Identification of Potential Habitats and Adjustment of Protected Area Boundaries for Large Wild Herbivores in the Yellow-River-Source National Park, China
Abstract
1. Introduction
2. Materials and Methods
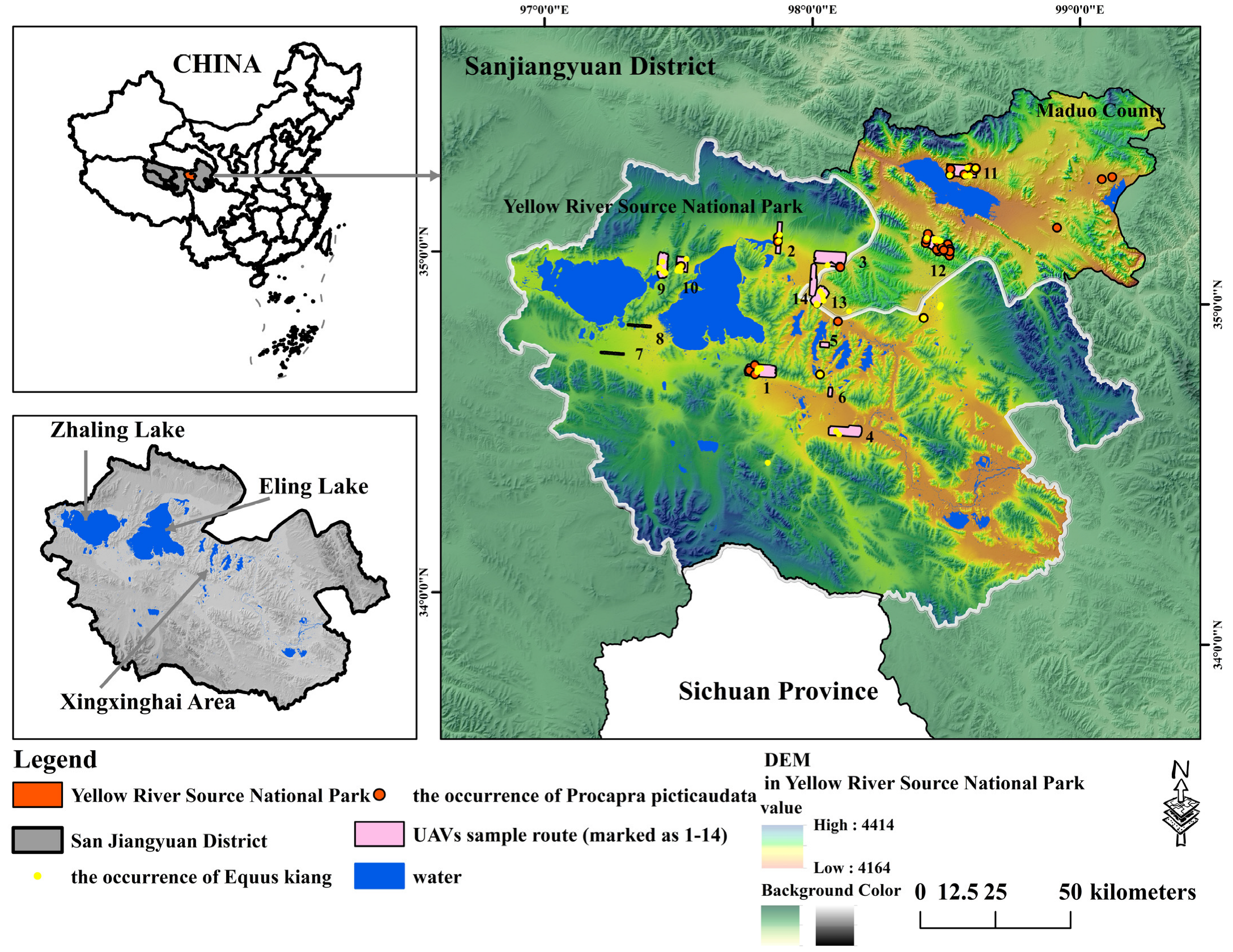
2.1. Study Area and Investigated Species
2.2. The Selection of Species Occurrence Point Data
2.3. Environmental Variables
| Category | Environment Factors | Abbreviation | Source | Resolution |
|---|---|---|---|---|
| Physical Geographical Factors | Digital Elevation Model | DEM [43] | Geospatial data cloud (https://www.gscloud.cn/, accessed on 23 July 2023) | 30 m |
| Slope | SLOPE | Extracted from DEM by arcgis 10.8 | 30 m | |
| Land Use and Land Cover Change | LUCC | China Land Cover Dataset [44] | 30 m | |
| Climatic Factors | Precipitation | PRE | National Earth System Science Data Center (http://www.geodata.cn/, accessed on 23 July 2023) | 1000 m |
| Temperature | TEM | National Earth System Science Data Center (http://www.geodata.cn/, accessed on 23 July 2023) | 1000 m | |
| Food Source Factors | Normalized Differential Vegetation Index | NDVI | National Science & Technology infrastructure (http://www.nesdc.org.cn/, accessed on 23 July 2023) | 30 m |
| Distance to Water | DW | Extracted from LUCC by arcgis 10.8 | 30 m | |
| Human Interference Factors | Human Footprint Index | HFP | A dataset of human footprint over the Qinghai-Tibet Plateau [45] | 1000 m |
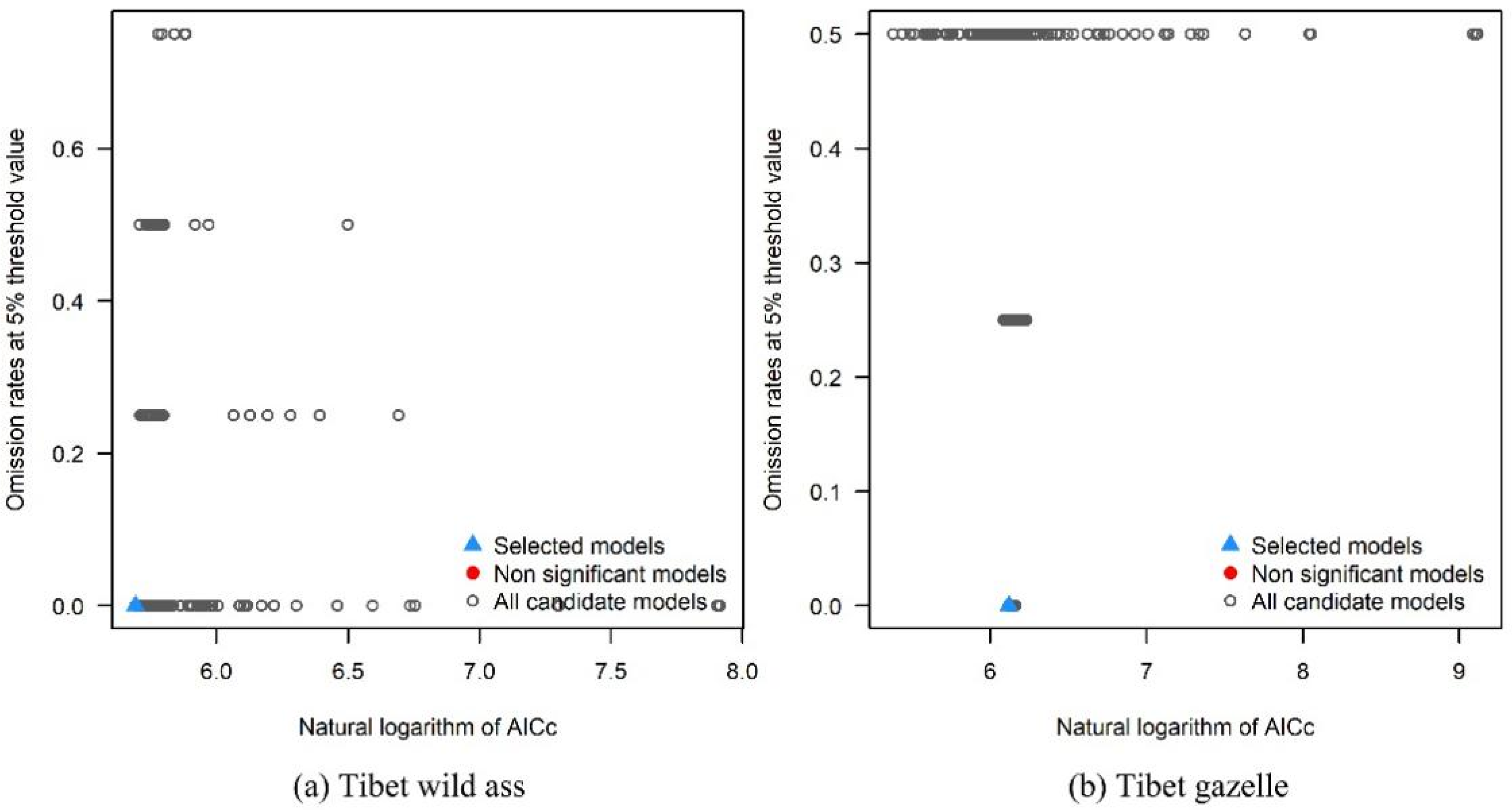
2.4. Parameters Optimization of MaxEnt Model
2.5. The Evaluation of Suitable Habitat in Different Phases of the ECRPSR
2.6. Assessment of Migration Path of Large Wild Herbivores in Different Phases of the ECRPSR
3. Results
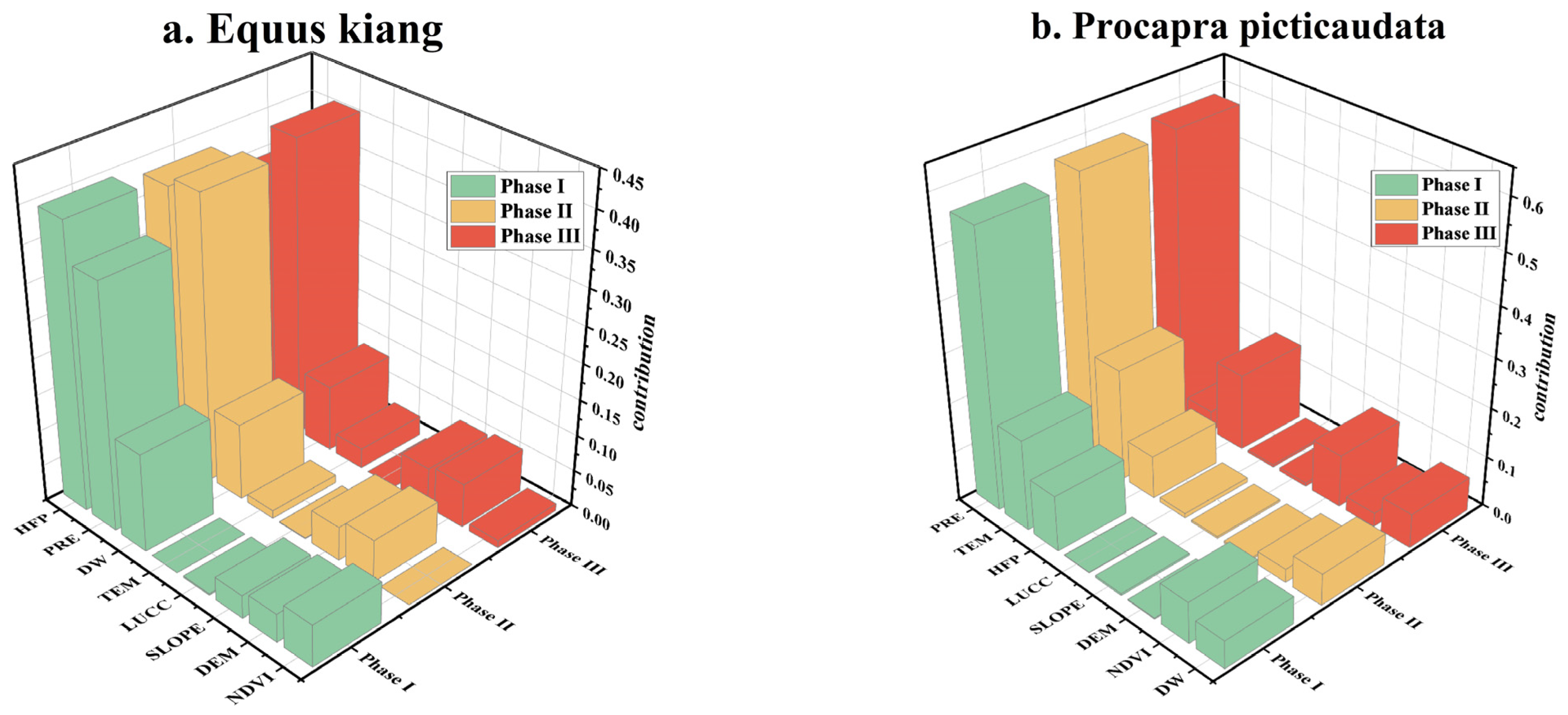
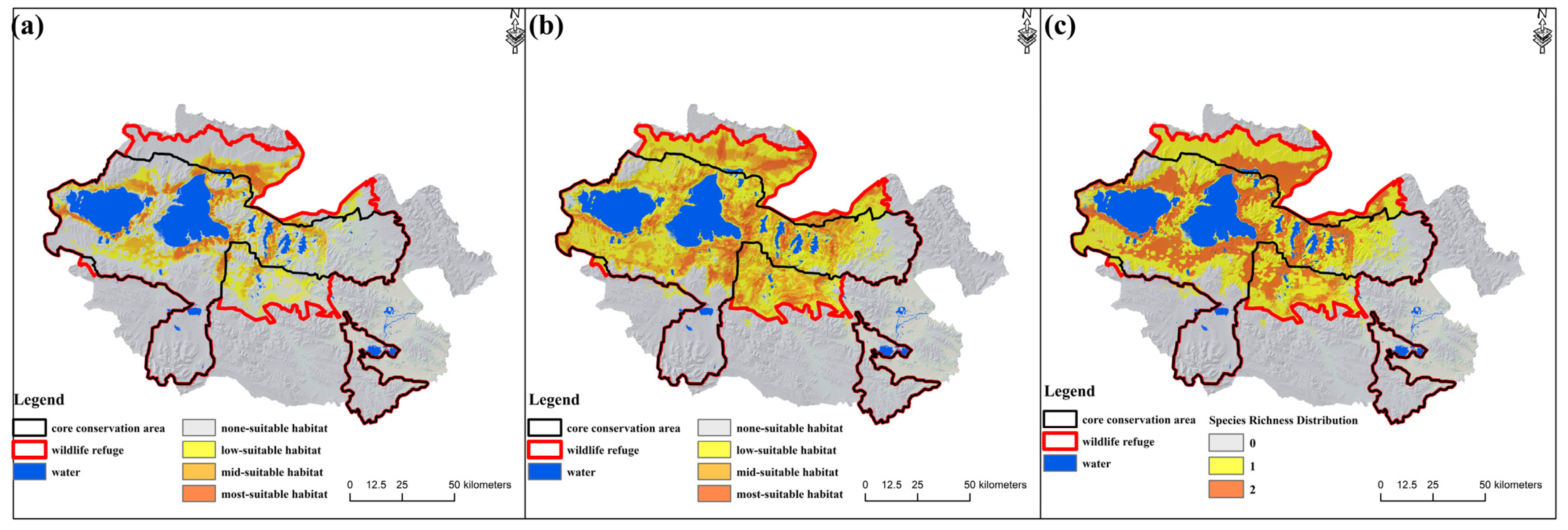
3.1. The Spatial–Temporal Evolution of Potential Suitable Habitat of Large Wild Herbivores
3.2. The Positive and Negative Change in Species-Suitable Habitat
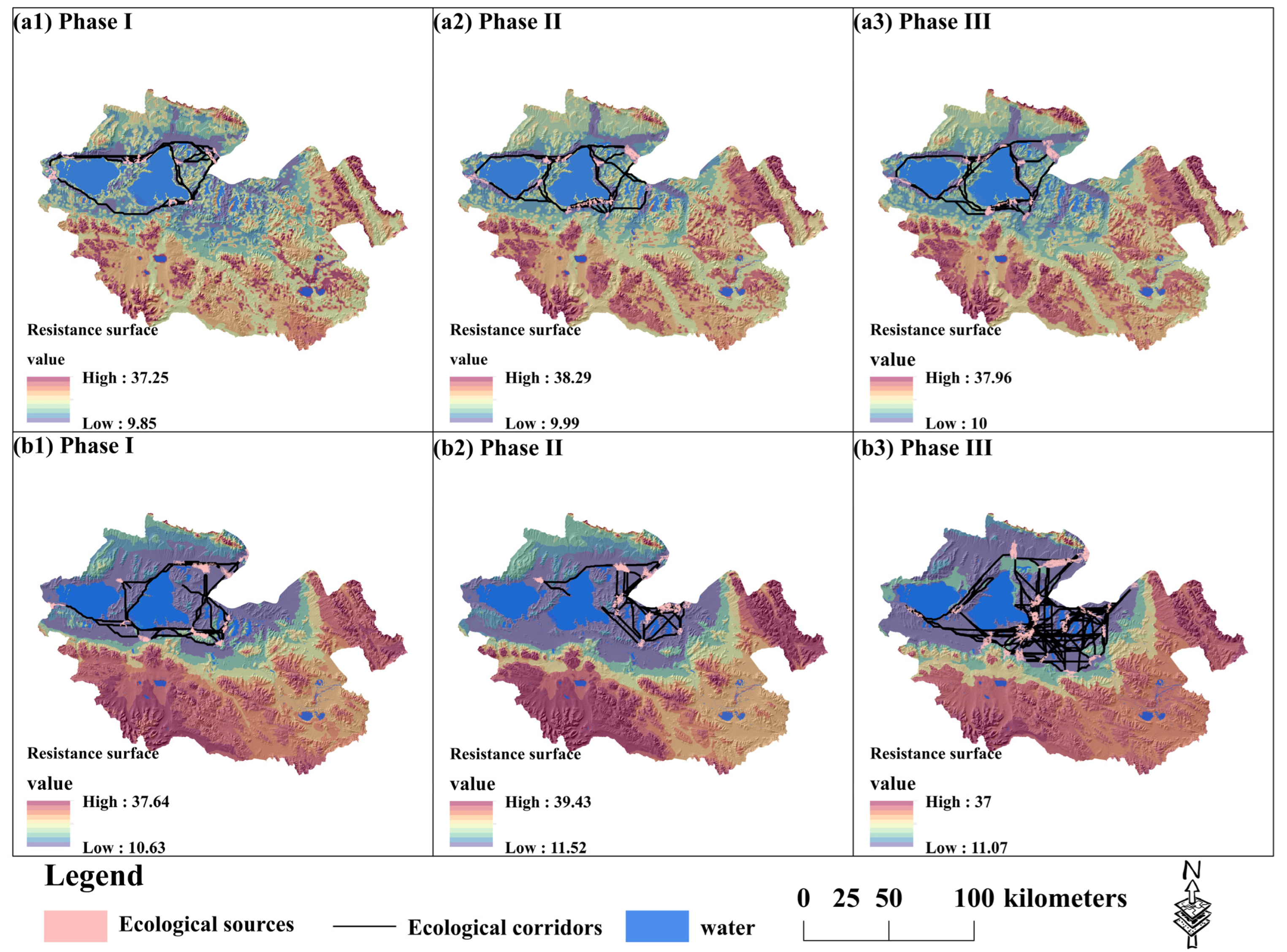
3.3. The Construction of Ecological Corridors in Different Phases of the ECRPSR
4. Discussion
4.1. Comparison of Species-suitable Habitat with Previous Studies
4.2. Adjustment of Protected Area Boundaries by Setting a Wildlife Refuge
4.3. Suggestions for Protecting Biodiversity in the YRSNP
4.4. Innovations, Limitations, and Prospects
5. Conclusions
- In light of the influence of the ECRPSR and SNP, the ecological corridors primarily concentrate within the core areas of the Zhaling–Eling Lake Reserve and the Xingxinghai Reserve, whereas the buffer area and test area lack such corridors. It is necessary to undertake ecological restoration efforts within these buffer and test areas to ensure the preservation of habitat distribution for large wild herbivores.
- The clarification of wildlife protection rights and responsibilities, as well as the implementation of targeted control measures for biodiversity conservation and focus on the marginal areas of species-suitable habitats which are particularly susceptible and prone to loss, can be effectively achieved through the adjustment of wildlife natural reserve boundaries based on the core conservation area of the YRSNP. The intervention of the ECRPSR becomes necessary to protect ecological sources.
- Given the significant impacts of climate change, which have led to alarming declines and extinctions of species, it is crucial to stabilize the changing climate and establish ecological corridors that align with its trajectory.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Pimm, S.L. Biodiversity: Climate change or habitat loss—Which will kill more species? Curr. Biol. 2008, 18, R117–R119. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.F.; Hellmann, J.J.; Boggs, C.L.; Ehrlich, P.R. Climate change hastens population extinctions. Proc. Natl. Acad. Sci. USA 2002, 99, 6070–6074. [Google Scholar] [CrossRef] [PubMed]
- Colwell, R.K.; Brehm, G.; Cardelus, C.L.; Gilman, A.C.; Longino, J.T. Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science 2008, 322, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Both, C. Shifts in phenology due to global climate change: The need for a yardstick. Proc. R. Soc. B Biol. Sci. 2005, 272, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Agudo, R. The future of species under climate change: Resilience or decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.A.; Cabeza, M.; Rahbek, C.; Araujo, M.B. Multiple dimensions of climate change and their implications for biodiversity. Science 2014, 344, 1247579. [Google Scholar] [CrossRef]
- Pecl, G.T.; Araujo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengard, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Panetta, A.M.; Stanton, M.L.; Harte, J. Climate warming drives local extinction: Evidence from observation and experimentation. Sci. Adv. 2018, 4, eaaq1819. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef]
- Fordham, D.A.; Jackson, S.T.; Brown, S.C.; Huntley, B.; Brook, B.W.; Dahl-Jensen, D.; Gilbert, M.T.P.; Otto-Bliesner, B.L.; Svensson, A.; Theodoridis, S.; et al. Using paleo-archives to safeguard biodiversity under climate change. Science 2020, 369, eabc5654. [Google Scholar] [CrossRef]
- Tu, W.; Du, Y.; Yi, J.; Liang, F.; Wang, N.; Qian, J.; Huang, S.; Luo, P.; Wang, X. Assessment of the dynamic ecological networks on the Qinghai-Tibet Plateau using human’s digital footprints. Ecol. Indic. 2023, 147, 109954. [Google Scholar] [CrossRef]
- Salmona, J.; Heller, R.; Quemere, E.; Chikhi, L. Climate change and human colonization triggered habitat loss and fragmentation in Madagascar. Mol. Ecol. 2017, 26, 5203–5222. [Google Scholar] [CrossRef]
- Rinaud, T.; Harmange, C.; Pays, O.; Sarasa, M.; Saillard, M.; Bretagnolle, V. Interspecific competition between two partridges in farmland landscapes. Anim. Behav. 2020, 165, 23–34. [Google Scholar] [CrossRef]
- Li, J.; Yin, H.; Wang, D.; Jiagong, Z.; Lu, Z. Human-snow leopard conflicts in the Sanjiangyuan Region of the Tibetan Plateau. Biol. Conserv. 2013, 166, 118–123. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Y.; Zhai, T.; He, T.; Qi, Y.; Jin, Z.; Cai, Y. The role of human activity in decreasing ecologically sound land use in China. Land Degrad. Dev. 2018, 29, 446–460. [Google Scholar] [CrossRef]
- Luck, G.W.; Ricketts, T.H.; Daily, G.C.; Imhoff, M. Alleviating spatial conflict between people and biodiversity. Proc. Natl. Acad. Sci. USA 2004, 101, 182–186. [Google Scholar] [CrossRef]
- Li, S.; Yu, D.; Huang, T.; Hao, R. Identifying priority conservation areas based on comprehensive consideration of biodiversity and ecosystem services in the Three-River Headwaters Region, China. J. Clean. Prod. 2022, 359, 132082. [Google Scholar] [CrossRef]
- Luo, Z.; Jiang, Z.; Tang, S. Impacts of climate change on distributions and diversity of ungulates on the Tibetan Plateau. Ecol. Appl. 2015, 25, 24–38. [Google Scholar] [CrossRef]
- Dai, Y.; Hacker, C.E.; Cao, Y.; Cao, H.; Xue, Y.; Ma, X.; Liu, H.; Zahoor, B.; Zhang, Y.; Li, D. Implementing a comprehensive approach to study the causes of human-bear (Ursus arctos pruinosus) conflicts in the Sanjiangyuan region, China. Sci. Total Environ. 2021, 772, 145012. [Google Scholar] [CrossRef]
- Ma, B.; Zeng, W.; Xie, Y.; Wang, Z.; Hu, G.; Li, Q.; Cao, R.; Zhuo, Y.; Zhang, T. Boundary delineation and grading functional zoning of Sanjiangyuan National Park based on biodiversity importance evaluations. Sci. Total Environ. 2022, 825, 154068. [Google Scholar] [CrossRef]
- Pekor, A.; Miller, J.R.B.; Flyman, M.V.; Kasiki, S.; Kesch, M.K.; Miller, S.M.; Uiseb, K.; van der Merve, V.; Lindsey, P.A. Fencing Africa’s protected areas: Costs, benefits, and management issues. Biol. Conserv. 2019, 229, 67–75. [Google Scholar] [CrossRef]
- Yi, S.; Wu, P.; Peng, X.; Tang, Z.; Bai, F.; Sun, X.; Gao, Y.; Qin, H.; Yu, X.; Wang, R.; et al. Biodiversity, environmental context and structural attributes as drivers of aboveground biomass in shrublands at the middle and lower reaches of the Yellow River basin. Sci. Total Environ. 2021, 774, 145198. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, S.; An, Y.; Sun, Y.; Zhao, S.; Liu, Y.; Li, M. Climatic factors and human disturbance influence ungulate species distribution on the Qinghai-Tibet Plateau. Sci. Total Environ. 2023, 869, 161681. [Google Scholar] [CrossRef]
- Miller, K.M.; McGill, B.J. Land use and life history limit migration capacity of eastern tree species. Glob. Ecol. Biogeogr. 2018, 27, 57–67. [Google Scholar] [CrossRef]
- Guo, X.; Shao, Q.; Li, Y.; Wang, Y.; Wang, D.; Liu, J.; Fan, J.; Yang, F. Application of UAV Remote Sensing for a Population Census of Large Wild Herbivores—Taking the Headwater Region of the Yellow River as an Example. Remote Sens. 2018, 10, 1041. [Google Scholar] [CrossRef]
- Yang, F.; Shao, Q.; Guo, X.; Tang, Y.; Li, Y.; Wang, D.; Wang, Y.; Fan, J. Effect of Large Wild Herbivore Populations on the Forage-Livestock Balance in the Source Region of the Yellow River. Sustainability 2018, 10, 340. [Google Scholar] [CrossRef]
- Boulanger-Lapointe, N.; Agustsdottir, K.; Barrio, I.C.; Defourneaux, M.; Finnsdottir, R.; Jonsdottir, I.S.; Marteinsdottir, B.; Mitchell, C.; Moller, M.; Nielsen, O.K.; et al. Herbivore species coexistence in changing rangeland ecosystems: First high resolution national open-source and open-access ensemble models for Iceland. Sci. Total Environ. 2022, 845, 157140. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Xian, Y.; Lu, Y.; Liu, G. Is climate change threatening or beneficial to the habitat distribution of global pangolin species? Evidence from species distribution modeling. Sci. Total Environ. 2022, 811, 151385. [Google Scholar] [CrossRef]
- Mukul, S.A.; Alamgir, M.; Sohel, M.S.I.; Pert, P.L.; Herbohn, J.; Turton, S.M.; Khan, M.S.I.; Munim, S.A.; Reza, A.; Laurance, W.F. Combined effects of climate change and sea-level rise project dramatic habitat loss of the globally endangered Bengal tiger in the Bangladesh Sundarbans. Sci. Total Environ. 2019, 663, 830–840. [Google Scholar] [CrossRef]
- St-Louis, A.; Côté, S.D. Equus kiang (Perissodactyla: Equidae). Mamm. Species 2009, 835, 1–11. [Google Scholar] [CrossRef]
- Leslie, D.M. Procapra picticaudata (Artiodactyla: Bovidae). Mamm. Species 2010, 42, 138–148. [Google Scholar] [CrossRef]
- Ma, B.; Xie, Y.; Zhang, T.; Zeng, W.; Hu, G. Identification of conflict between wildlife living spaces and human activity spaces and adjustments in/around protected areas under climate change: A case study in the Three-River Source Region. J. Environ. Manag. 2020, 262, 110322. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Peng, G.; Wen, C.; Zahoor, B.; Ma, X.; Hacker, C.E.; Xue, Y. Climate and land use changes shift the distribution and dispersal of two umbrella species in the Hindu Kush Himalayan region. Sci. Total Environ. 2021, 777, 146207. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, S.; Zhang, L.; Liu, J. Spatial variation of climatology monthly crop reference evapotranspiration and sensitivity coefficients in Shiyang river basin of northwest China. Agric. Water Manag. 2010, 97, 1506–1516. [Google Scholar] [CrossRef]
- Guo, B.; Zhang, J.; Meng, X.; Xu, T.; Song, Y. Long-term spatio-temporal precipitation variations in China with precipitation surface interpolated by ANUSPLIN. Sci. Rep. 2020, 10, 81. [Google Scholar] [CrossRef]
- Marinoni, O. Improving geological models using a combined ordinary–indicator kriging approach. Eng. Geol. 2003, 69, 37–45. [Google Scholar] [CrossRef]
- Bao, S.; Yang, F. Influences of Climate Change and Land Use Change on the Habitat Suitability of Bharal in the Sanjiangyuan District, China. Int. J. Environ. Res. Public Health 2022, 19, 17082. [Google Scholar] [CrossRef]
- Yang, J.; Huang, X. The 30 m annual land cover dataset and its dynamics in China from 1990 to 2019. Earth Syst. Sci. Data 2021, 13, 3907–3925. [Google Scholar] [CrossRef]
- Duan, Q.L.; Luo, L.H. Human footprints dataset of the Qinghai-Tibet Plateau during 1990–2015. Sci. Data Bank 2019. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P.; Araújo, M. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2013, 41, 629–643. [Google Scholar] [CrossRef]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. kuenm: An R package for detailed development of ecological niche models using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zheng, Q.; Shen, L.; Wang, G.; Liu, R.; Zhang, X.; Qi, J.; Zhang, A.; Meng, X. Assessment of habitat suitability in autumn for wild alpine musk deer in Xinglongshan National Nature Reserve with MaxEnt model. Chin. J. Ecol. 2024, 43, 299–304. [Google Scholar] [CrossRef]
- Yu, K. Security patterns and surface model in landscape ecological planning. Landsc. Urban Plan. 1996, 36, 1–17. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Wasof, S.; Decocq, G. Using process-based indicator species to evaluate ecological corridors in fragmented landscapes. Biol. Conserv. 2016, 201, 152–159. [Google Scholar] [CrossRef]
- Huang, C.; Hou, X.; Li, H. An improved minimum cumulative resistance model for risk assessment of agricultural non-point source pollution in the coastal zone. Environ. Pollut. 2022, 312, 120036. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Kou, X.; Ouyang, Z. Distributions of two native ungulates at the third pole are highly sensitive to global warming. Glob. Ecol. Conserv. 2022, 39, e02292. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, F.; Chi, X.; Li, G.; Cai, Z.; Qin, W.; Zhang, J.; Wu, T.; Zhang, T. The carrying pressure of livestock is higher than that of large wild herbivores in Yellow River source area, China. Ecol. Model. 2020, 431, 109163. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, Z.; Zhang, Y.; Zhong, W.; Xia, H.; Hao, Z.; Zhang, C.; Li, H. Predicting the impact of climate change on the distribution of two relict Liriodendron species by coupling the MaxEnt model and actual physiological indicators in relation to stress tolerance. J. Environ. Manag. 2022, 322, 116024. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, F.; Li, G.; Qin, W.; Wu, T.; Xu, F.; Hou, Y.; Song, P.; Cai, Z.; Zhang, T. The four antelope species on the Qinghai-Tibet plateau face habitat loss and redistribution to higher latitudes under climate change. Ecol. Indic. 2021, 123, 107337. [Google Scholar] [CrossRef]
- Jiang, F.; Li, G.; Qin, W.; Zhang, J.; Lin, G.; Cai, Z.; Gao, H.; Zhang, T. Setting priority conservation areas of wild Tibetan gazelle (Procapra picticaudata) in China’s first national park. Glob. Ecol. Conserv. 2019, 20, e00725. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Sillero, N. Cross-scale monitoring of habitat suitability changes using satellite time series and ecological niche models. Sci. Total Environ. 2021, 784, 147172. [Google Scholar] [CrossRef]
- Sun, X.; Long, Z.; Jia, J. A multi-scale Maxent approach to model habitat suitability for the giant pandas in the Qionglai mountain, China. Glob. Ecol. Conserv. 2021, 30, e01766. [Google Scholar] [CrossRef]
- Shao, Q.; Cao, W.; Fan, J.; Huang, L.; Xu, X. Effects of an ecological conservation and restoration project in the Three-River Source Region, China. J. Geogr. Sci. 2016, 27, 183–204. [Google Scholar] [CrossRef]
- Mantyka-pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Chang. Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Shao, Q.Q.; Guo, X.J.; Li, Y.Z.; Wang, Y.C.; Wang, D.L.; Liu, J.Y.; Fan, J.; Yang, F. Using UAV remote sensing to analyze the population and distribution of large wild herbivores. Natl. Remote Sens. Bull. 2018, 22, 497–507. [Google Scholar] [CrossRef]
- Ma, B.; Xie, Y.; Zhang, T.; Zeng, W.; Xue, Y. Construction of a human-wildlife spatial interaction index in the Three-River Source Region, China. Ecol. Indic. 2021, 129, 107986. [Google Scholar] [CrossRef]
- Carvalho, S.B.; Brito, J.C.; Crespo, E.G.; Watts, M.E.; Possingham, H.P. Conservation planning under climate change: Toward accounting for uncertainty in predicted species distributions to increase confidence in conservation investments in space and time. Biol. Conserv. 2011, 144, 2020–2030. [Google Scholar] [CrossRef]
- Hodgson, J.A.; Thomas, C.D.; Wintle, B.A.; Moilanen, A. Climate change, connectivity and conservation decision making: Back to basics. J. Appl. Ecol. 2009, 46, 964–969. [Google Scholar] [CrossRef]
- Feeley, K.J.; Silman, M.R. Land-use and climate change effects on population size and extinction risk of Andean plants. Glob. Chang. Biol. 2010, 16, 3215–3222. [Google Scholar] [CrossRef]
- Schloss, C.A.; Nunez, T.A.; Lawler, J.J. Dispersal will limit ability of mammals to track climate change in the Western Hemisphere. Proc. Natl. Acad. Sci. USA 2012, 109, 8606–8611. [Google Scholar] [CrossRef]
- Maciel, G.A.; Kraenkel, R.A. How population loss through habitat boundaries determines the dynamics of a predator–prey system. Ecol. Complex. 2014, 20, 33–42. [Google Scholar] [CrossRef]
- Searle, K.D.; van Vuuren, J.H. An abstract mathematical model for sustainable harvesting of a biological species on the boundary of a protected habitat. Ecol. Model. 2021, 452, 109591. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Walden-Schreiner, C.; Leung, Y.F.; Kuhn, T.; Newburger, T.; Tsai, W.L. Environmental and managerial factors associated with pack stock distribution in high elevation meadows: Case study from Yosemite National Park. J. Environ. Manag. 2017, 193, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Harun-or-Rashid, S.; Biswas, S.R.; Böcker, R.; Kruse, M. Mangrove community recovery potential after catastrophic disturbances in Bangladesh. For. Ecol. Manag. 2009, 257, 923–930. [Google Scholar] [CrossRef]




| Parameters | Electric Fixed-Wing UAV | Fuel-Powered Fixed-Wing UAV | Feima F1000 UAV |
|---|---|---|---|
| Number of aircrafts | 1 | 2 | 1 |
| Wingspan (meters) | 1.6 | 2.7 | 1.6 |
| Payload (kilograms) | 0.5 | 1.5 | 1 |
| Maximum takeoff weight (kilograms) | 3 | 17 | 3 |
| engine type | Electric | Fuel | Electric |
| Flight time (minutes) | 90 | 120 | 60 |
| Camera model | ILCE-5100 | ILCE-5100 | ILCE-5100 |
| Number of integrated cameras | 2 | 2 | 1 |
| Focal length (mm) | 30 | 30 | 30 |
| Camera resolution (pixel) | 6000 × 4000 | 6000 × 4000 | 6000 × 4000 |
| Camera model | ILCE-5100 | ILCE-5100 | ILCE-5100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, S.; Yang, F. Identification of Potential Habitats and Adjustment of Protected Area Boundaries for Large Wild Herbivores in the Yellow-River-Source National Park, China. Land 2024, 13, 186. https://doi.org/10.3390/land13020186
Bao S, Yang F. Identification of Potential Habitats and Adjustment of Protected Area Boundaries for Large Wild Herbivores in the Yellow-River-Source National Park, China. Land. 2024; 13(2):186. https://doi.org/10.3390/land13020186
Chicago/Turabian StyleBao, Shengwang, and Fan Yang. 2024. "Identification of Potential Habitats and Adjustment of Protected Area Boundaries for Large Wild Herbivores in the Yellow-River-Source National Park, China" Land 13, no. 2: 186. https://doi.org/10.3390/land13020186
APA StyleBao, S., & Yang, F. (2024). Identification of Potential Habitats and Adjustment of Protected Area Boundaries for Large Wild Herbivores in the Yellow-River-Source National Park, China. Land, 13(2), 186. https://doi.org/10.3390/land13020186







