Effects of Vegetation Restoration on Soil Fungal Communities During Early Post-Construction Phase of a Desert Steppe Photovoltaic Power Station
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Experimental Design and Sample Collection
2.3. Soil Enzymatic Activity
2.4. DNA Extraction, PCR Amplification, Libraries Preparation and Sequencing
2.5. Sequencing Data Analysis
3. Results
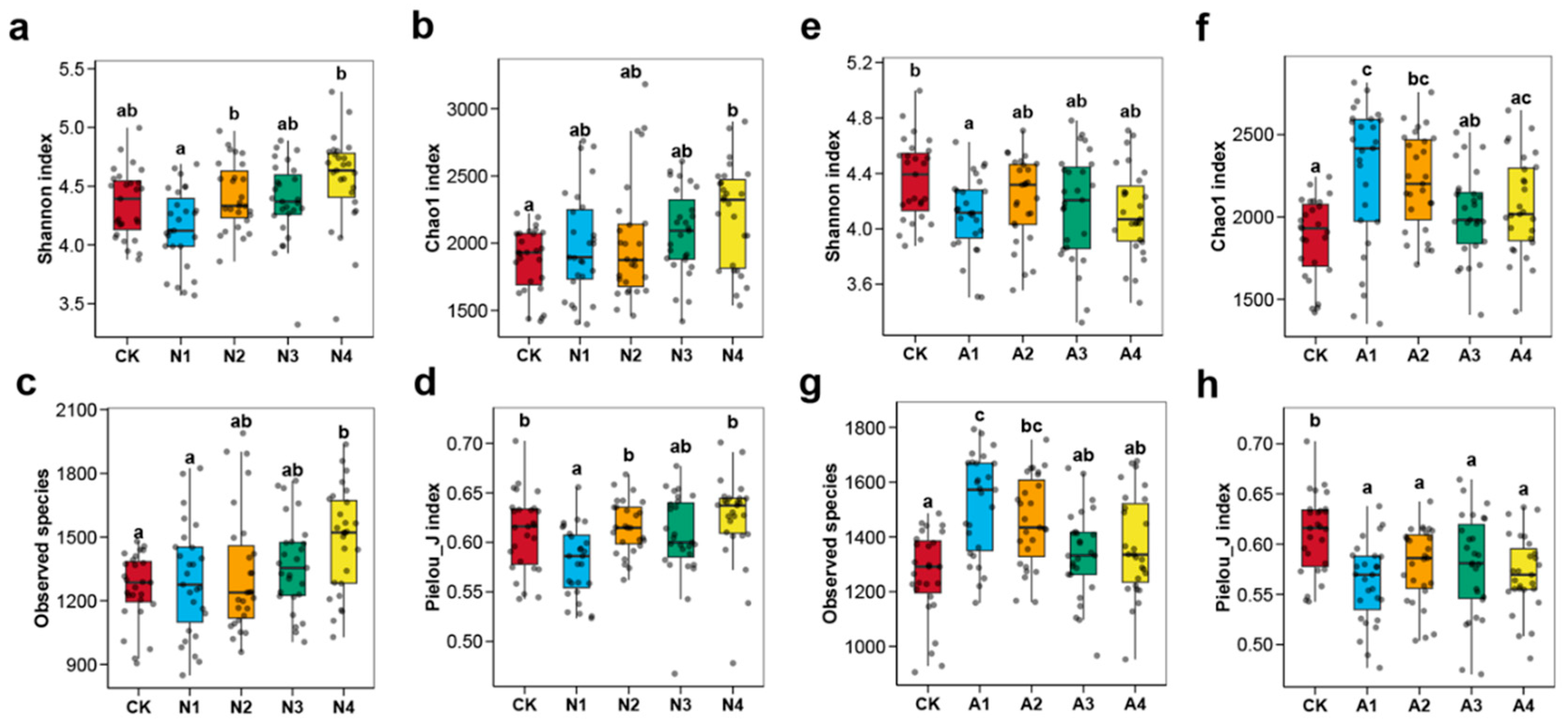
3.1. Effects of Restoration Measures and Solar Panel Shading on Soil Fungal Diversity
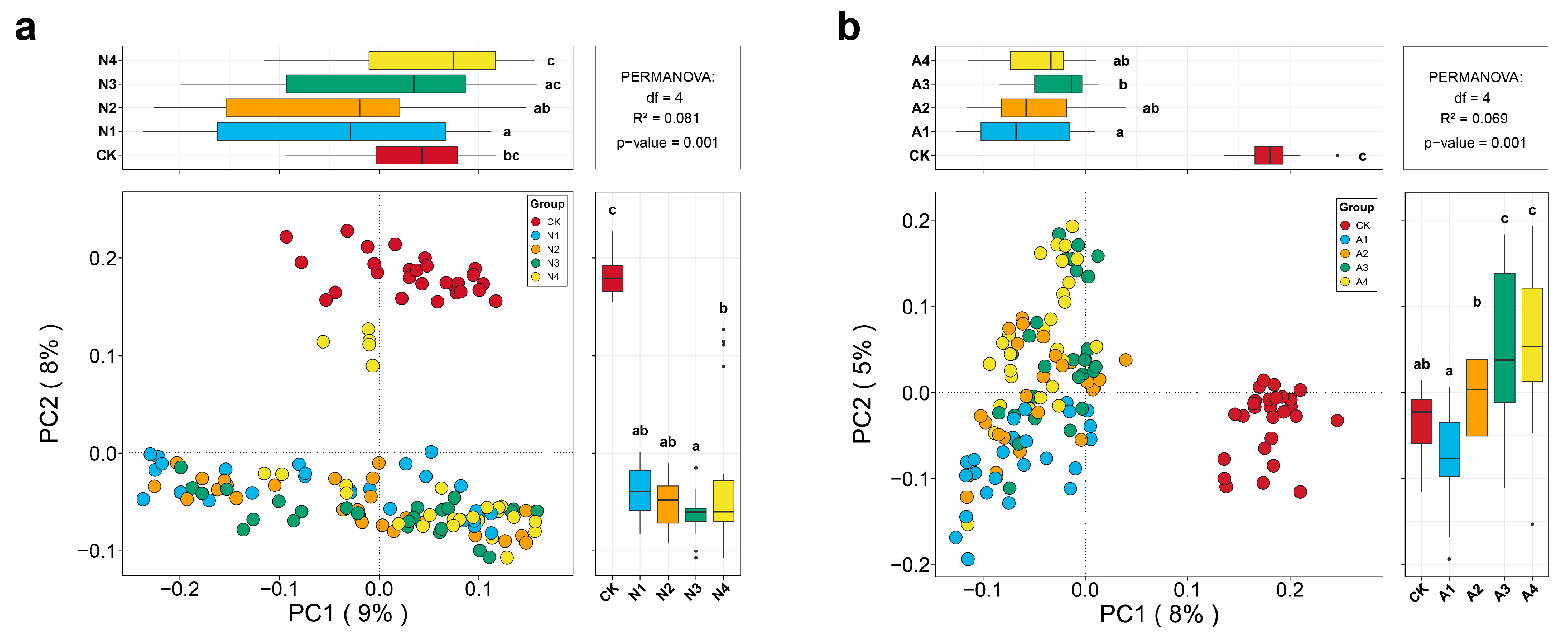
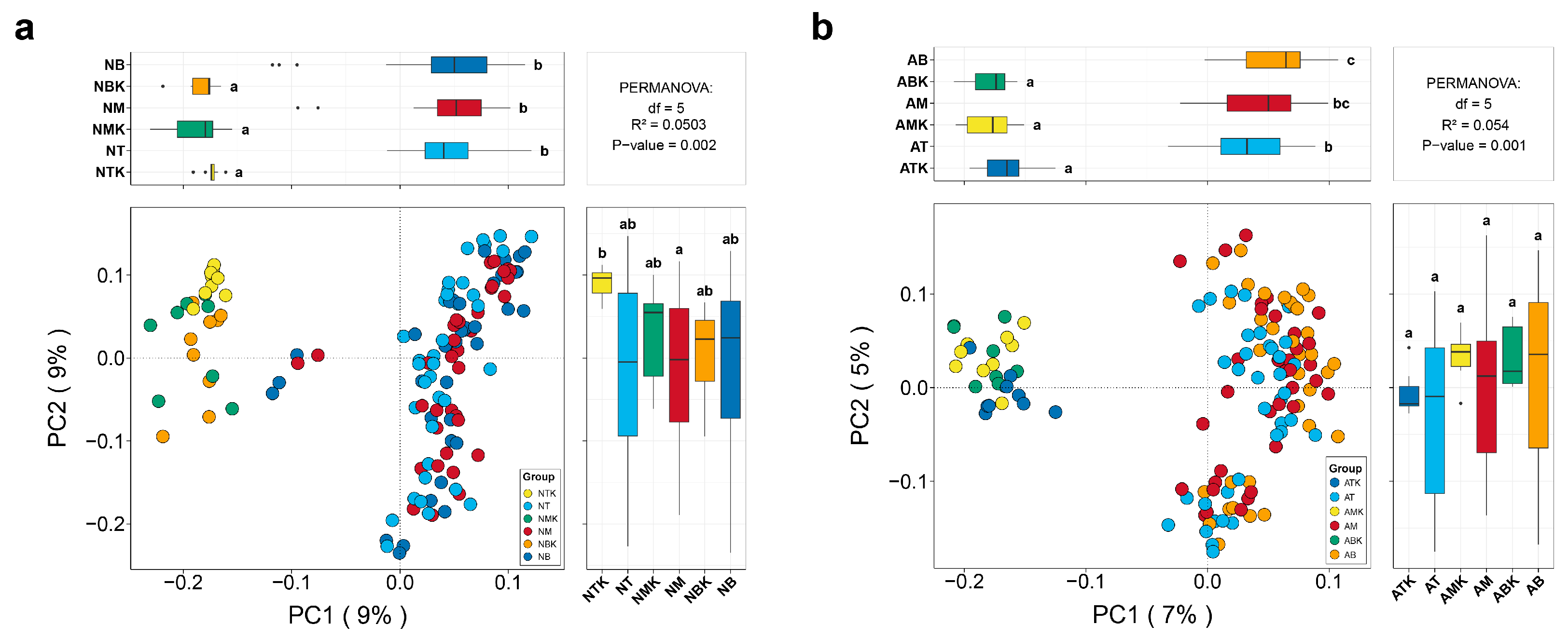
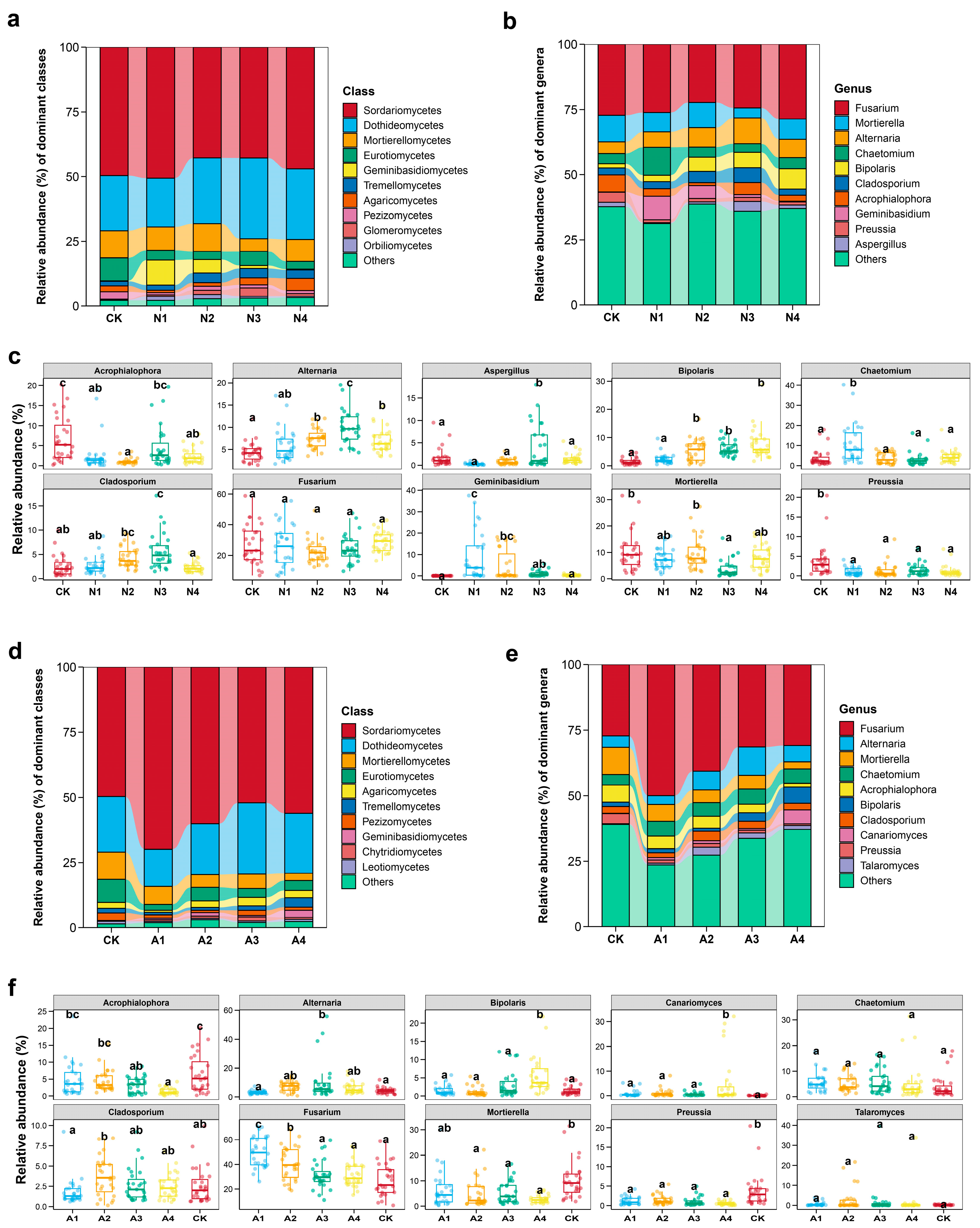
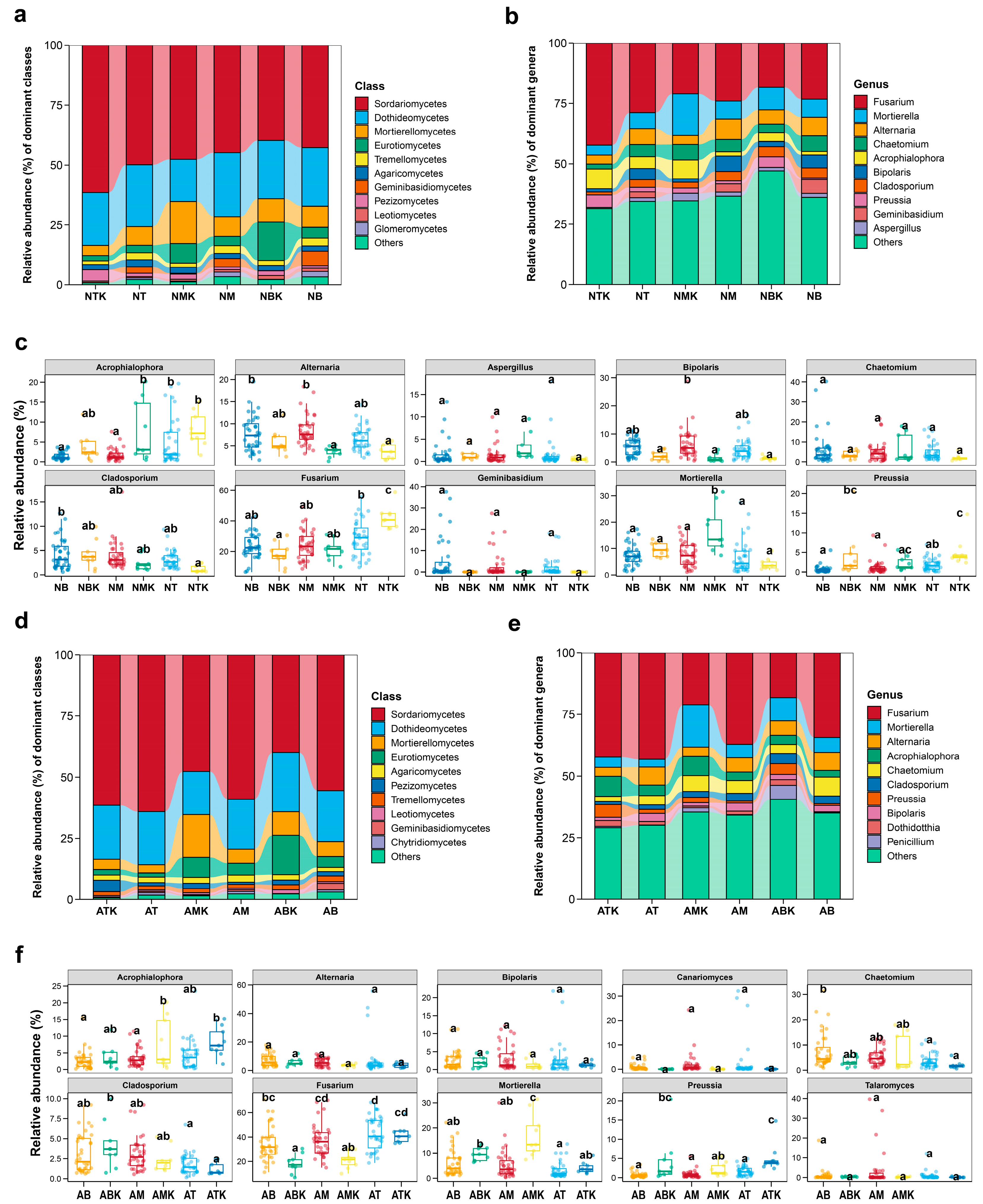
3.2. Effects of Restoration Measures and Solar Panel Shadings on the Compositions and Structures of Soil Fungal Communities in Semi-Arid Area Ecosystem
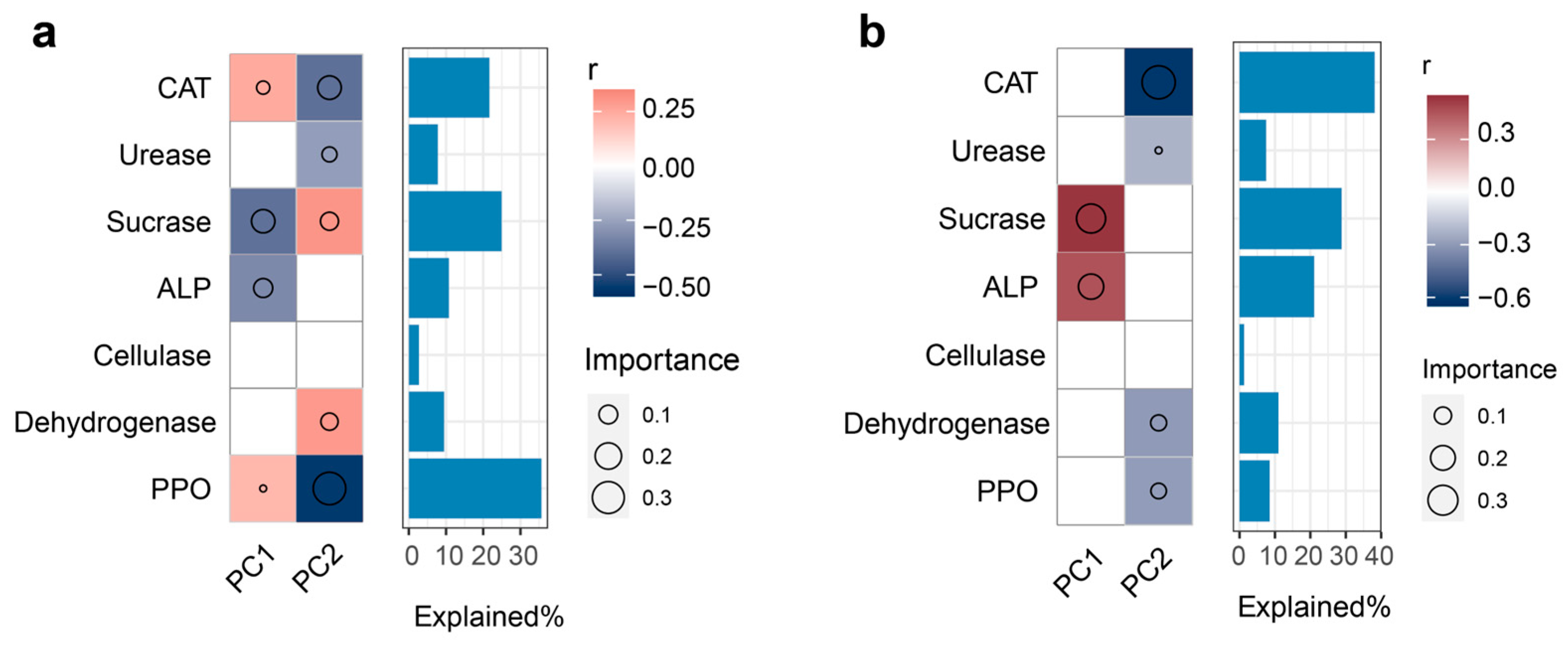
3.3. Key Enzyme Drivers of Fungal Community Dynamics Under Different Restoration Regimes
3.4. Correlations Between the Predicted Biomarkers and the Soil Enzymatic Activities
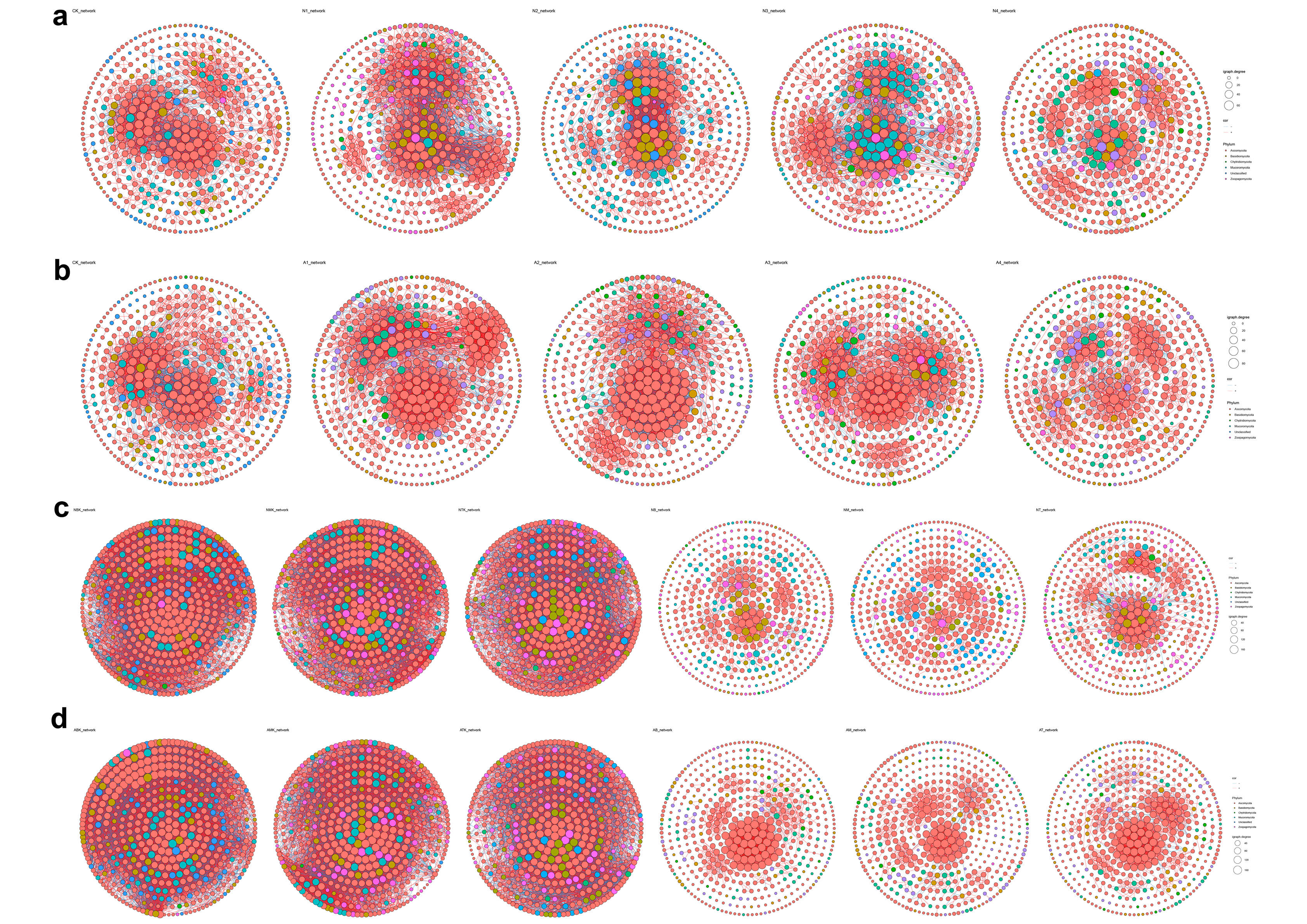
3.5. Impacts of Restoration Measures and Solar Panel Shadings on Soil Microbial Co-Occurrence Networks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Peng, T.; Ma, S.; Qi, C.; Song, Y.; Zhang, C.; Li, K.; Gao, N.; Pu, M.; Wang, X.; et al. Potential benefits and risks of solar photovoltaic power plants on arid and semi-arid ecosystems: An assessment of soil microbial and plant communities. Front. Microbiol. 2023, 14, 1190650. [Google Scholar] [CrossRef]
- Turney, D.; Fthenakis, V. Environmental impacts from the installation and operation of large-scale solar power plants. Renew. Sustain. Energy Rev. 2011, 15, 3261–3270. [Google Scholar] [CrossRef]
- Wu, C.; Liu, H.; Yu, Y.; Zhao, W.; Liu, J.; Yu, H.; Yetemen, O. Ecohydrological effects of photovoltaic solar farms on soil microclimates and moisture regimes in arid Northwest China: A modeling study. Sci. Total Environ. 2022, 802, 149946. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Mu, R.; Wang, D.; Wang, Z.; An, J.; Li, X. Effects of Two Ecological Governance Measures for Photovoltaic Power Stations on Plant Growth and Soil Nutrients. Plants 2025, 14, 797. [Google Scholar] [CrossRef]
- Lambert, Q.; Bischoff, A.; Cueff, S.; Cluchier, A.; Gros, R. Effects of solar park construction and solar panels on soil quality, microclimate, CO effluxes, and vegetation under a Mediterranean climate. Land Degrad. Dev. 2021, 32, 5190–5202. [Google Scholar] [CrossRef]
- Huang, Y.; Yesilonis, I.; Szlavecz, K. Soil microarthropod communities of urban green spaces in Baltimore, Maryland, USA. Urban For. Urban Green. 2020, 53, 126676. [Google Scholar] [CrossRef]
- Li, Y.; Armstrong, A.; Simmons, C.; Krasner, N.Z.; Hernandez, R.R. Ecological impacts of single-axis photovoltaic solar energy with periodic mowing on microclimate and vegetation. Front. Sustain. 2025, 6, 1497256. [Google Scholar] [CrossRef]
- Armstrong, A.; Waldron, S.; Whitaker, J.; Ostle, N.J. Wind farm and solar park effects on plant-soil carbon cycling: Uncertain impacts of changes in ground-level microclimate. Glob. Chang. Biol. 2014, 20, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Suuronen, A.; Muñoz-Escobar, C.; Lensu, A.; Kuitunen, M.; Guajardo Celis, N.; Espinoza Astudillo, P.; Ferrú, M.; Taucare-Ríos, A.; Miranda, M.; Kukkonen, J.V.K. The Influence of Solar Power Plants on Microclimatic Conditions and the Biotic Community in Chilean Desert Environments. Environ. Manag. 2017, 60, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kalnay, E.; Motesharrei, S.; Rivas, J.; Kucharski, F.; Kirk-Davidoff, D.; Bach, E.; Zeng, N. Climate model shows large-scale wind and solar farms in the Sahara increase rain and vegetation. Science 2018, 361, 1019–1022. [Google Scholar] [CrossRef]
- Yavari, R.; Zaliwciw, D.; Cibin, R.; McPhillips, L. Minimizing environmental impacts of solar farms: A review of current science on landscape hydrology and guidance on stormwater management. Environ. Res. Infrastruct. Sustain. 2022, 2, 032002. [Google Scholar] [CrossRef]
- Tanner, K.E.; Moore-O’Leary, K.A.; Parker, I.M.; Pavlik, B.M.; Hernandez, R.R. Simulated solar panels create altered microhabitats in desert landforms. Ecosphere 2020, 11, e03089. [Google Scholar] [CrossRef]
- Lioubimtseva, E.; Cole, R. Uncertainties of Climate Change in Arid Environments of Central Asia. Rev. Fish. Sci. 2006, 14, 29–49. [Google Scholar] [CrossRef]
- Wang, Z.; Xiong, H.; Zhang, F.; Qiu, Y.; Ma, C. Sustainable development assessment of ecological vulnerability in arid areas under the influence of multiple indicators. J. Clean. Prod. 2024, 436, 140629. [Google Scholar] [CrossRef]
- Bhaduri, D.; Sihi, D.; Bhowmik, A.; Verma, B.C.; Munda, S.; Dari, B. A review on effective soil health bio-indicators for ecosystem restoration and sustainability. Front. Microbiol. 2022, 13, 938481. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, H.; Li, C.; Lu, G.; Ye, D.; Ma, C.; Ren, L.; Li, G. Assessment of the ecological and environmental effects of large-scale photovoltaic development in desert areas. Sci. Rep. 2024, 14, 22456. [Google Scholar] [CrossRef]
- Dilixiati, B.; Wang, H.; Gong, L.; Wei, J.; Lei, C.; Dang, L.; Zhang, X.; Gu, W.; Zhang, H.; Zhang, J. Land Use Dynamics and Ecological Effects of Photovoltaic Development in Xinjiang: A Remote Sensing and Geospatial Analysis. Land 2025, 14, 1294. [Google Scholar] [CrossRef]
- Marrou, H.; Guilioni, L.; Dufour, L.; Dupraz, C.; Wery, J. Microclimate under agrivoltaic systems: Is crop growth rate affected in the partial shade of solar panels? Agric. For. Meteorol. 2013, 177, 117–132. [Google Scholar] [CrossRef]
- AL-agele, H.A.; Proctor, K.; Murthy, G.; Higgins, C. A Case Study of Tomato (Solanum lycopersicon var. Legend) Production and Water Productivity in Agrivoltaic Systems. Sustainability 2021, 13, 2850. [Google Scholar] [CrossRef]
- Barron-Gafford, G.A.; Pavao-Zuckerman, M.A.; Minor, R.L.; Sutter, L.F.; Barnett-Moreno, I.; Blackett, D.T.; Thompson, M.; Dimond, K.; Gerlak, A.K.; Nabhan, G.P.; et al. Agrivoltaics provide mutual benefits across the food–energy–water nexus in drylands. Nat. Sustain. 2019, 2, 848–855. [Google Scholar] [CrossRef]
- Fagnano, M.; Fiorentino, N.; Visconti, D.; Baldi, G.M.; Falce, M.; Acutis, M.; Genovese, M.; Di Blasi, M. Effects of a Photovoltaic Plant on Microclimate and Crops’ Growth in a Mediterranean Area. Agronomy 2024, 14, 466. [Google Scholar] [CrossRef]
- Zheng, J.; Luo, Y.; Chang, R.; Gao, X. An observational study on the microclimate and soil thermal regimes under solar photovoltaic arrays. Sol. Energy 2023, 266, 112159. [Google Scholar] [CrossRef]
- Yue, S.; Guo, M.; Zou, P.; Wu, W.; Zhou, X. Effects of photovoltaic panels on soil temperature and moisture in desert areas. Environ. Sci. Pollut. Res. 2021, 28, 17506–17518. [Google Scholar] [CrossRef]
- Wallace, J.S. Increasing agricultural water use efficiency to meet future food production. Agric. Ecosyst. Environ. 2000, 82, 105–119. [Google Scholar] [CrossRef]
- Zhang, C.; Lei, S.; Wu, H.; Liao, L.; Wang, X.; Zhang, L.; Liu, G.; Wang, G.; Fang, L.; Song, Z. Simplified microbial network reduced microbial structure stability and soil functionality in alpine grassland along a natural aridity gradient. Soil Biol. Biochem. 2024, 191, 109366. [Google Scholar] [CrossRef]
- Luo, S.-P.; He, B.-H.; Zeng, Q.-P.; Li, N.-J.; Yang, L. Effects of seasonal variation on soil microbial community structure and enzyme activity in a Masson pine forest in Southwest China. J. Mt. Sci. 2020, 17, 1398–1409. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; de Araujo Pereira, A.P.; Araujo, A.S.F.; Vaishnav, A.; Karpouzas, D.G.; Singh, B.K. Soil microbial diversity plays an important role in resisting and restoring degraded ecosystems. Plant Soil 2024, 500, 325–349. [Google Scholar] [CrossRef]
- Bissett, A.; Brown, M.V.; Siciliano, S.D.; Thrall, P.H. Microbial community responses to anthropogenically induced environmental change: Towards a systems approach. Ecol. Lett. 2013, 16, 128–139. [Google Scholar] [CrossRef]
- Wagg, C.; Hautier, Y.; Pellkofer, S.; Banerjee, S.; Schmid, B.; van der Heijden, M.G. Diversity and asynchrony in soil microbial communities stabilizes ecosystem functioning. eLife 2021, 10, e62813. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Mori, A.S.; Isbell, F.; Fujii, S.; Makoto, K.; Matsuoka, S.; Osono, T. Low multifunctional redundancy of soil fungal diversity at multiple scales. Ecol. Lett. 2016, 19, 249–259. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Handa, I.T.; Aerts, R.; Berendse, F.; Berg, M.P.; Bruder, A.; Butenschoen, O.; Chauvet, E.; Gessner, M.O.; Jabiol, J.; Makkonen, M.; et al. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B. Colloquium paper: Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. S1), 11512–11519. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Wyszkowski, M. Activity of Soil Dehydrogenases, Urease, and Acid and Alkaline Phosphatases in Soil Polluted with Petroleum. J. Toxicol. Environ. Health Part A 2010, 73, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Karaca, A.; Cetin, S.C.; Turgay, O.C.; Kizilkaya, R. Soil Enzymes as Indication of Soil Quality. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 119–148. [Google Scholar]
- Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef]
- Bueis, T.; Turrión, M.B.; Bravo, F.; Pando, V.; Muscolo, A. Factors determining enzyme activities in soils under Pinus halepensis and Pinus sylvestris plantations in Spain: A basis for establishing sustainable forest management strategies. Ann. For. Sci. 2018, 75, 34. [Google Scholar] [CrossRef]
- Hu, R.; Wang, X.-P.; Zhang, Y.-F.; Shi, W.; Jin, Y.-X.; Chen, N. Insight into the influence of sand-stabilizing shrubs on soil enzyme activity in a temperate desert. Catena 2016, 137, 526–535. [Google Scholar] [CrossRef]
- Wolińska, A.M.; Stępniewska, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases; Canuto, R.A., Ed.; IntechOpen: London, UK, 2012. [Google Scholar]
- Zhang, X.; Zheng, Y.; Yang, Y.; Ren, H.; Liu, J. Spatiotemporal evolution of ecological vulnerability on the Loess Plateau. Ecol. Indic. 2025, 170, 113060. [Google Scholar] [CrossRef]
- Chen, H.; Wu, W.; Li, C.; Lu, G.; Ye, D.; Ma, C.; Ren, L.; Li, G. Ecological and environmental effects of global photovoltaic power plants: A meta-analysis. J. Environ. Manag. 2025, 373, 123785. [Google Scholar] [CrossRef]
- Cui, Y.; Fang, L.; Guo, X.; Han, F.; Ju, W.; Ye, L.; Wang, X.; Tan, W.; Zhang, X. Natural grassland as the optimal pattern of vegetation restoration in arid and semi-arid regions: Evidence from nutrient limitation of soil microbes. Sci. Total Environ. 2019, 648, 388–397. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. 7—Enzyme activities. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 311–373. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Kadhum, M.A.; Hadwan, M.H. A precise and simple method for measuring catalase activity in biological samples. Chem. Pap. 2021, 75, 1669–1678. [Google Scholar] [CrossRef]
- Semenov, A.M.; Batomunkueva, B.P.; Nizovtseva, D.V.; Panikov, N.S. Method of determination of cellulase activity in soils and in microbial cultures, and its calibration. J. Microbiol. Methods 1996, 24, 259–267. [Google Scholar] [CrossRef]
- Gong, P. Dehydrogenase activity in soil: A comparison between the TTC and INT assay under their optimum conditions. Soil Biol. Biochem. 1997, 29, 211–214. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Zhu, J.; Lin, X.; Qi, J. Soil Carbon Sequestration: Role of Fe Oxides and Polyphenol Oxidase Across Temperature and Cultivation Systems. Plants 2025, 14, 927. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.X.; Chen, L.; Ma, T.; Li, X.; Zheng, M.; Zhou, X.; Chen, L.; Qian, X.; Xi, J.; Lu, H.; et al. EasyAmplicon: An easy-to-use, open-source, reproducible, and community-based pipeline for amplicon data analysis in microbiome research. Imeta 2023, 2, e83. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package; R package Version 2.5-6; Community Ecol. Package Version. 2019. Available online: https://www.mcglinnlab.org/publication/2019-01-01_oksanen_vegan_2019/ (accessed on 19 November 2025).
- RStudioTeam. RStudio: Integrated Development Environment for R; RstudioTeam: Boston, MA, USA, 2023. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. R Package “ggplot2”, v3.4.4. Elegant Graphics for Data Analysis; The R Foundation: Vienna, Austria, 2023.
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Wen, T.; Xie, P.; Yang, S.; Niu, G.; Liu, X.; Ding, Z.; Xue, C.; Liu, Y.-X.; Shen, Q.; Yuan, J. ggClusterNet: An R package for microbiome network analysis and modularity-based multiple network layouts. iMeta 2022, 1, e32. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Schloter, M.; Pecyna, M.J.; Kapturska, D.; Hofrichter, M.; Krüger, D.; Buscot, F. Life in leaf litter: Novel insights into community dynamics of bacteria and fungi during litter decomposition. Mol. Ecol. 2016, 25, 4059–4074. [Google Scholar] [CrossRef]
- Lambert, Q.; Gros, R.; Bischoff, A. Ecological restoration of solar park plant communities and the effect of solar panels. Ecol. Eng. 2022, 182, 106722. [Google Scholar] [CrossRef]
- Bai, Z.; Jia, A.; Bai, Z.; Qu, S.; Zhang, M.; Kong, L.; Sun, R.; Wang, M. Photovoltaic panels have altered grassland plant biodiversity and soil microbial diversity. Front. Microbiol. 2022, 13, 1065899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Xu, S.; Zhao, J.; Chen, S.; Liu, X.; Zheng, X.; Wang, X.; Zhu, Z.; Gao, F.; Fu, B.; et al. Soil microbial networks’ complexity as a primary driver of multifunctionality in photovoltaic power plants in the northwest region of China. Front. Microbiol. 2025, 16, 1579497. [Google Scholar] [CrossRef]
- Huang, C.; Wu, X.; Liu, X.; Fang, Y.; Liu, L.; Wu, C. Functional fungal communities dominate wood decomposition and are modified by wood traits in a subtropical forest. Sci. Total Environ. 2022, 806, 151377. [Google Scholar] [CrossRef]
- Sterkenburg, E.; Bahr, A.; Brandström Durling, M.; Clemmensen, K.E.; Lindahl, B.D. Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol. 2015, 207, 1145–1158. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, D.; Cai, X.; Xia, L.; Luo, Y.; Cheng, X.; An, S. Significant alterations in soil fungal communities along a chronosequence of Spartina alterniflora invasion in a Chinese Yellow Sea coastal wetland. Sci. Total Environ. 2019, 693, 133548. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Diao, L.; Wang, Y.; Yang, X.; Zhang, H.; Wang, J.; Luo, Y.; An, S.; Cheng, X. Responses of soil fungal communities and functional guilds to ~160 years of natural revegetation in the Loess Plateau of China. Front. Microbiol. 2022, 13, 967565. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, M.; Hagedorn, F.; Wipf, S.; Donhauser, J.; Vittoz, P.; Rixen, C.; Frossard, A.; Theurillat, J.-P.; Frey, B. The Soil Microbiome of GLORIA Mountain Summits in the Swiss Alps. Front. Microbiol. 2019, 10, 1080. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Frey, B.; Rime, T.; Phillips, M.; Stierli, B.; Hajdas, I.; Widmer, F.; Hartmann, M. Microbial diversity in European alpine permafrost and active layers. FEMS Microbiol. Ecol. 2016, 92, fiw018. [Google Scholar] [CrossRef]
- Bonfante, P.; Venice, F. Mucoromycota: Going to the roots of plant-interacting fungi. Fungal Biol. Rev. 2020, 34, 100–113. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Bayman, P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef]
- Ndinga-Muniania, C.; Mueller, R.C.; Kuske, C.R.; Porras-Alfaro, A. Seasonal variation and potential roles of dark septate fungi in an arid grassland. Mycologia 2021, 113, 1181–1198. [Google Scholar] [CrossRef]
- Legeay, J.; Basiru, S.; Ziami, A.; Errafii, K.; Hijri, M. Response of Alternaria and Fusarium Species to Low Precipitation in a Drought-Tolerant Plant in Morocco. Microb. Ecol. 2024, 87, 127. [Google Scholar] [CrossRef]
- Nguyen, H.D.T.; Nickerson, N.L.; Seifert, K.A. Basidioascus and Geminibasidium: A new lineage of heat-resistant and xerotolerant basidiomycetes. Mycologia 2013, 105, 1231–1250. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.; Cho, H.U.; Kim, S.H. Preussia jejuensis sp. nov., P. koreensis sp. nov., and P. isomera, Coprophilous Fungi Isolated from Horse Dung in Seopjikoji, Jeju Island in the Republic of Korea. Mycobiology 2025, 53, 200–213. [Google Scholar] [CrossRef]
- Branco, S.; Schauster, A.; Liao, H.-L.; Ruytinx, J. Mechanisms of stress tolerance and their effects on the ecology and evolution of mycorrhizal fungi. New Phytol. 2022, 235, 2158–2175. [Google Scholar] [CrossRef] [PubMed]
- Helal, G.A.; Khalil, R.R.; Galal, Y.G.; Soliman, S.M.; Abd Elkader, R.S. Studies on cellulases of some cellulose-degrading soil fungi. Arch. Microbiol. 2021, 204, 65. [Google Scholar] [CrossRef] [PubMed]
- Asemoloye, M.D.; Ahmad, R.; Jonathan, S.G. Transcriptomic responses of catalase, peroxidase and laccase encoding genes and enzymatic activities of oil spill inhabiting rhizospheric fungal strains. Environ. Pollut. 2018, 235, 55–64. [Google Scholar] [CrossRef]
- Neemisha; Sharma, S. Soil Enzymes and Their Role in Nutrient Cycling. In Structure and Functions of Pedosphere; Giri, B., Kapoor, R., Wu, Q.-S., Varma, A., Eds.; Springer Nature: Singapore, 2022; pp. 173–188. [Google Scholar]
- Tang, X.; Yang, J.; Lin, D.; Lin, H.; Xiao, X.; Chen, S.; Huang, Y.; Qian, X. Community assembly of ectomycorrhizal fungal communities in pure and mixed Pinus massoniana forests. J. Environ. Manag. 2024, 362, 121312. [Google Scholar] [CrossRef]
- Guo, H.; Liu, W.; Xie, Y.; Wang, Z.; Huang, C.; Yi, J.; Yang, Z.; Zhao, J.; Yu, X.; Sibirina, L.A. Soil microbiome of shiro reveals the symbiotic relationship between Tricholoma bakamatsutake and Quercus mongolica. Front. Microbiol. 2024, 15, 1361117. [Google Scholar] [CrossRef]
- Ge, S.; Gao, J.; Chang, D.; He, T.; Cai, H.; Wang, M.; Li, C.; Luo, Z.; E, Y.; Meng, J.; et al. Biochar contributes to resistance against root rot disease by stimulating soil polyphenol oxidase. Biochar 2023, 5, 55. [Google Scholar] [CrossRef]
- Niu, X.-M.; Zhang, K.-Q. Arthrobotrys oligospora: A model organism for understanding the interaction between fungi and nematodes. Mycology 2011, 2, 59–78. [Google Scholar] [CrossRef]
- Zhang, F.; Boonmee, S.; Bhat, J.D.; Xiao, W.; Yang, X.-Y. New Arthrobotrys Nematode-Trapping Species (Orbiliaceae) from Terrestrial Soils and Freshwater Sediments in China. J. Fungi 2022, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- García-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Kaushal, J.; Mehandia, S.; Singh, G.; Raina, A.; Arya, S.K. Catalase enzyme: Application in bioremediation and food industry. Biocatal. Agric. Biotechnol. 2018, 16, 192–199. [Google Scholar] [CrossRef]
- Sun, J.; Cui, W.; Wang, W.; Yang, X. The microclimatic and ecohydrological effects of photovoltaic facilities in arid/semi-arid regions of China: An integrated modeling study. J. Environ. Manag. 2025, 382, 125395. [Google Scholar] [CrossRef]
- Chen, S.; Wang, W.; Xu, W.; Wang, Y.; Wan, H.; Chen, D.; Tang, Z.; Tang, X.; Zhou, G.; Xie, Z.; et al. Plant diversity enhances productivity and soil carbon storage. Proc. Natl. Acad. Sci. USA 2018, 115, 4027–4032. [Google Scholar] [CrossRef]
- Lladó, S.; López-Mondéjar, R.; Baldrian, P. Drivers of microbial community structure in forest soils. Appl. Microbiol. Biotechnol. 2018, 102, 4331–4338. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, H.; Liu, L.; Dou, Y.; An, S. Comparison of soil microbial community between planted woodland and natural grass vegetation on the Loess Plateau. For. Ecol. Manag. 2020, 460, 117817. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, H.; Zhao, P.; Wei, X.; Ding, G.; Gao, G.; Shi, M. Vegetation Restoration Alters Fungal Community Composition and Functional Groups in a Desert Ecosystem. Front. Environ. Sci. 2021, 9, 589068. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Zobeck, T.M.; Gill, T.E.; Kennedy, A.C. Enzyme activities and microbial community structure in semiarid agricultural soils. Biol. Fertil. Soils 2003, 38, 216–227. [Google Scholar] [CrossRef]
- Zhang, Z.-S.; Dong, X.-J.; Liu, Y.-B.; Li, X.-R.; Jia, R.-L.; Hu, Y.-G.; He, M.-Z.; Huang, L. Soil oxidases recovered faster than hydrolases in a 50-year chronosequence of desert revegetation. Plant Soil 2012, 358, 275–287. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Costa, F. Potential use of dehydrogenase activity as an index of microbial activity in degraded soils. Commun. Soil Sci. Plant Anal. 1997, 28, 123–134. [Google Scholar] [CrossRef]
- Camiña, F.; Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, C. Measurement of dehydrogenase activity in acid soils rich in organic matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, K.; Wu, N.; Zhang, X.; Sun, F.; Chen, D.; Gao, W.; Zhou, J.; Zhao, J.; You, C.; et al. Variation in physicochemical and biochemical soil properties among different plant species treatments early in the restoration of a desertified alpine meadow. Land Degrad. Dev. 2019, 30, 1889–1903. [Google Scholar] [CrossRef]
- Zhao, M.; Jiang, M.; Qin, L.; Hu, N.; Meng, J.; Wang, M.; Wang, G. The recovery of soil eukaryotic alpha and beta diversity after wetland restoration. Sci. Total Environ. 2024, 925, 171814. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B.; et al. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef]
- Zai, X.; Cordovez, V.; Zhu, F.; Zhao, M.; Diao, X.; Zhang, F.; Raaijmakers, J.M.; Song, C. C4 cereal and biofuel crop microbiomes. Trends Microbiol. 2024, 32, 1119–1131. [Google Scholar] [CrossRef]
- Wei-Ye, L.; Hong-Bo, G.; Ke-Xin, B.; Alekseevna, S.L.; Xiao-Jian, Q.; Xiao-Dan, Y. Determining why continuous cropping reduces the production of the morel Morchella sextelata. Front. Microbiol. 2022, 13, 903983. [Google Scholar] [CrossRef]
- Li, X.; Chen, D.; Carrión, V.J.; Revillini, D.; Yin, S.; Dong, Y.; Zhang, T.; Wang, X.; Delgado-Baquerizo, M. Acidification suppresses the natural capacity of soil microbiome to fight pathogenic Fusarium infections. Nat. Commun. 2023, 14, 5090. [Google Scholar] [CrossRef] [PubMed]
- Büttner, H.; Rassbach, J.; Schultz, C.; Popp, J.; Gressler, M.; Hertweck, C. Beneficial Soil Fungus Kills Predatory Nematodes with Dehydropeptides Translocating into the Animal Gut. J. Am. Chem. Soc. 2024, 146, 34702–34710. [Google Scholar] [CrossRef] [PubMed]
- Büttner, H.; Niehs, S.P.; Vandelannoote, K.; Cseresnyés, Z.; Dose, B.; Richter, I.; Gerst, R.; Figge, M.T.; Stinear, T.P.; Pidot, S.J.; et al. Bacterial endosymbionts protect beneficial soil fungus from nematode attack. Proc. Natl. Acad. Sci. USA 2021, 118, e2110669118. [Google Scholar] [CrossRef]
- Wang, J.; Tian, D.; Knapp, A.K.; Chen, H.Y.H.; Luo, Y.; Li, Z.; Hou, E.; Huang, X.; Jiang, L.; Niu, S. Precipitation manipulation and terrestrial carbon cycling: The roles of treatment magnitude, experimental duration and local climate. Glob. Ecol. Biogeogr. 2021, 30, 1909–1921. [Google Scholar] [CrossRef]
- Sun, L.-J.; Qi, Y.-C.; Dong, Y.-S.; He, Y.-T.; Peng, Q.; Liu, X.-C.; Jia, J.-Q.; Guo, S.-F.; Cao, C.-C. Interactions of water and nitrogen addition on soil microbial community composition and functional diversity depending on the inter-annual precipitation in a Chinese steppe. J. Integr. Agric. 2015, 14, 788–799. [Google Scholar] [CrossRef]
- Araújo, M.B.; Rozenfeld, A.; Rahbek, C.; Marquet, P.A. Using species co-occurrence networks to assess the impacts of climate change. Ecography 2011, 34, 897–908. [Google Scholar] [CrossRef]
- Tu, Q.; Yan, Q.; Deng, Y.; Michaletz, S.T.; Buzzard, V.; Weiser, M.D.; Waide, R.; Ning, D.; Wu, L.; He, Z.; et al. Biogeographic patterns of microbial co-occurrence ecological networks in six American forests. Soil Biol. Biochem. 2020, 148, 107897. [Google Scholar] [CrossRef]
- de Vries, F.T.; Liiri, M.E.; Bjørnlund, L.; Bowker, M.A.; Christensen, S.; Setälä, H.M.; Bardgett, R.D. Land use alters the resistance and resilience of soil food webs to drought. Nat. Clim. Change 2012, 2, 276–280. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, Y.; Wu, S.; Gao, X.; He, X.; Dong, S. Core microbes regulate plant-soil resilience by maintaining network resilience during long-term restoration of alpine grasslands. Nat. Commun. 2025, 16, 3116. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Change 2021, 11, 343–348. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Niu, G.; Ji, B.; Wang, Z.; Jiang, Q. Effects of Vegetation Restoration on Soil Fungal Communities During Early Post-Construction Phase of a Desert Steppe Photovoltaic Power Station. Land 2025, 14, 2306. https://doi.org/10.3390/land14122306
Zhou W, Niu G, Ji B, Wang Z, Jiang Q. Effects of Vegetation Restoration on Soil Fungal Communities During Early Post-Construction Phase of a Desert Steppe Photovoltaic Power Station. Land. 2025; 14(12):2306. https://doi.org/10.3390/land14122306
Chicago/Turabian StyleZhou, Wenqing, Guoqing Niu, Bo Ji, Zhanjun Wang, and Qi Jiang. 2025. "Effects of Vegetation Restoration on Soil Fungal Communities During Early Post-Construction Phase of a Desert Steppe Photovoltaic Power Station" Land 14, no. 12: 2306. https://doi.org/10.3390/land14122306
APA StyleZhou, W., Niu, G., Ji, B., Wang, Z., & Jiang, Q. (2025). Effects of Vegetation Restoration on Soil Fungal Communities During Early Post-Construction Phase of a Desert Steppe Photovoltaic Power Station. Land, 14(12), 2306. https://doi.org/10.3390/land14122306





