Assessing Electrical Conductivity and Sodium Adsorption Ratio as Soil Salinity Indicators in Reclaimed Well Sites
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Sites
2.2. Soil Sampling and Analyses
2.3. Data Analyses
3. Results
3.1. Soil Properties
3.2. Site Factors and Soil Salinity
3.3. Relationship Between EC, SAR and Other Salt Ions
4. Discussion
4.1. Ecoregion and Soil Type Variation
4.2. EC, SAR, and Other Salt Ions as Reclamation Criteria
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Depth (m) | SAR | PH | Sodium (mg kg−1) | Chloride (mg kg−1) | Sulfate (mg kg−1) | Calcium (mg kg−1) | Magnesium (mg kg−1) | Potassium (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|---|
| 0–0.15 | EC | 0.62 *** | 0.61 *** | 0.73 *** | 0.63 *** | 0.78 *** | 0.65 *** | 0.72 *** | 0.38 ** |
| SAR | 0.36 *** | 0.77 *** | 0.47 *** | 0.53 *** | 0.28 * | ||||
| PH | 0.44 *** | 0.26 * | 0.46 *** | 0.44 ** | 0.42 ** | 0.34 * | |||
| Sodium | 0.66 *** | 0.78 *** | 0.51 *** | ||||||
| Chloride | 0.47 *** | 0.27 * | 0.45 *** | 0.32 * | |||||
| Sulfate | 0.54 *** | 0.69 *** | |||||||
| Calcium | 0.80 *** | 0.34 * | |||||||
| Magnesium | 0.24 * | ||||||||
| 0.16–0.30 | EC | 0.72 *** | 0.67 *** | 0.86 *** | 0.82 *** | 0.89 *** | 0.77 *** | 0.84 *** | 0.67 *** |
| SAR | 0.63 *** | 0.94 *** | 0.69 *** | 0.71 *** | 0.39 ** | 0.24 * | |||
| PH | 0.68 *** | 0.51 *** | 0.69 *** | 0.44 *** | 0.57 *** | 0.41 ** | |||
| Sodium | 0.77 *** | 0.84 *** | 0.47 *** | 0.63 *** | 0.35 * | ||||
| Chloride | 0.64 *** | 0.49 *** | 0.60 *** | 0.62 *** | |||||
| Sulfate | 0.69 *** | 0.81 *** | 0.46 *** | ||||||
| Calcium | 0.88 *** | 0.63 *** | |||||||
| Magnesium | 0.57 *** | ||||||||
| 0.31–0.45 | EC | 0.78 *** | 0.70 *** | 0.91 *** | 0.80 *** | 0.89 *** | 0.85 *** | 0.87 *** | 0.79 *** |
| SAR | 0.68 *** | 0.93 *** | 0.55 *** | 0.75 *** | 0.47 *** | 0.54 *** | 0.41 ** | ||
| PH | 0.73 *** | 0.49 *** | 0.77 *** | 0.54 *** | 0.64 *** | 0.50 *** | |||
| Sodium | 0.62 *** | 0.91 *** | 0.74 *** | 0.80 *** | 0.55 *** | ||||
| Chloride | 0.57 *** | 0.65 *** | 0.64 *** | 0.80 *** | |||||
| Sulfate | 0.83 *** | 0.88 *** | 0.63 *** | ||||||
| Calcium | 0.94 *** | 0.72 *** | |||||||
| Magnesium | 0.67 *** | ||||||||
| 0.46–0.60 | EC | 0.65 *** | 0.65 *** | 0.87 *** | 0.80 *** | 0.86 *** | 0.87 *** | 0.89 *** | 0.81 *** |
| SAR | 0.76 *** | 0.88 *** | 0.38 ** | 0.71 *** | 0.37 ** | 0.42 ** | 0.35 * | ||
| PH | 0.75 *** | 0.46 *** | 0.71 *** | 0.40 ** | 0.52 *** | 0.41 ** | |||
| Sodium | 0.50 *** | 0.93 *** | 0.73 *** | 0.78 *** | 0.59 *** | ||||
| Chloride | 0.46 ** | 0.62 *** | 0.64 *** | 0.74 *** | |||||
| Sulfate | 0.82 *** | 0.86 *** | 0.63 *** | ||||||
| Calcium | 0.93 *** | 0.76 *** | |||||||
| Magnesium | 0.74 *** | ||||||||
| 0.61–1.50 | EC | 0.54 *** | 0.55 *** | 0.83 *** | 0.68 *** | 0.70 *** | 0.84 *** | 0.92 *** | 0.70 *** |
| SAR | 0.65 *** | 0.88 *** | 0.71 *** | 0.20 * | 0.36 *** | 0.18 * | |||
| PH | 0.66 *** | 0.27 ** | 0.53 *** | 0.18 * | 0.48 *** | 0.29 *** | |||
| Sodium | 0.36 *** | 0.84 *** | 0.60 *** | 0.71 *** | 0.45 *** | ||||
| Chloride | 0.66 *** | 0.61 *** | 0.68 *** | ||||||
| Sulfate | 0.53 *** | 0.69 *** | 0.44 *** | ||||||
| Calcium | 0.86 *** | 0.68 *** | |||||||
| Magnesium | 0.68 *** |
| Depth (m) | SAR | PH | Sodium (mg kg−1) | Chloride (mg kg−1) | Sulfate (mg kg−1) | Calcium (mg kg−1) | Magnesium (mg kg−1) | Potassium (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|---|
| 0–0.15 | EC | 0.68 *** | 0.44 * | 0.75 *** | 0.58 *** | 0.83 *** | 0.66 *** | 0.78 *** | 0.32 * |
| SAR | 0.88 *** | 0.59 *** | 0.52 ** | 0.43 * | |||||
| PH | 0.33 * | 0.51 ** | 0.46 ** | ||||||
| Sodium | 0.66 *** | 0.76 *** | 0.59 *** | ||||||
| Chloride | 0.37 * | 0.47 ** | 0.41 * | ||||||
| Sulfate | 0.65 *** | 0.77 *** | |||||||
| Calcium | 0.80 *** | 0.44 * | |||||||
| Magnesium | 0.56 *** | 0.39 * | 0.78 *** | 0.70 *** | 0.79 *** | 0.78 *** | 0.85 *** | 0.47 ** | |
| 0.16–0.30 | EC | 0.44 * | 0.90 *** | 0.53 ** | 0.52 ** | ||||
| SAR | 0.50 ** | 0.49 ** | 0.30 * | 0.48 ** | |||||
| PH | 0.60 *** | 0.72 *** | 0.39 * | 0.60 *** | |||||
| Sodium | 0.29 * | 0.35 * | 0.46 ** | 0.56 *** | |||||
| Chloride | 0.68 *** | 0.81 *** | |||||||
| Sulfate | 0.88 *** | 0.47 ** | |||||||
| Calcium | 0.35 * | ||||||||
| Magnesium | 0.52 ** | 0.40 * | 0.78 *** | 0.64 *** | 0.71 *** | 0.76 *** | 0.85 *** | 0.54 ** | |
| 0.31–0.45 | EC | 0.51 ** | 0.84 *** | 0.52 ** | |||||
| SAR | 0.59 *** | 0.63 *** | 0.45 ** | ||||||
| PH | 0.85 *** | 0.53 ** | 0.67 *** | ||||||
| Sodium | 0.46 ** | 0.46 ** | 0.67 *** | ||||||
| Chloride | 0.67 *** | 0.79 *** | |||||||
| Sulfate | 0.90 *** | 0.50 ** | |||||||
| Calcium | 0.43 * | ||||||||
| Magnesium | 0.36 * | 0.70 *** | 0.69 *** | 0.62 *** | 0.74 *** | 0.86 *** | 0.68 *** | ||
| 0.46–0.60 | EC | 0.65 *** | 0.76 *** | 0.53 ** | |||||
| SAR | 0.46 ** | 0.39 * | |||||||
| PH | 0.88 *** | 0.55 ** | 0.66 *** | ||||||
| Sodium | 0.42 * | 0.50 ** | 0.74 *** | ||||||
| Chloride | 0.63 *** | 0.72 *** | |||||||
| Sulfate | 0.83 *** | 0.58 *** | |||||||
| Calcium | 0.56 ** | ||||||||
| Magnesium | 0.38 * | 0.61 *** | 0.75 *** | 0.78 *** | 0.53 ** | ||||
| 0.61–1.50 | EC | 0.56 *** | 0.88 *** | −0.33 * | 0.70 *** | −0.45 ** | |||
| SAR | 0.42* | −0.38 * | |||||||
| PH | 0.88 *** | ||||||||
| Sodium | 0.50 ** | 0.54 ** | 0.73 *** | ||||||
| Chloride | 0.73 *** | 0.61 *** | |||||||
| Sulfate | 0.44 * | ||||||||
| Calcium | 0.68 *** | 0.44 * | 0.75 *** | 0.58 *** | 0.83 *** | 0.66 *** | 0.78 *** | 0.32 * | |
| Magnesium | 0.88 *** | 0.59 *** | 0.52 ** | 0.43* |
| Depth (m) | SAR | PH | Sodium (mg kg−1) | Chloride (mg kg−1) | Sulfate (mg kg−1) | Calcium (mg kg−1) | Magnesium (mg kg−1) | Potassium (mg kg−1) | |
|---|---|---|---|---|---|---|---|---|---|
| 0–0.15 | EC | 0.64 ** | 0.45 * | 0.94 *** | 0.77 *** | ||||
| SAR | 0.49 * | ||||||||
| PH | 0.52 * | 0.45 * | 0.50 * | ||||||
| Sodium | 0.47 * | 0.59 * | |||||||
| Chloride | 0.59 * | 0.47 * | |||||||
| Sulfate | 0.54 * | ||||||||
| Calcium | 0.82 *** | ||||||||
| Magnesium | 0.47 * | ||||||||
| 0.16–0.30 | EC | 0.44 * | 0.59 * | 0.70 ** | 0.54 * | 0.77 *** | 0.67 ** | 0.72 ** | 0.75 *** |
| SAR | 0.65 ** | 0.85 *** | 0.45 * | 0.57 * | |||||
| PH | 0.68 ** | 0.60 * | 0.50 * | ||||||
| Sodium | 0.71 ** | 0.76 ** | 0.51 * | ||||||
| Chloride | 0.58 * | 0.56 * | 0.56 * | ||||||
| Sulfate | 0.71 ** | 0.48 * | |||||||
| Calcium | 0.69 ** | 0.63 ** | |||||||
| Magnesium | 0.70 ** | ||||||||
| 0.31–0.45 | EC | 0.66 ** | 0.84 *** | 0.60 * | 0.82 *** | 0.53 * | 0.68 ** | ||
| SAR | 0.86 *** | ||||||||
| Sodium | 0.64 ** | 0.57 * | |||||||
| Chloride | 0.48 * | ||||||||
| Sulfate | 0.54 * | 0.79 *** | 0.55 * | ||||||
| Calcium | 0.85 *** | 0.46 * | |||||||
| Magnesium | 0.60 * | ||||||||
| 0.46–0.60 | EC | 0.90 *** | 0.69 ** | 0.62 * | 0.47 * | ||||
| SAR | 0.89 *** | −0.53 * | −0.49 * | ||||||
| Chloride | 0.67 ** | 0.56 * | |||||||
| Sulfate | 0.54 * | 0.63 * | 0.60 * | ||||||
| Calcium | 0.95 *** | ||||||||
| Magnesium | 0.45 * | ||||||||
| 0.61–1.50 | EC | 0.71 ** | 0.94 *** | 0.80 *** | 0.77 *** | 0.77 *** | |||
| SAR | 0.44 * | ||||||||
| Sodium | 0.54 * | 0.54 * | 0.46 * | 0.51 * | |||||
| Chloride | 0.70 ** | 0.69 ** | 0.67 ** | ||||||
| Calcium | 0.88 *** | 0.68 ** | |||||||
| Magnesium | 0.59 * |
References
- Wang, J.; Liu, Y.; Wang, S.; Liu, H.; Fu, G.; Xiong, Y. Spatial distribution of soil salinity and potential implications for soil management in the Manas River watershed, China. Soil Use Manag. 2020, 36, 93–103. [Google Scholar] [CrossRef]
- Singh, A. Soil salinity: A global threat to sustainable development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Bertrand, A.; Gatzke, C.; Bipfubusa, M.; Lévesque, V.; Chalifour, F.P.; Claessens, A.; Rocher, S.; Tremblay, G.F.; Beauchamp, C.J. Physiological and biochemical responses to salt stress of alfalfa populations selected for salinity tolerance and grown in symbiosis with salt-tolerant rhizobium. Agronomy 2020, 10, 569. [Google Scholar] [CrossRef]
- Hammam, A.A.; Mohamed, E.S. Mapping soil salinity in the East Nile Delta using several methodological approaches of salinity assessment. Egypt. J. Remote Sens. Space Sci. 2020, 23, 125–131. [Google Scholar] [CrossRef]
- Aldabaa, A.; Weindorf, D.C.; Chakraborty, S.; Sharma, A.; Li, B. Combination of proximal and remote sensing methods for rapid soil salinity quantification. Geoderma 2015, 239, 34–46. [Google Scholar] [CrossRef]
- Decock, C.; Lee, J.; Necpalova, M.; Pereira, E.I.P.; Tendall, D.M.; Six, J. Mitigating N2O emissions from soil: From patching leaks to transformative action. Soil 2015, 1, 687–694. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef]
- Brevik, E.; Cerdà, A.; Mataix-Solera, J.; Pereg, L.; Quinton, J.; Six, J.; Van Oost, K. The interdisciplinary nature of soil. Soil 2015, 1, 117–129. [Google Scholar] [CrossRef]
- Alberta Energy Regulator. Statistical Reports. 2025. Available online: https://www.aer.ca/data-and-performance-reports/statistical-reports (accessed on 12 May 2025).
- Alberta Environment. Salt Contamination Assessment and Remediation Guidelines. Alberta Environment, Environmental Sciences Division Environmental Service. 2001. Available online: https://archive.org/details/saltcontaminatio00albe (accessed on 22 May 2025).
- Essington, M.E. Soil and Water Chemistry: An Integrated Approach, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 499–521. [Google Scholar]
- Alberta Environment and Parks. Alberta Tier 1 Soil and Groundwater Remediation Guidelines. 2024. Available online: https://open.alberta.ca/dataset/842becf6-dc0c-4cc7-8b29-e3f383133ddc/resource/b6b28b89-f0db-49c5-b3bd-6046943af610/download/epa-alberta-tier-1-soil-groundwater-remediation-guidelines-2024-06.pdf (accessed on 22 April 2025).
- Alberta Environment and Parks. Alberta Tier 2 Soil and Groundwater Remediation Guidelines. 2024. Available online: https://open.alberta.ca/dataset/aa212afe-2916-4be9-8094-42708c950313/resource/8e603ce2-4314-495c-a892-bcd496e9a54b/download/epa-alberta-tier-2-soil-groundwater-remediation-guidelines-2024-06.pdf (accessed on 22 April 2025).
- Saskatchewan Petroleum Industry/Government Environmental Committee (SPIGC). Saskatchewan Upstream Petroleum Sites Remediation Guidelines; Guideline No. 4; 2009. Available online: https://publications.saskatchewan.ca/#/products/75538 (accessed on 24 April 2025).
- Naeth, M.A.; Wilkinson, S.R.; Jobson, A.; Murata, A.; Fluker, M. Salt Affected Soils Well Site Reclamation Implications; Technical Report for Petroleum Technology Alliance of Canada (PTAC); Petroleum Technology Alliance of Canada (PTAC): Calgary, AB, Canada, 2016; p. 80. [Google Scholar]
- Hossain, M.S.; Rahman, G.K.M.M.; Solaiman, A.R.M.; Alam, M.S.; Rahman, M.M.; Mia, M.A.B. Estimating electrical conductivity for soil salinity monitoring using various soil–water ratios depending on soil texture. Commun. Soil Sci. Plant Anal. 2020, 51, 635–644. [Google Scholar] [CrossRef]
- Stavi, I.; Thevs, N.; Priori, S. Soil salinity and sodicity in drylands: A review of causes, effects, monitoring, and restoration measures. Front. Environ. Sci. 2021, 9, 712831. [Google Scholar] [CrossRef]
- McNeill, J.D. Rapid, accurate mapping of soil salinity by electromagnetic ground conductivity meters. In Advances in Measurement of Soil Physical Properties: Bringing Theory into Practice; Topp, G.C., Reynolds, W.D., Green, R.E., Eds.; SSSA Book Series; Willey: Madison, WI, USA, 1992; Volume 30, pp. 209–229. [Google Scholar]
- McKenzie, R.C.; George, R.J.; Woods, S.A.; Cannon, M.E.; Bennett, D.L. Use of the electromagnetic-induction meter (EM38) as a tool in managing salinisation. Hydrogeol. J. 1997, 5, 37–50. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 1264. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 17 September 2023).
- Peterson, B.G.; Carl, P.; Boudt, K.; Bennett, R.; Ulrich, J.; Zivot, E.; Wuertz, D. Package ‘PerformanceAnalytics’. 2020. Available online: https://cran.r-project.org/web/packages/PerformanceAnalytics/PerformanceAnalytics.pdf (accessed on 9 October 2022).
- Asuero, A.G.; Sayago, A.; González, A.G. The correlation coefficient: An overview. Crit. Rev. Anal. Chem. 2007, 36, 41–59. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Package ‘Vegan’: Community Ecology Package; Version 2.5-6. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 7 March 2022).
- Gavier-Pizarro, G.I.; Radeloff, V.C.; Stewart, S.I.; Huebner, C.D.; Keuler, N.S. Housing is positively associated with invasive exotic plant species richness in New England, USA. Ecol. Appl. 2010, 20, 1913–1925. [Google Scholar] [CrossRef] [PubMed]
- McLeod, A.I.; Xu, C.; McLeod, M.A. Package Bestglm. 2020. Available online: https://cran.r-project.org/web/packages/bestglm/bestglm.pdf (accessed on 12 March 2022).
- Nally, R.M. Hierarchical partitioning as an interpretative tool in multivariate inference. Aust. Ecol. 1996, 21, 224–228. [Google Scholar] [CrossRef]
- Walsh, C.; Mac Nally, R.; Walsh, M.C. The Hier.Part Package: Hierarchical Partitioning; R Project. 2003. Available online: http://cran.r-project.org (accessed on 12 March 2022).
- Natural Regions Committee. Natural Regions and Sub-Regions of Alberta; Pub. No. T/852; Government of Alberta: Edmonton, AB, Canada, 2006. Available online: https://open.alberta.ca/publications/0778545725 (accessed on 14 May 2025).
- Soil Classification Working Group. The Canadian System of Soil Classification, 3rd ed.; Agriculture and Agri-Food Canada Publication 1646; Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1998; p. 187. Available online: https://sis.agr.gc.ca/cansis/publications/manuals/1998-cssc-ed3/index.html (accessed on 19 May 2025).
- Pennock, D.; Bedard-Haughn, A.; Viaud, V. Chernozemic soils of Canada: Genesis, distribution, and classification. Can. J. Soil Sci. 2011, 91, 719–747. [Google Scholar] [CrossRef]
- Chang, C.; Sommerfeldt, T.C.; Carefoot, J.M.; Schaalje, C.B.; Chen, C.; Ano, S. Relationships of electrical conductivity with total dissolved salts and cation concentration of sulfate-dominant soil extracts. Can. J. Soil Sci. 1983, 63, 79–86. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef]
- Meehan, M.; Sedivec, K.; Desutter, T.; Augustin, C.; Daigh, A. Environmental Impacts of Brine (Produced Water); NDSU Extension Publication R1850; NDSU: Fargo, ND, USA, 2017; pp. 1–4. Available online: https://www.ag.ndsu.edu/publications/environment-natural-resources/environmental-impacts-of-brine-produced-water/r1850.pdf (accessed on 14 May 2025).
- Reich, M.; Aghajanzadeh, T.; Helm, J.; Parmar, S.; Hawkesford, M.J.; De Kok, L.J. Chloride and sulfate salinity differently affect biomass, mineral nutrient composition and expression of sulfate transport and assimilation genes in Brassica rapa. Plant Soil 2017, 411, 319–332. [Google Scholar] [CrossRef]
- Shirokova, Y.; Forkutsa, I.; Sharafutdinova, N. Use of electrical conductivity instead of soluble salts for soil salinity monitoring in Central Asia. Irrig. Drain. Syst. 2000, 14, 199–205. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. Application of soil electrical conductivity to precision agriculture: Theory, principles, and guidelines. Agron. J. 2003, 95, 455–471. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Crop and irrigation management strategies for saline–sodic soils and waters aimed at environmentally sustainable agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rahman, W.A.; Rowell, D.L. The influence of magnesium in saline and sodic soils: A specific effect or a problem of cation exchange? Eur. J. Soil Sci. 1979, 30, 535–546. [Google Scholar] [CrossRef]
- Andreeva, N. Determination of magnesium salinity in belozem region, Bulgaria. Bulg. J. Soil Sci. 2020, 5, 11–22. [Google Scholar]
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant-based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef]
- Robinson, M.J.C.; Dhar, A.; Naeth, M.A.; Nichol, C.K. Phosphogypsum impacts on soil chemical properties and vegetation tissue following reclamation. Environ. Monit. Assess. 2023, 195, 769. [Google Scholar] [CrossRef]
- Rojas, J.A.; Dhar, A.; Naeth, M.A. Urban green spaces restoration using native forbs, site preparation and soil amendments—A case study. Land 2022, 11, 498. [Google Scholar] [CrossRef]
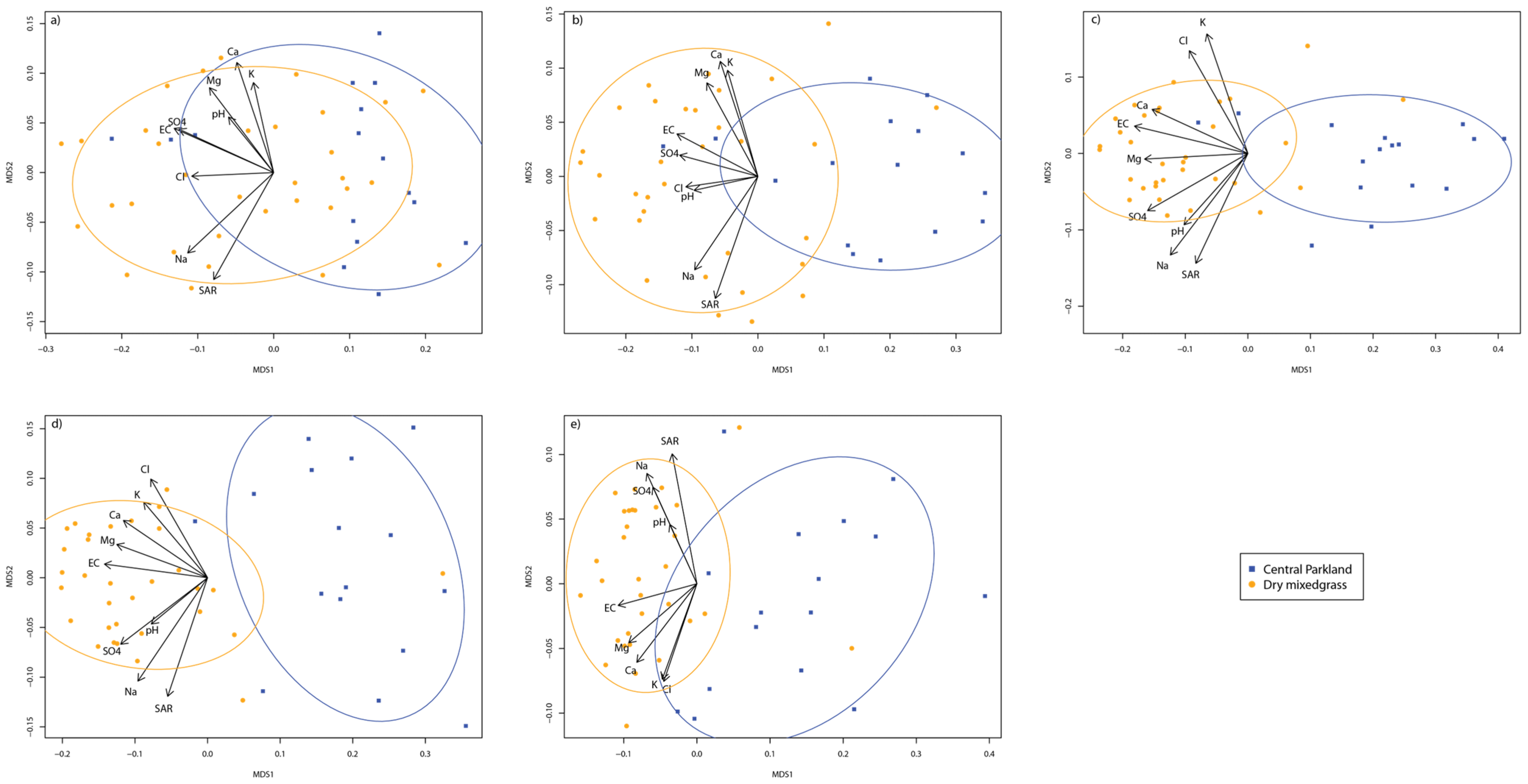
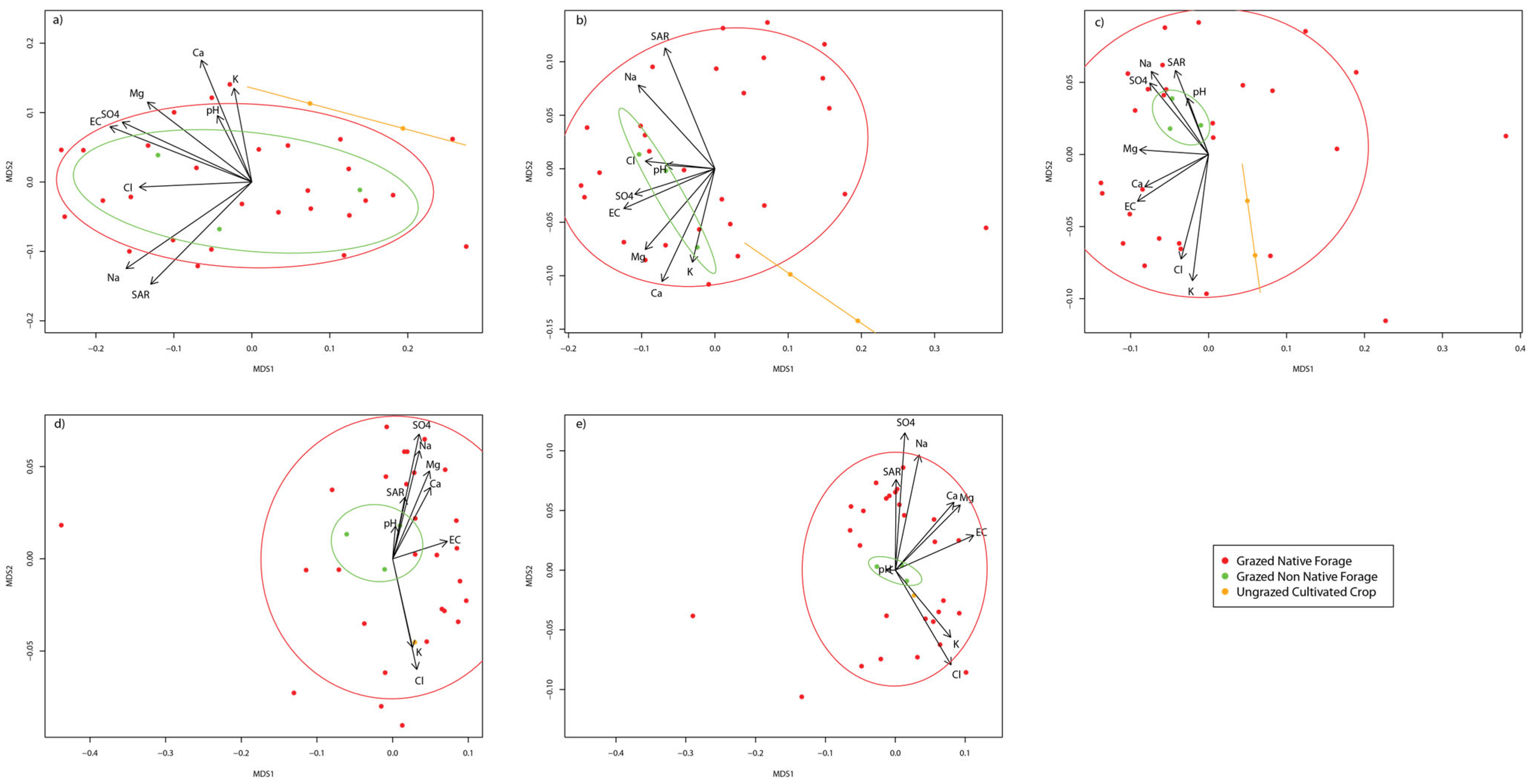

| Well Site | Ecoregion | Land Use | Drilling Year | Reclaim Year | Soil Texture |
|---|---|---|---|---|---|
| 1 | DMG | Grazed, native forage | 1978 | 2016–2018 | Fine |
| 2 | DMG | Grazed, native forage | 1978 | 2012–2018 | Fine |
| 3 | DMG | Grazed, native forage | 1978 | 2012–2016 | Fine |
| 4 | DMG | Grazed, native forage | 1975 | 2016 | Fine |
| 5 | DMG | Grazed, native forage | 1979 | 2014–2018 | Coarse |
| 6 | DMG | Grazed, native forage | 1983 | 2013 | Fine |
| 7 | DMG | Grazed, native forage | 1975 | 2013 | Fine |
| 8 | DMG | Grazed, native forage | 1978 | 2014–2018 | Fine |
| 9 | DMG | Grazed, native forage | 2003 | 2014–2016 | Fine |
| 10 | DMG | Grazed, native forage | 2002 | 2014 | Coarse |
| 11 | DMG | Grazed, native forage | 1973/2002 | 2009 | Fine |
| 12 | DMG | Grazed, native forage | 1981/2002 | 1997 | Fine |
| 13 | DMG | Grazed, native forage | 1978/2002 | 1999–2014 | Fine |
| 14 | DMG | Grazed, non-native forage | 1977 | 2014–2015 | Fine |
| 15 | DMG | Grazed, non-native forage | 1970 | 2016–2018 | Fine |
| 16 | CP | Grazed, non-native forage | 1980 | 2018 | Fine |
| 17 | CP | Grazed, non-native forage | 1975 | 2003 | Fine |
| 18 | CP | Ungrazed, non-native forage | 1951 | 1983–1993 | Fine |
| 19 | DMG | Cultivated | 1980/2002 | 2014–2015 | Fine |
| 20 | CP | Cultivated | 1969 | 2018 | Coarse |
| 21 | CP | Cultivated | 1976 | 1998–2000 | Mixed |
| 22 | CP | Cultivated | 1993 | 2006–2007 | Coarse |
| Depth (m) | EC (dS m−1) | SAR | PH | Sodium (mg kg−1) | Chloride (mg kg−1) | Sulfate (mg kg−1) | Calcium (mg kg−1) | Magnesium (mg kg−1) | Potassium (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| Dry Mixed Grass | |||||||||
| 0.0–0.15 | 3.5(1.9) | 3.4(0.8) | 7.5(0.05) | 94.2(24.9) | 90.0(28.7) | 294.4(73.8) | 84.0(14.9) | 22.5(3.9) | 45.0(11.8) |
| 0.16–0.30 | 3.6(0.5) | 5.7(1.0) | 7.8(0.06) | 258.3(64.1) | 243.7(67.6) | 828.6(205.9) | 38.1(19.0) | 74.7(15.6) | 67.5(17.9) |
| 0.31–0.45 | 5.7(0.7) | 7.3(1.0) | 8.0(0.08) | 403.8(74.8) | 444.2(108.3) | 1273.1(232) | 206.3(30.0) | 132.7(21.4) | 85.8(21.3) |
| 0.46–0.60 | 7.4(0.8) | 8.5(1.0) | 8.0(0.04) | 505.8(83.8) | 654.4(137.1) | 1516.3(277.5) | 250.9(37.0) | 176.7(29.5) | 105.7(28.2) |
| 0.61–1.5 | 8.4(0.6) | 9.1(1.1) | 8.1(0.02) | 590.7(85.4) | 739.8(130.0) | 1804.3(287.9) | 261.3(22.1) | 208.5(17.3) | 147.0(50.4) |
| Central Parkland | |||||||||
| 0.0–0.15 | 1.0(0.2) | 0.7(0.2) | 7.0(0.20) | 14.2(5.3) | 23.1(7.5) | 133.8(71.0) | 74.1(17.0) | 23.3(6.8) | 12.5(2.5) |
| 0.16–0.30 | 0.9(0.2) | 0.7(0.1) | 7.3(0.09) | 16.3(5.3) | 26.1(11.0) | 106.1(60.1) | 54.8(14.4) | 19.4(5.9) | 6.7(1.4) |
| 0.31–0.45 | 0.8(0.2) | 1.2(0.3) | 7.5(0.04) | 20.6(4.7) | 34.1(14.2) | 58.0(27.0) | 36.6(7.7) | 14.8(3.6) | 6.8(2.7) |
| 0.46–0.60 | 1.0(0.2) | 2.1(0.7) | 8.1(0.04) | 31.2(8.0) | 58.9(16.9) | 44.4(147.0) | 35.7(5.6) | 14.6(2.5) | 6.9(3.1) |
| 0.61–1.5 | 2.3(0.5) | 1.1(2.1) | 8.1(0.03) | 95.2(40.4) | 343.4(125.7) | 95.8(33.7) | 120.7(45.1) | 43.2(12.0) | 9.8(2.7) |
| Depth (m) | Variables | EC | SAR | PH | Sodium | Chloride | Sulfate |
|---|---|---|---|---|---|---|---|
| 0–0.15 | EC | 16.4 * | 7.2 | 22.4 * | 19.0 * | 35.0 * | |
| SAR | 26.1 * | 2.3 | 44.6 * | 12.2 | 14.7 | ||
| PH | 55.5* | 10.8 | 7.6 | 8.0 | 18.1 | ||
| Sodium | 27.4 * | 34.9 * | 2.2 | 13.4 | 22.1 * | ||
| Chloride | 43.8 * | 13.8 | 3.6 | 21.5 * | 17.3 * | ||
| Sulfate | 50.6 * | 12.4 * | 5.1 | 24.3 * | 7.7 | ||
| 0.15–0.30 | EC | 15.4 * | 5.2 | 31.3 * | 25.0 * | 23.1 * | |
| SAR | 16.4 * | 2.8 | 59.0 * | 10.2 | 11.5 * | ||
| PH | 19.4 | 13.9 | 28.2 | 5.2 | 33.4 | ||
| Sodium | 30.0 * | 27.7 * | 8.4 | 9.2 | 24.7 * | ||
| Chloride | 52.2 * | 8.6 | 1.6 | 17.6 * | 20 | ||
| Sulfate | 31.1 * | 15.1 | 9.5 | 33.7 * | 10.5 | ||
| 0.30–0.45 | EC | 12.7 * | 3.9 | 30.3 * | 34.6 * | 18.4 * | |
| SAR | 13.5 * | 2.9 | 65.9 * | 3.6 | 14.1 * | ||
| PH | 15.2 | 11.1 | 24.5 | 3.3 | 45.9 * | ||
| Sodium | 22.5 * | 31.6 * | 17.4 * | 3.4 | 24.5 * | ||
| Chloride | 56.4 * | 9.5 | 1.6 | 16.4* | 16.1 * | ||
| Sulfate | 23.2 * | 14.5 * | 20.9 * | 32.2 * | 9.2 | ||
| 0.45–0.60 | EC | 10.3 | 1.2 | 32.8 * | 35.4 * | 20.2 * | |
| SAR | 8.0 | 18.3 * | 55.1 * | 2.0 | 16.7 * | ||
| PH | 10.0 | 47.8 * | 23.8 * | 5.0 | 13.5 | ||
| Sodium | 22.8 * | 30.1 * | 10.3 | 4.0 | 32.6 * | ||
| Chloride | 49.0 * | 14.8 * | 4.2 | 16.2 * | 15.7 * | ||
| Sulfate | 22.1 * | 15.2 * | 8.8 | 40.5 * | 13.5 * | ||
| 0.60–1.50 | EC | 11.8 | 2.4 | 28.5 * | 47.9 * | 9.4 | |
| SAR | 6.4 | 22.4* | 50.4 * | 4.0 | 16.8 * | ||
| PH | 4.1 | 57.2 * | 24.7 * | 4.5 | 9.5 | ||
| Sodium | 12.5 | 43.8 * | 4.6 | 4.1 | 35.0 * | ||
| Chloride | 47.6 * | 16.6 * | 3.5 | 13.6 | 18.8 * | ||
| Sulfate | 11.4 | 27.0 * | 2.5 | 41.5 * | 17.5 * |
| Depth (m) | Variables | EC | SAR | PH | Sodium | Chloride | Sulfate |
|---|---|---|---|---|---|---|---|
| 0–0.15 | EC | 4.4 | 7.1 | 21.3 | 25.4 * | 41.8 * | |
| SAR | 12.4 | 21.0 | 18.9 | 21.6 | 26 | ||
| PH | 37.9 * | 13.6 | 14.3 | 19.7 | 14.3 | ||
| Sodium | 31.7 * | 5.9 | 6.1 | 19.9 | 36.4 * | ||
| Chloride | 34.2 * | 2.5 | 3.9 | 21.6 | 37.9 * | ||
| Sulfate | 42.7 * | 5.5 | 5.9 | 23.5 * | 22.2 * | ||
| 0.15–0.30 | EC | 12.9 | 4.5 | 26.4 | 25.6 | 30.6* | |
| SAR | 10.3 | 15.6 | 53.0 * | 9.0 | 12.0 | ||
| PH | 14.4 | 35.6 * | 25.7 | 10.8 | 13.4 | ||
| Sodium | 30.9 * | 20.5 * | 7.0 | 18.8 | 22.8 | ||
| Chloride | 34.7 * | 9.6 | 4.8 | 21.0 * | 29.9 * | ||
| Sulfate | 36.4 * | 11.0 | 4.6 | 24.1 | 24.0 | ||
| 0.30–0.45 | EC | 7.4 | 1.3 | 21.5 | 35.6 * | 34.1 * | |
| SAR | 9.3 | 7.7 | 64.5* | 4.0 | 14.5 | ||
| PH | 9.7 | 36.1 | 23.1 | 23.5 | 7.6 | ||
| Sodium | 25.2 | 47.5 * | 2.2 | 13.9 | 11.2 | ||
| Chloride | 49.0 * | 5.5 | 1.1 | 16.1 | 28.8 * | ||
| Sulfate | 45.1 * | 7.8 | 1.5 | 19.2 | 26.4 * | ||
| 0.45–0.60 | EC | 5.2 | 7.9 | 9.6 | 51.0 * | 26.4 * | |
| SAR | 3.9 | 5.3 | 80.2 * | 2.6 | 8.0 | ||
| PH | 14.8 | 20.4 | 22.7 | 30.3 * | 11.7 | ||
| Sodium | 8.0 | 82.4 * | 0.9 | 7.1 | 1.7 | ||
| Chloride | 64.9 * | 4.4 | 14.2 | 7.2 | 9.3 | ||
| Sulfate | 45.8 * | 10.3 | 4.8 | 18.8 | 20.3 | ||
| 0.60–1.50 | EC | 11.7 | 1.9 | 32.8 * | 47.2 * | 6.3 | |
| SAR | 7.6 | 4.1 | 74.6 * | 5.1 | 8.5 | ||
| PH | 11.6 | 30.1 | 14.8 | 20.9 | 22.7 | ||
| Sodium | 13.1 | 71.8 * | 3.0 | 9.3 | 2.9 | ||
| Chloride | 51.0 * | 8.2 | 2.6 | 25.5 * | 12.7 | ||
| Sulfate | 15.0 | 22.2 | 2.7 | 44.7 * | 15.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bony, L.; Dhar, A.; Wilkinson, S.R.; Naeth, M.A. Assessing Electrical Conductivity and Sodium Adsorption Ratio as Soil Salinity Indicators in Reclaimed Well Sites. Land 2025, 14, 2125. https://doi.org/10.3390/land14112125
Bony L, Dhar A, Wilkinson SR, Naeth MA. Assessing Electrical Conductivity and Sodium Adsorption Ratio as Soil Salinity Indicators in Reclaimed Well Sites. Land. 2025; 14(11):2125. https://doi.org/10.3390/land14112125
Chicago/Turabian StyleBony, Laura, Amalesh Dhar, Sarah R. Wilkinson, and M. Anne Naeth. 2025. "Assessing Electrical Conductivity and Sodium Adsorption Ratio as Soil Salinity Indicators in Reclaimed Well Sites" Land 14, no. 11: 2125. https://doi.org/10.3390/land14112125
APA StyleBony, L., Dhar, A., Wilkinson, S. R., & Naeth, M. A. (2025). Assessing Electrical Conductivity and Sodium Adsorption Ratio as Soil Salinity Indicators in Reclaimed Well Sites. Land, 14(11), 2125. https://doi.org/10.3390/land14112125






