Abstract
The conservation of dry sandy grasslands is a global issue because of the restoration and conservation of endangered ecosystems to provide a sufficient amount of forage under warming and drying climatic conditions. Our aim was to explore the impact of military activity on sandy grasslands in Hungary. The sample areas chosen were the Little Hungarian Plain (I. and II.) and the Great Hungarian Plain (III.), consisting abandoned, restored areas; still-active military exercise fields; and shooting ranges. In each sample area, six to ten coenological surveys were made. Based on our survey, the closed natural sandy grassland was documented only in the Little Hungarian Plain and were found rich in species. The open sandy grassland was described in all studied sites, Festuca vaginata appeared in all of them, while Festuca pseudovaginata was observed only in the Great Hungarian Plain. In the open sandy grassland, the natural vegetation had the highest cover value (78.8%), the sowed grassland area had the least cover value (53.3%), while the III. sample area was also poor in coverage (56.5%) but consisted of a natural species composition favorable for restoration. Our results confirmed the indirect role of military activity in the successful habitat conservation of Pannonian dry sandy grassland ecosystems.
1. Introduction
The conservation of dry sandy grasslands is a global issue, since their biodiversity is critically vulnerable to fragmentation. The effects of climate change have resulted in increasing significance of these drought-tolerant ecosystems. They represent an important genetic pool too, as biodiversity has been declining in grasslands of the Pannonian region [1,2,3]. Biodiversity loss is caused by anthropogenic influences, but also by the improper use or lack of land management [1]. Global warming necessitates the restoration and conservation of endangered ecosystems which are still able to provide a sufficient amount of forage under warming and drying climatic conditions too. The Pannonian endemic dry, sandy grasslands have a low nutrient content in general. Nevertheless, despite their low nutrient content and biomass value, these areas are used for grazing in the long term. The ecosystem of these habitats can provide nutrients even in the context of global warming. However, the application of sustainable management practices is necessary for their maintenance, as complete abandonment can cause biodiversity reduction and a decreased number of species. According to several research studies, this decrease in biodiversity is often accompanied by a decrease in plant biomass [2,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Appropriate grazing or mowing are important management practices for grasslands, especially in low-quality Pannonian grasslands, as the amount of surface phytomass has a positive effect on the number of species even with a slight disturbance [14]. The enhancement in productivity can contribute to species richness, contingent upon various factors such as geographical location [13], habitat expansion [11], fragmentation [7], biocoenosis type [6], and habitat successional condition [11].
As in the study of sandy grassland restoration [16], the amount of target species in sandy grassland was found to be higher in older than in younger fallows. By definition, fallows are arable areas that have been abandoned for less than 10 years, whereas grasslands are areas that have been treated as grassland for more than 10 years. The species are considered “target species” that can be dominant characteristic species in natural grasslands and that are also important for grassland and nature conservation management purposes. Most of the target species were already sown in young fallows, but only a few new target species appeared in older fallows [9]. According to the study by Rehounková and Prach [16], the appearance of new target species in sandy areas is probably a propagule-limited process, which is influenced by the species composition of adjacent grasslands [5,6]. This statement is confirmed by the experiences of restoration of the Homoktövis Nature Conservation Area in Hungary [7] too. Therefore, activities of habitat restoration are important to reduce isolation and increase the expansion of patches of grassland habitats. The final aim of restoration is to reach the habitats’ original condition to the closest extent possible [19]. The relevance of habitat restorations by active intervention has been increasing [20,21,22], especially in natural and semi-natural areas of highly transformed environments [21].
In the process of grassland restorations, sown grasslands has gained more importance [2,5], representing one of the most common interventions of habitat reconstruction today. However, the sowing of grasslands can be carried out in various ways, including the adding of hay or the application of seed mixtures of different diversities [23,24,25]. The selection of seed mixtures is an important issue for nature conservation, as in ideal cases, species compatible with the landscape and the most suitable for the environmental conditions of the area are collected [26,27]. When seed mixtures are sown, different seed mixtures with low diversities (2–8 species) [28,29] or high diversities (9–40 species) are used [25,29], if the determination of dominant species is conducted in controlled conditions. In addition, burning has also become common in regeneration maintenance works [30,31,32].
Countries worldwide dedicate vast areas to military exercises. Due to the nature of the activity, these military training grounds often exceed 100,000 hectares individually. The perception of these areas is Janus-faced, as on the one hand, the destructive nature of military training can cause significant environmental damage, while on the other hand, the restrictions applied to these areas makes it impossible to pursue other (non-military) human activities [33]. Based on the literature, evaluation of the harmful environmental effects of activities on training grounds can be grouped into the following main research areas: ground water pollution, soil degradation (compaction) and soil pollution (especially due to heavy metal contamination), the entry of pollutants into the food web, and ecotoxicology [34]. Soil compaction and structure degradation are central issues regarding vegetation, as decreased pore space and the related reduced hydraulic conductivity directly affect plant life [35]. Furthermore, the uptake of toxic metals by the roots and shoots of the grass, forb, and shrub species increases the risk of these substances entering the food chain [35,36]. The biomass of the vegetation can also be reduced significantly on military training grounds by the off-road maneuvers of tanks and other heavy military vehicles, and the bare surfaces may enhance soil erosion 30 times higher than reference plots [37].
However, the wide variety of damages caused by military training, coupled with their irregular distribution in space and time, creates a wide variety of microhabitats with diverse local ecological conditions [38,39]. A striking example of microhabitat diversification is related to explosion craters that increased species diversity in the Nowa Dęba (SE Poland) military training area [40]. This increased plant diversity on military training grounds was documented in other localities as well, and these vegetation units often harbored populations of endangered plant species [33,39]. This special phenomenon is explained by the “heterogeneous disturbance hypothesis”, which is related to Connell’s intermediate disturbance hypothesis [40]. Warren’s hypothesis emphasizes the importance of multiple kinds and frequencies of disturbance, as well as its varying severity, periodicity, and size. These factors are also suggested to be considered during conservation activities on abandoned military training grounds.
In the present research, we investigated vegetation types that grow on sandy soils with poor nutrient content and with questionable economic potential. However, as they are well adapted to drought, their potential expansion in the future may increase their economic importance as pasture and forage provider habitats. Among these vegetation types, the most typical ones are the calcareous perennial open sandy grasslands (Festucetum vaginatae Rapaics ex Soó 1929 em. Borhidi 1996), which grow in very dry and nutrient-poor areas.
Based on the reviewed literature, we sought to answer the following question in our research: Can military activity serve as a special option of habitat conservation in natural and semi-natural sandy grasslands of Hungary? This option can be considered special, as vegetation conservation does not belong to the task of military activities, so nature conservation in military areas is conducted in an indirect way. Thus, our aim was to investigate the impact of military activities on highly variable endemic arid vegetation types in the context of climate change using coenological surveys.
2. Materials and Methods
2.1. Description of the Study Areas
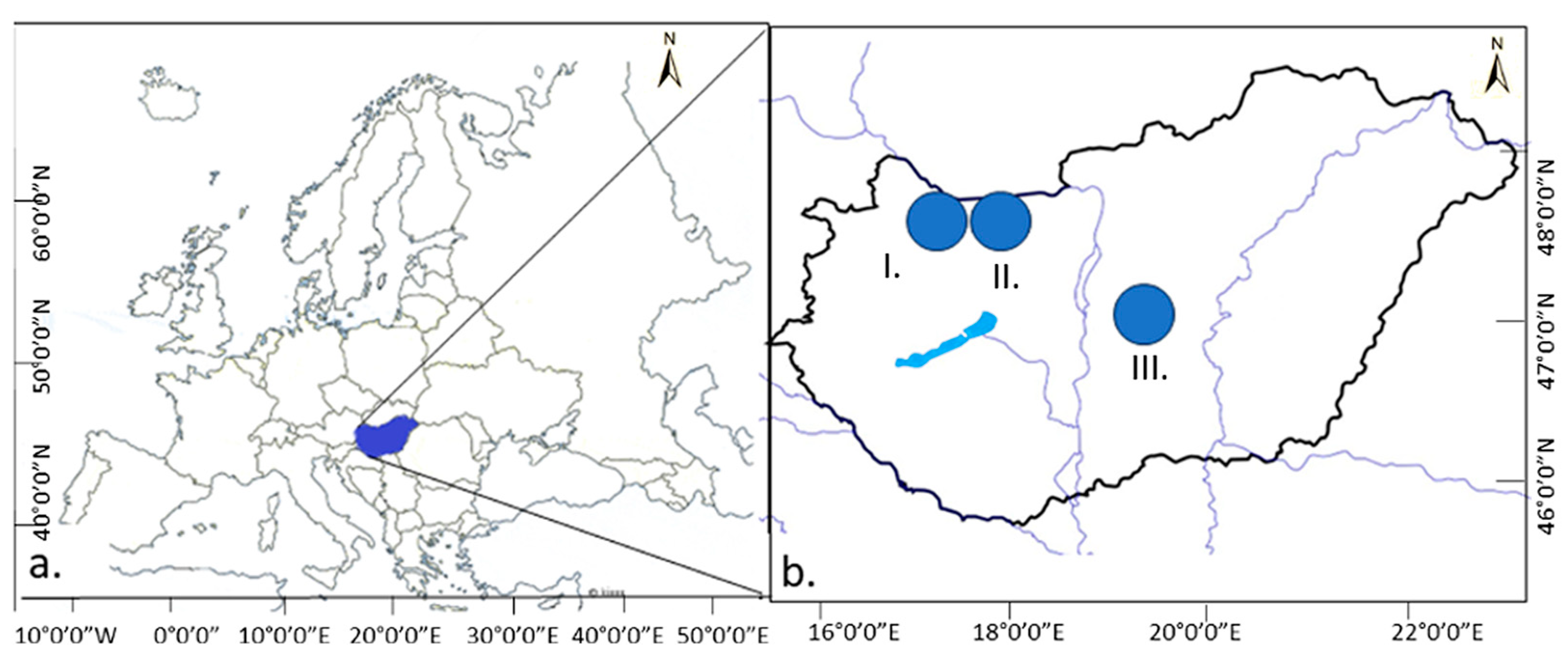
The surveys were carried out in two locations in the Little Hungarian Plain region (I. Military Shooting Range of Gönyű, and II. Military Exercise Area of Győrszentiván) and in one location in the Great Hungarian Plain (III. Tatárszentgyörgy) (Figure 1).
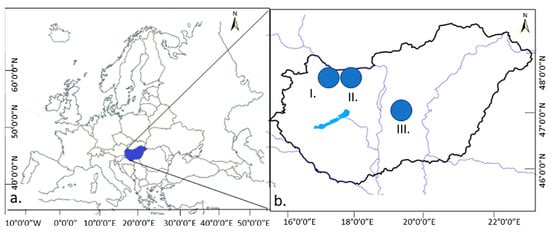
Figure 1.
Sample areas (I.: Little Hungarian Plain Gönyű (above sea level: 103 m), II.: Little Hungarian Plain Győrszentiván (above sea level: 116 m), III.: Great Hungarian Plain Tatárszentgyörgy (above sea level: 95 m)). (a). Europe, (b). Hungary.
The selected study areas are particularly valuable because they belong to the Pannonian endemic grasslands [3].
In the two military areas of the Little Hungarian Plain (Gönyű, localization: 47.733300, 17.833300; Győrszentiván, localization: 47.697863, 17.736483), habitat reconstruction and grassland establishment were carried out using different methods, and military activity is inactive at present. In the area of the Great Hungarian Plain (Tatárszentgyörgy, localization: 47.081500, 19.369669), military activities are still active.
In the Military Shooting Range of Gönyű, three vegetation types were surveyed: an undisturbed vegetation type, a burnt area (which was burnt for 3 years and abandoned 5 years before the time of the survey), and a manually sown grassland.
In the Military Exercise Area in Győrszentiván, a sown grassland patch was established with a dual purpose. One of the aims was to establish a grassland with a high nature conservation value, supplemented with increased grassland management utilization as well.
In the sample areas, the studied vegetation types (associations) were the following with the objectives of the grassland management:
- I.
- Military Shooting Range of Gönyű. Its main purpose was the habitat restoration and establishment of Festucetum vaginatae.
- I.1.
- FvGE: Festucetum vaginatae, an original, natural, calcareous, open sandy grassland, unmanaged.
- I.2.
- FvGEÉ: Festucetum vaginatae, an original, natural, calcareous, open sandy grassland, but burnt for 3 years and abandoned 5 years before this study, unmanaged.
- I.3.
- FvGS: Festucetum vaginatae, a manually sown semi-natural grassland, where an artificial sandy ridge, rampart, was established.
- II.
- Military Exercise Area of Győrszentiván. Its main purpose was to establish a habitat through grassland establishment for economic and nature conservation values.
- II.1.
- FvGYV: Festucetum vaginatae, a sown semi-natural grassland.
- II.2.
- FrGYV: Festucetum rupicolae, a sown semi-natural grassland.
- II.3.
- FrGYI: Festucetum rupicolae, a natural grassland.
- III.
- Military Exercise Area of Tatárszentgyörgy. There is still active military activity (military exercises) here.
- III.1.
- FpT: Festucetum pseudovaginatae, natural or semi-natural grassland.
- III.2.
- FvT: Festucetum vaginatae, natural or semi-natural grassland.
In the areas of the Little Hungarian Plain (Gönyű, Győrszentiván), military activities were terminated in 1989. The restoration of the area was planned in 2006, and then the reclamation of the grasslands started in 2008 and was carried out in two phases, using two 5-year plans. Our survey was performed in 2018, 5 years after the termination of the restoration project. Based on this, the natural sandy grassland (FvGE) was already a natural grassland before the initial works in 2006. In the natural grassland that was burnt for 3 years (2010–2013), the activity had been abandoned for 5 years by 2018. On the other hand, in the two studied sown grassland areas (Gönyű—FvGS, Győrszentiván—FvGYV, FrGYV), the intervention was carried out in 2015, 3 years before the study period. Deforestation was applied in the Festucetum rupicolae natural grassland (FrGYI) and the invasive woody species were also removed in 2015. The only still-active military exercise area of our study is situated in the Great Hungarian Plain (Tatárszentgyörgy).
Among the natural vegetation types in the sample areas, the calcareous open sandy grassland is Festucetum vaginatae Rapaics ex Soó 1929 em. Borhidi 1996, which can be found in all sample areas of the three studied locations. Thus, a comparison of this vegetation type was also conducted, including the effects of the different management practices.
2.2. Field Methodology
In the field surveys, we used 2 × 2 m quadrats in each sample area and in each vegetation type in 2018. During the vegetation period, the modified method of Braun–Blanquet was followed [41], where vegetation cover is given as a percentage. Each sampling quadrat was chosen randomly in typical patches of the studied vegetation type. In each patch, 6–10 coenological surveys were carried out in each vegetation period and repeated at the same sample location. All occurring vascular plant species were noted down and their cover values were estimated. Finally, the cover values of the species in the three vegetation periods (May, June, September) were analyzed together. Species names were provided based on the nomenclature of Király [42].
2.3. Statistical Analysis
To describe the mean and deviation of vegetation cover of the studied areas, we averaged the cover of species in each sample area and then we worked with these data in further steps. For each treatment, we ordered the plant species into different categories (characteristic species in open sandy grassland, characteristic species in closed natural sandy grassland, weeds, disturbance-tolerant species) in order to prepare clear diagrams. The differences in species number between territories were analyzed using one-way ANOVA. We used an agglomerative cluster analysis technique (Ward’s method by PAST) with a fusion algorithm as a combinatorial method (minimizing increase in variance), and the correlation was used as the comparative function. In the analyses, the Kruskal–Wallis test was used for the variables of the one-way analysis of variance (ANOVA). Tukey’s HSD test (post hoc test) was used to perform pair-wise multiple comparisons. The data structure of the coenological surveys was analyzed with detrended correspondence analysis (DCA) ordination using Bray–Curtis Distance Indices to visualize the species composition of the F. vaginata, F. rupicola, and F. pseudovaginata series in the three sites. All statistical analyses were performed using the PAST program [43,44].
3. Results
In order to analyze data easily, we classified the surveyed data into four coenosystematic groups (Table 1) [3]:

Table 1.
The recorded species of the four coenosystematic groups.
- Characteristic species in open sandy grassland (Festucion vaginatae, Corynephoretalia, Festucetalia vaginatae & rupicolae, and Festucetalia vaginatae & valesiacae).
- Characteristic species in closed natural sandy grassland (Brometea, Festuco-Brometea, Festucetalia valesiacae) refer to the natural condition of the grasslands.
The other two groups include the species that indicate disturbance:
- 3.
- Weeds.
- 4.
- Disturbance-tolerant species (Figure 2).
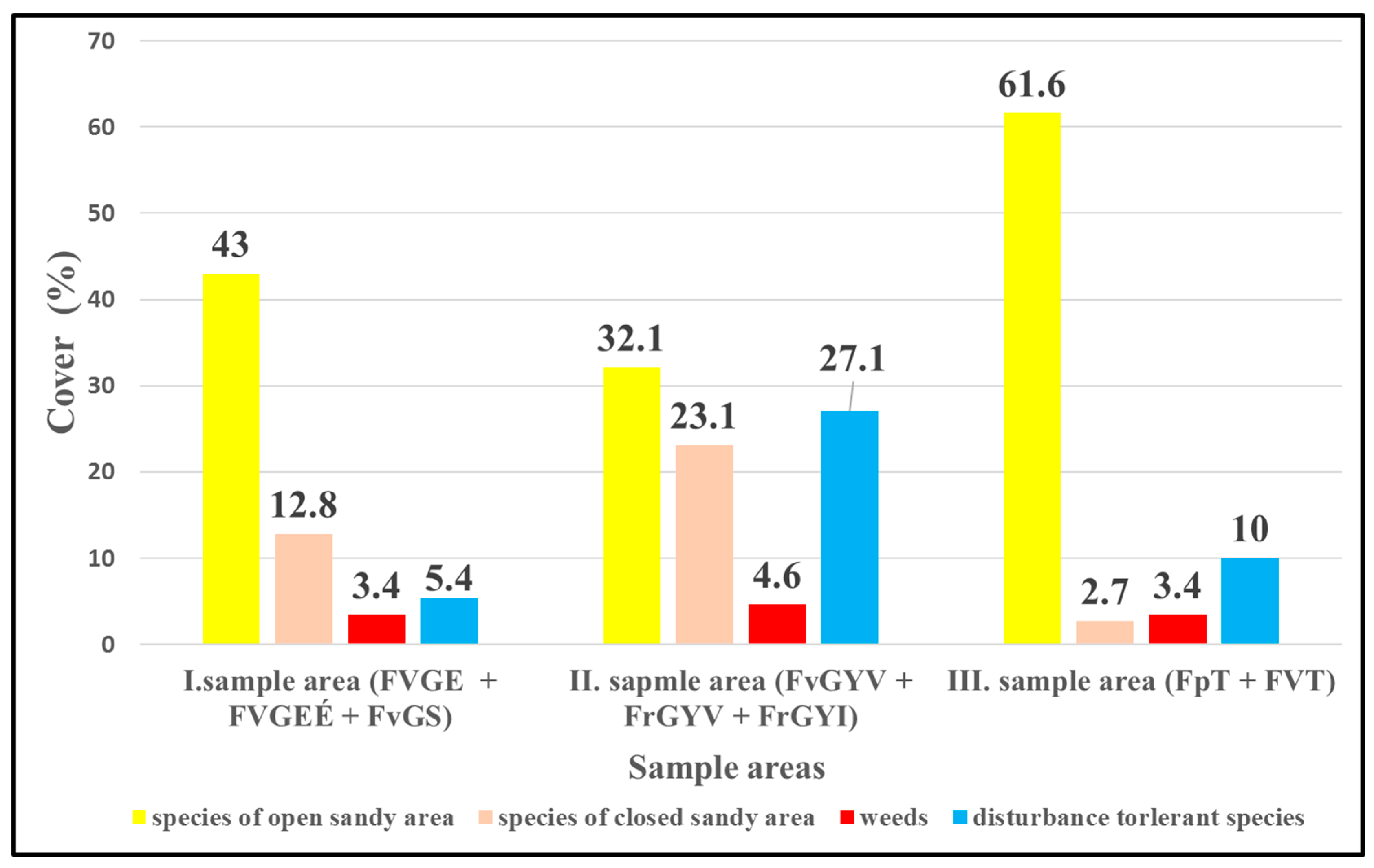
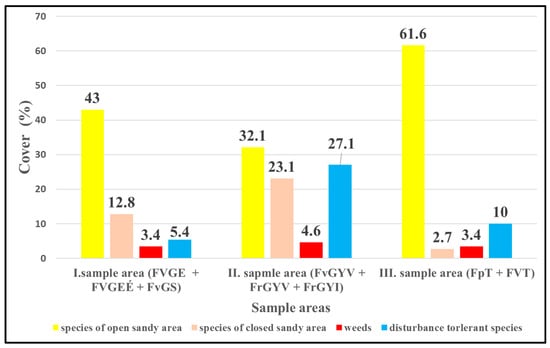 Figure 2. Comparison of the sample areas of the three locations based on the mean cover in % of species belonging to the four vegetation categories.
Figure 2. Comparison of the sample areas of the three locations based on the mean cover in % of species belonging to the four vegetation categories.
Among the study sites, the I. sample area had the lowest mean cover value. Examining all vegetation types of sample areas of the three locations together, the III. sample area, where military activity is still active, belongs mainly to the open grassland category (61.6%). At the same time, characteristic species of open sandy grassland were dominant in the I. and II. sample areas, too. The I. sample area had a quite high mean cover (43%), and it was followed by the II. sample areas with 32.1%. The closed grasslands of I. and II. sample areas were rich in species. The proportion of species of natural closed grassland was highest in the II. sample area (23.1%) but was quite high in the I. sample area (12.8%) as well.
Weeds were found with low cover (3.5–4.6%) in all three sample areas, reaching the highest proportion in the II. sample area (4.6%). The proportion of disturbance-tolerant species was low in the I. and III. sample areas. The highest mean cover value of the disturbance-tolerant species (27.1%) was found in the sown grassland areas of the II. sample area, followed by the III. sample area with around 10% and the recultivated area of the I. sample area (FvGS) with only 5.4%.
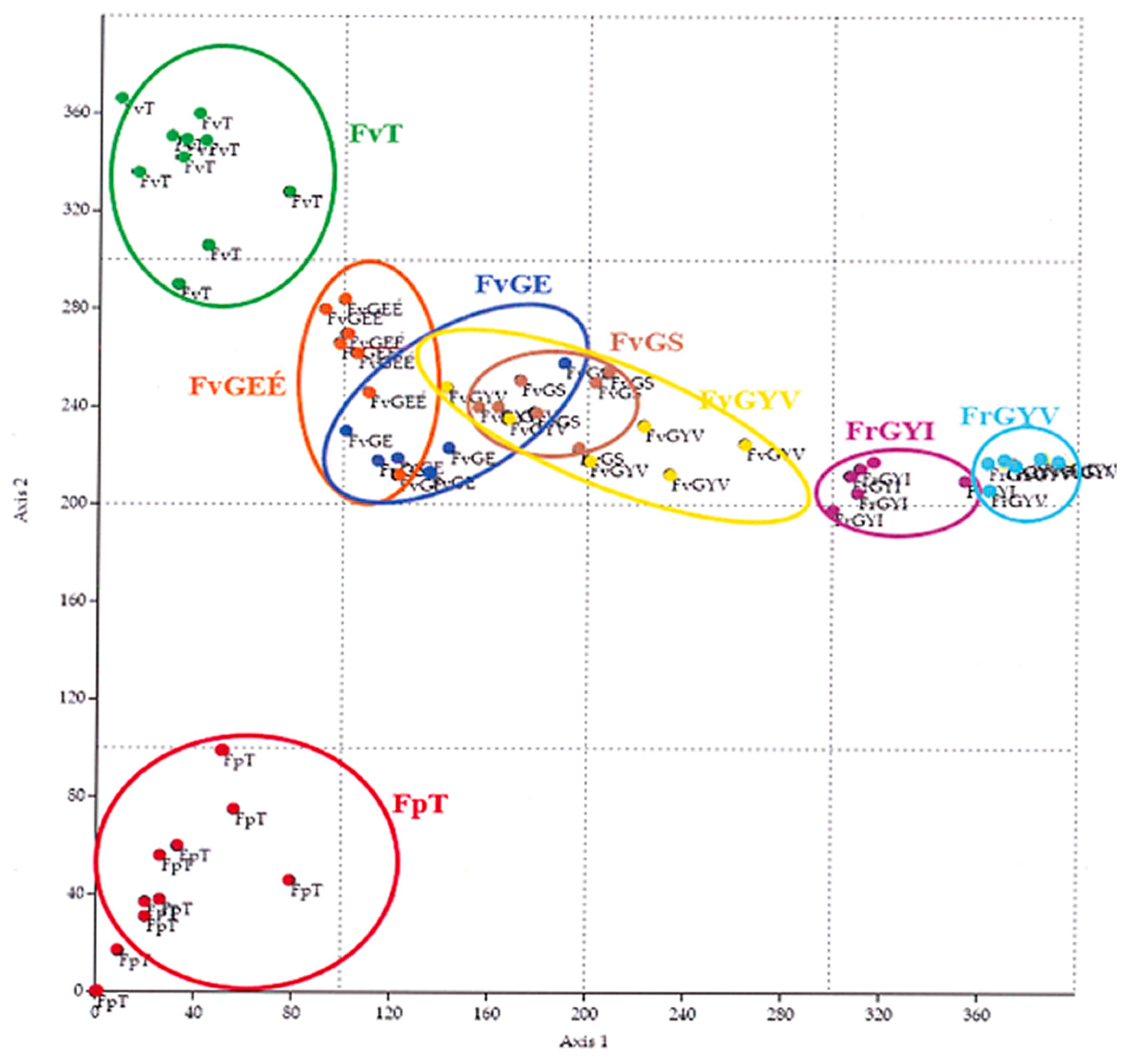
Based on the sample areas and the coenological records, it can be seen from the figure of DCA (Figure 3) that the records of the III. sample area are well distinguished. Among these, the most distinguishable are the records of the Festucetum pseudovaginatae vegetation type of area III (FpT). The records of the Festucetum vaginatae (FvT) of area III. are close to but also distinguishable from the sample areas (I. and II.) of the Little Hungarian Plain. The original sown Festucetum vaginatae (FvGE) and the original but burnt open Festucetum vaginatae (FvGEÉ) grassland records form a complete group, whereas the records of the closed grassland Festucetum rupicolae (FrGYV and FrGYI) are separated significantly in terms of number of species, vegetation cover, and statistical comparison.
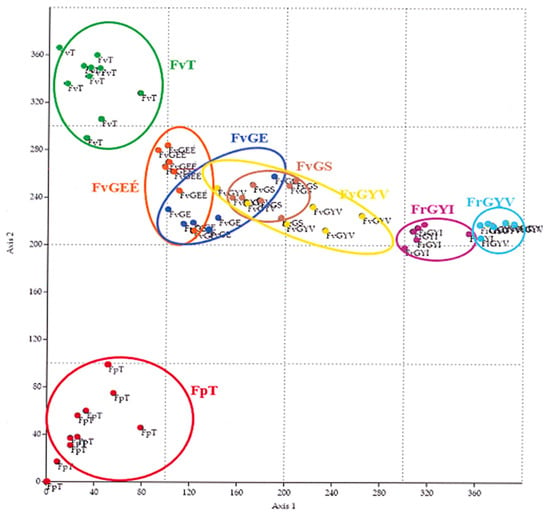
Figure 3.
DCA analysis of the coenological records.
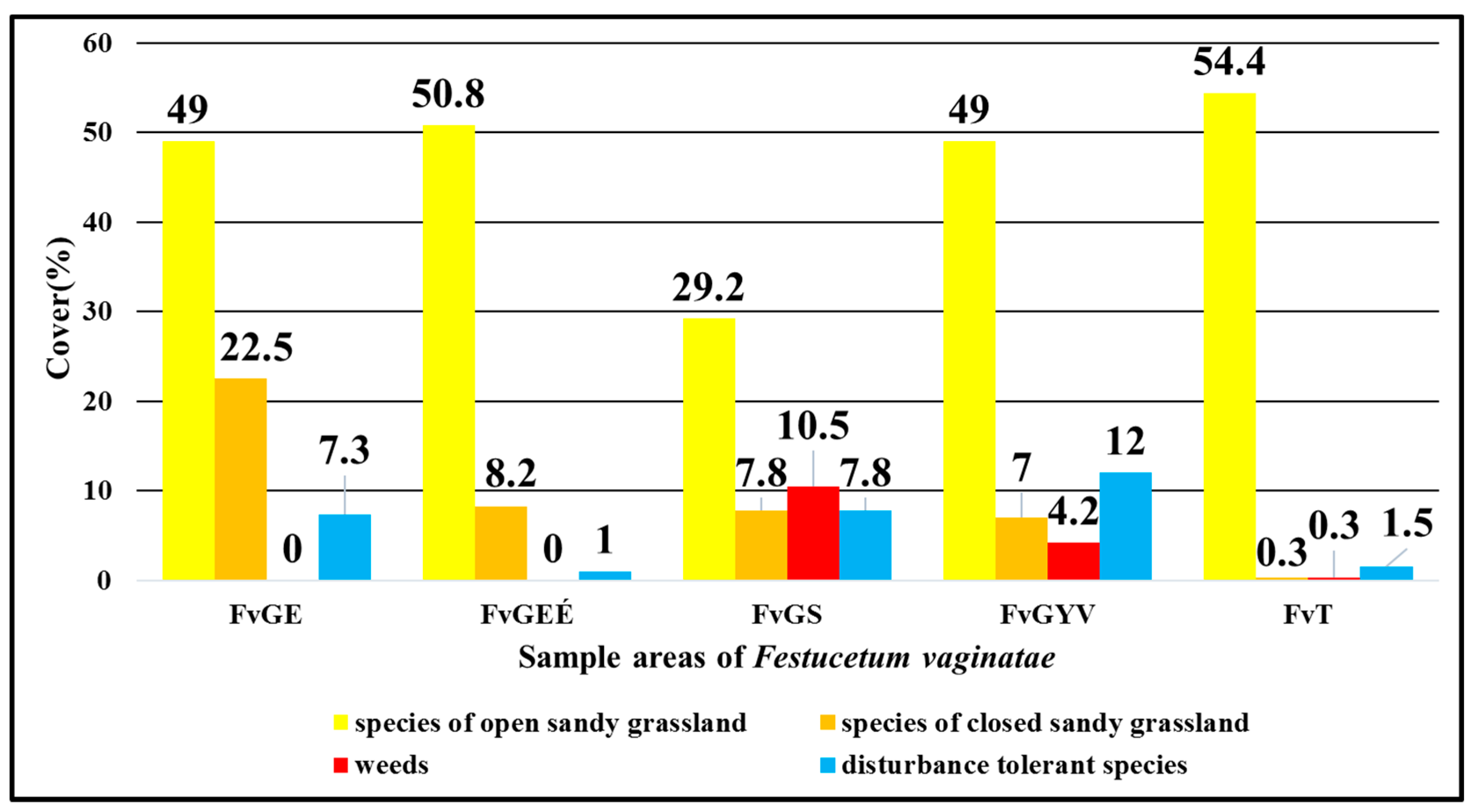
Focusing on the five areas with the vegetation type classification of Festucetum vaginatae association showed that the sown rampart of the I. sample area (FvGS) had the highest proportion of weed species with 10.5% (Figure 4). It was followed by the sown grassland of the II. sample area (FvGYV) with 4.2%. However, only a minimal mean cover value (0.3%) can be seen in the III. of weeds. The proportion of disturbance-tolerant species was high in the sown rampart of the I. sample area (7.8%) (FvGS) and in the original vegetation of the I. sample area (7.3%) (FvGE), but the highest mean cover value (12%) was in the sown grassland of the II. sample area (FvGYV). The number of species that belonged to the open grassland category was generally high, around 50%, except for the vegetation type of the sown rampart of the I. sample area (29.2%) (FvGS), and it was highest in the III. sample area, where military activities are still active (54.4%). In terms of species of closed grassland, the richest were the original vegetation of the I. sample area (22.5%). It was followed by the burnt grassland of the I. sample area with 8.2% (FvGEÉ), and then the sown rampart of the I. sample area (FvGS) with 7.8%. The sown grassland of the II. sample area (FvGYV) had a lower cover value in terms of the characteristic species of closed grassland (7%), but the III. sample area (FvT) had almost no species that are characteristic of closed grasslands (0.3%).
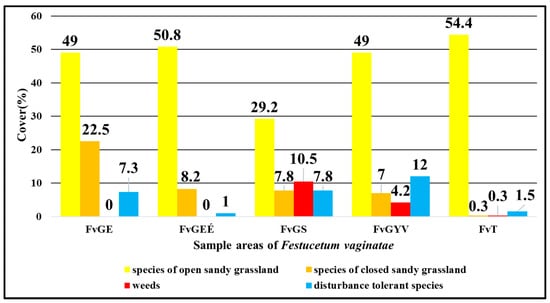
Figure 4.
Comparison of Festucetum vaginatae vegetation types in the three sample areas based on the mean cover in % of the species belonging to the four vegetation categories.
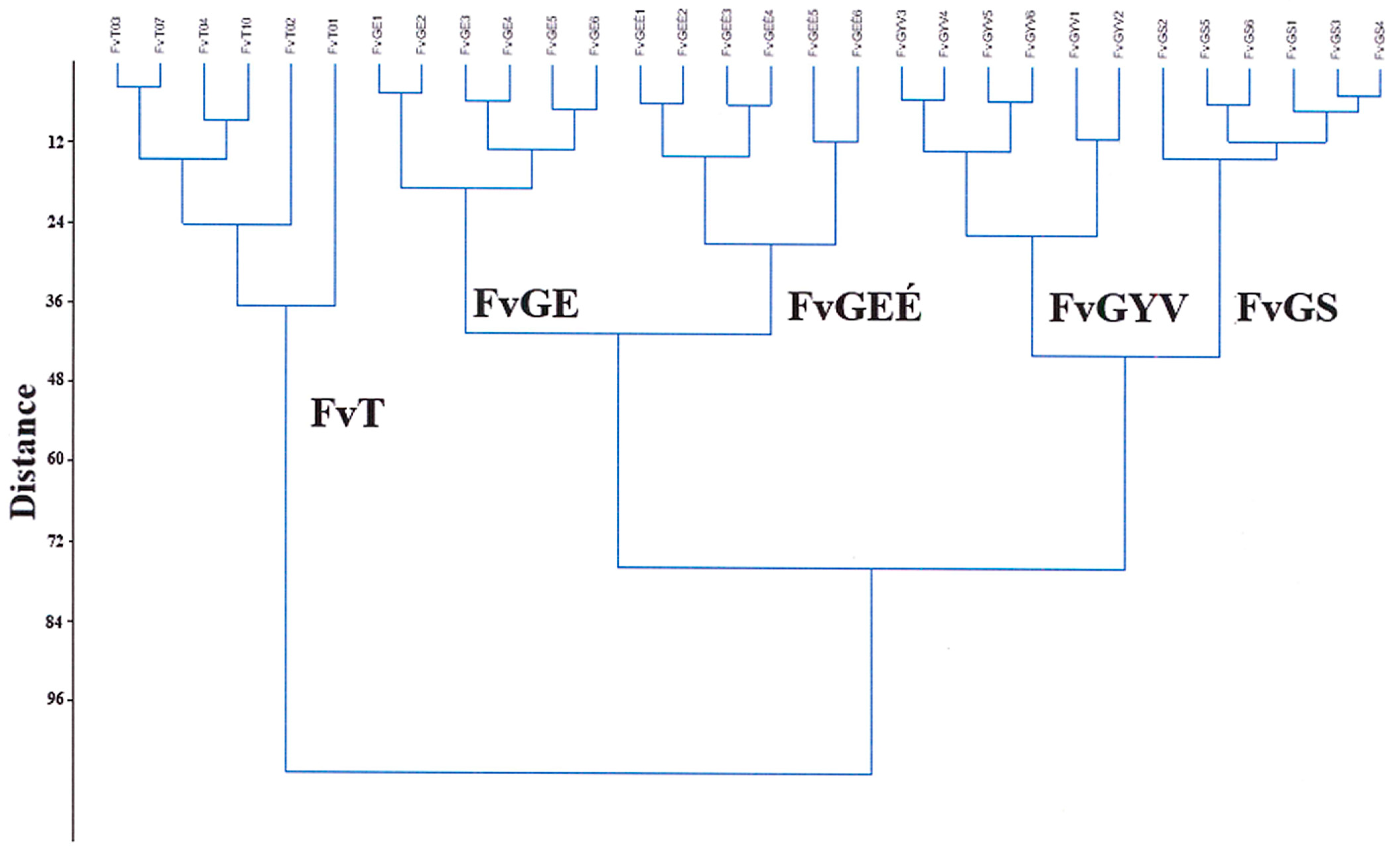
Based on the classification analysis of the Festucetum vaginatae vegetation types (Figure 5), the groups of records that belonged to each sample area were organized into individual clades. The clade of the Great Hungarian Plain area in Tatárszentgyörgy (FvT) was distinctly separated from the other studied locations. Based on the clade, the two sown grasslands (FvGYS and FvGI) were close to each other, and the two areas of the Little Hungarian Plain (Gönyű, Györszentiván) were not classified by geographical separation, but by management. The lowest difference between the quadrats was shown by the sown rampart of area 1 in Gönyű (FvGS).
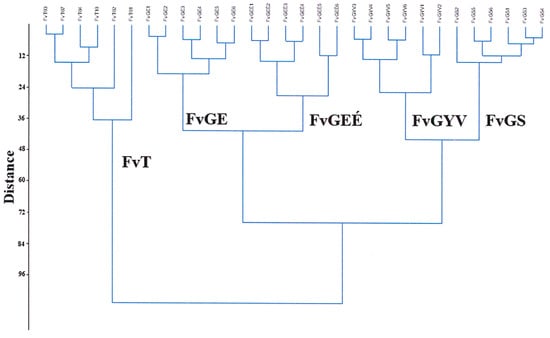
Figure 5.
Hierarchical classification of the records of the vegetation type Festucetum vaginatae in the three sample areas.
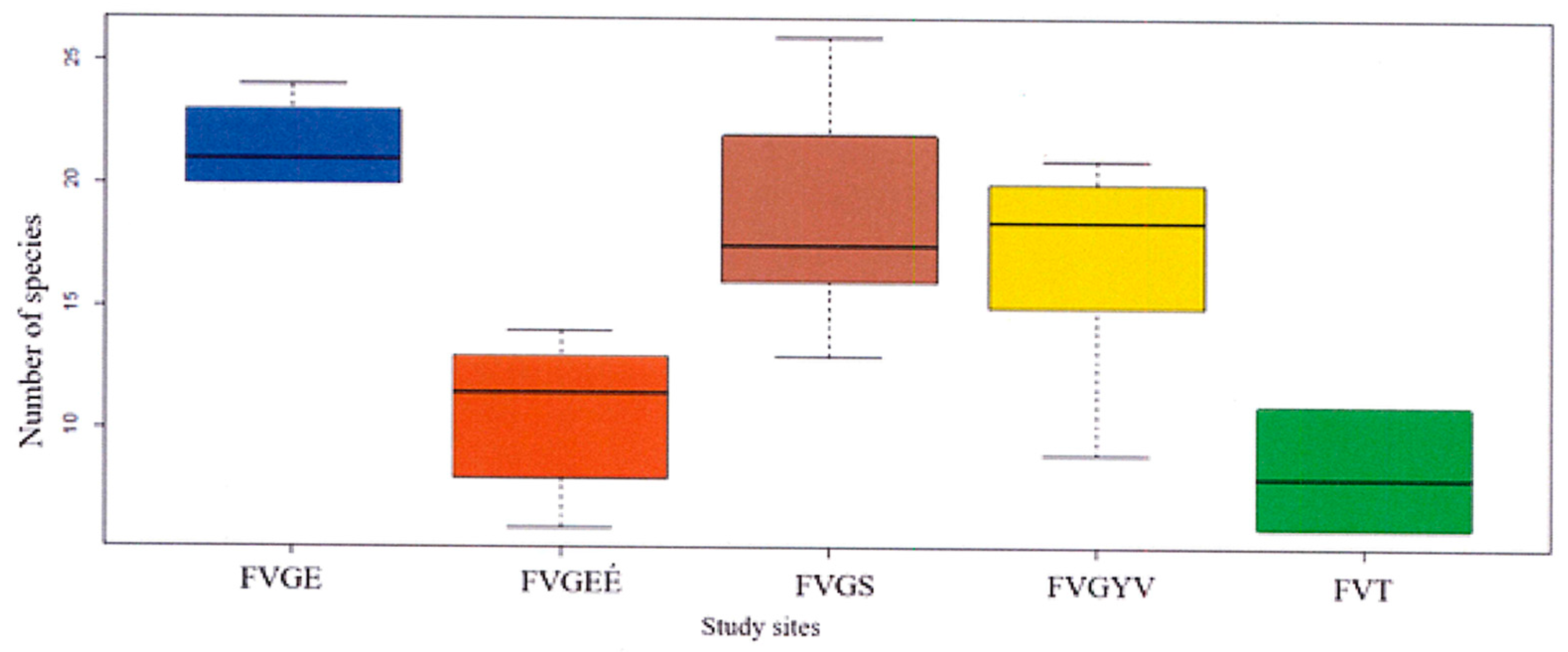
According to the recorded data of the Festucetum vaginatae vegetation type, the vegetation of Tatárszentgyörgy (III. sample area) was distinguishable from the other sample areas (Figure 2, Figure 3 and Figure 4). The highest average number of species was found among the records of natural grassland at 23. The average number of species in the burnt area (FVGEÉ) was significantly lower at 12, and the average number of species of the III. sample area in Tatárszentgyörgy (FvT) was the lowest at 7, whereas the number of species in the manually sown rampart (FvGS) in Gönyű was 22. Similarly, the number of species in the sown grassland (FvGYV) of the II. sample area in Győrszentiván was lower at 15 (Figure 6).
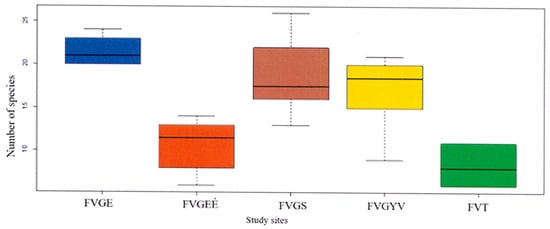
Figure 6.
Number of species of the surveyed vegetation types.
4. Discussion
Among the three sample areas, the I. sample area had the lowest mean cover values in the vegetation, reflecting the fact that among the studied vegetation types, the manually sown areas with low species cover reduce the value of total cover of the area. In the Little Hungarian Plain, the abundance of species in closed grasslands is higher than the species richness in the sample area of the Great Hungarian Plain. This can be the result of the specific species composition of closed grasslands (Poa angustifolia, Festuca rubra, Festuca arundinacea) that were used for grassland sowing [3,45]. In addition, there is also a climatic context, because the grasslands of the Little Hungarian Plain have more mesophilic species and vegetation types, indicating Atlantic environmental conditions [1]. The reason is that the climate of the Little Hungarian Plain within the Pannonian Basin is cooler than in the Great Hungarian Plain [46].
Among the studied grasslands, the sown rampart of the I. sample area (FvGS) and the sown grassland of the II. sample area (FvGYV) are very similar to the study of sandy fallows, where the amount of target species (plant species of open sandy vegetation) is greater in older fallows than in younger fallows. Moreover, the target species that are typical in open sandy grasslands (Festucion vaginatae, Corynephoretalia, Festucetalia vaginatae & rupicolae, and Festucetalia vaginatae & valesiacae) include most of the species at the start of succession. On the contrary, in our study target species were already documented at the beginning of the restoration period [9]. The results of the study by Rehounková and Prach [16] are similar to the results of other studies on grasslands [9,22,47]. These studies suggest that the appearance of open sandy grassland species in the sandy grassland is probably a propagule-limited process, which can be influenced by the species composition of adjacent grasslands. Therefore, characteristic species could appear rapidly in our sample areas.
During the military activity, significant natural propagule sources and patches of native grassland survived from where species could grow again easily and rapidly. The fallows which do not have suitable propagule sources can provide target species that may grow very slowly. Furthermore, it can impede the growing of target species by microhabitat limitation due to the growth of perennial biomass [33,36]. Our present studies rejected the possibility of microhabitat limitation, because both fires and active military activity resulted in low numbers of species, but the occurring species were composed of characteristic plant species of open sandy grassland vegetation.
The dominant grass species (Festuca, Stipa) have made the territorial conquest. Their short generation time and faster adaptability are confirmed in the present study. Hence, they may become dominant at the start of succession, where they occupy mainly open, disturbed areas first [48]. This is due to species with a short life stage responding quickly to changes and disturbances, reacting much faster than perennial species. This result refers to natural annual species and pioneer species. The present study also confirms the results that during succession, all of these species with short life stages are replaced over time by perennials [49] due to their better competitive ability [45,47].
Looking at the species numbers, the effects of the current active military activity would indicate a decline in the vegetation. However, based on the data and the analysis of the vegetation composition, the conserved open sandy grassland in the Great Hungarian Plain (FvT) shows natural conditions. It provides the occurrence of pioneer species of the open surface. In addition, it supports the appearance and maintenance of an endemic association of Festucetum pseudovaginatae [50], which is unique to the Great Hungarian Plain. It contains high proportions of species of open sandy grasslands, so it is achieved to conserve the naturalness of the vegetation, although not as an intended result of the military activity.
The investigation of species that occur in critical habitats in extreme conditions is also important in the context of climate change as a major vulnerability to species extinction [51,52]. The rise in global temperature may lead to various risks [51,53], and the best prediction for the near-future is 1.5 °C (2021–2040) [54]. The climatic factors significantly influence the composition of vegetation, which reflects global climate change [55]. The vegetation of extreme arid habitats is important in terms of climate change because these habitat types will probably expand, and their conservation is an international priority [56]. In the investigated habitat type, mechanical factors also affect the vegetation [57,58], degrading the area, but nevertheless, based on the investigations, the vegetation of the III. sample area tolerated the active military activities well. The role of the dominant species in the open sandy grasslands (Festuca vaginata and the endemic Festuca pseudovaginata) might be enhanced by this condition, as endemic species can adapt better to climate change [59,60,61,62,63]. Based on our study, we were able to confirm that these endemic, extreme-drought-tolerant, perennial Festuca species and the studied vegetation types were well adapted to extreme drought and tolerated the extreme human disturbance, with changes in soil and climate in the micro-conditions.
In the year of our study, after the 5 years of restoration works were finished, the vegetation in the two sample areas (I. and II. sample areas) in the recultivated and sown Little Hungarian Plain became more natural. This is due to the propagule sources near the study areas, from where the natural grassland could spread and grow. So, overall, to answer the question, the military activity indirectly supported the regeneration of the open sandy grassland vegetation of the studied area. In summary, active military activity resulted in the conservation of open grassland conditions, which might help the growth of the relatively low number of species of the potential, native, rare, and endemic open sandy grasslands of the Pannonian Basin.
5. Conclusions
Despite the previous hypothesis that military activity can cause only vegetation degradation, based on the results of our research, the activities in military areas can beneficially affect the dry, sandy grassland habitats. The military exercise areas in the open sandy grasslands of the Pannonian region played a significant role in the vegetation survival, as these areas were isolated from potentially damaging land uses that often cause their certain disappearance. Despite their relative undisturbedness, these areas may be threatened by numerous factors (invasive species, weeding, off-road motorcycling, etc.) that could destroy their remnant natural values over a few decades.
Long term surveys and well-established nature conservation management plans of the subjected areas can help to restore and maintain these valuable, endemic vegetation types. Cooperation between nature conservation and national defense agencies can define the requirements for military land use in the future and set objectives for nature conservation in the affected areas. Despite the different practices applied, all management methods were suitable for conserving and restoring the natural condition of the studied grasslands. The results of the coenological records will provide a useful part of long-term monitoring databases.
Moreover, the present work indicates that these types of grasslands in different regions around the world will tackle the challenges related to food supply requirements due to the effect of global warming that will significantly influence the production capacity of grasslands. Thus, the present results on grasslands’ vegetation components and species ratios can be subject to caveats. Hence, based on the results and conclusions, the sample areas are affected by several dependent factors. With all of the aspects considered carefully, these data are suitable to support the development of nature conservation practices in the dry sandy grasslands of Hungary in the future.
Author Contributions
Conceptualization, K.P., M.B. and J.H.; methodology, K.P. and S.S.; software, P.P., C.M. and J.H.; formal analysis, Z.W. and F.S.; investigation, K.P., A.F., E.S.-F., S.S., M.F., D.B. and J.H.; writing—original draft preparation, M.B., A.F., E.S.-F., K.P., J.H. and P.C.; writing—review and editing, M.B., A.F., K.P., P.C. and J.H.; supervision, Z.W. and K.P.; funding acquisition, K.P. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by OTKA K-125423 and OTKA K-147342.
Data Availability Statement
Data are contained within the article..
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bischoff, A.; Auge, H.; Mahn, E.G. Seasonal changes in the relationship between plant species richness and community biomass in early succession. Basic Appl. Ecol. 2005, 6, 385–394. [Google Scholar] [CrossRef]
- Guo, Q. The diversity–biomass–productivity relationships in grassland management and restoration. Basic Appl. Ecol. 2007, 8, 199–208. [Google Scholar] [CrossRef]
- Penksza, K.; Saláta, D.; Pápay, G.; Péter, N.; Bajor, Z.; Lisztes-Szabó, Z.; Fűrész, A.; Fuchs, M.; Michéli, E. Do Sandy Grasslands along the Danube in the Carpathian Basin Preserve the Memory of Forest-Steppes? Forests 2021, 12, 114. [Google Scholar] [CrossRef]
- Albert, Á.J.; Kelemen, A.; Valkó, O.; Miglécz, T.; Csecserits, A.; Rédei, T.; Deák, B.; Tóthmérész, B.; Török, P. Secondary succession in sandy old-fields: A promising example of spontaneous grassland recovery. Appl. Veg. Sci. 2014, 17, 214–224. [Google Scholar] [CrossRef]
- Bajor, Z.; Zimmermann, Z.; Szabó, G.; Fehér, Z.; Járdi, I.; Lampert, R.; Kerény-Nagy, V.; Penksza, P.; Lisztes-Szabó, Z.; Székely, Z.; et al. Effect of conservation management practices on sand grassland vegetation in Budapest, Hungary. Appl. Ecol. Environ. Res. 2016, 14, 233–247. [Google Scholar] [CrossRef]
- Catorci, A.; Piermarteri, K.; Penksza, K.; Házi, J.; Tardella, F.M. Filtering effect of temporal niche fluctuation and amplitude of environmental variations on the trait-related flowering patterns: Lesson from sub-Mediterranean grasslands. Sci. Rep. 2017, 7, 12034. [Google Scholar] [CrossRef] [PubMed]
- Clevell, A.F. Restoring for natural authenticity. Ecol. Restor. 2000, 18, 216–217. [Google Scholar] [CrossRef][Green Version]
- Cornwell, W.K.; Grubb, P.J. Regional and local patterns in plant species richness with respect to resource availability. Oikos 2003, 100, 417–428. [Google Scholar] [CrossRef]
- Coulson, T.; Mace, G.M.; Hudson, E.; Possingham, H. The use and abuse of population viability analysis. Trends Ecol. Evol. 2001, 16, 219–221. [Google Scholar] [CrossRef]
- Critchley, C.N.R.; Burke, M.J.W.; Stevens, D.P. Conservation of lowland semi-natural grasslands in the UK: A review of botanical monitoring results from agri-environment schemes. Biol. Conserv. 2004, 115, 263–278. [Google Scholar] [CrossRef]
- Csecserits, A.; Czúcz, B.; Halassy, M.; Kröel-Dulay, G.; Rédei, T.; Szabó, K.; Szitár, R.; Török, K. Regeneration of sandy old-fields in the forest steppe region of Hungary. Plant Biosyst. 2011, 145, 715–729. [Google Scholar] [CrossRef]
- Deák, B.; Valkó, O.; Kelemen, A.; Török, P.; Miglécz, T.; Ölvedi, T.; Lengyel, S.; Tóthmérész, B. Litter and graminoid biomass accumulation suppresses weedy forbs in grassland restoration. Plant Biosyst. 2011, 145, 730–737. [Google Scholar] [CrossRef]
- Dolt, C.; Goverde, M.; Baur, B. Effects of experimental small-scale habitat fragmentation on above- and below-ground plant biomass in calcareous grasslands. Acta Oecologica 2005, 27, 49–56. [Google Scholar] [CrossRef]
- Gillman, L.N.; Wright, S.D. The influence of productivity on the species richness of plants. a critical assessment. Ecology 2006, 87, 234–1243. [Google Scholar] [CrossRef]
- Mittelbach, G.G.; Steiner, C.F.; Scheiner, S.M.; Gross, K.L.; Reynolds, H.L.; Waide, R.B.; Willig, M.R.; Dodson, S.I.; Gough, L. What is the observed relationship between species richness and productivity? Ecology 2001, 82, 2381–2396. [Google Scholar] [CrossRef]
- Rehounková, K.; Prach, K. Spontaneous vegetation succession in disused gravel-sand pits: Role of local site and landscape factors. J. Veg. Sci. 2006, 17, 583–590. [Google Scholar] [CrossRef]
- Schaffers, A.P. Soil, biomass, and management of semi-natural vegetation—Part II. Factors controlling species diversity. Plant Ecol. 2002, 158, 247–268. [Google Scholar] [CrossRef]
- Valkó, O.; Deák, B.; Török, P.; Kirmer, A.; Tischew, S.; Kelemen, A.; Tóth, K.; Miglécz, T.; Radócz, S.; Sonkoly, J.; et al. High-diversity sowing in establishment gaps: A promising new tool for enhancing grassland biodiversity. Tuexenia 2016, 36, 359–378. [Google Scholar] [CrossRef]
- Hölzel, N.; Otte, A. Restoration of a species-rich flood meadow by topsoil removal and diaspore transfer with plant material. Appl. Veg. Sci. 2003, 6, 131–140. [Google Scholar] [CrossRef]
- Jongepierová, I.; Mitchley, J.; Tzanopoulos, J. A field experiment to recreate species rich hay meadows using regional seed mixtures. Biol. Conserv. 2007, 139, 297–305. [Google Scholar] [CrossRef]
- Lepŝ, J.; Doleẑal, J.; Bezemer, T.M.; Brown, V.K.; Hedlund, K.; Igual Arroyo, M.; Jörgensen, H.B.; Lawson, C.S.; Mortimer, S.R.; Peix Geldart, A.; et al. Long-term effectiveness of sowing high and low diversity seed mixtures to enhance plant community development on ex-arable fields. Appl. Veg. Sci. 2007, 10, 97–110. [Google Scholar] [CrossRef]
- Török, P.; Miglécz, T.; Valkó, O.; Kelemen, A.; Deák, B.; Lengyel, S.; Tóthmérész, B. Recovery of native grass biodiversity by sowing on former croplands: Is weed suppression a feasible goal for grassland restoration? J. Nat. Conserv. 2012, 20, 41–48. [Google Scholar] [CrossRef]
- Mijnsbrugge, V.K.; Bischoff, A.; Smith, B. A question of origin: Where and how to collect seed for ecological restoration. Basic Appl. Ecol. 2010, 11, 300–311. [Google Scholar] [CrossRef]
- Pywell, R.F.; Bullock, J.M.; Hopkins, A.; Walker, K.J.; Sparks, T.H.; Burke, M.J.W.; Peel, S. Restoration of species, rich grassland on arable land: Assessing the limiting processes using a multi, site experiment. J. Appl. Ecol. 2002, 39, 294–309. [Google Scholar] [CrossRef]
- Rasran, l.; Vogt, K.; Jensen, K. Seed content and conservation evaluation of hay material of fen grasslands. J. Nat. Conserv. 2006, 14, 34–45. [Google Scholar] [CrossRef]
- Omidi, M.; Heydari, M.; Abedi, M.; Kohzadean, M.; Valkó, O.; Prévosto, B. Evaluating the restoration potential of soil seed banks in degraded semi-arid oak forests: Influence of canopy cover types and fire-related cues on seed germination. Forest Ecol. Manag. 2022, 524, 120534. [Google Scholar] [CrossRef]
- Valkó, O.; Kelemen, A.; Miglécz, T.; Török, P.; Deák, B.; Tóth, K.; Tóth, J.P.; Tóthmérész, B. Litter removal does not compensate detrimental fire effects on biodiversity in regularly burned semi-natural grasslands. Sci. Total Environ. 2018, 622–623, 783–789. [Google Scholar] [CrossRef]
- Centeri, C.; Herczeg, E.; Vona, M.; Balázs, K.; Penksza, K. The effects of land-use change on plant-soil-erosion relations, Nyereg Hill, Hungary. J. Plant Nutr. Soil Sci. 2009, 172, 586–592. [Google Scholar] [CrossRef]
- Deák, B.; Valkó, O.; Török, P.; Végvári, Z.; Hartel, T.; Schmotzer, A.; Kapocsi, I.; Tóthmérész, B. Grassland fires in Hungary—Experiences of nature conservationists on the effects of fire on biodiversity. Appl. Ecol. Environ. Res. 2014, 12, 267–283. [Google Scholar] [CrossRef]
- Broomandi, P.; Guney, M.; Kim, J.R.; Karaca, F. Soil Contamination in Areas Impacted by Military Activities: A Critical Review. Sustainability 2020, 12, 9002. [Google Scholar] [CrossRef]
- Stadler, T.; Temesi, Á.; Lakner, Z. Soil Chemical Pollution and Military Actions: A Bibliometric Analysis. Sustainability 2022, 14, 7138. [Google Scholar] [CrossRef]
- Warren, S.D.; Holbrook, S.W.; Dale, D.A.; Whelan, N.L.; Elyn, M.; Grimm, W.; Jentsch, A. Biodiversity and the Heterogeneous Disturbance Regime on Military Training Lands. Restor. Ecol. 2007, 15, 606–612. [Google Scholar] [CrossRef]
- Whitecotton, R.C.A.; David, M.B.; Darmody, R.G.; Price, D.L. Impact of Foot Traffic from Military Training on Soil and Vegetation Properties. Environ. Manag. 2000, 26, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Milchunas, D.G.; Schulz, K.A.; Shaw, R.B. Plant Community Structure in Relation to Long-Term Disturbance by Mechanized Military Maneuvers in a Semiarid Region. Environ. Manag. 2000, 25, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Cizek, O.; Vrba, P.; Benes, J.; Hrazsky, Z.; Koptik, J.; Kucera, T.; Marhoul, P.; Zamecnik, J.; Konvicka, M. Conservation Potential of Abandoned Military Areas Matches That of Established Reserves: Plants and Butterflies in the Czech Republic. PLoS ONE 2013, 8, e53124. [Google Scholar] [CrossRef]
- Ellwanger, G.; Reiter, K. Nature conservation on decommissioned military training areas—German approaches and experiences. J. Nat. Conserv. 2019, 49, 1–8. [Google Scholar] [CrossRef]
- Krawczyk, R.; Zubel, R.; Komsta, Ł. Military Training Areas and Vegetation—The Effect of Explosion Craters on Species Diversity along a Moisture Gradient. Pol. J. Ecol. 2019, 67, 194–205. [Google Scholar] [CrossRef]
- French, K.; Pellow, B.; Henderson, M. Vegetation of the Holsworthy Military Area. Cunninghamia 2001, 6, 893–940. [Google Scholar]
- Douglas, P.P.; Shaw, R.B. Rediscovery of Tetramolopium arenarium Subsp. arenarium var. arenarium (Asteraceae: Astereae) on the Pohakuloa Training Area, Hawaii. Ann. Mo. Bot. Gard. 1989, 76, 1182–1185. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie; Wien: New York, NY, USA, 1964; pp. 2–865. [Google Scholar] [CrossRef]
- Király, G. (Ed.) Új Magyar Füvészkönyv. Magyarország Hajtásos Növényei. Határozókulcsok [New Hungarian Herbal. The Vascular Plants of Hungary. Identification Key]; ANP Igazgatóság: Jósvafő, Hungary, 2009; pp. 3–456.
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 20 February 2023).
- Addinsoft XLSTAT 2016: Data Analysis and Statistical Solution for Microsoft Excel. Long Island, NY, USA. Available online: https://www.xlstat.com/fr/ (accessed on 5 March 2023).
- Prach, K.; Pyšek, P.; Šmilauer, P. Changes in Species Traits during Succession: A Search for Pattern. Oikos 1997, 79, 201–205. [Google Scholar] [CrossRef]
- Fábián, Á.P.; Matyasovszky, I. Analysis of climate change in Hungary according to an extended Köppen classification system, 1971–2060. Időjárás 2010, 114, 251–263. [Google Scholar]
- Szentes, S.; Sutyinszki, Z.; Kiss, T.; Fűrész, A.; Saláta, D.; Harkányiné Székely, Z.; Penksza, K. Verges as Fragments of Loess Grasslands in the Carpathian Basin and Their Festuca Species. Diversity 2022, 14, 510. [Google Scholar] [CrossRef]
- Süveges, K. Data on the flora of the Danube–Tisza Interfluve (Hungary). Bot. Commun. 2023, 110, 111–154. [Google Scholar] [CrossRef]
- Latzel, V.; Klimešová, J.; Doležal, J.; Pyšek, P.; Tackenberg, O.; Prach, K. The Association of Dispersal and Persistence Traits of Plants with Different Stages of Succession in Central European Man-Made Habitats. Folia Geobot. 2011, 46, 289–302. [Google Scholar] [CrossRef]
- Penksza, K.; Pápay, G.; Csontos, P. Syntaxonomical analysis of sandy grassland vegetation dominated by Festuca vaginata and F. pseudovaginata in the Pannonian basin. Hacquetia 2021, 20, 217–224. [Google Scholar] [CrossRef]
- Nzei, J.M.; Ngarega, B.K.; Mwanzia, V.M.; Musili, P.M.; Wang, Q.F.; Chen, J.M. The past, current, and future distribution modeling of four water lilies (Nymphaea) in Africa indicates varying suitable habitats and distribution in climate change. Aquat. Bot. 2021, 173, 103416. [Google Scholar] [CrossRef]
- Mkala, E.M.; Mutinda, E.S.; Wanga, V.O.; Oulo, M.A.; Oluoch, W.A.; Nzei, J.; Wasva, E.N.; Odago, W.; Nanjala, C.; Mwachala, G.; et al. Modeling impacts of climate change on the potential distribution of three endemic Aloe species critically endangered in East Africa. Ecol. Inf. 2022, 71, 101765. [Google Scholar] [CrossRef]
- Wan, J.N.; Mbari, N.J.; Wang, S.W.; Liu, B.; Mwangi, B.N.; Rasoarahona, J.R.E.; Xin, H.P.; Zhou, Y.D.; Wang, Q.F. Modeling impacts of climate change on the potential distribution of six endemic baobab species in Madagascar. Plant Divers. 2021, 43, 117–124. [Google Scholar] [CrossRef] [PubMed]
- IPCC Sixth Assessment Report—Climate Change 2023 [EB/OL]. Available online: https://www.ipcc.ch/ (accessed on 26 May 2023).
- Yan, X.; Wang, S.; Duan, Y.; Han, J.; Huang, D.; Zhou, J. Current and future distribution of the deciduous shrub Hydrangea macrophylla in China estimated by MaxEnt. Ecol. Evol. 2021, 11, 16099–16112. [Google Scholar] [CrossRef] [PubMed]
- Wanga, V.O.; Ngarega, B.K.; Oulo, M.A.; Mkala, E.M.; Ngumbau, V.M.; Onjalalaina, G.E.; Odago, W.O.; Nanjala, C.; Ochieng, C.O.; Gichua, M.K.; et al. Projected impacts of climate change on the habitat of Xerophyta species in Africa. Plant Divers. 2024, 46, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Rybansky, M. Determination the ability of military vehicles to override vegetation. J. Terramech 2020, 91, 129–138. [Google Scholar] [CrossRef]
- Gilewitch, D.A.; King, W.C.; Palka, E.J.; Harmon, R.S.; McDonald, E.V.; Doe III, W.W. Characterizing the desert environment for Army operations. In Military Geosciences in the Twenty-First Century: Geological Society of America Reviews in Engineering Geology; Harmon, R.S., Baker, S.E., McDonald, E.V., Eds.; Geological Society of America: Boulder, CO, USA, 2014; Volume 22, pp. 57–68. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, B.; Zhang, M.; Jie, M.; Guo, S.; Wang, Y. Relict Plants Are Better Able to Adapt to Climate Change: Evidence from Desert Shrub Communities. Plants 2023, 12, 4065. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.A.; Fayaz, M.; Qadir, R.; Rafiq, S.; Guleria, K.; Quadir, J.; Wani, T.A.; Kaloo, Z.A. Predicting potential distribution and range dynamics of Aquilegia fragrans under climate change: Insights from ensemble species distribution modelling. Environ. Monit. Assess. 2023, 195, 623. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, S.; Feng, J.; Ge, J.; Wang, T. Free-ranging livestock changes the acoustic properties of summer soundscapes in a Northeast Asian temperate forest. Biol. Conserv. 2023, 282, 110123. [Google Scholar] [CrossRef]
- Fausett, S.R.; Sandjak, A.; Billard, B.; Braendle, C. Higher-order epistasis shapes natural variation in germ stem cell niche activity. Nat. Commun. 2023, 14, 2824. [Google Scholar] [CrossRef]
- Varol, T.; Cetin, M.; Ozel, H.B.; Sevik, H.; Zeren Cetin, I. The Effects of Climate Change Scenarios on Carpinus betulus and Carpinus orientalis in Europe. Water Air Soil. Pollut. 2022, 233, 45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).