Highlights
- We examined climate change and land use and disturbance changes in the eastern U.S.
- Climate has not warmed but precipitation has increased.
- Climate and land use have interacted to reduce wildfire frequency and increase tree growth.
- Human activities facilitated the expansion of native tree species distribution, non-native species invasion, and damaging native species outbreaks.
- Recent climate change and land use have not influenced deer herbivory levels.
- A warmer and drier climate may reverse interactions with land use, varying by species.
- Management can correct non-climate stressors and support ecosystems against climate change.
Abstract
Applying an interaction framework, we examined whether climate change and combined land use and disturbance changes were synergistic, antagonistic, or neutral for forest issues of wildfires, tree growth, tree species distributions, species invasions and outbreaks, and deer herbivory, focused on the eastern United States generally since the 1800s and the development of instrumental records (1895). Climate largely has not warmed during 1981–2020 compared to 1895–1980, but precipitation has increased. Increased precipitation and land use (encompassing fire exclusion and forestation, with coarse fuel accumulation due to increased tree densities) have interacted synergistically to dampen wildfire frequency in the humid eastern U.S. For overall tree growth, increased precipitation, carbon fertilization, and land use (i.e., young, fast-growing dense stands) likely have been positive, generating a synergistic interaction. Human activities created conditions for expanding native tree species distributions, non-native species invasions, and damaging native species outbreaks. No strong evidence appears to exist for recent climate change or land use influences on deer populations and associated herbivory levels. In the future, a warmer and effectively drier climate may reverse synergistic and neutral interactions with land use, although effects of climate interactions with land use will vary by species. Management can help correct non-climate stressors due to land use and support resilient structures and species against climate change.
1. Introduction to Climate Change and Land Use
Ecosystem dynamics are governed by a variety of interacting factors that operate over time and space, including climate change and the combination of land use and disturbance factors, which, whether natural or human-mediated, produce differential impacts on the overall composition, structure, and function of ecosystems [1]. During the past 50 years, habitat loss due to agricultural conversion and intensification, urbanization, and resource extraction had the greatest direct influence on loss of biodiversity, ecosystem functions, and ecosystem services from local to regional scales in all biomes [2]. Nonetheless, published work has documented that recent climate change forced by human use of fossil fuels has altered a suite of ecosystem processes worldwide [3].
Although climate has been variable during the Holocene (about 12,000 years BP), atmospheric CO2 concentration has increased from 280 ppm prior to the Industrial Revolution (about year 1750) to 420 ppm present day, chiefly as the result of fossil fuel consumption [3]. The average temperature has increased about 1 °C in the northern hemisphere since 1900, with most of the warming concentrated during the last 40 years [3]. Even though anthropomorphically driven, recent temperature increases are within the range of historical variation during the Holocene, albeit on a trajectory to be exceeded in the foreseeable future [3].
Long-term global warming is accompanied by regional variability in both rate and magnitude of change. Specifically, the eastern U.S. displays an overall lack of warming along with increased precipitation during the last forty years of climate relative to climate earlier during the 1900s, albeit with complex spatiotemporal dynamics (Figure 1; [4,5]). Exceptions to neutral temperature change include the Northeast and mid-Atlantic regions that have experienced mild warming of about 0.25–0.75 °C, whereas portions of the Central Plains and Southeast have cooled 0.25–0.75 °C (Figure 1A). Most of the eastern U.S. has experienced an increase in annual precipitation of 2–25 cm (Figure 1B), which combined with stable temperatures, results in reduced evapotranspiration (Figure 1C,D). Conversely, a large extent in the Southeast has received 2–10 cm less precipitation relative to 1895–1980. Lack of warming in the eastern U.S. extends into the Atlantic Ocean, with additional cooling in the North Atlantic Ocean, and remains under investigation [5,6]. While the future is unknown, general circulation models as a group predict continued precipitation gains in the eastern U.S. with temperatures warming by 3 to 6 °C [7].
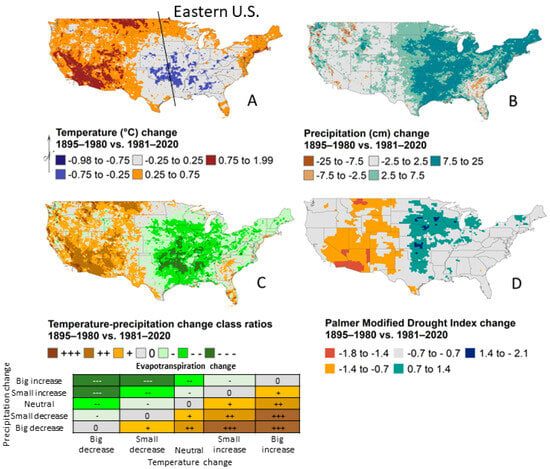
Figure 1.
Changes in the mean annual temperature (A), precipitation (B), temperature-precipitation change class ratios (C), and Palmer Modified Drought Index (D) between 1895–1980 and 1981–2020 for the United States. The temperature-precipitation change class ratios were calculated by applying a simple classification to mean temperature and precipitation maps but presented similar changes of stable or decreased evapotranspiration in the eastern U.S. as the Palmer Modified Drought Index, which applied tree-ring reconstructions of available water and instrumental data. Data are modified [4,8].
Vegetation dynamics are intricately tied to human history, through the influences of hunting, agriculture, and land management in the form of fire [9,10,11]. Humans have been an agent of change since their permanent establishment across the North American continent about 15 thousand years ago; for example, numerous megaherbivores became extinct after human establishment [12]. Human populations and their role as disturbance agents on vegetation have changed appreciably during the Holocene, particularly since Euro-American settlement at the expense of Indigenous populations [10,13,14,15]. A suite of changes occurred at a rapid rate in the type, extent, and magnitude of land-use practices by timber harvest and agricultural conversion: abrupt shifts in fire regimes to eventual fire exclusion; overexploitation of native species; introduction and spread of non-native plants, animals, and diseases; and climate change due to fossil fuel extraction and use [11,16,17,18,19]. For example, prior to Euro-American settlement, vast areas of the U.S. were savannas and woodlands, which were dominated by warm-adapted, pyrogenic tree species, such as oak (Quercus) and pine (Pinus), that provided foodways in the form of plants and wildlife [11,20]. Under fire exclusion, these ecosystems have transitioned to closed forests, which are composed of diverse fire-sensitive tree species, that supply forest products [20,21,22].
Land use since Euro-American settlement is long-standing, whereas climate change is developing in the eastern U.S., with trends of increasing precipitation and overall stable temperatures during the past 40 years that are not yet outside of the range of historical variation. Nonetheless, climate change acts as another axis of disturbance on species and ecosystems that works within an interactive framework with land use and disturbance (hereafter, land use). Depending on the scenario, land use and climate change can (1) amplify or facilitate effects through synergistic interactions, (2) simply add effects with no (neutral) interactions, or (3) have antagonistic interactions, which can have a canceling effect (where factor strength is similar) or overriding effect (where factor strength is imbalanced and one factor diminishes the other). A range of outcomes are possible but the interactive framework between land use and climate change is not clearly specified by evidence from realized outcomes of the past, for guiding predictions to the future as warming increases beyond historical temperatures. Comprehensive studies typically are not available, research shows variation within regions due to different contexts and species, and publication bias for significant findings exists. Additionally for published research, while a driver may be associated with study results, typically both climate and land use are not examined simultaneously in studies, and climate and land use may be confounded, that is, the effects of one ascribed to the other [23]. Kane et al. [24] thoroughly examined fire interactions with drought, bark beetles, and pathogens in the western U.S. Seidl et al. [25] partitioned direct, indirect, and interaction effects of climate change on fire, drought, wind, snow and ice, insects, and pathogens in an analysis of reported pathways globally.
As a first step to incorporate land use effects into an interactive framework with climate change for a subset of forest issues, we explicitly (1) specified primary mechanisms and realized evidence by which climate and land use may have been influential since becoming operational (i.e., land use changes resulting from Euro-American settlement mainly since the late 1800s and climate change of the past 40 years relative to instrumental record networks since 1895), (2) synthesized interactions between climate and land use to determine their type and strength of interactions, and (3) suggested how climate, land use, and interactions may develop as warming progresses in the eastern U.S. (largely defined as Minnesota, Iowa, Missouri, Arkansas, and Louisiana and all states eastward; Figure 1A). Future work then could advance and test the conceptual framework through comprehensive literature reviews, meta-analyses, and framing of hypotheses for empirical studies with modeling and experimental approaches [25]. Defining land use, we mean direct changes to ecosystems through land conversion from wildlands to intensively and extensively human-controlled environments, associated with change from Indigenous to European management, which leads to compositional and structural changes in ecosystems and alterations in how disturbance regimes, such as fire and insects, operate. We also included pollution such as nitrogen deposition, non-native species introductions, and overexploitation of megaherbivores into the disturbance categories caused directly by humans. Our questions, including some contrast with the western U.S., encompassed the following forest topics:
- What are the dynamics of wildfire in response to climate and land use?
- What are the dynamics of tree growth and distributions in response to climate and land use?
- What are the dynamics of non-native species, damaging native insects and disease, and white-tailed deer (Odocoileus virginianus, one of the last remaining widespread large herbivores) in response to climate and land use?
- How can ecological and adaptive management mitigate negative impacts of climate change and land use to maintain forest resiliency?
For this examination, we focused principally on direct effects, rather than slower acting indirect effects that are less influential [25]. We did not include forest issues that have not been detected to change in severity over recent time or are predicted to decrease, such as wind events or snow and ice, or that are restricted in extent, such as sea level rise [26]. As for effects of land use on climate, although tree planting and new forestation have been advocated to increase carbon storage and reduce climate change, this is an unreliable and limited approach that may cause damage to intact ecosystems and Indigenous peoples, while avoiding the solution to climate change, which is to reduce the amount of carbon emissions [27].
2. Fire
2.1. Climate Change Effects
Drier and warmer climate increases the number of extreme wildfire weather days by reducing fuel moisture and increasing the likelihood of ignition [24,25]. Greater precipitation typically fosters vegetation production, generating fuel accumulation (see section on tree growth). Most of the eastern U.S. has experienced climate change of increased precipitation with stable temperatures, albeit with slight warming along the northern tier and mid-Atlantic coast, which likely has reduced extreme wildfire weather days and the chance of severe wildfires, while accumulating coarse woody fuels (i.e., trees; Figure 1).
2.2. Land Use Effects
Land use also influences fires, which in turn interact with weather, primarily by affecting fuel type of fine herbaceous fuels or coarse woody fuels from trees. Land use has trajectories to surface fires or severe forest fires, defined by mortality to overstory trees, depending on the height of dominant vegetation. Historically, frequent surface fires reduced tree density and promoted herbaceous vegetation. Herbaceous vegetation in turn promoted surface fires that were limited in flame length by small-statured vegetation but relatively frequent because fine herbaceous vegetation ignites more readily than coarse fuels. Without surface fires, tree densities increase and accumulate, reducing probability of ignition but creating fuels for severe fire behavior, with long flame lengths due to travel up tree boles into canopies (green ladders) and then spread within continuous canopies. Currently, due to widespread forestation in wildlands following Euro-American settlement, land use resulted in less frequent but more severe fires in ecosystems, due to greater tree densities [28]. Shifts in tree species composition from fire-tolerant to fire-sensitive species and wetter microclimates also altered the leaf litter and fuel bed properties and pyrogenicity tendencies of the understory, making most forest less conducive to burning (i.e., mesophication; [21]). Moreover, fire is limited by fragmentation through land use conversion. Agricultural areas of crops and grazing remove fuels, whereas suburban and urban areas have sparse vegetation and abundant firebreaks (i.e., concrete and impervious surfaces; [15]).
2.3. Interactions and Relative Factor Strength
A wetter climate due to climate change combined with continuous coarse fuels due to land use change to forests will reduce the frequency of fires. A drier climate allows coarse fuels to ignite more readily in closed forests, increasing the frequency of severe fires. Conversely, a wetter climate with seasonal drying increases herbaceous fuel production in open ecosystems and consequently encourages frequent surface fires. When surface fires were frequent in grasslands, savannas, and woodlands before Euro-American land use, drier climate likely suppressed fire frequency by limiting herbaceous growth, which is the primary surface fire fuel [29]. Fine herbaceous fuel development requires seasonal precipitation to grow vegetation followed by seasonal drying, which results in tinder for easy ignition, as opposed to coarse fuels, which generally resist ignition without extended drying.
In terms of relative factor strength, land use of fire exclusion and subsequent coarse fuel accumulation in high density forests has led to divergent wildfire pathways based on humid or dry climates, with wildfire frequency dampened in the humid eastern U.S. and wildfire frequency increasing in the dry western U.S. (Figure 2). While forestation and fuel accumulation have occurred throughout the U.S., trends of area burned by wildfire, excluding prescribed burns, over time are different when comparing the humid eastern U.S. to the dry western U.S. Predominantly coniferous tree species common in the western U.S may be more flammable relative to the greater proportion of mixed and broadleaf forests in the eastern U.S. Nonetheless, based on historical records, the eastern U.S. demonstrates that wildfires are rare after land use change resulting in forestation in a humid temperate region with moderate, and indeed increasing, precipitation.
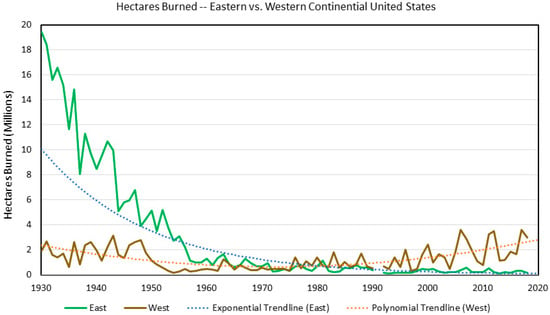
Figure 2.
Area (hectares) burned and trendlines by region based on fire records provided by the USDA Forest Service, Washington Office, and Short [30]. The eastern region is composed of Minnesota, Iowa, Missouri, Arkansas, and Louisiana and all states eastward. The western region is composed of all states west of the eastern region states. In 1930, ten-fold more hectares burned in the east than the west based on long-term USDA Forest Service fire records (19 million vs. 1.9 million hectares, respectively; Figure 2). Some of this difference might be due to underreporting in the west associated with lower human population densities, hence fewer fire detections due to remoteness. At any rate, annual hectares burned held steady in the west until a noticeable drop occurred around 1950. In contrast, annual hectares burned by wildfire dropped sharply in the east until the mid-1950s, with slow decreases thereafter. The east and the west had similar total area burned by wildfire during the 1960s to the early 1980s, after which trendlines crossed, with the west surpassing the east in the extent of burning by wildfires. This upward trend separates two regions into the future, as the west is currently burning by wildfires at a similar rate as during the 1930s.
2.4. Summary and Future Prediction
Increased precipitation in the humid eastern U.S. probably has interacted synergistically with land use to reduce the frequency of severe fires. Overall, land use resulting in tree densification and forestation reduces frequency of fires but switches to severe forest fires compared to land use leading to frequent surface fires in ecosystems with herbaceous vegetation. When land use promotes forests, with a switch to severe forest fires, then a wet climate decreases (severe) fire frequency relative to dry climate. In contrast, a drier climate allows accumulated coarse fuels to ignite more readily, increasing the frequency of severe fires and overcoming land use conversion to infrequent severe fires in ecosystems with high tree density. Therefore, future warming in the eastern U.S., without concurrent offsetting increased precipitation, may flip the direction of the interaction, with the climate eventually canceling or reversing the dampening effects on (severe) fire frequency by land use in an increasingly antagonistic interaction, such that warming increases severe wildfire frequency.
3. Tree Growth
3.1. Climate Change Effects
Climate change encompasses elevated temperature and carbon dioxide concentration and shifts in precipitation patterns, which can affect tree reproduction (through both fecundity and seedling establishment), growth, survival, and mortality, and overall forest productivity. Direct effects of climate change on tree growth and mortality may be more related to temperature in cold-limited forests and moisture in water-limited forests [31]. Boisvenue and Running [32] concluded that climate change has generally increased forest productivity where water was not limiting, describing the ‘greening of the biosphere’. Way and Oren (2010) reviewed the generally positive temperature effects on tree physiology and growth, with greater growth by deciduous species than evergreen species and in cold-limited temperate and boreal regions rather than tropical regions, where warming may induce growth declines. The growing season has been extended by 10–20 days in temperate regions [3,33]. Despite the lengthening growing season, temperature increases become detrimental to tree regeneration, growth, and survival when compounded by worsening drought conditions, particularly for vulnerable seedlings before root establishment [34,35]. While temperature increases may appear beneficial for cold-limited forests, maximum temperatures and exceeding an optimal temperature range can be particularly impactful on tree regeneration, growth, and survival, especially for boreal species that are sensitive to high temperatures [35]. Additionally, increased temperatures allow entry of tree species from warmer regions, that is, expected poleward shifts in distributions that disrupt existing ecosystems.
The effects of enriched atmospheric CO2 on tree growth have been investigated during the last 50 years [36,37]. Elevated CO2 has positive effects, within limits, on tree growth because CO2 uptake and fixation are the primary functions of photosynthesis [38,39]. Elevated CO2 and photosynthetic enhancement improve water-use efficiency in trees, as stomata do not need to stay open as long to replenish CO2 inside the leaf, thus reducing leaf transpiration [40,41]. Nevertheless, at some point photosynthesis will become CO2-saturated, after which increased atmospheric CO2 will not augment photosynthesis and growth [42,43].
Greater water availability, mild warming to date in cold-limited northern forests, and carbon fertilization on photosynthesis overall may have accelerated tree growth in the eastern U.S. [31,44,45,46,47]. Many portions of the eastern U.S. are now receiving more precipitation (see Figure 1), hence falling into the “greening biosphere” category. Voelker et al. [44] reported CO2 growth enhancement in oak and pine in the central U.S., but growth declined with tree age. Data from 120 journal articles describing physiology and production in the 12 large-scale free air CO2 enrichment experiments (FACE; 475–600 ppm) confirmed increased light-saturated carbon uptake and assimilation, growth, and aboveground production, whereas specific leaf area and stomatal conductance diminished under elevated CO2 treatments [37]. In recent decades, net ecosystem productivity in hemlock-dominated forests increased 93%, which was attributed in part to an increase in mean annual temperature and growing season length [48].
Some studies show either neutral or negative effects of climate change on tree growth, with negative effects due to components of climate [49]. Canham and Murphy [50] reported little or no statistical relationship between growth and precipitation for 40 of the 50 most common tree species in the eastern U.S., but that tree species of colder environs experienced reduced survival in warmer climates. In the Adirondack Mountains of New York, most sugar maple (Acer saccharum) trees exhibited negative basal area growth trends in the last several decades [51], which was inconsistent with increased moisture availability and precipitation pH (less acidity) in the region [52]. Recent studies have shown that both timing and intensity of drought, especially during early season, can affect growth [53,54]. Increased forest mortality has occurred in some locations in the eastern U.S. related to drought and high temperatures, reducing any gains in productivity [55,56].
3.2. Land Use Effects
Regarding land use, young and fully stocked stands composed of fast-growing tree species overall have greatest annual growth and production, resulting in the basis of production forestry [47,49,57]. Widespread forest cutting has replaced old-growth forests with younger, faster-growing forests [57,58]. Tree growth rates slow with age [57]. Indeed, tree species that exhibit rapid early growth particularly may slow in growth after a few decades, as they divert resources to reproduction, allowing some slow-growing long-lived species to eventually surpass the overall growth of tree species with fast life strategies; that is, the greatest annual rate of production may not yield the greatest long-term production, or carbon storage, for a stand [57,59,60]. Overstory tree removal remains frequent at 25 to 80 years in the eastern U.S. [61]. Fire exclusion had increased tree densities (see fire section above). Additionally, composition generally has shifted from a few relatively slow-growing, long-lived tree species that are stress-tolerant to numerous early-seral species that grow quickly in response to severe disturbance, reproduce young, and die within 100 years in the eastern U.S. [62]. Perhaps in consequence, mortality is seven-fold greater in diverse stands than monospecific stands [63]. Moreover, nitrogen deposition may enhance the growth of some tree species [35,64].
3.3. Interactions and Relative Factor Strength
Both climate change and land use have mechanisms and evidence for increasing annual growth in the eastern U.S. Land use trades maximizing total production in old-growth stands for maximizing annual growth rates in young stands. Climate change of increased precipitation, with warming in northern forests and carbon fertilization, has encouraged overall growth. In terms of the relative factor strengths of climate change and land use change, a study of forest responses to climate change for 38 eastern tree species over a recent 6-to-18-year period concluded that the effect of competition on growth and mortality risk exceeded the effects of climate variation in space or time for almost all species [65]. Moreover, forest recovery from past human disturbance is predicted to supersede climate change effects well into the future [66]. For trees located in Kentucky, New York, Ohio, and Pennsylvania, tree mortality between 1959 and 1985 was more affected by competition than climate factors [67]. Lines et al. [68] reported that tree diameter had the greatest relationship with mortality rate across the eastern U.S., due to lower mortality rates for trees of intermediate size than smaller or larger trees. Furthermore, Dietze and Moorcroft [35] reported that atmospheric pollutants, specifically acid deposition and ozone, and stand-level biotic dynamics, of moderate diameters, were the main drivers of tree mortality in the eastern U.S.
3.4. Summary and Future Prediction
We have presented mechanisms and evidence by which climate change and land use change may affect tree growth. In the eastern U.S., the overall direct effects of climate change on tree growth have likely been at least slightly positive due to elevated CO2 and the predominance of available water, with warming in cold-limited northern forests, while land use has likely increased growth rates through a change to productive young forests composed of fast-growing tree species at high densities. Although climate and land use interactions related to tree growth probably cover the full spectrum of synergistic to antagonistic, both of these drivers are likely to have overall positive effects on tree growth rates in the eastern U.S, with synergy in that climate conditions support the growth rate of fast-growing trees in fully stocked stands. In terms of carbon, fast carbon sequestration in young, small diameter trees that have short lifespans is not to be confused with long-term carbon storage potential of old, large diameter trees [69]. In the future, it is possible that the lack of stress tolerance in the fast-growing tree species of dense forests will result in increased die-offs due to flash droughts, heat waves, and insect outbreaks, as diverse stands already exhibit greater mortality rates than less diverse stands in temperate regions [63]. These mortality events may eliminate any gains in productivity due to warm and wet conditions and result in uncontrolled boom and bust cycles of growth and mortality. Eventually, warming temperatures may exceed tolerances for tree species adapted to current temperatures, producing disordered growth, survival, and reproduction, but with replacement by southern tree species that may be more productive, due to allocation of resources for growth rather than frost tolerance.
4. Tree Species Distributions
4.1. Climate Change Effects
Climate provides an outline of potential distributions. However, most species have not achieved their full potential distributions based on climate, even across contiguous space within the potential distribution [70]. Species are restricted in distributions due to different barriers to migration, from physical (e.g., mountains, deserts) to biological (e.g., interactions and metapopulation dynamics) to disturbance regimes (e.g., frequent fire). Indeed, due to land use through fire exclusion that changed competition for growing space in the eastern U.S., some historically dominant fire-tolerant tree species have contracted in range (e.g., longleaf pine, Pinus palustris; shortleaf pine, Pinus echinata) while fire-sensitive species have expanded, demonstrating examples of both reduced and increased filling of climate space [15]. Moreover, species have naturalized outside of their native ranges, leading to increases in the known suitable climate space [70].
Expectations are that most species will move poleward to higher, cooler latitudes to maintain their ideal climate envelope in response to warming climate [71]. Many tree species have expanded distributions in the U.S. (e.g., 15 studies located by Taheri et al. [72]). Nonetheless, causation and whether species may be moving in multiple directions is not determined in most studies [72]. Instead, expected patterns are interpreted as evidence for species migration in response to climate change, despite an overall lack of warming in the eastern U.S. The most definitive examples to date of climate change effects on tree distributions are observed outside the eastern U.S., specifically at high latitudes and altitudes [73] with tree line advancement [74], although land (pasture) abandonment may also influence the latter [75]. Recent upward shifts in forest ecotones in mountainous regions have been attributed to climate change in eastern forests [76]. Equally, phenological changes have been equivocal in this region, given relatively stable temperatures, with anomalous delayed start of season dates [77,78], but phenological changes may display the first climate signal before tree species distribution shifts.
4.2. Land Use Effects
Attribution of climate change to movement of tree species is challenging, given that tree distribution and density changes followed Euro-American settlement, regardless of climate variation [20]. Historical accounts since the 1600s from across the U.S. have consistently documented rapid tree growth and expansion following the wave of Euro-American land use change [15,79,80]. Trees invaded the westernmost grasslands in the eastern U.S. by 1800 and migrated since 1850 from eastern forests into the central Great Plains grasslands. Fire-sensitive tree species expanded from fire-protected wetlands, rocky outcrops and barren land cover, or high elevations. Inconsistent changes in moisture availability, which are within the range of natural variation, have not provided correlations with comprehensive tree expansion, but land use change has corresponded with tree changes based on timing, magnitude and direction of change, and mechanism [15,22]. Native tree species have increased within U.S. forests and grasslands, arguably with much greater effects, by replacing historical ecosystems, than non-native invasive species [62]. Shifts in tree species composition occurred due to land use change, from a regime of frequent surface fires combined with infrequent overstory disturbance to a regime of fire exclusion combined with chronic overstory disturbance.
4.3. Interactions and Relative Factor Strength
Currently, limited evidence supports shifts to higher latitudes or elevations in tree species distributions under increased precipitation with only slight warming in northern forests, but strong evidence supports that tree species have expanded due to land use. Expansion has been multi-directional, with prominent westward expansion into central Great Plains grasslands. Therefore, no interaction currently occurs.
4.4. Summary and Future Prediction
In summary, no strong evidence appears to exist for recent climate driving changes in species distributions because species began to shift following the front of Euro-American settlement, generally with the timing of agricultural development. However, climate does delineate overall potential distributions, with land use allowing trees to fill any treeless growing space in wildlands within suitable climate space. In the future, warmer temperature will facilitate poleward shifts in species distributions, producing compositional changes from boreal to temperate species, temperate to subtropical species, and subtropical to tropical species. Due to the strong yet varied influence by land use disturbance, this may result in a range of interactions and divergent outcomes, including rapid movement, lags, and direction reversals, varying by species. Land use pathways may guide species into warmer or drier locations, such as North American grasslands, regardless of the expectation that species will follow climate change.
5. Invasions and Outbreaks: Non-Native Plants and Non-Native and Native Insects and Diseases
5.1. Non-Native Species
Species typically are most successful at survival, growth, and reproduction within an ecological context or niche, including broad climate envelopes of temperature and precipitation. Nevertheless, species are not distributed throughout their global climate envelopes, due to dispersion barriers, competition, consumers (i.e., predators, herbivores, humans), disease, disturbance regimes, and other abiotic and biotic factors. Equally, because of constraining factors, species may have a broader climate tolerance than realized by current occurrences, because the physiological range is unknown.
Non-native plant species richness is greatest in the northeastern U.S. and along western coastal states, whereas the southeastern and interior U.S. have fewer non-native plant species [81]. Therefore, effects of non-native plants on forests may vary regionally and proportionally to the number of invasive species. Norway maple (Acer platanoides), tree of heaven (Ailanthus altissima), and Chinese tallow tree (Triadica sebifera) are examples of invasive non-native tree species. Garlic mustard (Alliaria petiolata), Japanese barberry (Berberis thunbergia), honeysuckles (Lonicera maackii and L. japonica), Japanese stilt grass (Microstegium vimineum), multiflora rose (Rosa multiflora), cogongrass (Imperata cylindrica), Chinese privet (Ligustrum sinense), and kudzu (Pueraria montana var. lobata) are some invasive non-native upland plant species. Direct changes in temperature and water availability do not appear overall to benefit invasive plant species [82].
Invasive forest insects in the eastern U.S. include the spongy moth (Lymantria dispar dispar), hemlock woolly adelgid (Adelges tsugae), Asian longhorned beetle (Anoplophora glabripennis), and emerald ash borer (Agrilus planipennis). Increased temperatures may be most beneficial for non-native insects as a group because warming increases mobility, growth, reproduction, and overwinter survival, albeit mortal thermal thresholds may occur [83,84,85]. Species such as the hemlock woolly adelgid and emerald ash borer are currently limited by cold winter temperatures but predicted future warming likely will allow expansion throughout the range of the host species in North America [86,87]. Frost-sensitive and non-diapausing species experience increased survival during warm winters [83,85]. However, warming reduces the amount and duration of insulating snow cover, perhaps resulting in greater over-winter mortality [85]. The decoupling of temperature and photoperiod cues may be disruptive for both insects and host plants [85] and increased temperatures could disconnect synchrony with native host plants [83], which are tracking climate change less closely than invasive plants [88,89]. Changes in plant defense chemistry due to climate may produce variable effects in plant susceptibility to infection, depending on interacting species and chemicals, while plant palatability and predators of insects also may be affected by climate change [86,90].
Invasive diseases of trees include chestnut blight (Cryphonectria parasitica), beech bark disease (Neonectria) that enters through wounds created by Cryptococcus fagisuga, Dutch elm disease (Ascomycota), white pine blister rust (Cronartium ribicola), and oak wilt (Bretziella fagacearum). Pathogens may be most sensitive to timing and quantity of precipitation, relative humidity, and factors that influence moisture [86,91]. Fungi in particular are very sensitive to factors that influence leaf-surface or soil moisture, and benefit from increased precipitation [91]. Pathogens may become more or less virulent under changed precipitation patterns, and disease epizootics generally will be unexpected, in part due to hybridization and other genetic changes [86,92].
Non-native species introductions into new distributions arose through human agency, not climate, by and large during the past centuries. Since Euro-American settlement, humans have moved thousands of species beyond their native ranges and dispersion barriers, including 2600 plants, 600 insects, and 100 diseases commonly observed in the U.S. [93]. Most non-native plants have been introduced deliberately for ornamental or agricultural purposes but imported nursery stock is a common pathway for non-native disease and insect introductions [94].
Regarding interactions between non-native species introductions, climate, and the future, plants introduced for ornamental or agricultural purposes are generally easy to propagate, show rapid growth, and tolerate wide climatic conditions [82]. Colonizing traits of rapid growth, less pressure from biotic constraints (e.g., release from parasites and consumers), and pre-selection to wide climate conditions position non-native species at a competitive advantage to increase and expand compared to native species, under many climates. Non-native species overall may be limited to climate envelopes of their native ranges, but as many as 15% of all non-native species are adapting to climate conditions outside of native climate envelopes [95]. For changing climate, non-native species overall appear to be tracking climate change phenologically more closely than native species [88,89]. Nevertheless, few non-native species are considered invasive, as measured by effects on native species, communities, and ecosystems. Unlike introduced species, native populations contain the full array of genetic variation and phenotypic plasticity that will aid in adaptation to changing climate conditions. Furthermore, given that climate change and associated extreme weather events are stressors to survival and growth, it may be that stress-tolerant traits, such as slower aboveground growth, may be more beneficial than rapid growth, a trait of invasive species, under climate change [96].
5.2. Outbreaks of Native Insects and Fungi
Outbreaks of several species of native insects (e.g., southern pine beetle, Dendroctonus frontalis; red oak borer, Enaphalodes rufulus) and fungi (e.g., fusiform rust, Cronartium quercumm f.sp. fusiforme; oak wilt; Ceratocystis fagacearum) that attack trees consistently occur in current forests with high densities and basal area [97], which typically are greater than in historical forests that had low tree densities due to frequent fire. While increased tree densities occur regardless of climate variation, greater water availability ameliorates stress on trees, particularly in high density stands. Many predisposing long-term factors influence insect and disease outbreaks and tree susceptibility, which are related to land use and disturbance, such as previous disturbance, fire exclusion, stand structure, tree age, and site location. Inciting factors are short-term conditions such as drought, related to climate, that weakens trees, especially in high density stands. Outbreaks outside of historical variation have occurred in the western U.S., due to a combination of land use change, resulting in trees with reduced vigor in dense forests, and climate change of warm summers that accelerate insect growth and development and warm winters that allow insect larvae to survive, along with, in some locations, drought-stressed trees [98].
5.3. Summary and Future Prediction
In summary, invasions by non-native species were facilitated mainly by human vectors and native insect outbreaks likely are due to land use resulting in forests of greater basal area. However, future climate change may interact with land use change to amplify outbreaks outside the range of historical variation through drought-stressed trees, warm summers that accelerate insect growth and development, warm winters that allow insect larvae to survive, coupled with land use change that creates dense forest stands with reduced tree vigor. Non-native species responses to future climate change may be quite variable, counter to expectations, and complicated by land use disturbance, producing no general trends by taxa [99]. Land use may cancel expected climate change effects given that some species are able to be successful outside of climate envelopes. Therefore, climate interactions with land use span the range from synergistic effects on invasions and outbreaks to canceling climate change effects on invasions and outbreaks.
6. Deer, a Remaining Large Herbivore
6.1. Deer Herbivory as a Forest Health Issue
A diverse array of megaherbivores once roamed the North American continent, surviving through all Pleistocene ice age cycles until the last one. Quite tellingly, their demise occurred about 13,000 to 11,000 years ago, after human establishment [12]. Most of the approximately 30 megaherbivores in the eastern U.S. truly were mega-sized, with 10 species exceeding 1000 kg. In contrast, the only remaining widespread large herbivore in the eastern U.S. is the white-tailed deer at 45 kg, not qualifying as mega-sized by some thresholds. The population of white-tailed deer is unknown during megaherbivore extinction, but likely deer numbers increased after loss of competitors. Consequently, megaherbivore extinction probably reduced overall herbivory on vegetation while benefiting extant herbivore species. The disturbance regime switch from megaherbivores to surface fire was continuous in North America, with the importance of fire (as measured in charcoal) increasing proportionally with megafauna decrease (measured in dung fungal spores; [100,101]). Indeed, the changeover to fire as a disturbance likely lessened ecological changes expected from loss of megaherbivores as a disturbance.
Although deer are considered an overabundant, invasive forest health problem, best available evidence suggests that deer currently are within historical population limits after recovering from overexploitation during Euro-American settlement [102,103]. The influence of deer on trees is relatively minor compared to when megaherbivores were a major disturbance agent; for example, deer herbivory at current populations have not been able to shift ecosystem states from closed, dense forests back to open forests of savannas and woodlands [104]. Deer remain a natural disturbance on young trees, albeit minor except in localized areas, as demonstrated by problem areas of high densities even when deer populations were recovering after overharvesting during Euro-American settlement [105]. Tree species (e.g., Thuja occidentalis) browsed heavily by deer at local scales have increased at regional scales since Euro-American settlement, whereas historically dominant tree species have decreased due to land use changes of frequent overstory disturbance and limited understory disturbance [106].
6.2. Climate Change Effects
No distinct effect from climate is apparent on deer distributions, and deer populations generally remain with historical levels, with fluctuations. Deer have a wide range and an even larger climate envelope that remains unfilled for now. Climate change effects on deer distributions appear minimal, both in the recent past and for the future, based on modeling (Figure 3).
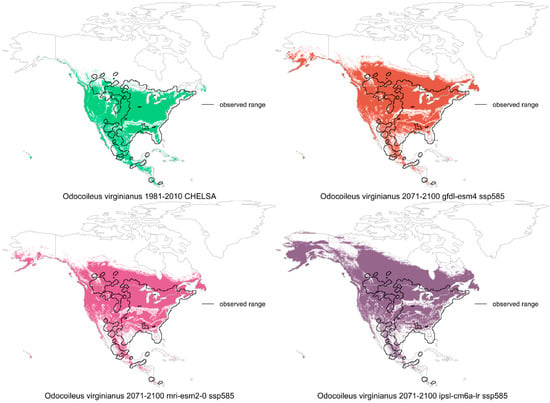
Figure 3.
Modeled species distributions of white-tailed deer that show observed range (outlined) from occurrence records, the climate envelope (temperature and precipitation of occurrences) during 1981–2010 (green), and the likely future climate envelopes during 2071–2100, under three general circulation models and high emissions (non-green colors; modeling followed [107]).
6.3. Land Use Effects
No distinct effect from land use is apparent on deer populations. After population reduction due to historical overexploitation, subsequent deer populations have recovered to within historical levels, with some fluctuation. Deer prefer forests to various land uses and while forests have increased in tree density over time, in spite of deer herbivory, deer are coping with current land use by browsing trees and using clearcuts and other forest openings [108].
6.4. Interactions, Relative Factor Strength, and Summary
Despite dynamics, we found no permanent effects on deer populations, and herbivory by extension, due to recent land use and climate change and, consequently, no interactions, but future effects and interactions may develop.
7. Ecological Forestry to Mitigate Climate Change
General recommendations for ecological forestry to lessen impacts of climate change include retaining genetic diversity and phenotypic plasticity and promoting warm-adapted species with wide amplitudes of climate tolerance and good dispersal ability. Nonetheless, differentiating the ability of species to survive, grow, disperse, and reproduce under climate change compared to whether a species is competitive under climate change is critical. That is, northern tree species may be able to complete their life cycles under warmer temperatures but may not be able to compete against faster-growing southern species, as no species can have the most competitive traits under all conditions [109]. Even so, spruce, aspen, pine, and other boreal tree species may be desirable and possible to maintain with active silviculture. Reducing additional stressors, such as risk of insect outbreaks, and competition for limited resources will help trees survive and thrive under climate change. This may be accomplished in part by restricting tree densities [96,97].
Assisted migration through management is an option to help tree species migrate, given that species will not be able to migrate at the same rate as projected warming and also that land use and other factors may drive tree species to warmer or drier locations, a conflicting or confounding outcome. Assisted migration and plantings may be desirable for species of conservation concern with limited ranges and dispersal abilities. Assisted migration is controversial, but species may be selected for tolerance of stresses, such as fire, drought, and insects, and be compatible with restoration objectives. For example, historically dominant oak species and longleaf and shortleaf pines are fire-tolerant, drought-tolerant, and windfirm, complementing the structural characteristics of open forests [110]. Fire-tolerant species such as oaks and pines that commit to early root development are likely to survive extreme weather events in early growth stages better than species committed to rapid early growth (e.g., longleaf pine height eventually may exceed loblolly pine [Pinus taeda] height, but after 25 to 30 years; [59]). Likewise, tolerant large diameter oaks and pines within open forests are more probable to survive droughts, fires, hurricanes, and insect outbreaks compared to fast-growing species in dense forests. Although oaks and pines are wide-ranging, they are not good dispersers compared to fast-growing species that produce abundant lightweight seeds. Therefore, oak and pine species are suitable candidates for assisted migration and plantings.
Conversely, assisted migration and plantings may be undesirable for native invasive species, such as red maple (Acer rubrum), that have been released by land use disturbance. Currently, many native tree species are expanding, sometimes with assistance through plantings such as for reforestation and tree initiatives. Indiscriminate planting of fast-growing and stress-intolerant species, with traits of fast growth and little reserves for fire and drought tolerance, at high densities, may foster forest vulnerability to climate change in terms of greater tree die-offs during extreme weather events. Also, caution may be needed in replanting after die-offs to not simply replace the same species with characteristics and structures that are not resistant to fire, drought, insects, and wind damage.
The historical open forest structure of understocked savannas and woodlands is ideally suited for tolerating climate change while restoring effects of Euro-American land use. The lower density structure means that trees require fewer resources, including less water requirement, resulting in greater ecosystem-level drought tolerance. Lower densities and understocking below maximum potential forest growth reduce competition for moisture and other resources, creating a “resource availability buffer” as a strategy for extreme climatic conditions and outbreaks of insects and disease [96]. Larger diameter trees are more fire-resistant and the lack of midstory and understory trees helps prevent fires from ascending to severe flame lengths, while discontinuous canopies also prevent canopy spread. The open structure is a firebreak and fuel treatment, which may prevent and reduce high severity fire, unlike the successional forest structure, with ladder fuels and dense canopies. The open structure is more windfirm to hurricanes and other severe wind events than high contrast edges of successional forests [111]. Open forests also support native species diversity, while providing a range of conditions that may supply refugia [112]. Additionally, for some damaging native species and a fire-sensitive subset of non-native species, open forests to reduce basal area and fire treatments may prevent outbreaks [113].
Open forest management is an alternative to conventional silvicultural systems, which concentrate on managing growth to optimize periodic yield and new overstory recruitment [112]. Instead, open forest management focuses on prevention of regeneration with rare recruitment of desired canopy dominants, maintenance of an understocked overstory, and treatments to encourage a more extensive groundlayer dominated by herbs. Open forest management involves multiple tools such as prescribed fire, herbicide use, and periodic harvests or deadenings to control stocking, permit recruitment of new canopy dominants, and financially support understory maintenance treatments [112]. Even with silvicultural targets that emulate the historic disturbance regime, achieving historical forest conditions is a challenge. Increased herbivory may be a necessary and missing component for restoration, particularly as propagule pressure from fire-sensitive tree species has increased. Thinning and burning treatments may be needed to reduce stand densities and fuel loads to help avert large-scale catastrophic canopy fires. In the eastern U.S., warming temperatures that effectively result in a drying climate may facilitate the success of prescribed burning to restore fire-tolerant communities by countering the influx of fire-sensitive, fire-dampening competitors.
8. Summary of Interactions
Climate change interacts with land use and disturbance, influencing ecosystem dynamics across time. Climate change and human practices have directly or indirectly impacted nearly every ecosystem in the eastern United States since continental deglaciation began approximately 15,000 years before present. When climate was cooler, boreal tree species extended into the southeastern U.S., and tree species have shifted northward over time as temperatures have warmed. The first humans to live on the continent affected ecosystems, including maintenance of fire-adapted tree genera such as oak, pine, and hickory in open forests that supply food resources. Due to European colonial expansion starting in the 16th century, land use practices changed, and open forests transitioned to closed forests, which are now used primarily for forest products. Despite these influences, some species such as deer have recovered from past overexploitation in the eastern U.S.
This synthesis of climate interactions with human activities, encompassing land use and anthropogenic disturbances, reveals a greater factor strength of land use than climate change on ecosystems during the past century or two in the eastern U.S. (Table 1). These findings concur with other studies that have determined land use disturbance plays a major role in ecosystems over time, at times overriding climate, especially during the latter portion of the Holocene, when human activities intensified [2,114,115,116]. Indeed, besides influencing terrestrial ecosystems, humans directly affect climate because current climate change is also an outcome of human activities. Specifying an interactive framework results in a more thorough understanding of forest ecosystem responses to a changing environment and ecosystem management for the future as warming increases beyond past temperatures. Incorporating the framework into explicit testable hypotheses could guide future research directions and inform management strategies aimed at mitigating the adverse effects of climate change and land use on forest ecosystems.

Table 1.
Mechanisms by which climate and land use may affect different forest issues, with the realized outcome and current interaction in the eastern United States, and prediction of the future interaction under warming climate.
Land use that causes an immediate reaction in terms of tree mortality or species migration has been a stronger and more immediate force than climate change to date. Climate change is a relatively slow, subtle process, with lag effects in combination with phenotypic plasticity in tree species and genetic traits due to past adaptation to similar temperature regimes [71]. The unusual aspect of climate change, due to human activities, has been overall lack of warming in the eastern U.S. combined with increased precipitation, albeit warming likely will occur in the future due to forcing by increased greenhouse gas levels [3]. Climate change since 1980 has been subtle, including warming in the Northeast and along the mid-Atlantic coast and a large dome of stable temperature over most of the remaining region with an overall increase in precipitation, except for decreased precipitation in the Southeast. Therefore, studies that attribute changes to warming temperatures in the eastern U.S. may be located in an area of exception or may have a suspect attribution to climate change.
Fire exclusion, resulting from changed land use and leading to continuous coarse fuel accumulation due to high density forests, has divergent ecological pathways, with wildfire dampened in the humid eastern U.S., along with increased precipitation. Fire has occurred in the eastern U.S. throughout a range of precipitation, but wildfire has been rare since land conversion to ecosystems with greater tree density, as fire regimes shifted in type from frequent surface fires in ecosystems with a dominant herbaceous layer to infrequent severe fires in closed forests. Conversely, climate change exerts a strong influence to enhance the frequency of severe fires in the dry western U.S., where tree densities also have increased; the western U.S. is currently burning with wildfire areas equivalent to those during the 1930s. If future warming generates effectively drier conditions in the eastern U.S., outside of historical ranges, then it is likely that severe fire frequency will increase in an antagonistic interaction between climate change and land use.
Climate and land use currently have a synergistic interaction for increasing tree growth rates in eastern forests. Climate change through increased available water, carbon fertilization, and slight warming in cold-limited northern forests generally is stimulatory to growth in forests. Land use probably has increased growth rates through a change to productive, fully stocked young forests composed of fast-growing tree species. In the future, it is possible that the lack of stress tolerance in the fast-growing tree species of fully stocked forests will result in increased die-offs due to flash droughts, heat waves, insect outbreaks, or simply even-aged stands of short-lived tree species reaching the typical mortality age.
Attribution of climate change effects on tree species distributions is difficult in the U.S. Rapid tree growth and expansion in multiple directions, but principally westwards to the grasslands of central North America, followed the wave of Euro-American land use change, regardless of climate variation. Inconsistent changes in moisture availability, which are within the range of natural variation, have not provided correlations with comprehensive tree increases, based on timing, magnitude and direction of change, and mechanism. Nonetheless, warming likely will direct species northwards, which for some species will be in the same direction as land use, but climate change and land use may be antagonistic interactions for other species.
Conditions that produced non-native species invasions and damaging native species outbreaks were created by human activities. No strong evidence appears to exist for climate change causing changes in non-native species distributions, because species were introduced through human vectors, with relative recency of information about non-native species distributions. After climate warms, combined with the strong but varied influence of land use disturbance, rapid movement and also lags and direction reversals by species may ensue. Species responses to climate change may be quite variable; therefore, climate interactions with land use disturbance span the range from synergistic to antagonistic effects on invasions and outbreaks.
Climate change is expected to become more influential in the future with warming and perhaps effectively a drier climate due to evapotranspiration. Depending on the characteristics of climate change and land use disturbance, synergistic interactions between climate change and land use disturbance may accelerate appropriate, expected responses by tree species to climate change, but these changes may not be desired, such as replacement of boreal tree species by temperate tree species. Antagonistic interactions between land use disturbance and climate change will generate surprising outcomes, which in some cases may be damaging, by overriding the appropriate, optimal response, in terms of survival, growth, reproduction, or productivity, to climate change. However, management may reduce land use and climate stressors on ecosystems. A resilient range of forest structures, with an abundant and diverse herbaceous component, and stress-tolerant tree species may be more stable under climate change than fast-growing tree species with short lifespans in high density forests. Future research and adaptive management can test the stipulated conceptual framework as climate warming unfolds in the eastern United States.
Author Contributions
Conceptualization, M.D.A. and B.B.H.; data curation, B.B.H. and G.J.N.; formal analysis, B.B.H. and G.J.N.; investigation, B.B.H., M.D.A. and G.J.N.; writing—original draft preparation, B.B.H., M.D.A. and G.J.N.; writing—B.B.H., M.D.A. and G.J.N.; visualization, B.B.H. and G.J.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
This research was supported by the USDA Forest Service, Rocky Mountain Research Station. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oliver, C.D.; Larson, B.C. Forest Stand Dynamics: Updated Edition; John Wiley and Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services [IPBES]. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; Available online: https://www.ipbes.net/global-assessment (accessed on 10 January 2022).
- Intergovernmental Panel on Climate Change [IPCC]. Summary for Policymakers. In Climate Change 2021, The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, S., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Monteiro, S., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; pp. 3–32. [Google Scholar]
- PRISM Climate Group. Oregon State University. 2021. Available online: https://prism.oregonstate.edu (accessed on 29 October 2021).
- Eischeid, J.K.; Hoerling, M.P.; Quan, X.W.; Kumar, A.; Barsugli, J.; Labe, Z.M.; Kunkel, K.E.; Schreck, I.I.I.C.J.; Easterling, D.R.; Zhang, T.; et al. Why has the summertime central US warming hole not disappeared? J. Clim. 2023, 36, 7319–7336. [Google Scholar] [CrossRef]
- Keil, P.; Mauritsen, T.; Jungclaus, J.; Hedemann, C.; Olonscheck, D.; Ghosh, R. Multiple drivers of the North Atlantic warming hole. Nat. Clim. Chang. 2020, 10, 667–671. [Google Scholar] [CrossRef]
- Abatzoglou, J.T. Development of gridded surface meteorological data for ecological applications and modelling. Int. J. Climatol. 2013, 33, 121–131. [Google Scholar] [CrossRef]
- Cook, E.R.; Seager, R.; Heim, R.R., Jr.; Vose, R.S.; Herweijer, C.; Woodhouse, C. Megadroughts in North America: Placing IPCC projections of hydroclimatic change in a long-term paleoclimate context. J. Quat. Sci. 2010, 25, 48–61. [Google Scholar] [CrossRef]
- Abrams, M.D. Fire and the development of oak forests. BioScience 1992, 42, 346–353. [Google Scholar] [CrossRef]
- Nevle, R.J.; Bird, D.K.; Ruddiman, W.F.; Dull, R.A. Neotropical human–landscape interactions, fire, and atmospheric CO2 during European conquest. Holocene 2011, 21, 853–864. [Google Scholar] [CrossRef]
- Abrams, M.D.; Nowacki, G.J.; Hanberry, B.B. Oak forests and woodlands as Indigenous landscapes in the Eastern United States. J. Torrey Bot. Soc. 2022, 149, 101–121. [Google Scholar] [CrossRef]
- Malhi, Y.; Doughty, C.E.; Galetti, M.; Smith, F.A.; Svenning, J.C.; Terborgh, J.W. Megafauna and ecosystem function from the Pleistocene to the Anthropocene. Proc. Natl. Acad. Sci. USA 2016, 113, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Nowacki, G.J.; MacCleery, D.W.; Lake, F.K. Native Americans, ecosystem development, and historical range of variation. In Historical Environmental Variation in Conservation and Natural Resource Management; Weins, J.A., Hayward, G.D., Safford, H.D., Giffen, C.M., Eds.; Wiley-Blackwell: West Sussex, UK, 2012; pp. 76–91. [Google Scholar]
- Hanberry, B.B. Timing of tree density increases, influence of climate change, and a land use proxy for tree density increases in the eastern United States. Land 2021, 10, 1121. [Google Scholar] [CrossRef]
- Denevan, W.M. The pristine myth: The landscape of the Americas in 1492. Ann. Assoc. Am. Geogr. 1992, 82, 369–385. [Google Scholar] [CrossRef]
- Foster, D.R. Land use history (1730–1990) and vegetation dynamics in central New England, USA. J. Ecol. 1992, 80, 753–771. [Google Scholar] [CrossRef]
- Whitney, G.G. From Coastal Wilderness to Fruited Plain: A History of Environmental Change in Temperate North America from 1500 to the Present; Cambridge University Press: New York, NY, USA, 1994. [Google Scholar]
- Munoz, S.E.; Gajewski, K.; Peros, M.C. Synchronous environmental and cultural change in the prehistory of the northeastern United States. Proc. Natl. Acad. Sci. USA 2010, 107, 22008–22013. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, G.J.; Abrams, M.D. Is climate an important driver of post European vegetation change in the eastern U.S.? Glob. Chang. Biol. 2015, 21, 314–334. [Google Scholar] [CrossRef] [PubMed]
- Nowacki, G.J.; Abrams, M.D. Demise of fire and mesophication of eastern U.S. forests. BioScience 2008, 58, 123–138. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Abrams, M.D.; Arthur, M.A.; Varner, J.M. Reviewing fire, climate, deer, and foundation species as drivers of historically open oak and pine forests and transition to closed forests. Front. For. Glob. Chang. 2020, 3, 56. [Google Scholar] [CrossRef]
- Davison, C.W.; Rahbek, C.; Morueta-Holme, N. Land-use change and biodiversity: Challenges for assembling evidence on the greatest threat to nature. Glob. Chang. Biol. 2021, 27, 5414–5429. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.M.; Varner, J.M.; Metz, M.R.; van Mantgem, P.J. Characterizing interactions between fire and other disturbances and their impacts on tree mortality in western US Forests. For. Ecol. Manag. 2017, 405, 188–199. [Google Scholar] [CrossRef]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Gensini, V.A.; Brooks, H.E. Spatial trends in United States tornado frequency. NPJ Clim. Atmos. Sci. 2018, 1, 38. [Google Scholar] [CrossRef]
- Holl, K.D.; Brancalion, P.H. Tree planting is not a simple solution. Science 2020, 368, 580–5811. [Google Scholar] [CrossRef]
- Reilly, M.J.; Norman, S.P.; O’Brien, J.J.; Loudermilk, E.L. Drivers and ecological impacts of a wildfire outbreak in the southern Appalachian Mountains after decades of fire exclusion. For. Ecol. Manag. 2022, 524, 120500. [Google Scholar] [CrossRef]
- Shuman, B.; Henderson, A.K.; Plank, C.; Stefanova, I.; Ziegler, S.S. Woodland-to-forest transition during prolonged drought in Minnesota after ca. AD 1300. Ecology 2009, 90, 2792–2807. [Google Scholar] [CrossRef]
- Short, K.C. Spatial Wildfire Occurrence Data for the United States, 1992–2020; Forest Service Research Data Archive: Fort Collins, CO, USA, 2022. [Google Scholar] [CrossRef]
- Way, D.A.; Oren, R. Differential responses to changes in growth temperature between trees from different functional groups and biomes: A review and synthesis of data. Tree Physiol. 2010, 30, 669–688. [Google Scholar] [CrossRef]
- Boisvenue, C.; Running, S.W. Impacts of climate change on natural forest productivity—Evidence since the middle of the 20th century. Glob. Chang. Biol. 2006, 12, 862–882. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- Dietze, M.C.; Moorcroft, P.R. Tree mortality in the eastern and central United States: Patterns and drivers. Glob. Chang. Biol. 2011, 17, 3312–3326. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The response of natural ecosystems to the rising global CO2 levels. Annu. Rev. Ecol. Syst. 1990, 21, 167–196. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2015, 165, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Karnosky, D.F. Impacts of elevated atmospheric CO2 on forest trees and forest ecosystems: Knowledge gaps. Environ. Int. 2003, 29, 161–169. [Google Scholar] [CrossRef]
- Norby, R.J.; Warren, J.M.; Iversen, C.M.; Medlyn, B.E.; McMurtrie, R.E. CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc. Natl. Acad. Sci. 2010, 107, 19368–19373. [Google Scholar] [CrossRef]
- Keenan, T.F.; Hollinger, D.Y.; Bohrer, G.; Dragoni, D.; Munger, J.W.; Schmid, H.P.; Richardson, A.D. Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 2013, 499, 324–327. [Google Scholar] [CrossRef]
- Mathias, J.M.; Thomas, R.B. Global tree intrinsic water use efficiency is enhanced by increased atmospheric CO2 and modulated by climate and plant functional types. Proc. Natl. Acad. Sci. USA 2021, 118, e2014286118. [Google Scholar] [CrossRef] [PubMed]
- Korner, C. Carbon limitation in trees. J. Ecol. 2003, 91, 4–17. [Google Scholar] [CrossRef]
- Sperry, J.S.; Venturas, M.D.; Todd, H.N.; Trugman, A.T.; Anderegg, W.R.; Wang, Y.; Tai, X. The impact of rising CO2 and acclimation on the response of US forests to global warming. Proc. Natl. Acad. Sci. USA 2019, 116, 25734–25744. [Google Scholar] [CrossRef]
- Voelker, S.L.; Muzika, R.; Guyette, R.P.; Stambaugh, M.C. Historical CO2 growth enhancement declines with age in Quercus and Pinus. Ecol. Monogr. 2006, 76, 549–564. [Google Scholar] [CrossRef]
- McMahon, S.M.; Parker, G.G.; Miller, D.R. Evidence for a recent increase in forest growth. Proc. Natl. Acad. Sci. USA 2010, 107, 3611–3615. [Google Scholar] [CrossRef]
- Davis, E.C.; Sohngen, B.; Lewis, D.J. The effect of carbon fertilization on naturally regenerated and planted US forests. Nat. Commun. 2022, 13, 5490. [Google Scholar] [CrossRef]
- Hogan, J.A.; Domke, G.M.; Zhu, K.; Johnson, D.J.; Lichstein, J.W. Climate change determines the sign of productivity trends in US forests. Proc. Natl. Acad. Sci. USA 2024, 121, e2311132121. [Google Scholar] [CrossRef] [PubMed]
- Finzi, A.C.; Giasson, M.A.; Barker Plotkin, A.A.; Aber, J.D.; Boose, E.R.; Davidson, E.A.; Dietze, M.C.; Ellison, A.M.; Frey, S.D.; Goldman, E.; et al. Carbon budget of the Harvard Forest Long-Term Ecological Research site: Pattern, process, and response to global change. Ecol. Monogr. 2020, 90, e01423. [Google Scholar] [CrossRef]
- Caspersen, J.P.; Pacala, S.W.; Jenkins, J.C.; Hurtt, G.C.; Moorcroft, P.R.; Birdsey, R.A. Contributions of land-use history to carbon accumulation in US forests. Science 2000, 290, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Canham, C.D.; Murphy, L. The demography of tree species response to climate: Sapling and canopy tree survival. Ecosphere 2017, 8, e01701. [Google Scholar] [CrossRef]
- Bishop, D.A.; Beier, C.M.; Pederson, N.; Lawrence, G.B.; Stella, J.C.; Sullivan, T.J. Regional growth decline of sugar maple (Acer saccharum) and its potential causes. Ecosphere 2015, 6, 179. [Google Scholar] [CrossRef]
- Oswald, E.M.; Pontius, J.; Rayback, S.A.; Schaberg, P.G.; Wilmot, S.H.; Dupigny-Giroux, L.A. The complex relationship between climate and sugar maple health: Climate change implications in Vermont for a key northern hardwood species. For. Ecol. Manag. 2018, 422, 303–312. [Google Scholar] [CrossRef]
- Peters, R.L.; Groenendijk, P.; Vlam, M.; Zuidema, P.A. Detecting long-term growth trends using tree rings: A critical evaluation of methods. Glob. Chang. Biol. 2015, 21, 2040–2054. [Google Scholar] [CrossRef]
- D’Orangeville, L.; Houle, D.; Duchesne, L.; Phillips, R.P.; Bergeron, Y.; Kneeshaw, D. Beneficial effects of climate warming on boreal tree growth may be transitory. Nat. Commun. 2018, 9, 3213. [Google Scholar] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.T.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Crosby, M.K.; Fan, Z.; Fan, X.; Leininger, T.D.; Spetich, M.A. Assessing forest mortality patterns using climate and FIA data at multiple scales. In Moving from Status to Trends: Forest Inventory and Analysis (FIA) Symposium 2012; Morin, R.S., Liknes, G.C., Eds.; U.S. Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2012; pp. 319–324. [Google Scholar]
- Johnson, S.; Abrams, M.D. Age class, longevity and growth rate relationships: Protracted growth increases in old trees in the eastern United States. Tree Physiol. 2009, 29, 1317–1328. [Google Scholar] [CrossRef]
- Abrams, M.D. Where has all the white oak gone? BioScience 2003, 53, 927–939. [Google Scholar] [CrossRef]
- Cram, M.M.; Outcalt, K.W.; Zarnoch, S.J. Growth of longleaf and loblolly pine planted on South Carolina sandhill sites. South. J. Appl. For. 2010, 34, 79–83. [Google Scholar] [CrossRef]
- Bragg, D.C. A reevaluation of superior tree performance after 48 years for a loblolly pine progeny test in southern Arkansas. Front. For. Glob. Chang. 2021, 4, 716443. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, J.M.; Birdsey, R.; McCullough, K.; He, L.; Deng, F. Age structure and disturbance legacy of North American forests. Biogeosciences 2011, 8, 715–732. [Google Scholar] [CrossRef]
- Hanberry, B.B. Confronting the issue of invasive native tree species due to land use change in the eastern United States. Land 2022, 11, 161. [Google Scholar] [CrossRef]
- Searle, E.B.; Chen, H.Y.; Paquette, A. Higher tree diversity is linked to higher tree mortality. Proc. Natl. Acad. Sci. USA 2022, 119, e2013171119. [Google Scholar] [CrossRef] [PubMed]
- Quinn Thomas, R.; Canham, C.D.; Weathers, K.C.; Goodale, C.L. Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 2010, 3, 13–17. [Google Scholar] [CrossRef]
- Clark, J.S.; Bell, D.M.; Hersh, M.H.; Nichols, L. Climate change vulnerability of forest biodiversity: Climate and competition tracking of demographic rates. Glob. Chang. Biol. 2011, 17, 1834–1849. [Google Scholar] [CrossRef]
- Duveneck, M.J.; Thompson, J.R.; Gustafson, E.J.; Liang, Y.; de Bruijn, A.M. Recovery dynamics and climate change effects to future New England forests. Landsc. Ecol. 2017, 32, 1385–1397. [Google Scholar] [CrossRef]
- Yaussy, D.A.; Iverson, L.R.; Matthews, S.N. Competition and climate affects US hardwood-forest tree mortality. For. Sci. 2013, 59, 416–430. [Google Scholar] [CrossRef]
- Lines, E.R.; Coomes, D.A.; Purves, D.W. Influences of forest structure, climate and species composition on tree mortality across the eastern US. PLoS ONE 2010, 5, e13212. [Google Scholar] [CrossRef]
- Brienen, R.J.; Caldwell, L.; Duchesne, L.; Voelker, S.; Barichivich, J.; Baliva, M.; Ceccantini, G.; Di Filippo, A.; Helama, S.; Locosselli, G.M.; et al. Forest carbon sink neutralized by pervasive growth-lifespan trade-offs. Nat. Commun. 2020, 11, 4241. [Google Scholar] [CrossRef]
- Perret, D.L.; Leslie, A.B.; Sax, D.F. Naturalized distributions show that climatic disequilibrium is structured by niche size in pines (Pinus L.). Glob. Ecol. Biogeogr. 2019, 28, 429–441. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Naimi, B.; Rahbek, C.; Araújo, M.B. Improvements in reports of species redistribution under climate change are required. Sci. Adv. 2021, 7, eabe1110. [Google Scholar] [CrossRef] [PubMed]
- Roe, G.H.; Baker, M.B.; Herla, F. Centennial glacier retreat as categorical evidence of regional climate change. Nat. Geosci. 2017, 10, 95–99. [Google Scholar] [CrossRef]
- Smith, W.K.; Germino, M.J.; Johnson, D.M.; Reinhardt, K. The altitude of alpine treeline: A bellwether of climate change effects. Bot. Rev. 2009, 75, 163–190. [Google Scholar] [CrossRef]
- Gehrig-Fasel, J.; Guisan, A.; Zimmermann, N.E. Tree line shifts in the Swiss Alps: Climate change or land abandonment? J. Veg. Sci. 2007, 18, 571–582. [Google Scholar] [CrossRef]
- Beckage, B.; Osborne, B.; Gavin, D.G.; Pucko, C.; Siccama, T.; Perkins, T. A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proc. Natl. Acad. Sci. USA 2008, 105, 4197–4202. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X. Effects of temperature variability and extremes on spring phenology across the contiguous United States from 1982 to 2016. Sci. Rep. 2020, 10, 17952. [Google Scholar] [CrossRef]
- Bórnez, K.; Verger, A.; Descals, A.; Peñuelas, J. Monitoring the responses of deciduous forest phenology to 2000–2018 climatic anomalies in the Northern Hemisphere. Remote Sens. 2021, 13, 2806. [Google Scholar] [CrossRef]
- Day, G.M. The Indian as an ecological factor in the northeastern forest. Ecology 1953, 34, 329–346. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Noss, R.F. Locating potential historical fire-maintained grasslands of the eastern United States based on topography and wind speed. Ecosphere 2022, 13, e4098. [Google Scholar] [CrossRef]
- Hanberry, B.B. Non-native plant species richness and influence of greenhouses and human populations in the conterminous United States. Ecol. Process. 2023, 12, 27. [Google Scholar] [CrossRef]
- Bradley, B.A.; Blumenthal, D.M.; Wilcove, D.S.; Ziska, L.H. Predicting plant invasions in an era of global change. Trends Ecol. Evol. 2010, 25, 310–318. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K.; Butterfield, J.; Buse, A.; Coulson, J.C.; Farrar, J.; et al. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Chang. Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 105, 6668–6672. [Google Scholar] [CrossRef]
- Bale, J.S.; Hayward, S.A.L. Insect overwintering in a changing climate. J. Exp. Biol. 2010, 213, 980–994. [Google Scholar] [CrossRef]
- Dukes, J.S.; Pontius, J.; Orwig, D.; Garnas, J.R.; Rodgers, V.L.; Brazee, N.; Cooke, B.; Theoharides, K.A.; Stange, E.E.; Harrington, R.; et al. Responses of insect pests, pathogens, and invasive plant species to climate change in the forests of northeastern North America: What can we predict? Can. J. For. Res. 2009, 39, 231–248. [Google Scholar] [CrossRef]
- Sobek-Swant, S.; Crosthwaite, J.C.; Lyons, D.B.; Sinclair, B.J. Could phenotypic plasticity limit an invasive species? Incomplete reversibility of mid-winter deacclimation in emerald ash borer. Biol. Invasions 2012, 14, 115–125. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cleland, E.E. Phenological niches and the future of invaded ecosystems with climate change. AoB Plants 2014, 6, plu013. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Davis, T.J.; Schaefer, H.; Cleland, E.E.; Cook, B.I.; Travers, S.E.; Willis, C.G.; Davis, C.C. Temperature-dependent shifts in phenology contribute to the success of exotic species with climate change. Am. J. Bot. 2013, 100, 1407–1421. [Google Scholar] [CrossRef]
- Robinson, E.A.; Ryan, G.D.; Newman, J.A. A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012, 194, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Meentemeyer, R.K.; Cunniffe, N.J.; Cook, A.R.; Filipe, J.A.; Hunter, R.D.; Rizzo, D.M.; Gilligan, C.A. Epidemiological modeling of invasion in heterogeneous landscapes: Spread of sudden oak death in California (1990–2030). Ecosphere 2011, 2, 1–24. [Google Scholar] [CrossRef]
- Brasier, C.M. Rapid evolution of introduced plant pathogens via interspecific hybridization. BioScience 2001, 51, 123–133. [Google Scholar] [CrossRef]
- EDDMapS. 2021. Available online: https://www.eddmaps.org/species/ (accessed on 19 November 2021).
- Liebhold, A.M.; Brockerhoff, E.G.; Garrett, L.J.; Parke, J.L.; Britton, K.O. Live plant imports: The major pathway for forest insect and pathogen invasions of the United States. Front. Ecol. Environ. 2012, 10, 135–143. [Google Scholar] [CrossRef]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1347. [Google Scholar] [CrossRef]
- McNulty, S.G.; Boggs, J.L.; Sun, G. The rise of the mediocre forest: Why chronically stressed trees may better survive extreme episodic climate variability. New For. 2014, 45, 403–415. [Google Scholar] [CrossRef]
- Asaro, C.; Koch, F.H.; Potter, K.M. Denser forests across the USA experience more damage from insects and pathogens. Sci. Rep. 2023, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Romme, W.H.; Clement, J.; Hicke, J.; Kulakowski, D.; MacDonald, L.H.; Schoennagel, T.L.; Veblen, T.T. Recent Forest Insect Outbreaks and Fire Risk in Colorado Forests: A Brief Synthesis of Relevant Research; Colorado Forest Restoration Institute: Fort Collins, CO, USA, 2006. [Google Scholar]
- De Chazal, J.; Rounsevell, M.D. Land-use and climate change within assessments of biodiversity change: A review. Glob. Environ. Chang. 2009, 19, 306–315. [Google Scholar] [CrossRef]
- Robinson, G.S.; Burney, L.P.; Burney, D.A. Landscape paleoecology and megafaunal extinction in southeastern New York State. Ecol. Monogr. 2005, 75, 295–315. [Google Scholar] [CrossRef]
- Gill, J.L.; Williams, J.W.; Jackson, S.T.; Lininger, K.B.; Robinson, G.S. Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 2009, 326, 1100–1103. [Google Scholar] [CrossRef]
- Seton, E.Y. Lives of Game Animals; Branford: Boston, MA, USA, 1927; Volume III, Part I; Available online: https://archive.org/details/livesofgameanima0003seto/page/n15/mode/2up (accessed on 19 June 2022).
- Hanberry, B.; Hanberry, P. Rapid digitization to reclaim thematic maps of white-tailed deer density from 1982 and 2003 in the conterminous US. PeerJ 2020, 8, e8262. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Abrams, M.D. Does white-tailed deer density affect tree stocking in forests of the eastern United States? Ecol. Process. 2019, 8, 30. [Google Scholar] [CrossRef]
- Leopold, A.; Sowls, L.K.; Spencer, D.L. A survey of over-populated deer ranges in the United States. J. Wildl. Manag. 1947, 11, 162–177. [Google Scholar] [CrossRef]
- Hanberry, B.B.; Faison, E.K. Re-framing deer herbivory as a natural disturbance regime with ecological and socioeconomic outcomes in the eastern United States. Sci. Total Environ. 2023, 868, 161669. [Google Scholar] [CrossRef]
- Hanberry, B.B. Practical guide for retaining correlated climate variables and unthinned samples in species distribution modeling, using random forests. Ecol. Inform. 2024, 79, 102406. [Google Scholar] [CrossRef]
- Hanberry, B.B. Addressing regional relationships between white-tailed deer densities and land classes. Ecol. Evol. 2021, 11, 13570–13578. [Google Scholar] [CrossRef] [PubMed]
- Loehle, C. Height growth rate tradeoffs determine northern and southern range limits for trees. J. Biogeogr. 1998, 25, 735–742. [Google Scholar] [CrossRef]
- Abrams, M.D. Adaptations and responses to drought in Quercus species of North America. Tree Physiol. 1990, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Stanturf, J.A.; Goodrick, S.L.; Outcalt, K.W. Disturbance and coastal forests: A strategic approach to forest management in hurricane impact zones. For. Ecol. Manag. 2007, 250, 119–135. [Google Scholar] [CrossRef]
- Bragg, D.C.; Hanberry, B.B.; Hutchinson, T.F.; Jack, S.B.; Kabrick, J.M. Silvicultural options for open forest management in eastern North America. For. Ecol. Manag. 2020, 474, 118383. [Google Scholar] [CrossRef]
- Holzmueller, E.J.; Jose, S.; Jenkins, M.A. The relationship between fire history and an exotic fungal disease in a deciduous forest. Oecologia 2008, 155, 347–356. [Google Scholar] [CrossRef]
- Danneyrolles, V.; Dupuis, S.; Fortin, G.; Leroyer, M.; de Römer, A.; Terrail, R.; Vellend, M.; Boucher, Y.; Laflamme, J.; Bergeron, Y.; et al. Stronger influence of anthropogenic disturbance than climate change on century-scale compositional changes in northern forests. Nat. Commun. 2019, 10, 1265. [Google Scholar] [CrossRef] [PubMed]
- Knight, C.A.; Anderson, L.; Bunting, M.J.; Champagne, M.; Clayburn, R.M.; Crawford, J.N.; Klimaszewski-Patterson, A.; Knapp, E.E.; Lake, F.K.; Mensing, S.A.; et al. Land management explains major trends in forest structure and composition over the last millennium in California’s Klamath Mountains. Proc. Natl. Acad. Sci. USA 2022, 119, e2116264119. [Google Scholar] [CrossRef] [PubMed]
- Napier, J.D.; Chipman, M.L. Emerging palaeoecological frameworks for elucidating plant dynamics in response to fire and other disturbance. Glob. Ecol. Biogeogr. 2022, 31, 138–154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).