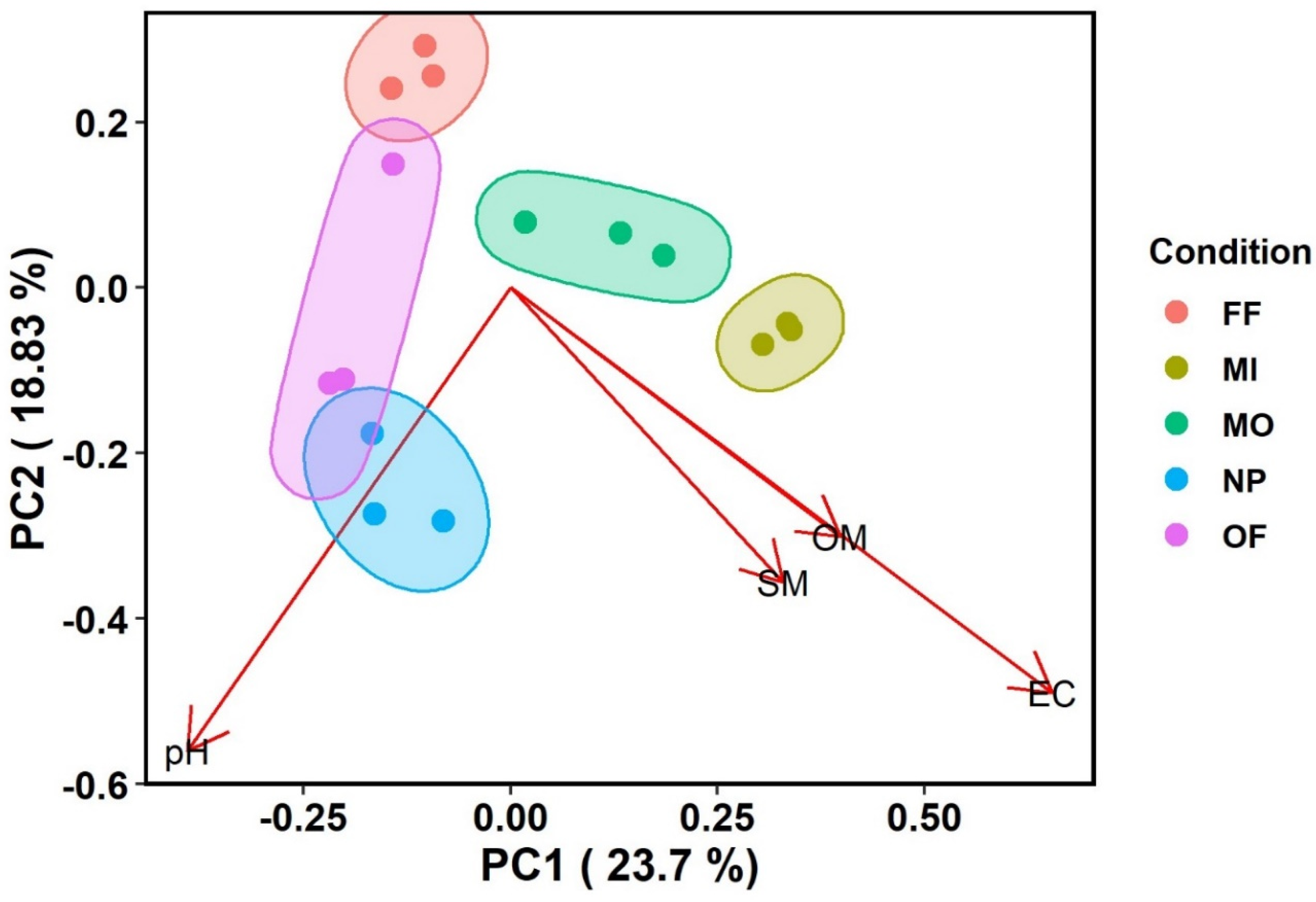
4. Results
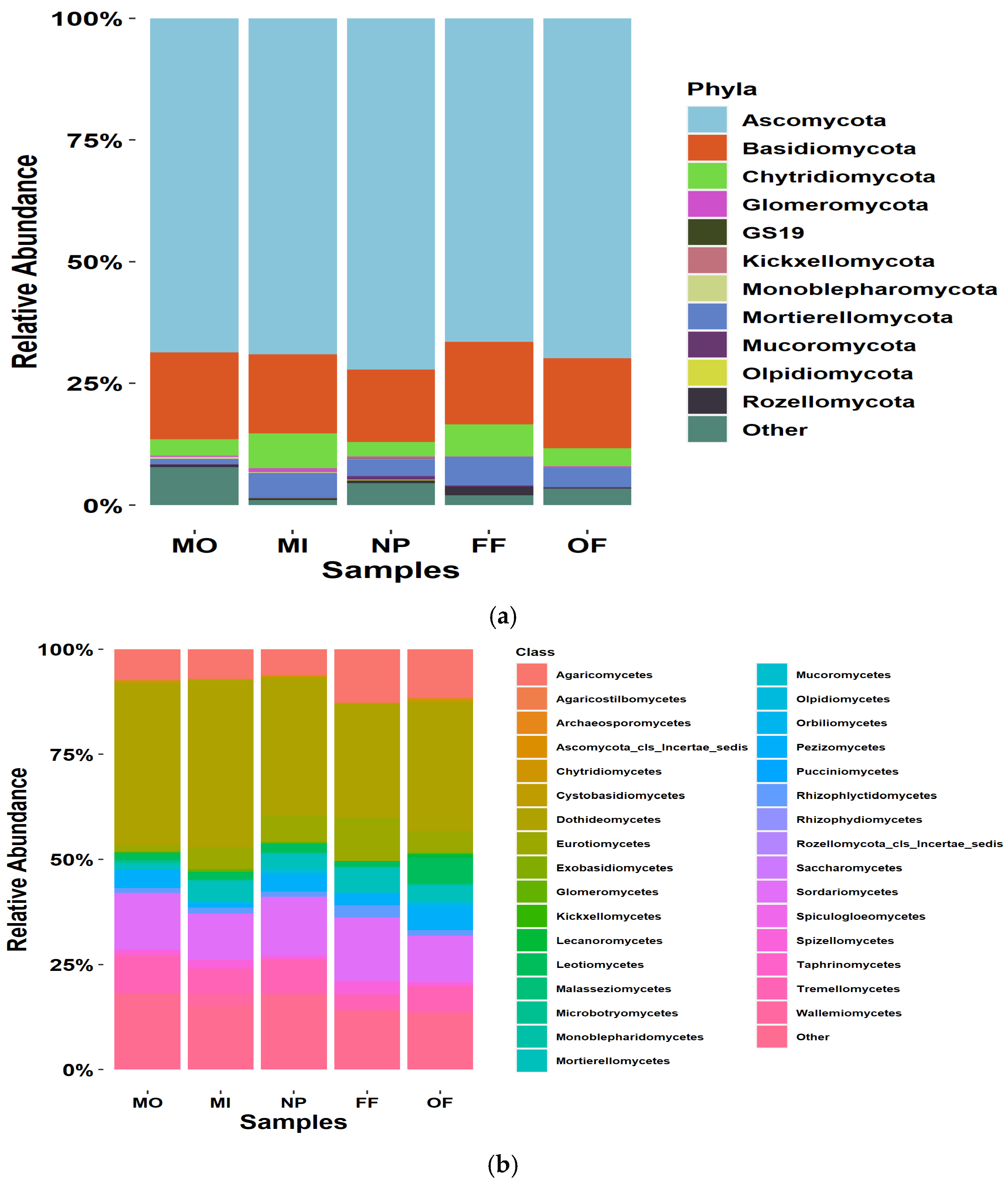
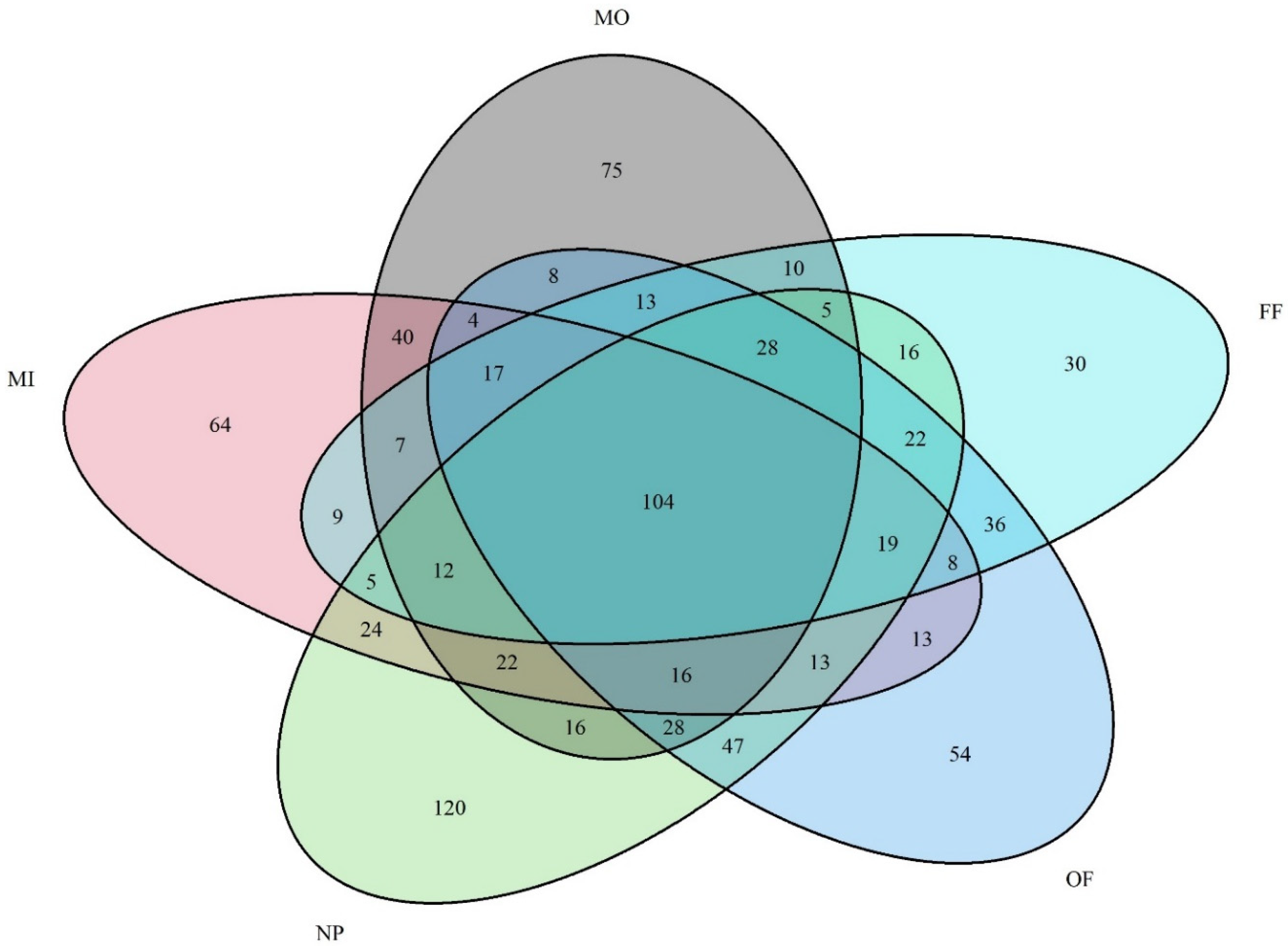
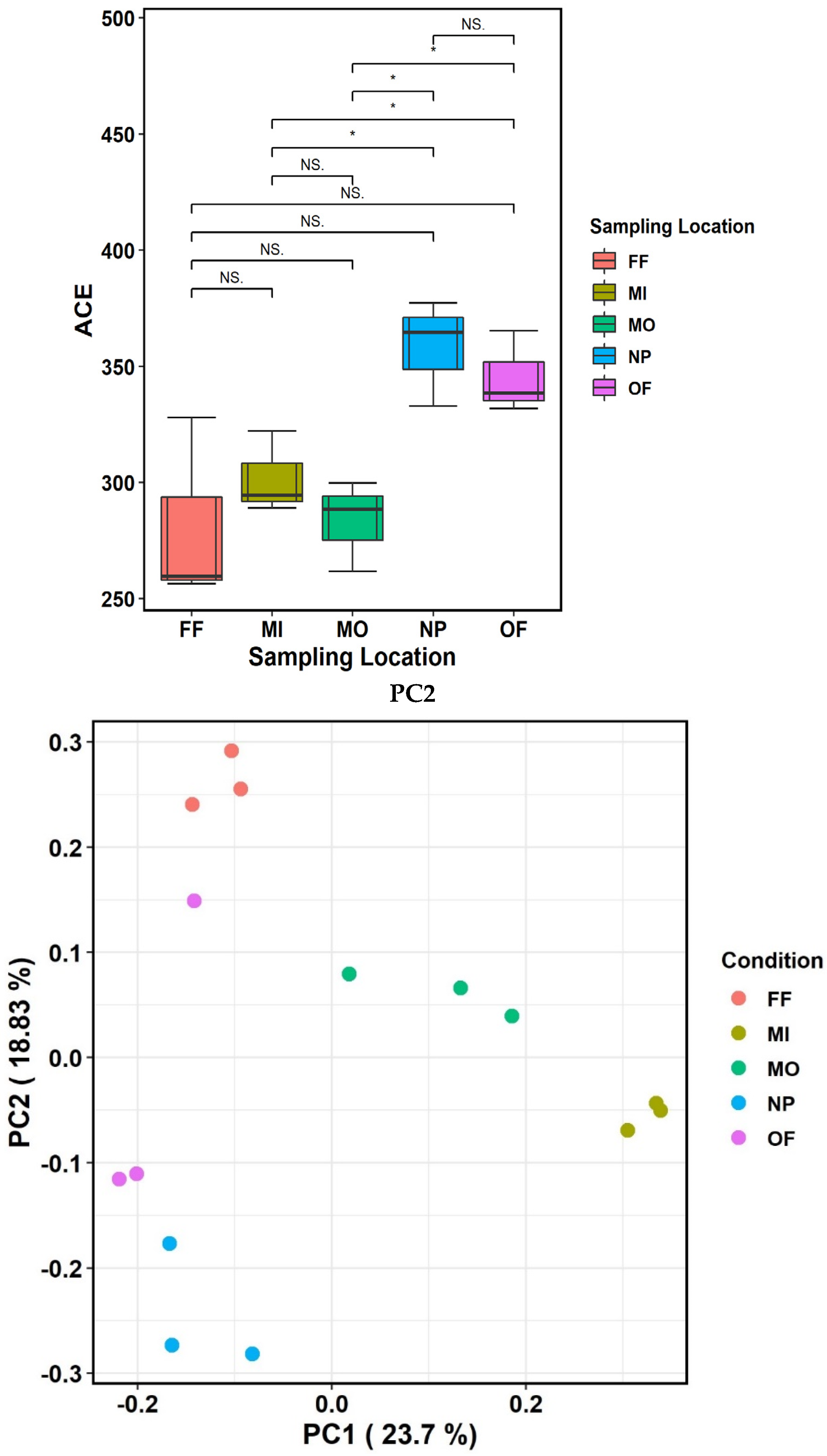
4.1. Phylogenetic Markers and Diversity
4.2. Fungal Trophic Group and Guild Assignment
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vega-Avila, A.D.; Gumiere, T.; Andrade, P.A.M. Bacterial communities in the rhizosphere of Vitis vinifera L. cultivated under distinct agricultural practices in Argentina. Antonie Leeuwenhoek 2015, 107, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; Bershaw, J.; Hugo, R. Volcanic Glass as a Proxy for Paleotopography Suggests New Features in Late-Miocene Oregon. Atmosphere 2024, 15, 561. [Google Scholar] [CrossRef]
- Ashley, R. Grape Wine Nutrition—An Australian Perspective; University of California Agriculture and Natural Resources: Davis, CA, USA, 2011. [Google Scholar]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef]
- Pogue, K.R. Folds, floods, and fine wine: Geologic influences on the terroir of the Columbia Basin. In Volcanoes to vineyards: Geologic field trips through the dynamic landscape of the Pacific Northwest; Geological Society of America: Boulder, CO, USA, 2009; Volume 15, p. 1. [Google Scholar]
- Berlanas, C.; Berbegal, M.; Elena, G.; Laidani, M.; Cibriain, J.F.; Sagües, A.; Gramaje, D. The fungal and bacterial rhizosphere microbiome associated with grapevine rootstock genotypes in mature and young vineyards. Front. Microbiol. 2019, 10, 1142. [Google Scholar] [CrossRef]
- Karakis, S. Terroir Studies in Washington and Wisconsin American Viticultural Areas. Ph.D. Thesis, The University of Wisconsin-Milwaukee, Milwaukee, WI, USA, 2017. [Google Scholar]
- Schreiner, R.P.; Lee, J.; Skinkis, P.A. N, P, and K supply to Pinot Noir grapevines: Impact on vine nutrient status, growth, physiology, and yield. Am. J. Enol. Vitic. 2013, 64, 26–38. [Google Scholar] [CrossRef]
- Adl, S.M. The Ecology of Soil Decomposition; CABI International: Wallingford, UK, 2003. [Google Scholar]
- Holland, T.C.; Reynolds, A.G.; Bowen, P.A.; Bogdanoff, C.P.; Marciniak, M.; Brown, R.B.; Hart, M.M. The response of soil biota to water availability in vineyards. Pedobiologia 2013, 56, 9–14. [Google Scholar] [CrossRef]
- Ren, X.; Cai, T.; Chen, X.; Zhang, P.; Jia, Z. Effect of rainfall concentration with different ridge widths on winter wheat production under semiarid climate. Eur. J. Agron. 2016, 77, 20–27. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, X.; Wu, J.; Zhao, J.; Zhao, M.; Chu, G.; Hui, D.; Zhou, G.; Deng, Q.; Zhang, D. Changes in soil microbial biomass, community composition, and enzyme activities after half-century forest restoration in degraded tropical lands. Forests 2019, 10, 1124. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Bünger, W.; Burbano, C.S.; Sabale, M.; Hurek, T. Roots shaping their microbiome: Global hotspots for microbial activity. Annu. Rev. Phytopathol. 2015, 53, 403–424. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Barron, E.S.; Boddy, L.; Dahlberg, A.; Griffith, G.W.; Nordén, J.; Ovaskainen, O.; Perini, C.; Senn-Irlet, B.; Halme, P. A fungal perspective on conservation biology. Conserv. Biol. 2015, 29, 61–68. [Google Scholar] [CrossRef]
- Weißbecker, C.; Wubet, T.; Lentendu, G.; Kühn, P.; Scholten, T.; Bruelheide, H.; Buscot, F. Experimental evidence of functional group-dependent effects of tree diversity on soil fungi in subtropical forests. Front. Microbiol. 2018, 9, 2312. [Google Scholar] [CrossRef]
- Marriott, E.E.; Wander, M.M. Total and labile soil organic matter in organic and conventional farming systems. Soil Sci. Soc. Am. J. 2006, 70, 950–959. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- van der Linde, S.; Suz, L.M.; Orme, C.D.L.; Cox, F.; Andreae, H.; Asi, E.; Atkinson, B.; Benham, S.; Carroll, C.; Cools, N.; et al. Environment and host as large-scale controls of ectomycorrhizal fungi. Nature 2018, 558, 243–248. [Google Scholar] [CrossRef]
- Lupatini, M.; Korthals, G.W.; de Hollander, M.; Janssens, T.K.S.; Kuramae, E.E. Soil microbiome is more heterogeneous in organic than in conventional farming system. Front. Microbiol. 2017, 7, 2064. [Google Scholar] [CrossRef]
- Potter, T.S.; Vereecke, L.; Lankau, R.A.; Sanford, G.R.; Silva, E.M.; Ruark, M.D. Long-term management drives divergence in soil microbial biomass, richness, and composition among upper Midwest, USA cropping systems. Agric. Ecosyst. Environ. 2022, 325, 107718. [Google Scholar] [CrossRef]
- van Rijssel, S.Q.; Veen, G.F.; Koorneef, G.J.; Bakx-Schotman, J.M.T.; Ten Hooven, F.C.; Geisen, S.; van der Putten, W.H. Soil microbial diversity and community composition during conversion from conventional to organic agriculture. Mol. Ecol. 2022, 31, 4017–4030. [Google Scholar] [CrossRef] [PubMed]
- Gat, Z.; Horovitz, T.; Chechik, F. Winter Climate Regime at the Golan Heights: Topoclimatology, Frost and Dynamic Model Chilling Portions; Agromet Rep. 2; Division of Agricultural Meteorology, Israel Meteorological Service: Bet Dagan, Israel, 2001.
- Ben-Asher, M.; Haviv, I.; Roering, J.J.; Crouvi, O. The influence of climate and microclimate aspect on soil creep efficiency: Cinder cone morphology and evolution along the eastern Mediterranean Golan Heights. Earth Surf. Process. Landf. 2017, 42, 2649–2662. [Google Scholar] [CrossRef]
- Curtis, M.; Michaeli, E.; Aburto, F.; Itkin, D. Soil classification as a tool for contributing to sustainability at the landscape scale and forecasting impacts of management practices in agriculture and forestry. Soil Tillage Res. 2024, 244, 10216. [Google Scholar]
- Singer, A.; Zarei, M.; Lange, F.M.; Stahr, K. Halloysite characteristics and formation in the northern Golan Heights. Geoderma 2004, 123, 279–295. [Google Scholar] [CrossRef]
- Steinberger, Y.; Doniger, T.; Applebaum, I.; Sherman, C.; Rotbart, N. Effects of Vineyard Agro-management Practices on Soil Bacterial Community Composition, and Diversity. Microb. Ecol. 2024, 87, 17. [Google Scholar] [CrossRef]
- Vestergaard, G.; Schulz, S.; Schöler, A.; Schloter, M. Making big data smart—How to use metagenomics to understand soil quality. Biol. Fertil. Soils 2017, 53, 479–484. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 18 March 2025).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P. Vegan: Community Ecology Package; R Package Version 2.0-2, 17 January 2017; R: Vienna, Austria, 2017. [Google Scholar]
- Day, N.J.; Dunfield, K.E.; Johnstone, J.F.; Mack, M.C.; Turetsky, M.R.; Walker, X.J.; White, A.L.; Baltzer, J.L. Wildfire severity reduces richness and alters composition of soil fungal communities in boreal forests of western Canada. Glob. Chang. Biol. 2019, 25, 2310–2324. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Kristiansson, E.; Ryberg, M.; Hallenberg, N.; Larsson, K.H. Intraspecific ITS variability in the Kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol. Bioinform. 2008, 2008, 193–201. [Google Scholar] [CrossRef]
- He, Y.; Zhou, B.J.; Deng, G.H.; Jiang, X.T.; Zhang, H.; Zhou, H.W. Comparison of microbial diversity determined with the same variable tag sequence extracted from two different PCR amplicons. BMC Microbiol. 2013, 13, 208. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R.E. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef]
- Hart, M.M. Evaluating the diversity of soil microbial communities in vineyards relative to adjacent native ecosystems. Appl. Soil. Ecol. 2016, 100, 91–103. [Google Scholar] [CrossRef]
- Likar, M.; Stres, B.; Rusjan, D.; Potisek, M.; Regvar, M. Ecological and conventional viticulture gives rise to distinct fungal and bacterial microbial communities in vineyard soils. Appl. Soil. Ecol. 2017, 113, 86–95. [Google Scholar] [CrossRef]
- Baldrian, P.; Bell-Dereske, L.; Lepinay, C.; Větrovský, T.; Kohout, P. Fungal communities in soils under global change. Stud. Mycol. 2022, 103, 1–24. [Google Scholar] [CrossRef]
- Frac, M.; Hannula, S.E.; Belka, M.; Jȩdryczka, M. Fungal biodiversity and their role in soil health. Front. Microbiol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Žifčáková, L.; Větrovský, T.; Howe, A.; Baldrian, P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ. Microbiol. 2016, 18, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Kleine, B.I.; Stefánsson, A.; Kjartansdóttir, R.; Prause, S.; Weisenberger, T.B.; Reynolds, H.I.; Sveinbjörnsdóttir, Á.E.; Jackson, M.D.; Gudmundsson, M.T. The Surtsey volcano geothermal system: An analogue for seawater-oceanic crust interaction with implications for the elemental budget of the oceanic crust. Chem. Geol. 2020, 550, 119702. [Google Scholar] [CrossRef]
- Gysi, A.P.; Stefánsson, A. CO2–water–basalt interaction. Numerical simulation of low temperature CO2 sequestration into basalts. Geochim. Cosmochim. Acta 2011, 75, 4728–4751. [Google Scholar] [CrossRef]
- Coleman, D.C.; Crossley, D.A., Jr.; Hendrix, P.F. Fundamentals of Soil Ecology, 2nd ed.; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Blomberg, A.; Adler, L. Physiology of osmotolerance in fungi. In Advances in Microbial Physiology; Academic Press Ltd.: San Diego, CA, USA, 1992; Volume 33. [Google Scholar]
- Schappe, T.; Albornoz, F.E.; Turner, B.L.; Jones, F.A. Co-occurring fungal functional groups respond differently to tree neighborhoods and soil properties across three tropical rainforests in Panama. Microb. Ecol. 2020, 79, 675–685. [Google Scholar] [CrossRef]
- Kutos, S.; Barnes, E.M.; Bhutada, A.; Lewis, J.D. Preferential associations of soil fungal taxa under mixed compositions of eastern American tree species. FEMS Microbiol. Ecol. 2022, 98, 1–12. [Google Scholar] [CrossRef]
- Talbot, J.M.; Bruns, T.D.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Peay, K.G. Independent roles of ectomycorrhizal and saprotrophic communities in soil organic matter decomposition. Soil. Biol. Biochem. 2013, 57, 282–291. [Google Scholar] [CrossRef]
- James, T.Y.; Kauff, F.; Schoch, C.L.; Matheny, P.B.; Hofstetter, V.; Cox, C.J.; Celio, G.; Gueidan, C.; Fraker, E.; Miadlikowska, J.; et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 2006, 443, 818–822. [Google Scholar] [CrossRef]
- Yin, Y.; Yuan, Y.; Zhang, X.; Huhe; Cheng, Y.; Borjigin, S. Comparison of the responses of soil fungal community to straw, inorganic fertilizer, and compost in a farmland in the loess plateau. Microbiol. Spectr. 2022, 10, e02230-21. [Google Scholar] [CrossRef] [PubMed]
- Soonvald, L.; Loit, K.; Runno-Paurson, E.; Astover, A.; Tedersoo, L. Characterising the effect of crop species and fertilisation treatment on root fungal communities. Sci. Rep. 2020, 10, 18741. [Google Scholar] [CrossRef]
- Fracchia, S.; Garcia-Romera, I.; Godeas, A.; Ocampo, J.A. Effect of the saprophytic fungus Fusarium oxysporum on arbuscular mycorrhizal colonization and growth of plants in greenhouse and field trials. Plant Soil 2000, 223, 177–186. [Google Scholar] [CrossRef]
- Cai, X.-B.; Peng, Y.-L.; Yang, M.-N.; Zhang, T.; Zhang, Q. Grassland degradation decrease the diversity of arbuscular mycorrhizal fungi species in Tibet Plateau. Not. Bot. Horti Agrobot. Cluj Napoca 2014, 42, 333–339. [Google Scholar] [CrossRef]
- Li, X.; Gai, J.; Cai, X.; Li, X.; Christie, P.; Zhang, F.; Zhang, J. Molecular diversity of arbuscular mycorrhizal fungi associated with two co-occurring perennial plant species on a Tibetan altitudinal gradient. Mycorrhiza 2014, 24, 95–107. [Google Scholar] [CrossRef] [PubMed]

| Site Name | Label | Coordination |
|---|---|---|
| Merom Golan Organic Vineyard | MO | 33°03′50.9″ N 35°45′10.6″ E |
| Merom-Golan Intensive Conventional Vineyard | MI | 33°03′29.5″ N 35°44′57.4″ E |
| Natural pasture | NP | 33°03′44.4″ N 35°45′06.9″ E |
| Foliar fertilization | FF | 33°04′45.8″ N 35°46′21.8″ E |
| Open field | OF | 33°04′45.6″ N 35°46′22.5″ E |
| Species Richness | Shannon Index | S. Chao1 | S.ACE | |
|---|---|---|---|---|
| MI | 234 ± 15.53 b | 4.04 ± 0.1 b | 313 ± 26.1 ab | 302 ± 17.7 bc |
| MO | 235 ± 17.21 b | 4.05 ± 0.12 b | 275 ± 23.0 b | 283 ± 19.5 c |
| OF | 269 ± 11.24 a | 4.22 ± 0.07 b | 343 ± 36.9 a | 345 ± 17.8 ab |
| FF | 209 ± 18.93 b | 3.72 ± 0.17 c | 274 ± 46.9 b | 281 ± 40.5 c |
| NP | 291 ± 17.47 a | 4.45 ± 0.12 a | 350 ± 32.4 a | 358 ± 22.9 a |
| Df | Sum of Squares | Mean Squares | F | R2 | Pr (>F) | |
|---|---|---|---|---|---|---|
| Sample location | 4 | 1.44 | 0.360 | 3.54 | 0.586 | 0.0001 |
| Residuals | 10 | 1.02 | 0.102 | 0.414 | ||
| Total | 14 | 2.46 | 1.00 |
| MO | MI | NP | FF | OF | |
|---|---|---|---|---|---|
| Pathotroph | 34.3 | 39.2 | 29.2 | 25.0 | 33.9 |
| Saprotroph (SA) | 64.5 | 60.3 | 68.8 | 74.9 | 65.3 |
| Symbiotroph (SY) | 1.2 | 0.5 | 2.0 | 0.1 | 0.8 |
| SY/SA | 1.82 | 0.810 | 2.95 | 0.164 | 1.17 |
| Trophic Mode | Confidence Ranking | MO | MI | NP | FF | OF |
|---|---|---|---|---|---|---|
| Unassigned | 136 | 125 | 169 | 107 | 132 | |
| Unassigned% | 32 | 30.7 | 32.4 | 30.4 | 28.3 | |
| Saptotrophs% of total | 39.2 | 39.8 | 39.0 | 43.4 | 41.5 | |
| Pathotroph | Highly Probable | 4 | 5 | 3 | 4 | 4 |
| Possible | 52 | 49 | 59 | 37 | 50 | |
| Probable | 49 | 53 | 76 | 39 | 57 | |
| Saprotroph | Highly Probable | 13 | 14 | 14 | 16 | 14 |
| Possible | 101 | 98 | 103 | 91 | 110 | |
| Probable | 106 | 100 | 152 | 92 | 124 | |
| Symbiotroph | Highly Probable | 17 | 9 | 16 | 6 | 14 |
| Possible | 72 | 67 | 78 | 62 | 78 | |
| Probable | 11 | 12 | 20 | 5 | 15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotbart, N.; Doniger, T.; Applebaum, I.; Steinberger, Y. Soil Fungal Communities in the Rhizosphere of Sauvignon Blanc Grapes Subjected to Various Agricultural Management Practices. Land 2025, 14, 667. https://doi.org/10.3390/land14040667
Rotbart N, Doniger T, Applebaum I, Steinberger Y. Soil Fungal Communities in the Rhizosphere of Sauvignon Blanc Grapes Subjected to Various Agricultural Management Practices. Land. 2025; 14(4):667. https://doi.org/10.3390/land14040667
Chicago/Turabian StyleRotbart, Nativ, Tirza Doniger, Itaii Applebaum, and Yosef Steinberger. 2025. "Soil Fungal Communities in the Rhizosphere of Sauvignon Blanc Grapes Subjected to Various Agricultural Management Practices" Land 14, no. 4: 667. https://doi.org/10.3390/land14040667
APA StyleRotbart, N., Doniger, T., Applebaum, I., & Steinberger, Y. (2025). Soil Fungal Communities in the Rhizosphere of Sauvignon Blanc Grapes Subjected to Various Agricultural Management Practices. Land, 14(4), 667. https://doi.org/10.3390/land14040667






