Analysis of Soil δ13C and δ15N Along Precipitation Gradient: Critical Insights into Tree–Grass Interactions and Soil C Sequestration in Savannas
Abstract
1. Introduction
2. Materials and Methods
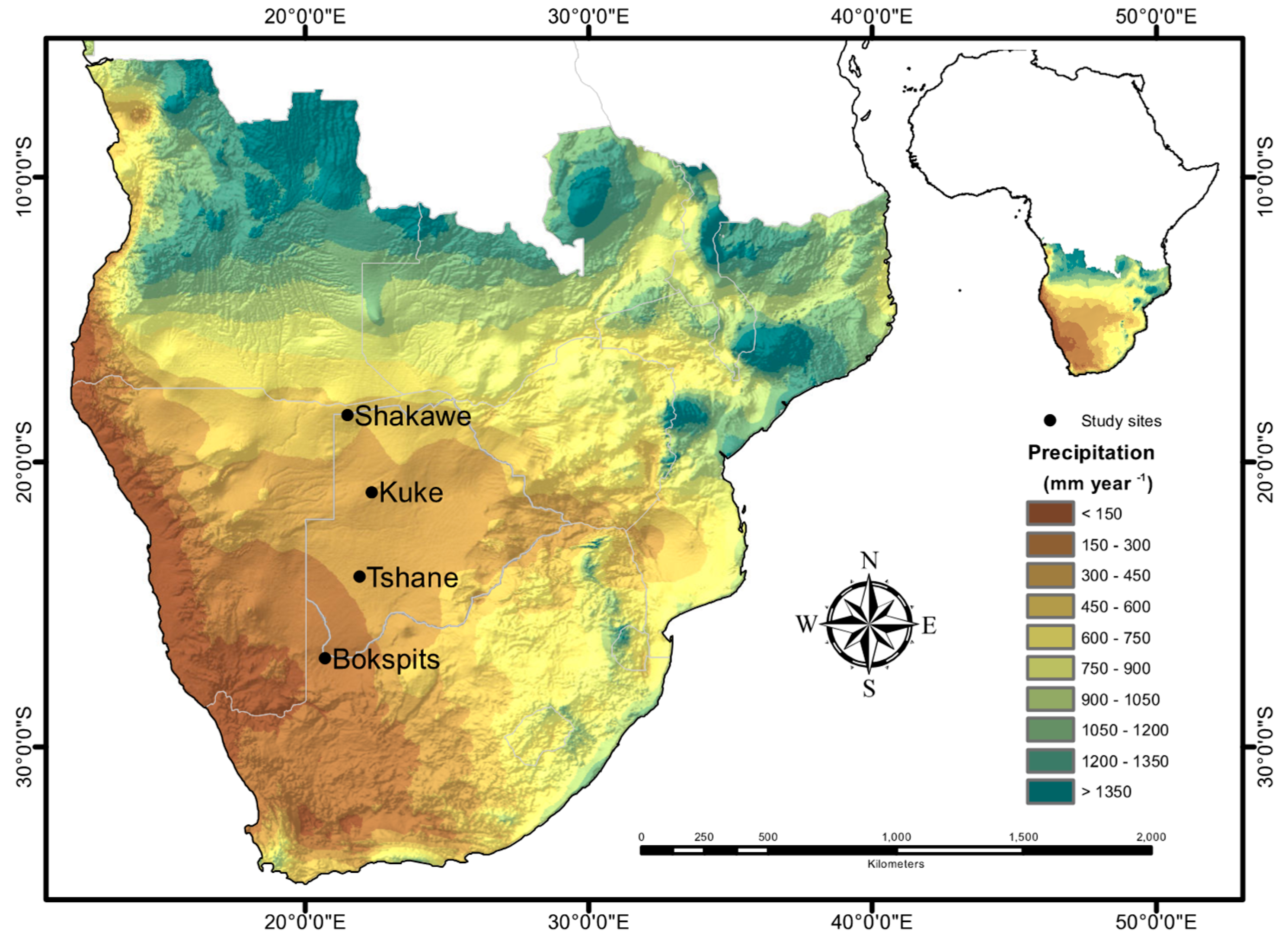
2.1. Study Site Description
2.2. Sample Design
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Soil Texture
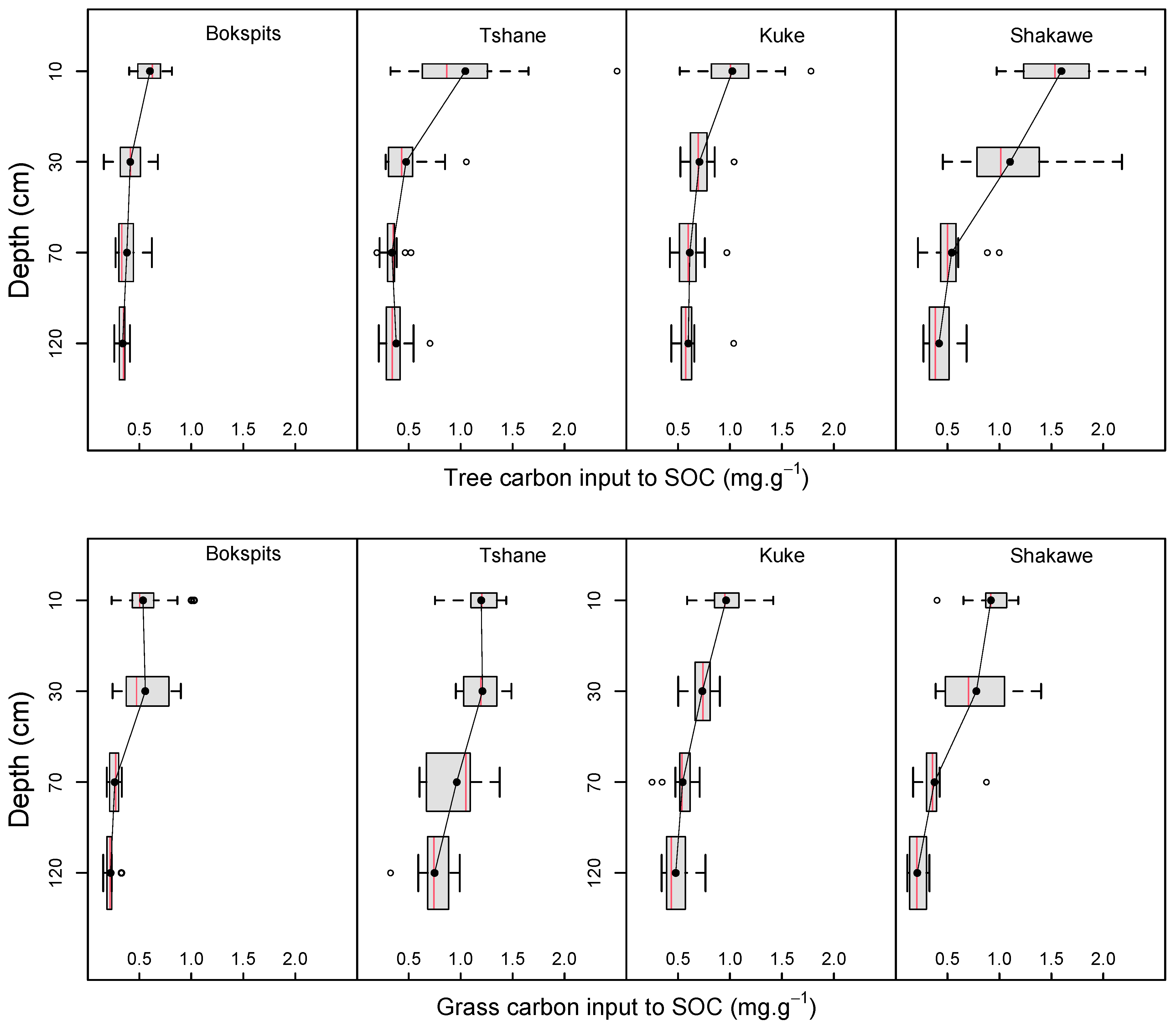
3.2. SOC Input from Trees and Grasses
3.3. Soil δ15N
4. Discussion
4.1. Soil Texture
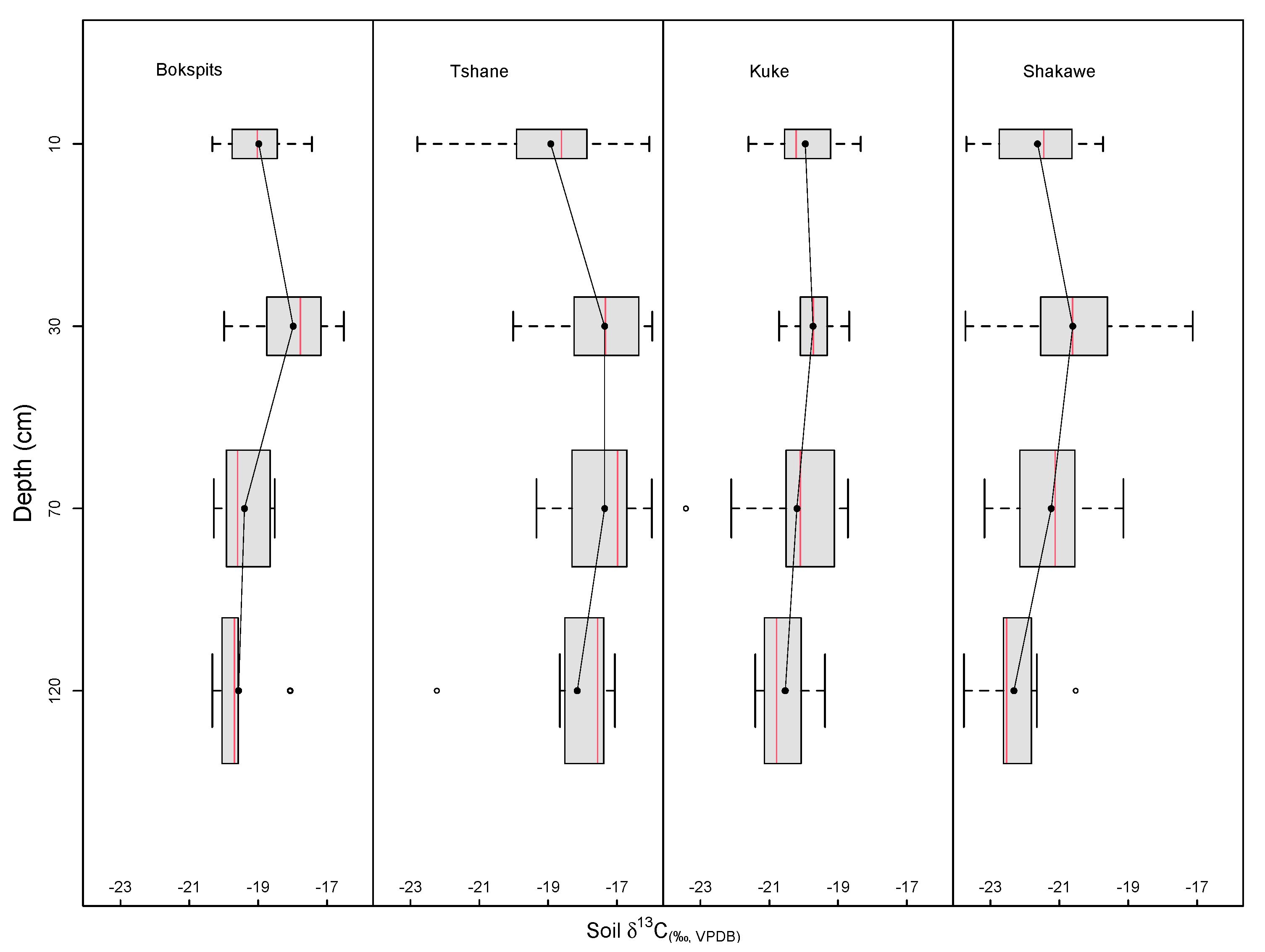
4.2. Soil Profile δ13C and Tree–Grass Belowground Interaction
4.3. Soil Profile δ13C and Precipitation Gradient
4.4. Soil Profile δ15N Along Precipitation Gradient
4.5. Potential Impacts of Climate Change on C Cycling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scholes, R.J.; Archer, S.R. Tree-Grass interactions in savannas. Annu. Rev. Ecol. Syst. 1997, 28, 517–544. [Google Scholar] [CrossRef]
- Sankaran, M.; Hanan, N.P.; Scholes, R.J.; Ratnam, J.; Augustine, D.J.; Cade, B.S.; Gignoux, J.; Higgins, S.I.; Le Roux, X.; Ludwig, F.; et al. Determinants of woody cover in African savannas. Nature 2005, 438, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Grunow, J.O.; Groeneveld, H.T.; Toit, S.H.C.D. Above-Ground Dry Matter Dynamics of the Grass Layer of a South African Tree Savanna. J. Ecol. 1980, 68, 877–889. [Google Scholar] [CrossRef]
- Belsky, A.J. Influences of Trees on Savanna Productivity: Tests of Shade, Nutrients, and Tree-Grass Competition. Ecology 1994, 75, 922–932. [Google Scholar] [CrossRef]
- Bond, W.J.; Parr, C.L. Beyond the forest edge: Ecology, diversity and conservation of the grassy biomes. Biol. Conserv. 2010, 143, 2395–2404. [Google Scholar] [CrossRef]
- Grubb, P.J. The Maintenance of Species-Richness in Plant Communities: The Importance of the Regeneration Niche. Biol. Rev. 1977, 52, 107–145. [Google Scholar] [CrossRef]
- Walker, B.H.; Noy-Meir, I. Aspects of the Stability and Resilience of Savanna Ecosystems. In Ecology of Tropical Savannas; Huntley, B.J., Walker, B.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 556–590. [Google Scholar]
- Bate, G.C.; Furniss, P.R.; Pendle, B.G. Water Relations of Southern African Savannas. In Ecology of Tropical Savannas; Huntley, B.J., Walker, B.H., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 336–358. [Google Scholar]
- Hipondoka, M.H.T.; Aranibar, J.N.; Chirara, C.; Lihavha, M.; Macko, S.A. Vertical distribution of grass and tree roots in arid ecosystems of Southern Africa: Niche differentiation or competition? J. Arid Environ. 2003, 54, 319–325. [Google Scholar] [CrossRef]
- Shmida, A.; Ellner, S. Coexistence of plant species with similar niches. Vegetatio 1984, 58, 29–55. [Google Scholar] [CrossRef]
- Glenn, E.; Squires, V.; Olsen, M.; Frye, R. Potential for carbon sequestration in the drylands. Water Air Soil Pollut. 1993, 70, 341–355. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Grace, J.; José, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Dintwe, K.; Okin, G.S.; D’Odorico, P.; Hrast, T.; Mladenov, N.; Handorean, A.; Bhattachan, A.; Caylor, K.K. Soil organic C and total N pools in the Kalahari: Potential impacts of climate change on C sequestration in savannas. Plant Soil 2014, 396, 27–44. [Google Scholar] [CrossRef]
- White II, D.A.; Welty-Bernard, A.; Rasmussen, C.; Schwartz, E. Vegetation controls on soil organic carbon dynamics in an arid, hyperthermic ecosystem. Geoderma 2009, 150, 214–223. [Google Scholar] [CrossRef]
- Nicholson, S.E.; Kim, J. The relationship of the El Niño-Southern Oscillation to African rainfall. Int. J. Climatol. 1997, 17, 117–135. [Google Scholar] [CrossRef]
- Nicholson, S.E. Climatic and environmental change in Africa during the last two centuries. Clim. Res. 2001, 17, 123–144. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.; Reichstein, M.; Smith, M.D.; Smith, S.D.; Bell, J.E.; Fay, P.A.; et al. Consequences of More Extreme Precipitation Regimes for Terrestrial Ecosystems. BioScience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Shongwe, M.E.; van Oldenborgh, G.J.; van den Hurk, B.J.J.M.; de Boer, B.; Coelho, C.A.S.; van Aalst, M.K. Projected Changes in Mean and Extreme Precipitation in Africa under Global Warming. Part I: Southern Africa. J. Clim. 2009, 22, 3819–3837. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Working Group I Contribution to the IPCC 5th Assessment Report; IPCC Secretariat: Geneva, Switzerland, 2013. [Google Scholar]
- Batisani, N.; Yarnal, B. Rainfall variability and trends in semi-arid Botswana: Implications for climate change adaptation policy. Appl. Geogr. 2010, 30, 483–489. [Google Scholar] [CrossRef]
- Dintwe, K.; Okin, G.S. Soil organic carbon in savannas decreases with anthropogenic climate change. Geoderma 2018, 309, 7–16. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, Q.; Shugart, H.H. Dynamic responses of African ecosystem carbon cycling to climate change. Clim. Res. 2001, 17, 183–193. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Reynolds, J.F.; Cunningham, G.L.; Huenneke, L.F.; Jarrell, W.M.; Virginia, R.A.; Whitford, W.G. Biological Feedbacks in Global Desertification. Science 1990, 247, 1043–1048. [Google Scholar] [CrossRef]
- Zeng, N.; Neelin, J.D. The Role of Vegetation–Climate Interaction and Interannual Variability in Shaping the African Savanna. J. Clim. 2000, 13, 2665–2670. [Google Scholar] [CrossRef]
- Grace, P.R.; Post, W.M.; Hennessy, K. The potential impact of climate change on Australia’s soil organic carbon resources. Carbon Balance Manag. 2006, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Bishop, T. Mapping change in key soil properties due to climate change over south-eastern Australia. Soil Res. 2019, 57, 467–481. [Google Scholar] [CrossRef]
- Ayarza, M.; Rao, I.; Vilela, L.; Lascano, C.; Vera, R. Soil carbon accumulation in crop–livestock systems in acid soil savannas of South America: A review. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Castellano, G.R.; Santos, L.A.; Menegário, A.A. Carbon Soil Storage and Technologies to Increase Soil Carbon Stocks in the South American Savanna. Sustainability 2022, 14, 5571. [Google Scholar] [CrossRef]
- Locatelli, J.L.; Grosso, S.D.; Santos, R.S.; Hong, M.; Gurung, R.; Stewart, C.E.; Cherubin, M.R.; Bayer, C.; Cerri, C.E.P. Modeling soil organic matter changes under crop diversification strategies and climate change scenarios in the Brazilian Cerrado. Agric. Ecosyst. Environ. 2025, 379, 109334. [Google Scholar] [CrossRef]
- Rittl, T.F.; Oliveira, D.; Cerri, C.E.P. Soil carbon stock changes under different land uses in the Amazon. Geoderma Reg. 2017, 10, 138–143. [Google Scholar] [CrossRef]
- Wei, X.; Shao, M.; Gale, W.; Li, L. Global pattern of soil carbon losses due to the conversion of forests to agricultural land. Sci. Rep. 2014, 4, 4062. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.G.; Schuster, M.J.; Dukes, J.S. Rainfall variability and nitrogen addition synergistically reduce plant diversity in a restored tallgrass prairie. J. Appl. Ecol. 2016, 53, 579–586. [Google Scholar] [CrossRef]
- Huang, J.; Wu, P.; Zhao, X. Effects of rainfall intensity, underlying surface and slope gradient on soil infiltration under simulated rainfall experiments. Catena 2013, 104, 93–102. [Google Scholar] [CrossRef]
- Thomas, B.F.; Behrangi, A.; Famiglietti, J.S. Precipitation Intensity Effects on Groundwater Recharge in the Southwestern United States. Water 2016, 8, 90. [Google Scholar] [CrossRef]
- Jin, Y.; Goulden, M.L. Ecological consequences of variation in precipitation: Separating short-versus long-term effects using satellite data: Ecological effects of precipitation variation. Glob. Ecol. Biogeogr. 2014, 23, 358–370. [Google Scholar] [CrossRef]
- Berry, R.S.; Kulmatiski, A. A savanna response to precipitation intensity. PLoS ONE 2017, 12, e0175402. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K.; Brehm, G.; Cardelús, C.L.; Gilman, A.C.; Longino, J.T. Global Warming, Elevational Range Shifts, and Lowland Biotic Attrition in the Wet Tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef]
- Bombelli, A.; Henry, M.; Castaldi, S.; Adu-Bredu, S.; Arneth, A.; de Grandcourt, A.; Grieco, E.; Kutsch, W.L.; Lehsten, V.; Rasile, A.; et al. An outlook on the Sub-Saharan Africa carbon balance RID A-7494-2011 RID D-1226-2010. Biogeosciences 2009, 6, 2193–2205. [Google Scholar] [CrossRef]
- Ciais, P.; Bombelli, A.; Williams, M.; Piao, S.L.; Chave, J.; Ryan, C.M.; Henry, M.; Brender, P.; Valentini, R. The Carbon Balance of Africa: Synthesis of Recent Research Studies. Philos. Trans. R. Soc. A 2011, 369, 2038–2057. [Google Scholar] [CrossRef]
- Ries, L.P.; Shugart, H.H. Nutrient limitations on understory grass productivity and carbon assimilation in an African woodland savanna. J. Arid Environ. 2008, 72, 1423–1430. [Google Scholar] [CrossRef]
- Bai, E.; Boutton, T.W.; Liu, F.; Wu, X.B.; Hallmark, C.T.; Archer, S.R. Spatial variation of soil δ13C and its relation to carbon input and soil texture in a subtropical lowland woodland. Soil Biol. Biochem. 2012, 44, 102–112. [Google Scholar] [CrossRef]
- Zhou, Y.; Mushinski, R.; Hyodo, A.; Wu, X.; Boutton, T. Vegetation change alters soil profile δ15N values at the landscape scale. Soil Biol. Biochem. 2018, 119, 110–120. [Google Scholar] [CrossRef]
- West, J.B.; Bowen, G.J.; Cerling, T.E.; Ehleringer, J.R. Stable isotopes as one of nature’s ecological recorders. Trends Ecol. Evol. 2006, 21, 408–414. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Buchmann, N.; Flanagan, L.B. Carbon Isotope Ratios in Belowground Carbon Cycle Processes. Ecol. Appl. 2000, 10, 412–422. [Google Scholar] [CrossRef]
- Boutton, T.W.; Archer, S.R.; Midwood, A.J.; Zitzer, S.F.; Bol, R. δ13C values of soil organic carbon and their use in documenting vegetation change in a subtropical savanna ecosystem. Geoderma 1998, 82, 5–41. [Google Scholar] [CrossRef]
- Wang, L.; D’Odorico, P.; Ries, L.; Macko, S.A. Patterns and implications of plant-soil δ13C and δ15N values in African savanna ecosystems. Quat. Res. 2010, 73, 77–83. [Google Scholar] [CrossRef]
- Ringrose, S.; Matheson, W.; Wolski, P.; Huntsman-Mapila, P. Vegetation cover trends along the Botswana Kalahari transect. J. Arid Environ. 2003, 54, 297–317. [Google Scholar] [CrossRef]
- Bird, M.I.; Veenendaal, E.M.; Lloyd, J.J. Soil carbon inventories and δ13C along a moisture gradient in Botswana. Glob. Change Biol. 2004, 10, 342–349. [Google Scholar] [CrossRef]
- Tyson, P.D.; Crimp, S.J. The Climate of the Kalahari Transect. Trans. R. Soc. S. Afr. 1998, 53, 93–112. [Google Scholar] [CrossRef]
- Thomas, A.D.; Shaw, P.A. The Kalahari Environment; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Hulme, M. Climate Change and Southern Africa: An Exploration of Some Potential Impacts and Implications in the SADC (Southern African Development Community) Region; Climatic Research Unit, University of East Anglia, World Wide Fund for Nature: Norwich, UK, 1996. [Google Scholar]
- Shugart, H.H.; Macko, S.A.; Lesolle, P.; Szuba, T.A.; Mukelabai, M.M.; Dowty, P.; Swap, R.J. The SAFARI 2000—Kalahari Transect Wet Season Campaign of year 2000. Glob. Change Biol. 2004, 10, 273–280. [Google Scholar] [CrossRef]
- Scholes, R.J.; Dowty, P.R.; Caylor, K.; Parsons, D.A.B.; Frost, P.G.H.; Shugart, H.H. Trends in Savanna Structure and Composition along an Aridity Gradient in the Kalahari. J. Veg. Sci. 2002, 13, 419–428. [Google Scholar] [CrossRef]
- Wang, L.; D’Odorico, P.; Ringrose, S.; Coetzee, S.; Macko, S.A. Biogeochemistry of Kalahari sands. J. Arid Environ. 2007, 71, 259–279. [Google Scholar] [CrossRef]
- de Vries, J.J.; Selaolo, E.T.; Beekman, H.E. Groundwater recharge in the Kalahari, with reference to paleo-hydrologic conditions. J. Hydrol. 2000, 238, 110–123. [Google Scholar] [CrossRef]
- Swap, R.J.; Aranibar, J.N.; Dowty, P.R.; Gilhooly Iii, W.P.; Macko, S.A. Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: Patterns and implications. Glob. Change Biol. 2004, 10, 350–358. [Google Scholar] [CrossRef]
- Aranibar, J.N.; Otter, L.; Macko, S.A.; Feral, C.J.W.; Epstein, H.E.; Dowty, P.R.; Eckardt, F.; Shugart, H.H.; Swap, R.J. Nitrogen cycling in the soil–plant system along a precipitation gradient in the Kalahari sands. Glob. Change Biol. 2004, 10, 359–373. [Google Scholar] [CrossRef]
- Caylor, K.K.; Shugart, H.H.; Dowty, P.R.; Smith, T.M. Tree spacing along the Kalahari transect in southern Africa. J. Arid Environ. 2003, 54, 281–296. [Google Scholar] [CrossRef]
- Okin, G.S.; Mladenov, N.; Wang, L.; Cassel, D.; Caylor, K.K.; Ringrose, S.; Macko, S.A. Spatial patterns of soil nutrients in two southern African savannas. J. Geophys. Res. 2008, 113. [Google Scholar] [CrossRef]
- Meyer, T.; D’Odorico, P.; Okin, G.S.; Shugart, H.H.; Caylor, K.K.; O’Donnell, F.C.; Bhattachan, A.; Dintwe, K. An analysis of structure: Biomass structure relationships for characteristic species of the western Kalahari, Botswana. Afr. J. Ecol. 2013, 52, 20–29. [Google Scholar] [CrossRef]
- Bhattachan, A.; D’Odorico, P.; Dintwe, K.; Okin, G.S.; Collins, S.L. Resilience and recovery potential of duneland vegetation in the southern Kalahari. Ecosphere 2014, 5, 1–14. [Google Scholar] [CrossRef]
- Bhattachan, A.; D’Odorico, P.; Okin, G.S.; Dintwe, K. Potential dust emissions from the southern Kalahari’s dunelands. J. Geophys. Res. Earth Surf. 2013, 118, 307–314. [Google Scholar] [CrossRef]
- Koch, G.W.; Vitousek, P.M.; Steffen, W.L.; Walker, B.H. Terrestrial transects for global change research. Plant Ecol. 1995, 121, 53–65. [Google Scholar] [CrossRef]
- Wang, L.; D’Odorico, P.; Okin, G.S.; Macko, S.A. Isotope composition and anion chemistry of soil profiles along the Kalahari Transect. J. Arid Environ. 2008, 73, 480–486. [Google Scholar] [CrossRef]
- Bird, B.W.; Kirby, M.E.; Howat, I.M.; Tulaczyk, S. Geophysical evidence for Holocene lake-level change in southern California (Dry Lake). Boreas 2010, 39, 131–144. [Google Scholar] [CrossRef]
- Kirby, M.E.; Knell, E.J.; Anderson, W.T.; Lachniet, M.S.; Palermo, J.; Eeg, H.; Lucero, R.; Murrieta, R.; Arevalo, A.; Silveira, E.; et al. Evidence for insolation and Pacific forcing of late glacial through Holocene climate in the Central Mojave Desert (Silver Lake, CA). Quat. Res. 2015, 84, 174–186. [Google Scholar] [CrossRef]
- Harris, D.; Horwath, W.R.; van Kessel, C. Acid fumigation of soils to remove carbonates prior to total organic carbon or carbon-13 isotopic analysis. Soil Sci. Soc. Am. J. 2001, 65, 1853–1856. [Google Scholar] [CrossRef]
- Olsson, I.U. Radiocarbon Variations and Absolute Chronology; Proceedings; Wiley Interscience Division, John Wiley & Sons: New York, NY, USA, 1970. [Google Scholar]
- Craig, H. The geochemistry of the stable carbon isotopes. Geochim. Cosmochim. Acta 1953, 3, 53–92. [Google Scholar] [CrossRef]
- Craig, H. Isotopic standards for carbon and oxygen and correction factors for mass-spectrometric analysis of carbon dioxide. Geochim. Cosmochim. Acta 1957, 12, 133–149. [Google Scholar] [CrossRef]
- Amundson, R.; Stern, L.; Baisden, T.; Wang, Y. The isotopic composition of soil and soil-respired CO2. Geoderma 1998, 82, 83–114. [Google Scholar] [CrossRef]
- Stuiver, M.; Polach, H.A. Reporting of 14C Data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef]
- Epstein, M.S.; Diamondstone, B.I.; Gills, T.E.; Adams, J.R. A New River Sediment Standard, Reference Material. J. Res. Natl. Bur. Stand. 1988, 93, 234. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable Isotopes in Ecosystem Studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Mariotti, A. Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 1983, 303, 685–687. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon Isotopes in Photosynthesis. BioScience 1988, 38, 328–336. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon isotope fractionation in plants. Phytochemistry 1981, 20, 553–567. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Keeling, C.D.; Piper, S.C.; Bacastow, R.B.; Wahlen, M.; Whorf, T.P.; Heimann, M.; Meijer, H.A. Atmospheric CO2 and 13CO2 Exchange with the Terrestrial Biosphere and Oceans from 1978 to 2000: Observations and Carbon Cycle Implications. In A History of Atmospheric CO2 and Its Effects on Plants, Animals, and Ecosystems; Baldwin, I.T., Caldwell, M.M., Heldmaier, G., Jackson, R.B., Lange, O.L., Mooney, H.A., Schulze, E.D., Sommer, U., Ehleringer, J.R., Dearing, M.D., Eds.; Springer: New York, NY, USA, 2005; pp. 83–113. [Google Scholar]
- Werner, R.A.; Brand, W.A. Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun. Mass Spectrom. 2001, 15, 501–519. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Koba, K.; Marin-Spiotta, E.; Wang, L. Ecological interpretations of nitrogen isotope ratios of terrestrial plants and soils. Plant Soil 2015, 396, 1–26. [Google Scholar] [CrossRef]
- Handley, L.L.; Raven, J.A. The use of natural abundance of nitrogen isotopes in plant physiology and ecology. Plant Cell Environ. 1992, 15, 965–985. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Macko, S.A.; Shugart, H.H. Interpretation of nitrogen isotope signatures using the NIFTE model. Oecologia 1999, 120, 405–415. [Google Scholar] [CrossRef]
- Amundson, R.; Austin, A.T.; Schuur, E.a.G.; Yoo, K.; Matzek, V.; Kendall, C.; Uebersax, A.; Brenner, D.; Baisden, W.T. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles 2003, 17, 1031. [Google Scholar] [CrossRef]
- Craine, J.M.; Elmore, A.J.; Wang, L.; Augusto, L.; Baisden, W.T.; Brookshire, E.N.J.; Cramer, M.D.; Hasselquist, N.J.; Hobbie, E.A.; Kahmen, A.; et al. Convergence of soil nitrogen isotopes across global climate gradients. Sci. Rep. 2015, 5, 8280. [Google Scholar] [CrossRef]
- Shearer, G.; Kohl, D.H.; Virginia, R.A.; Bryan, B.A.; Skeeters, J.L.; Nilsen, E.T.; Sharifi, M.R.; Rundel, P.W. Estimates of N2-fixation from variation in the natural abundance of 15N in Sonoran desert ecosystems. Oecologia 1983, 56, 365–373. [Google Scholar] [CrossRef]
- Virginia, R.A.; Jarrell, W.M.; Rundel, P.W.; Shearer, G.; Kohl, D.H. The Use of Variation in the Natural Abundance of 15N to Assess Symbiotic Nitrogen Fixation by Woody Plants. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Springer: New York, NY, USA, 1989; pp. 375–394. [Google Scholar]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable Isotopes in Plant Ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Phillips, D.L.; Gregg, J.W. Source partitioning using stable isotopes: Coping with too many sources. Oecologia 2003, 136, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L.; Newsome, S.D.; Gregg, J.W. Combining sources in stable isotope mixing models: Alternative methods. Oecologia 2005, 144, 520–527. [Google Scholar] [CrossRef]
- Setshogo, M.P.; Venter, F. Trees of Botswana: Names and Distribution; Southern African Botanical Diversity Network (SABONET): Pretoria, South Africa, 2003; p. 170. [Google Scholar]
- Bird, M.I.; Pousai, P. Variations of δ13C in the surface soil organic carbon pool. Glob. Biogeochem. Cycles 1997, 11, 313–322. [Google Scholar] [CrossRef]
- Weiguo, L.; Xiahong, F.; Youfeng, N.; Qingle, Z.; Yunning, C.; Zhisheng, A.N. δ13C variation of C3 and C4 plants across an Asian monsoon rainfall gradient in arid northwestern China. Glob. Change Biol. 2005, 11, 1094–1100. [Google Scholar] [CrossRef]
- Sanaiotti, T.M.; Martinelli, L.A.; Victoria, R.L.; Trumbore, S.E.; Camargo, P.B. Past Vegetation Changes in Amazon Savannas Determined Using Carbon Isotopes of Soil Organic Matter. Biotropica 2002, 34, 2–16. [Google Scholar] [CrossRef]
- Wang, L.; Okin, G.S.; Caylor, K.K.; Macko, S.A. Spatial heterogeneity and sources of soil carbon in southern African savannas. Geoderma 2009, 149, 402–408. [Google Scholar] [CrossRef]
- Amundson, R. The Use of Stable Isotopes in Assessing the Effect of Agriculture on Arid and Semi-Arid Soils. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Springer: New York, NY, USA, 1989; pp. 318–341. [Google Scholar]
- Ma, J.-Y.; Sun, W.; Liu, X.-N.; Chen, F.-H. Variation in the Stable Carbon and Nitrogen Isotope Composition of Plants and Soil along a Precipitation Gradient in Northern China. PLoS ONE 2012, 7, e51894. [Google Scholar] [CrossRef]
- Dougill, A.J.; Thomas, A.D. Kalahari sand soils: Spatial heterogeneity, biological soil crusts and land degradation. Land Degrad. Dev. 2004, 15, 233–242. [Google Scholar] [CrossRef]
- Bhattachan, A.; Tatlhego, M.; Dintwe, K.; O’Donnell, F.; Caylor, K.K.; Okin, G.S.; Perrot, D.O.; Ringrose, S.; D’Odorico, P. Evaluating Ecohydrological Theories of Woody Root Distribution in the Kalahari. PLoS ONE 2012, 7, e33996. [Google Scholar] [CrossRef]
- Walker, B.; Langridge, J. Predicting savanna vegetation structure on the basis of plant available moisture (PAM) and plant available nutrients (PAN): A case study from Australia. J. Biogeogr. 1997, 24, 813–825. [Google Scholar] [CrossRef]
- Gillson, L. Evidence of a tipping point in a southern African savanna? Ecol. Complex. 2015, 21, 78–86. [Google Scholar] [CrossRef]
- Wynn, J.G.; Bird, M.I.; Wong, V.N.L. Rayleigh distillation and the depth profile of 13C/12C ratios of soil organic carbon from soils of disparate texture in Iron Range National Park, Far North Queensland, Australia. Geochim. Cosmochim. Acta 2005, 69, 1961–1973. [Google Scholar] [CrossRef]
- Mordelet, P.; Menaut, J.-C.; Mariotti, A. Tree and Grass Rooting Patterns in an African Humid Savanna. J. Veg. Sci. 1997, 8, 65–70. [Google Scholar] [CrossRef]
- February, E.C.; Higgins, S.I. The distribution of tree and grass roots in savannas in relation to soil nitrogen and water. S. Afr. J. Bot. 2010, 76, 517–523. [Google Scholar] [CrossRef]
- February, E.C.; Cook, G.D.; Richards, A.E. Root dynamics influence tree–grass coexistence in an Australian savanna. Austral Ecol. 2013, 38, 66–75. [Google Scholar] [CrossRef]
- Nilsen, E.T.; Sharifi, M.R.; Rundel, P.W.; Jarrell, W.M.; Virginia, R.A. Diurnal and Seasonal Water Relations of the Desert Phreatophyte Prosopis Glandulosa (Honey Mesquite) in the Sonoran Desert of California. Ecology 1983, 64, 1381–1393. [Google Scholar] [CrossRef]
- Kambatuku, J.R.; Cramer, M.D.; Ward, D. Overlap in soil water sources of savanna woody seedlings and grasses. Ecohydrology 2013, 6, 464–473. [Google Scholar] [CrossRef]
- O’Donnell, F.C.; Caylor, K.K.; Bhattachan, A.; Dintwe, K.; D’Odorico, P.; Okin, G.S. A quantitative description of the interspecies diversity of belowground structure in savanna woody plants. Ecosphere 2015, 6, 1–15. [Google Scholar] [CrossRef]
- Scanlon, T.M.; Albertson, J.D.; Caylor, K.K.; Williams, C.A. Determining land surface fractional cover from NDVI and rainfall time series for a savanna ecosystem. Remote Sens. Environ. 2002, 82, 376–388. [Google Scholar] [CrossRef]
- Caylor, K.K.; D’Odorico, P.; Rodriguez-Iturbe, I. On the ecohydrology of structurally heterogeneous semiarid landscapes. Water Resour. Res. 2006, 42, 1–13. [Google Scholar] [CrossRef]
- Ringrose, S.; Matheson, W.; Vanderpost, C. Analysis of soil organic carbon and vegetation cover trends along the Botswana Kalahari Transect. J. Arid Environ. 1998, 38, 379–396. [Google Scholar] [CrossRef]
- Dohn, J.; Dembélé, F.; Karembé, M.; Moustakas, A.; Amévor, K.A.; Hanan, N.P. Tree effects on grass growth in savannas: Competition, facilitation and the stress-gradient hypothesis. J. Ecol. 2013, 101, 202–209. [Google Scholar] [CrossRef]
- Ludwig, F.; Dawson, T.E.; Prins, H.H.T.; Berendse, F.; Kroon, H. Below-ground competition between trees and grasses may overwhelm the facilitative effects of hydraulic lift. Ecol. Lett. 2004, 7, 623–631. [Google Scholar] [CrossRef]
- Ludwig, F.; de Kroon, H.; Berendse, F.; Prins, H.H.T. The influence of savanna trees on nutrient, water and light availability and the understorey vegetation. Plant Ecol. 2004, 170, 93–105. [Google Scholar] [CrossRef]
- Priyadarshini, K.V.R.; Prins, H.H.T.; Bie, S.d.; Heitkönig, I.M.A.; Woodborne, S.; Gort, G.; Kirkman, K.; Fry, B.; Kroon, H.d. Overlap in nitrogen sources and redistribution of nitrogen between trees and grasses in a semi-arid savanna. Oecologia 2013, 174, 1107–1116. [Google Scholar] [CrossRef]
- Hartmann, D.L.; Klein Tank, A.M.G.; Rusticucci, M.; Alexander, L.V.; Brönnimann, S.; Charabi, Y.; Dentener, F.J.; Dlugokencky, E.J.; Easterling, D.R.; Kaplan, A.; et al. Observations: Atmosphere and Surface. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 159–254. [Google Scholar]
- Willis, K.J.; Bennett, K.D.; Burrough, S.L.; Macias-Fauria, M.; Tovar, C. Determining the response of African biota to climate change: Using the past to model the future. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120491. [Google Scholar] [CrossRef]
- Anadón, J.D.; Sala, O.E.; Maestre, F.T. Climate change will increase savannas at the expense of forests and treeless vegetation in tropical and subtropical Americas. J. Ecol. 2014, 102, 1363–1373. [Google Scholar] [CrossRef]
- Higgins, S.I.; Scheiter, S. Atmospheric CO2 forces abrupt vegetation shifts locally, but not globally. Nature 2012, 488, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.; Schimel, D.S.; Holland, E.A. Mechanisms of shrubland expansion: Land use, climate or CO2? Clim. Change 1995, 29, 91–99. [Google Scholar] [CrossRef]
- Walter, H. Vegetation of the Earth in Relation to Climate and the Eco-Physiological Conditions, 1st ed.; Springer: London, UK; New York, NY, USA, 1973; p. 230. [Google Scholar]

| Site Feature | Bokspits | Tshane | Kuke | Shakawe |
|---|---|---|---|---|
| Geographic | 26°53′39″ S | 24°01′01″ S | 20°58′36″ S | 18°21′51″ S |
| Coordinates | 20°41′54″ E | 21°52′08″ E | 22°28′48″ E | 21°50′31″ E |
| Mean Annual Precipitation (mm) | 180 | 360 | 440 | 540 |
| Mean Min Temp (°C) | 2.1 | 3.9 | 4.4 | 5.8 |
| Mean Max Temp (°C) | 34.3 | 33.5 | 33.4 | 33.7 |
| Soil pH | 6.7 | 5.6 | 5.9 | 5.4 |
| Organic Carbon (g m−2) | 1397.0 | 2506.0 | 2136.0 | 1982.0 |
| Woody biomass (g m−2) | 0.15 | 0.31 | 0.40 | 0.52 |
| Nitrogen (g m−2) | 78.6 | 151.0 | 135.0 | 107.0 |
| C3 Foliar δ13CVPDB (‰) | −23.2 | −25.9 | −25.9 | −26.7 |
| C4 Foliar δ13CVPDB (‰) | −14.0 | −14.2 | −13.8 | −13.4 |
| C3 foliar δ15Natm (‰) | 7.40 | 6.82 | 7.27 | 3.50 |
| Site | Depth | Clay | Silt | Sand |
|---|---|---|---|---|
| (MAP) | (cm) | (% Composition) | ||
| Bokspits (180 mm) | 10 | 0.55 | 3.10 | 96.34 |
| 30 | 0.67 | 3.56 | 95.76 | |
| 70 | 0.81 | 4.36 | 94.82 | |
| 120 | 1.30 | 10.36 | 88.32 | |
| 0–120 | 0.97 | 6.62 | 92.39 | |
| Tshane (350 mm) | 10 | 1.54 | 5.01 | 93.43 |
| 30 | 1.13 | 6.30 | 92.55 | |
| 70 | 1.52 | 7.76 | 90.70 | |
| 120 | 1.58 | 8.17 | 90.22 | |
| 0–120 | 1.48 | 7.46 | 91.04 | |
| Kuke (440 mm) | 10 | 1.15 | 3.44 | 95.40 |
| 30 | 0.87 | 3.88 | 95.23 | |
| 70 | 1.05 | 4.56 | 94.38 | |
| 120 | 1.00 | 4.59 | 94.39 | |
| 0–120 | 1.01 | 4.37 | 94.61 | |
| Shakawe (540 mm) | 10 | 0.00 | 1.02 | 98.98 |
| 30 | 0.00 | 0.83 | 99.16 | |
| 70 | 0.00 | 0.00 | 99.99 | |
| 120 | 0.00 | 1.21 | 98.79 | |
| 0–120 | 0.00 | 0.73 | 99.27 | |
| Site | Trees | Grasses | ||||||
|---|---|---|---|---|---|---|---|---|
| Co (g m−3) | Tree Input (g m−2) | k (m) | e-Folding Depth (m) | Z50 (m) | Z95 (m) | Co (g m−3) | Grass Input (g m−2) | |
| Bokspits (180 mm) | 965.5 | 769.4 | 0.5 | 2.1 | 1.5 | 6.3 | 881.0 | 648.0 |
| Tshane (350 mm) | 1216.7 | 808.4 | 0.9 | 1.1 | 0.8 | 3.4 | 2084.4 | 1721.0 |
| Kuke (440 mm) | 1423.2 | 1121.9 | 0.5 | 2.0 | 1.4 | 6.0 | 1470.3 | 1073.5 |
| Shakawe (540 mm) | 2729.5 | 1288.1 | 1.8 | 0.6 | 0.4 | 1.7 | 1583.9 | 830.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dintwe, K.; Okin, G.S.; O’Donnell, F.; Gilhooly, W.P., III; Bhattachan, A.; Tatlhego, M.; Wang, L.; D’Odorico, P. Analysis of Soil δ13C and δ15N Along Precipitation Gradient: Critical Insights into Tree–Grass Interactions and Soil C Sequestration in Savannas. Land 2025, 14, 2328. https://doi.org/10.3390/land14122328
Dintwe K, Okin GS, O’Donnell F, Gilhooly WP III, Bhattachan A, Tatlhego M, Wang L, D’Odorico P. Analysis of Soil δ13C and δ15N Along Precipitation Gradient: Critical Insights into Tree–Grass Interactions and Soil C Sequestration in Savannas. Land. 2025; 14(12):2328. https://doi.org/10.3390/land14122328
Chicago/Turabian StyleDintwe, Kebonye, Gregory S. Okin, Frances O’Donnell, William P. Gilhooly, III, Abinash Bhattachan, Mokganedi Tatlhego, Lixin Wang, and Paolo D’Odorico. 2025. "Analysis of Soil δ13C and δ15N Along Precipitation Gradient: Critical Insights into Tree–Grass Interactions and Soil C Sequestration in Savannas" Land 14, no. 12: 2328. https://doi.org/10.3390/land14122328
APA StyleDintwe, K., Okin, G. S., O’Donnell, F., Gilhooly, W. P., III, Bhattachan, A., Tatlhego, M., Wang, L., & D’Odorico, P. (2025). Analysis of Soil δ13C and δ15N Along Precipitation Gradient: Critical Insights into Tree–Grass Interactions and Soil C Sequestration in Savannas. Land, 14(12), 2328. https://doi.org/10.3390/land14122328







