Abstract
The impact of the active hostilities associated with Russia’s large-scale armed invasion of the territory of Ukraine on soil degradation as a result of military actions has resulted in soil damage due to heavy military armored vehicles. Debris from destroyed military equipment, ammunition, and fuel remnants lead to multi-factor damage to the soil system, causing local and global pollution and losses of soil resources. In all the studied cases, mechanical, chemical, and physical soil degradation were observed. This was manifested in changes in granulometric fractions at explosion sites, burning areas, and locations with heavy-metal contamination. Equipment incineration has resulted in an increase in the sand fraction (2.0–0.05 mm) by 1.2–1.8 times and a decrease in the clay fraction (<0.002 mm) by 1.1–1.2 times. The soil contamination levels with regard to heavy metals significantly surpass health standards, with the highest pollution levels observed for Pb, Zn, and Cd. Across all affected areas, changes occurred in the microbiome structure (a 20.5-fold increase in the proportion of mycelial organisms), microbiological process activity was suppressed (a 1.2-fold decrease), microbial biomass (a 2.1-fold decrease) was reduced, and high soil toxicity (99.8%) was observed. Explosions and the pyrolysis of armored vehicles have a significant impact on soil mesobiota and plants. The results indicate the existence of complex interactions between various factors in the soil environment post-explosion, significantly affecting soil health.
1. Introduction
Armed conflicts induce disruptions of ecosystems [1,2,3,4] and contribute to the development of degradation processes [5], the loss of biodiversity [6,7], and the pollution of soil [8,9] and water resources [10,11,12], negatively impacting the climate system [13,14,15]. Increasing attention is being devoted to the carbon footprint because of warfare and militarization. Specifically, the Russian–Ukrainian war is exacerbating environmental deterioration, causing an increase in the carbon footprint over time in both countries, with Ukraine experiencing the greatest environmental impact [16]. Over the past century, more than 30 wars have occurred worldwide, significantly impacting not only societal development but also the planet as a whole [17,18,19,20]. All these instances have collectively hindered the achievement of the Sustainable Development Goals [21] and Food and Agriculture Organization goals regarding the conservation and sustainable use of soil resources for food security [22,23,24,25,26] and entail various temporally undefined adverse phenomena for the economic and social sectors. The deployment of various types of weaponry releases numerous chemical compounds and gases, metallic fragments from projectiles and destroyed military equipment, and fuel and lubricant materials, which, directly or through chemical reactions with environmental elements, induce various degradation processes. Therefore, areas of military operations, military training zones, and shooting ranges, as well as sites of explosive material production and utilization, are considered major sources of contamination for terrestrial ecosystems and have an impact on human health [27,28]. These areas require monitoring studies for the development of rehabilitation measures and the mitigation of negative environmental consequences.
Recently, the number of publications devoted to the ecological consequences of military actions in Ukraine has increased significantly, including their impact on the soil system and the determination of economic losses inflicted on the land by armed aggression [3,9,29,30,31,32,33]. The actual consequences of modern large-scale wars, such as the Russian–Ukrainian war, have an impact on almost all components of nature [34]. A proposal has been made to introduce a new type of soil degradation—degradation caused by armed aggression—which encompasses mechanical, physical, chemical, physico-chemical, and biological degradation [9]. Despite the relevance and importance of the impact of armed conflicts and their consequences for ecosystems, there is currently insufficient scientific research and publications related to the multifaceted influence of armed conflicts on soil resources, particularly with respect to destroyed military equipment.
Soil bears the brunt of armed conflicts, experiencing the most significant contamination, and it preserves the consequences of war for an extended period. Therefore, assessing the degree of soil destruction and contamination allows the evaluation of environmental risks, future adverse consequences for terrestrial ecosystems, and the loss of ecosystem services [35,36,37,38]. Researchers primarily focus on the mechanical destruction of soil cover, alterations in the physico-chemical properties of soils after military actions, contamination with toxicants (heavy metals, organic compounds, etc.) and their migration, and the determination of potential adverse impacts and soil restoration methods [8,39,40].
One of the most prevalent hazardous consequences during armed conflicts and caused by the use of weaponry is the chemical contamination of the soil [8,27,41,42,43,44,45]. Specifically, during combat, a range of toxic compounds present in various calibers of ammunition, products of the destruction and burning of heavy machinery, fuel from fuel spills, technical lubricants, organic solvents, etc., enter the soil. The behavior of most of these compounds in Ukrainian soils is inadequately researched, and there are no established regulatory limits for their concentrations in soils [9]. Meanwhile, an increasing number of research findings are confirming that potential sources of emissions of various potentially toxic substances into the natural environment are related to military activities and pollution with heavy metals and determining the pathways of their migration and the associated risks for human health and biota [43,46].
It has been established that soils within the territory of military facilities remain significantly contaminated with toxic compounds from ammunition and their residues for many decades, containing harmful substances, including antimony (Sb), lead (Pb), uranium (U), 2,4-dinitrotoluene, 2,4,6-trinitrotoluene (TNT), 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX), and others [8,28,43,47,48]. Most of these compounds are resistant to biological degradation or treatment and thus persist in the biosphere and become a source of pollution, potentially harming human health and the environment due to their toxic impact [43,48,49].
The release of heavy metals into the natural environment during ground combat and bombings is precipitated by weapon residues containing Pb, Cu, Cd, Sb, Cr, Ni, and Zn, which subsequently migrate into water sources, thereby increasing the risk of a human impact. Biomonitoring studies have shown the accumulation of heavy metals in plants, invertebrates, and vertebrate species [28,50,51,52,53].
It is known that catastrophic concentrations of chemical elements and various compounds resulting from the use of explosive weapons lead to disruptions in physiological and biochemical processes in living organisms. For instance, elevated levels of heavy metals in the soil not only have a direct toxic effect but also indirectly negatively impact the reproduction and bioproduction of soil microorganisms [54,55,56,57].
When assessing the impact of potentially toxic substances on the soil system due to various military actions, attention is primarily paid to the study of bacterial communities, with less focus on archaea [58]. The main research efforts are dedicated to identifying active microorganisms for conducting bioremediation on contaminated soils [59,60,61,62,63].
It has been determined that chemical compound type, soil pH, and moisture content are critical factors influencing bacterial communities in contaminated military sites. These factors significantly alter bacterial diversity and community structure at military–industrial sites and ammunition disposal areas [64,65,66].
The high toxicity of explosive compounds [67,68], coupled with the stability of their chemical structures and their ability to bind to organic matter in the soil, prevents and significantly hinders soil restoration efforts [69]. Explosive substances have a significant impact on plants, and their concentration is directly proportional to consequences such as delayed seed germination, reduced plant biomass, fertility, anatomical and morphological aberrations, disruption of biological rhythms, and more [68,70]. Increased sporadic development of teratogenesis also occurs, leading to anomalies in photosynthesis processes and overall organism functioning [71,72].
The soil mesofauna is equally susceptible to the influence of explosive substances and, in some cases, serves as a better, more indicative biomarker. These organisms accumulate xenobiotics by directly consuming nutrient substrates and absorbing them through their surface coverings. Research indicates that Eisenia andrei [73], Enchytraeus crypticus, and Folsomia candida [74] are sensitive to explosive contamination, with LC50 concentrations ranging from 143 to 365 mg kg−1, depending on the soil type [75]. Explosive substances such as trinitrotoluene, hexogen, octogen, and others are not lethal to earthworms; however, they are known to reduce earthworm biomass and reproductive capacity (leading to reductions in the number of cocoons and young worms) [74,76,77].
Therefore, the aim of this study is to assess the potential environmental risks to ecosystems arising from soil contamination due to the use of weapons in armed conflicts. Many aspects of the consequences of military actions still require attention. Specifically, research is needed on the impact on physical parameters (the granulometric state), chemical parameters (concentrations of heavy metals), and biological parameters (the soil microbiome and mesofauna) in soil systems disrupted by military equipment, which remains on battlefields in substantial quantities (thousands of tons) and enters ecosystems.
2. Materials and Methods
2.1. Experimental Site and Investigation Design
This study was conducted in the territory of Bohodukhivskyi district, Kharkiv Oblast, Ukraine, where areas impacted by heavy armored vehicles were identified following combat operations, located at the following coordinates: a—50°15′25.2″ N, 36°02′12.6″ E; b—50°09′32.0″ N, 36°10′46.0″ E; c—50°16′58.8″ N, 36°04′15.8″ E (Figure 1). The general appearances of the fragments of the destroyed vehicles, a T72B3 tank, a T80 tank, and a thermobaric rocket launcher, i.e., the TOS-1 “Solntsepek”, are depicted in Figure 1a, b, and c, respectively.
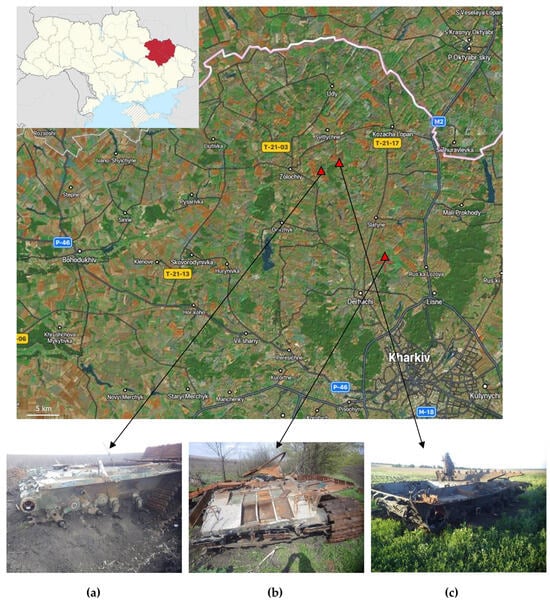
Figure 1.
Map of the studied area (Bogodukhiv district, Kharkiv region, Ukraine) presented as a satellite image from Google Earth [78], its location on a map of Ukraine (insert), and images of the sample collection places: fragments of a T-72B3 tank (a), a T-80 tank (b) that exploded due to the detonation of the ammunition, and a TOS-1 “Solntsepek” thermobaric rocket launcher (c) after the detonation of the ammunition due to the impact of cassette ammunition. Red triangles show the sampling localities.
2.2. Description of Sites and Soil Sampling
Soil sampling was performed according to the standardized methods of the State Standard of Ukraine ISO 10381 series [79,80,81,82,83,84,85,86,87,88,89] after each tank fragment was examined separately. The general approach consisted of collecting soil samples at the explosion sites and control samples in an undamaged area (at least 50 m from the epicenter). However, there are common features of sample collection in these cases. The most important feature, in our opinion, is determining where the armored vehicle was detonated. In the area of the most significant explosion, the largest soil damage areas were visually identified (via changes in soil color and structure, odor) even after a year of the fragments being in the detonation site.
In the detonation areas, vehicles and armor fragments typically have a crater and are bent towards the detonation site (Figure 2 and Figure 3). An exception was the thermobaric rocket launcher based on the TOS-1, where fuel tanks were damaged by subsequent rocket detonation (Figure 3b).

Figure 2.
Images of the damaged transport-loading vehicles: detonated T-72 tank (a) after the explosion of the ammunition in the middle of the tank (where the ammunition was stored); (b) detonated front part of a T-80 tank (the ruptured right side of the tank, near the place where the driver sits).

Figure 3.
Images of the damaged transport-loading vehicles: the points of impact on the TOS-1 “Solntsepek” on the upper part of the hull on the left (a) and right (b) sides (the fuel tanks).
Based on these facts about the damage to the vehicles, soil samples were collected precisely at the points of explosions, which, in our opinion, are the most contaminated and suffered the greatest damage. Soil sampling was carried out at a depth of 0–20 cm. As controls (which, in relation to these research samples, are referred to as Controls 1, 2, and 3), soil samples were taken at no more than 50 m from each object, considering the local topography.
The investigated soils are classified as Chernic Phaeozems according to the WRB classification (Table 1).

Table 1.
Agrochemical characteristics of the soil in the research area (at a depth of 0–20 cm).
2.3. Soil Sample Characterization
2.3.1. Soil Sample Preparation
The collected soil samples were processed in a laboratory by removing plant residues and non-natural debris. They were dried in ovens at temperatures not exceeding 40 °C. During the drying process, large clumps were manually broken down [83]. The air-dried sample was thoroughly mixed, and a portion of the sample was taken for physical analysis using the quartering method, while the rest was allocated for chemical analysis. For the determination of mobile compounds of phosphorus, potassium, sulfur, and pH, the samples were ground and sieved through a sieve with a 1 mm aperture. For the determination of organic matter carbon, the samples were ground and sieved through a sieve with a 0.25 mm aperture.
2.3.2. Determination of Soil Agrochemical Indicators and Contents of Microelements and Heavy Metals (HMs)
The pH of the water extract was determined by extracting water-soluble salts from 10 g of air-dried soil (previously ground and sieved through a 1 mm sieve) with 50 cm3 of distilled water [84]. The suspension was stirred for 5 min, and the pH was measured using a pH meter after 5 h of settling.
The soil organic matter (SOM) content determination was carried out using the oxidometric method [85]. Air-dried soil (0.2 g) samples were crushed and sieved (using a pore diameter of 0.25 mm) and then treated with 10 cm3 of a chromic mixture for 20 min at 150–160 °C. Titration was carried out with a Mohr salt solution in the presence of a 0.2% solution of phenylanthranilic acid. The organic matter content in the soil was calculated by multiplying the organic carbon content by a conversion factor of 1.97.
The content of P2O5 and K2O in the soil was determined via spectrophotometry after extracting phosphorus and potassium mobile compounds from the soil with a solution of ammonium bicarbonate [86]. The content of potassium was determined using flame atomic emission spectroscopy.
Mobile sulfur determination was carried out by extracting sulfur compounds from the air-dried soil sample, weighing 30 g (previously crushed and sieved through a sieve with a pore diameter of 1 mm), with 75 mL of 1 mol dm−3 of potassium chloride solution; precipitating sulfates with a precipitating solution (containing 20 g of barium chloride, 60 mL of 1 mol dm−3 hydrochloric acid solution, and 5 g of starch per 1 dm3); and measuring the optical density of the suspension using a spectrophotometer at a wavelength of 520 nm [87].
The content of trace elements and heavy metals (Cd, Co, Cr, Cu, Fe, Mn, Ni, Pb, and Zn) was determined according to a previously published method [88] in a buffered ammonium acetate extract with a pH of 4.8 using the atomic absorption spectrophotometry method via a SATURN-4 spectrophotometer (Ukraine).
2.3.3. Determination of the Granulometric Composition of the Soil
To determine the soil’s granulometric composition, at least 100 g of each soil sample was ground and sieved through a 1 mm hole sieve (considering the methodological aspects of DSTU 4730-2007) [89] and measured using a Malvern Instruments Mastersizer 3000E instrument (Westborough, MA, USA). Carbonates were removed with 0.2 mol dm−3 of hydrochloric acid. Disaggregation was carried out by using 1 mol dm−3 of sodium hydroxide solution. The determination of the particle size of the soil was carried out using the laser diffraction method via a Mastersizer 3000E particle analyzer produced by Malvern Instruments (USA) with a HydroEV liquid dispersion module, using the following parameters: dispersant–distilled water with a refractive index of 1.33; stirrer speed, 2250 rpm; background and sample measurement time, 15 s; number of measurements, 6; and mathematical calculation model: Mie theory with a refractive index of 1.40 and an absorption of 0.01.
2.3.4. Microbiological Studies of Soil and Emission of CO2
- Microbiological Study of Soil Microorganisms
For the microbiological analyses, we selected the soil samples from each variant of the experiment and an abandoned field in a test with 5-fold replication and prepared an average sample. Batches of 10 g each were put on sterile mortar, and then the microorganisms were separated from the soil particles using a previously published method [90]. Tenfold solutions of the soil suspensions were prepared, which were used for inoculations in the media selected for each ecological-trophic or taxonomic group of microorganisms. The quantitative amounts of the microorganisms of the main ecological-trophic and taxonomic groups in soil were as determined using a method consisting of inoculating the soil suspension with standard growth media, which is generally accepted in soil microbiology and described as follows: all the microorganisms were inoculated on peptone–glucose agar (PGA) with soil extraction, bacteria that use organic nitrogen (Norg) were inoculated onto meat infusion agar (MIA), streptomyces and bacteria that use mineral nitrogen (Nmin) were inoculated on starch-and-ammonia agar (SAA), all pedotrophs were inoculated on soil agar (SA), nitrogen-fixing microorganisms were inoculated on the non-nitrogenous media developed by Ashby and Vinogradsky, oligotrophs were inoculated on purified agar (PA), and micromycetes were inoculated on Czapek–Dox agar. After the inoculation of the media, the bacteria were incubated at a temperature of 28 °C for 5–14 days. The colonies that grew in these media were counted under the assumption that each colony originated from a single viable cell. The number of microorganisms grown on the nutrient media was expressed in Colony-Forming Units (CFU) per gram of dry soil. For this purpose, we determined the moisture of the soil samples for the experiments using thermostat-gravimetric analysis and recalculated the obtained number of colonies, taking into consideration the coefficient of moisture and the solution of the soil suspension. The inoculations were repeated three times, and the resulting data were analyzed using statistical methods. A confidence interval was calculated for the number of microorganisms to ensure the results were reliable and accurate.
The taxonomic structures of the microbial communities were determined as a percentage of taxa: bacteria, streptomyces, and micromycetes [91,92].
- Direction of the Soil Microbiological Process
The direction of microbiological processes in the soil was determined using the appropriate coefficients [92] (Equations (1)–(4)):
− The coefficient of mineralization (Kmin) was calculated using the ratio
where CSAA is number of microorganisms immobilizing the mineral forms of nitrogen, and CMIA is the number of organotrophs;
− The coefficient of oligotrophity (Kol) was calculated as the ratio
where CPA is the number of microorganisms that are able to absorb nutrients from very rarefied solutions over the total number of eutrophic microorganisms, CSAA and CMIA;
− The coefficient of pedotrophity (Kped) was calculated as the ratio
where CSA is the number of pedotrophic microorganisms and CMIA is the number of microorganisms using organic nitrogen;
− The coefficient of the transformation of organic matter (Ktom) was calculated using the following equation:
- Diversity of Soil Microbiomes
The diversity of soil microbiocenosis was calculated according to the Shannon index and the Simpson index (Shannon index, Simpson’s index) [93].
- Content of Total Microbial Biomass (Cmic)
The content of general microbial biomass (Cmic) in the soil was determined using the rehydration method through the gentle drying of soil samples at a temperature of 65–70 °C over 24 h with further extraction with 0.5 mol dm−3 K2SO4 solution [94,95]. The content of carbon of the organic compounds in the soil (Corg) was calculated with consideration of humus content using a 1.724 coefficient, accepting that humus is 58% carbon on average [85].
- Emission of CO2
Determination of the production of CO2 by the soil was carried out under laboratory conditions (temperature, 24–25 °C; moisture, 60% of total capacity; incubation time, 24 h) using the adsorption method (after alkaline adsorption) and the titration method with an HCl solution [96,97].
2.3.5. Soil Toxicity
The toxicity of the soil was determined under laboratory conditions using test cultures, namely, Raphanus sativus and Lepidium sativum [98]. This study was conducted six months after combat had taken place. Only untreated seeds with germination abilities greater than 90% were used in this test. Twenty seeds were sown in each pot (containing 10 g of soil). The soil was watered with distilled water; the soil moisture level was determined to be 40%. Each sample was prepared in five replicates. This experiment was carried out in a plant growth chamber at a temperature of 21 °C/18 °C (day/night). Air humidity in the chamber was kept at 80%; light intensity was 25,000 lm m−2 surface in an hourly cycle of 14/10 (day/night). Seven days after the beginning of the test, germinated seeds were counted in each sample. Subsequently, the five most representative seedlings in each sample were selected, and the remaining seedlings were removed. After 14 days, plants were collected, the lengths of shoots and roots were measured, and the biomass of fresh shoots was weighed.
Acute earthworm toxicity tests were conducted according to standard DSTU ISO 11268-1:2003 Soil quality [99]. Effects of pollutants were tested on earthworms (Eisenia fetida). Plastic pots were filled with 500 g of contaminated soil, and the moisture level was maintained at 40% using a manure-and-water solution (with manure serving as food for the earthworms). Control samples, which were not spiked, consisted of soil with manure and water solutions. Each sample was prepared in five replicates. Ten washed earthworms were introduced into each container, which were then covered with gauze to prevent the earthworms from escaping.
The test was conducted for 10 days at room temperature and in stable soil moisture conditions. After 10 days, living organisms were counted (constituting a mortality assessment) and weighed (to determine the effect of chemicals on the biomass).
Toxicity endpoints such as LC50 were calculated using the linear regression best-fitting model. Statistically significant differences between the results were evaluated based on determining the standard deviation (p ≤ 0.05).
2.3.6. Statistical Analysis of Data
The experimental results were statistically analyzed using Statistica 10 software. The tests were performed in 5 repetitions. Mean values () and their standard deviations (SDs) were calculated. The level of significance selected for the study was p < 0.05. Dispersion analysis and the Tukey test were used to compare the averages of the independent samples.
3. Results and Discussion
3.1. Destroyed Heavy Armored Vehicles Act as a Factor Influencing Soil Ecosystems
During military operations, a significant amount of military and special equipment, as well as the remnants of ammunition, including unexploded ordnance, are left on the battlefield. According to current data from WarSpotting [100], hundreds of units of destroyed military equipment remain in combat zones and liberated territories, posing environmental hazards [6,9,12,101,102]. Current statistics indicate that the armed forces of Russia and Ukraine use tens of thousands of projectiles daily, resulting in the destruction of military equipment and military and civilian objects and generating a large amount of solid/liquid waste derived from ammunition in cultivated soil systems [103,104]. The Ministry of Defense of Ukraine is gradually removing these fragments from the battle sites without documenting the impact locations, creating a future risk for the agricultural sector.
The cumulative impact of fragments from destroyed military equipment, ammunition residues, and fuel and lubricant materials, along with the burning processes, inflicts multifaceted damage to the soil system, directly and indirectly leading to local and global pollution and a loss of soil resources [32,105].
Based on an analysis of available scientific publications and the authors’ extensive experience, a cause-and-effect diagram of the impact of destroyed heavy armored vehicles on the soil has been developed, illustrating potential global ecological consequences (Figure 4).
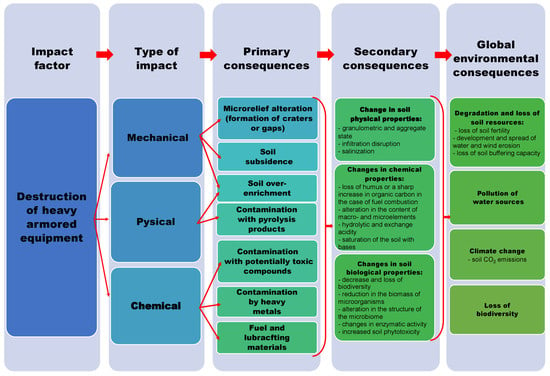
Figure 4.
Scheme depicting the cause-and-effect relationships of soil degradation due to the destruction (or detonation) of heavy armored vehicles.
3.2. Effects on Soil Chemistry
The results indicate changes in the chemical properties of the soil in the areas in which detonation has occurred and where military equipment debris is present (Table 2).

Table 2.
Agrichemical indicators of soil in the zones of detonation and burning of heavy armored vehicles.
Changes in the content of phosphorus and potassium were detected (Table 2). Upon comparing Control 1 (T72B3) with the site of the soil affected by the fragments of a T72B3 (bottom), it was observed that phosphorus levels decreased 1.34-fold, and potassium levels increased 0.5-fold. Similar changes occurred at the site of the impact on the T72B3 tank (stern) at the location of the engine and transmission: phosphorus levels increased 0.43-fold, and those of potassium increased 0.56-fold. Another object of study, the T80 tank, shows a 1.69-fold decrease in phosphorous and a 1.53-fold decrease in potassium compared to Control 2. The fragments of the TOS-1 “Solntsepek” indicated a 0.42-fold increase in phosphorus and a 0.74-fold increase in potassium.
All these changes are not anomalous and are quite typical of soil properties, indicating fluctuations in phosphorus from the average content (16–30 mg kg−1) or that for the control for the T72B3 tank to a high phosphorus content (31–45 mg kg−1). Similar conclusions can be arrived at for potassium, showing an increase from the average value of the Control, 1–171.11 mg kg−1 (corresponding to an average content of 101–200), to a high content of 337.4 mg kg−1 (corresponding to a high potassium compound content equal to 301–400 mg kg−1). One can assume that based on indicators such as phosphorus and potassium, the soil received such “fertilizers” and improved its condition.
Changes in organic matter in all the research samples practically did not occur and fluctuated from elevated levels of 3.1–4.0% (Control 3) to high levels (4.1–5.0%) for the fragments of the T72 (bottom, stern), the fragments of the T80 (bottom), and their controls. The fragments of the TOS-1 “Solntsepek” represent an anomaly in these soil samples, wherein the organic matter is 8.28%, which is practically never encountered in soils under natural conditions in Ukraine. These changes and fluctuations indicate the different chemical natures of the substances that were in these armored vehicles at the time of the explosion.
Significant changes in mobile sulfur content were observed for all the samples. In the control samples, the mobile sulfur content did not exceed 3.4 mg kg−1, while in the samples taken at the sites of armored vehicle explosions, it increased by 16.5–65.0-fold. In the authors’ opinion, this increase in mobile sulfur content is associated with the acceleration of mineralization due to the increase in soil temperature during the explosion and burning of armored vehicles. Another source of sulfur is tank fuel, which contains sulfur-heterocycles [106]. The gross sulfur content in chernozem soils is 0.2–0.5% on average, so this increase does not exceed the gross content. According to regulatory documents [107], the maximum permissible concentration (MPC), considering the background (clarke) for sulfur, is 160.0 mg kg−1. Thus, according to our data, there is no exceedance, but sulfur, which is present in the products of burning technology, may be partially present in an insoluble state, and it is also present in fuel and lubricants (in organic form) and not determined or considered when entering the soil. Therefore, over time, this “reserve” may lead to the exceedance of permissible norms.
During the analysis of the collected samples for their content of microelements and heavy metals, it was found that at the sampling site (bottom) for the T72B3, there was an exceedance of MPC for elements such as Cd (8.5 times), Cu (4 times), Pb (38.9 times), and Zn (7.4 times) (Table 3). There was also an increase in the background content, specifically for the following elements: Cr, 2 times; Ni, 2.7 times; Fe, 151 times; and Co, 174 times. In the soil at the site of the wreckage of the T72B3, there was no exceedance of the MPC, but there was an increase in the background content: Cr, 4.6 times; Ni and Pb, 5.6 times; Fe, 10.5 times; Cu, 19 times; Co, 76 times; and Zn, 605.5 times.

Table 3.
Reference values of concentrations of heavy metals in the studied soil (average values) for the studied metals.
In the study of the soil samples from under the wreckage of the T80 tank, it was found that the exceedance of MPC for Pb was equal to 78.8 times (Table 3), and there was also an increase in background content for Fe and Co (1.4 times), Cd (1.5 times), Ni (2.1 times), Cr (7.8 times), and Zn (8.7 times).
In the investigation of the soil under the wreckage of the TOS-1 “Solntsepek”, it was found that the exceedance of MPC for Zn was 1.4 times the threshold, and there was also an increase in the background content: Cu—1.3 times, Ni—1.4 times, Cd—1.5 times, Cr—1.8 times, Co—8.3 times, Fe—20.9 times, and Pb—54.4 times (Table 3).
The highest level of soil contamination was noted for Pb, Zn, and Cd, with the highest concentrations of cadmium, lead, and zinc being observed in the soil at the bottom of the T72B3 tank. Based on the obtained data, the contamination impacts of the destroyed vehicles (tanks) on the soil have the following distribution: Pb > Zn > Cd > Cu.
3.3. Changes in the Granulometric Composition of the Soil
When projectiles strike and armored vehicles are destroyed, mechanical and physical damage to the soil occur. To determine the nature of mechanical damage to the soil, changes in the granulometric composition of the soil were investigated (Table 4).

Table 4.
The granulometric composition of the soil.
In all the samples studied, there were slight changes in the granulometric fractions at the sites of explosions and burning of the machinery. The burning of the machinery led to an increase in the sand fraction (2.0–0.05 mm) by 1.2–1.8 times and a decrease in the clay fraction (<0.002 mm) by 1.1–1.2 times. These changes did not result in a modification of the soil class based on the granulometric composition, so they will not significantly impact the soil’s air–water balance. In our opinion, the increase in the sand fraction (2.0–0.05 mm) and the decrease in the clay fraction (<0.002 mm) are associated with the entry of fuel and lubricants into the soil during the explosions, leading to the partial cementation of particles.
3.4. Changes in the Microbiological Status of the Soil
Research on the soil microbiome under the influence of military actions (regarding various forms and types of turbulence, emissions of potentially toxic elements, pyrolysis, etc.) is currently especially important, as soil microorganisms constitute a significant part of any ecosystem and are highly sensitive to anthropogenic stress. The qualitative and quantitative compositions of soil microorganisms are indicators of environmental pollution, reflecting the ecological consequences of various factors, including military actions. Studying soil microbial diversity, through the identification and counting of morphocultural types of ecologically trophic groups in areas affected by military activities (in this case, due to the burning and explosion of the ammunition of a T72B3 tank), revealed that such factors contributed to changes in the structures of indigenous microbial communities, leading to significant alterations in their functioning. Conversely, significant fluctuations in the structures of microbiocenoses in soils of natural ecosystems were not observed.
It was found that the number of ammonifiers in the soil samples significantly affected by thermal, physical, and chemical factors increased demonstratively (by 10.3 times, on average) compared to the control sample taken from an agroecosystem (the field was not under cultivation during the military actions) (Table 5). This indicates the presence of stressful conditions, such as contamination with various organic and inorganic toxic compounds, and, as a result, the adaptation of microorganisms to these conditions and the performance of the function of organic compound transformation.

Table 5.
Total pool of microorganisms in soil.
Alongside ammonifiers, with an increase in the distance from the epicenter of the explosion and the destruction of heavy armored vehicles, a significant increase in the number of actinomycetes was observed. Actinomycetes play an active role in the synthesis of biologically active substances, indicating the soil ecosystem’s activity in self-regulation and environmental purification with respect to toxic substances.
However, the presence of nitrogen-mineralizing bacteria was reduced in the soil contaminated as a result of the explosion and subsequent pyrolysis of the T72B3 tank and its ammunition. Their quantity was inversely proportional to their distance from the explosion’s epicenter (r = 0.86), which can be explained by the significant changes in the physical and chemical parameters of the soil (Table 5). Additionally, at a distance of 4 m from the explosion’s epicenter, a high number of nitrogen-mineralizing bacteria were found. Although the increase in this group of bacteria indicates their stability, the resilience of the agroecosystem, and soil fertility, in this case, the presence of such a large number of microorganisms in this group suggests that there are many sources of organic matter in this area. Considering the near absence of plant cover, it can be assumed that xenobiotics are the source of organic matter. There was also a significant increase in the number of oligotrophs and pedotrophs, indicating the activation of self-regulation, self-reproduction, and purification processes with respect to chemical substances.
Furthermore, it has been demonstrated that a significant change in the dominant microbial species occurred in almost all the ecological-trophic groups. The dominant microbiota in the soil contaminated due to the pyrolysis of heavy armored vehicles along with their ammunition included Proteobacteria (15.2–67.3%), Bacteroidetes (3.8–59.2%), Firmicutes (1.0–25.8%), and Actinobacteria (0.2–11.5%).
It is important to emphasize that statistically significant increases in the quantity of micromycetes in the soil at the epicenter of the explosion and subsequent burning of the ammunition at a distance of 4.5 m were established: 20.5 times and 7.7 times, respectively (Figure 5). The number of colony-forming units (CFUs) of micromycetes in the soil at the explosion’s epicenter was 206.3 thousand CFU/g; at a distance of 2 m, it was 114.3 thousand CFU/g; and at 4 m away, it was 77.3 thousand CFU/g. It should be noted that in the control samples, the quantities ranged from 5.6 to 10.3 thousand CFU/g. Such a significant increase in the number of micromycetes indicates the presence of a large amount of organic matter, along with hydrocarbon metabolism, nutrient cycling, and interaction with plants and other microorganisms.
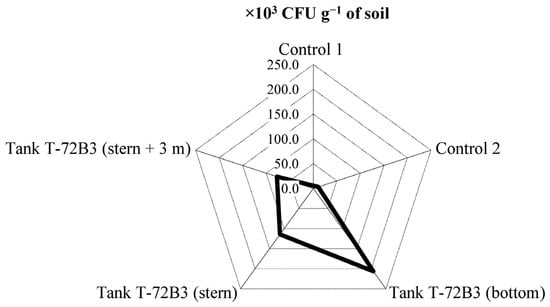
Figure 5.
Quantitative composition of micromycetes under the influence of an explosion followed by the pyrolysis of the T72B3 and its ammunition.
The research conducted on the biodiversity levels in the selected soil samples affected by military actions indicates different levels of biodiversity depending on the type of the studied object. The high Shannon index value for the control sample indicates significant diversity in natural ecosystems, while lower values were observed in agrocenosis. Despite the higher quantity of microorganisms, the biodiversity of soil affected by heavy armored vehicles and the products of their combustion decreases. The Simpson index also indicates the degree of species evenness, where low values signify higher diversity. We have proven that despite the increase in the number of microorganisms in the soil due to the detonation of heavy armored vehicles and the combustion of their products, there was a significant reduction in the level of microbial species diversity (Table 6).

Table 6.
Diversity of microbial communities in the soil.
The determination of the coefficients for the interaction of organic matter with soil and its mineralization showed that the processes of organic matter’s decomposition and transition into a mineral form in the soil at the epicenter of the T72B3 tank and its ammunition explosion were slower compared to that of the control sample. However, at a distance of 4 m from the epicenter, these processes occurred quite intensively and even surpassed the levels of the same processes in the natural ecosystem (Table 7). Nevertheless, the level of biological activity and the availability of nutrients in such soil (as shown by the oligotrophy coefficient) were reduced, indicating that these soils lose their properties as living matter and nutrients transition into a form that is inaccessible to living organisms.

Table 7.
Direction of microbiological processes in soil.
While investigating the pedotrophy coefficient, it was observed that the soil microorganisms at the epicenter of the explosion and burning products, as well as those within a radius of no more than 2 m, exhibited a relatively high level of activity and adaptation to environmental conditions.
The coefficient of organic matter transformation indicates the efficiency of processes transforming organic substances in soil. It was established that in the soils affected by the explosion and burning products, the transformation of nutrients occurred at a slower pace, which, in our opinion, was caused by the extensive contamination with toxic substances, heavy metals, etc. The parallel accumulation of organic matter will likely contribute to the development of various negative impacts on ecosystems in the future.
The burning of heavy armored vehicles, along with their ammunition, can have a pronounced effect on the microbial biomass of the soil. However, the measurements did not confirm significant changes in the control variant (agroecosystem) and in the presence of military equipment at the explosion epicenter (Figure 6).
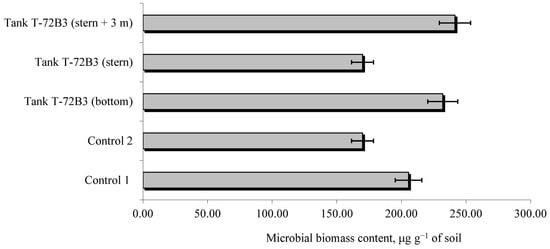
Figure 6.
The impact of heavy armored vehicles and the products of their combustion on the microbial biomass content in the soil.
Our research on soil carbon dioxide emission (Figure 7), influenced by the pyrolysis of the T72B3 tank’s ammunition, demonstrates that CO2 emission is closely related to the total soil microbial biomass. This is indicated by a significant directly proportional correlation coefficient between these indicators in the soil of the natural ecosystem (Control 1, R = 0.82) and in the soil of the agroecosystem (Control 2, R = 0.95). However, in the soils that underwent pyrolysis with the release and transformation of various toxic elements, an inversely proportional correlation between CO2 emissions and the total microbial biomass was observed. This is evidenced by the R value of −0.79 at points distant from the epicenter of the explosion and burning and of −0.93 at the epicenter. This inverse correlation analysis suggests that a significant portion of CO2 emissions in such soil occurs not due to the ecotrophic activity of microorganisms but rather through so-called “heterotrophic respiration”, as confirmed by other researchers [69,108].
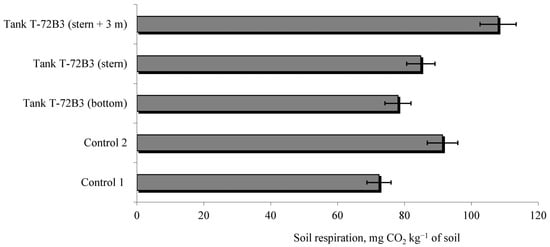
Figure 7.
The influence of the explosion and subsequent pyrolysis of the T-72B3 tank and its ammunition on soil respiration.
We established that the pyrolysis of the T72B3 tank along with its ammunition stimulated the release of carbon dioxide from the soil. However, it is currently not possible to reliably determine whether heterotrophic or autotrophic respiration predominates at this stage of soil functioning or which will prevail in the future. In our opinion, additional research is needed to accurately determine the predominant mechanisms and interactions between heterotrophic and autotrophic respiration under the influence of pyrolysis to obtain a deeper understanding of soil self-renewal and self-regulation in the future.
3.5. Assessment of Soil Phytotoxicity and Changes in the Soil Mesofauna
Currently, phytotoxicity is assessed using various parameters, such as the seed germination rate, plant biomass, and root growth. Research on areas affected by heavy armored vehicles, explosions, and the pyrolysis of both equipment and its ammunition, as well as the introduction of colossal amounts of chemical substances into the soil, has revealed significant areas of seed germination inhibition for both wild and cultivated plants in areas affected by this technology. A similar phenomenon of high soil toxicity due to explosive substances (TNT, RDX, and HMX) has been reported in previous studies [55,109]. Synergistic effects of explosive mixtures have been identified, and it has been established that the toxic effects depend not only on the concentration of explosive substances but also on the soil type.
In assessing the impact of contamination via the burning and explosion of the T72B3 tank’s ammunition on soil phytotoxicity, a zone of complete toxicity (100%) was identified at a distance of 1.5 m from the center of impact. At a distance of 1.6–4.5 m from the epicenter of the explosion, pioneer plant populations were observed after a six-month recovery period, showing a patchy character. The phytotoxicity of this zone ranged from 96.2 to 81.5%. Only at a distance greater than 5 m was the absence of phytotoxicity observed, with the colonization of agrocenoses by diverse types of vegetation (Figure 8).

Figure 8.
Impact of the pyrolysis of the T72B3 tank on soil phytotoxicity.
Samples of soil contaminated with explosive substances and those subjected to pyrolysis processes were further investigated in regard to the inhibition processes of plant bioindicators (Raphanus sativus and Lepidium sativum), their biomasses, and roots. It was found that soil samples from the epicenter of the T72B3 tank were extremely toxic, as neither Raphanus sativus nor Lepidium sativum germinated. The soil collected at a distance of 2 m from the epicenter was characterized as inhibitory, as only 85.1% of Raphanus sativus and 81.2% of Lepidium sativum germinated, with a significant loss of their biomasses and root systems. Our results for the phytotoxicity assessment are consistent with the findings of other researchers [55,68,69]. Additionally, it has been demonstrated that the particular plant species also influences the sensitivity of soil to a complex of explosive substances [110]. Similar studies have been conducted on Lepidium sativum, Brassica rapa, Avena sativa [111], Lolium perenne, and Medicago sativa [71].
The next stage of our work involved studying the response of soil biota (using Eisenia fetida as an example) to the selected soil samples. The test was considered valid since no mortality was observed in the control group (48 h), and the decrease in the average body weights of the earthworms in the control group was 1.6%, which corresponds to international standards [112]. Laboratory studies revealed that soil samples from the epicenter of the explosion and its vicinity, up to a distance of 4.5 m, significantly affected the activity and behavior of the worms. Out of the 50 individuals introduced into the substrate for each repetition of the variant over 120 h (5 days), 94.9% mortality of Eisenia fetida was observed. The surviving individuals showed signs of growth inhibition, confirmed by statistical errors. It is also worth noting that Eisenia fetida exhibited signs of acute toxicity upon contact with soil samples on the skin, manifesting physiological (loss of fluid, a filamentous appearance, disruption of the clitellum) and behavioral changes (Table 8).

Table 8.
The impact of burning and the explosion of the ammunition of the T72B3 tank on soil toxicity as determined using the test object Eisenia fetida.
It is known that various toxic substances, as well as heavy metals (including Zn, Cu, Pb, and Cr) [113], affect the growth, development, and reproductive function of earthworms [114,115]. Lukkary et al. have demonstrated that there is a direct proportional relationship between pollutant concentration and distance from the source. Key indicators such as biomass, survival, and diversity are crucial for assessing toxicity [116].
The entry of various toxic substances into the soil, even in the smallest concentrations, induces various behavioral reactions in earthworms [114]. They demonstrate the ability to detect and avoid contaminated areas and show various morphological changes (twisting, slowing down and speeding up movements, spiralization, body shortening, and an increase or reduction in preclitellar growth) [117]. Researchers have reported that an increase in the content of Cu, Ni, and Cd in soil causes morphological changes (ulcers, vesicles, segmentation, fragmentation, and swelling of cells) in A. chlorotica [118]. Some studies showed that earthworms not only react to the influence of pollutants but also have the ability to develop protective mechanisms against adverse environmental conditions [118,119,120]. The entry of various toxic substances into soil, even in minimal concentrations, triggers different behavioral reactions in earthworms [114]. Earthworms exhibit the ability to detect and avoid contaminated areas, and they display a range of morphological changes such as twisting, altering movement speed, spiralization, body shortening, and the swelling or shrinking of the preclitellar region [117,121]. These responses highlight the potential of earthworms as bioindicators for soil health and the importance of monitoring and mitigating soil contamination.
4. Conclusions
It was established that the tanks with diesel engines, such as the T72B3 and the thermobaric rocket launcher TOS-1 “Solntsepek”, cause the greatest contamination. The T-80 tank, featuring a gas turbine engine, has a relatively smaller impact on the soil. Considering that the areas affected by armored vehicles are usually located on agricultural land, there is a need for their mandatory monitoring and scientific support for further reclamation.
The highest soil pollution levels were noted for Pb, Zn, and Cd. According to the obtained data, the contamination effects of tanks on soils have the following distribution: Pb > Zn > Cd > Cu.
In all the studied samples, insignificant changes in granulometric fractions occurred at the explosion and burning sites of the vehicles. Burning vehicles led to an increase in the sand fraction (2.0–0.05 mm) by 1.2–1.8 times and a decrease in the clay fraction (<0.002 mm) by 1.1–1.2 times. The impact of thermal, physical, and chemical effects on the soil ecosystem caused by the explosion and pyrolysis of armored vehicles has been thoroughly studied. The results of this study show a significant increase in the quantities of micromycetes, pedotrophs, and oligotrophs, indicating contamination with various organic and inorganic toxic compounds. These stress conditions led to the active adaptation of microorganisms and their involvement in the transformation of organic residues.
It has been proven that explosions and pyrolysis of armored vehicles have a significant impact on soil mesobiota (with toxicity for soil worms at a level of 94.9% mortality over 5 days) and plants (soil phytotoxicity—99.8%). Thus, the obtained results indicate there are complex interactions between various factors in the soil environment after an explosion, which can cause unpredictable changes in the structure and function of microbial communities and mesofauna. Further research into these processes is crucial to attain a deeper understanding of their impacts and potential consequences for the environment.
Author Contributions
Conceptualization, M.S. and O.D.; methodology, S.M., N.V., and K.S.; software, S.M.; validation, L.S. and R.M.; formal analysis, M.S.; investigation, M.S.; resources, M.S., S.M., L.S., and R.M.; data curation, O.D.; writing—original draft preparation, O.D.; writing—review and editing, L.S. and R.M.; visualization, L.S. and R.M.; supervision, L.S. and R.M.; project administration, L.S. and R.M.; funding acquisition, L.S. and R.M. All authors have read and agreed to the published version of the manuscript.
Funding
Authors are grateful to the National Research Fund of Ukraine for support within the project “Assessment of the impact of armed aggression on the state of chernozems and the development of activities for the accelerated restoration of soil fertility in the context of ensuring food security” No. 2022.01/0031 in the framework of the program “Science for the reconstruction of Ukraine in the war and post-war periods”.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank the Armed Forces of Ukraine for providing permission, support, and protection during sample collection in the territory of the region of Kharkiv, where active military operations are taking place.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qayyum, U.; Anjum, S.; Sabir, S. Armed Conflict, Militarization and Ecological Footprint: Empirical Evidence from South Asia. J. Clean. Prod. 2021, 281, 125299. [Google Scholar] [CrossRef]
- Pereira, P.; Bašić, F.; Bogunovic, I.; Barcelo, D. Russian-Ukrainian War Impacts the Total Environment. Sci. Total Environ. 2022, 837, 155865. [Google Scholar] [CrossRef] [PubMed]
- Solokha, M.; Pereira, P.; Symochko, L.; Vynokurova, N.; Demyanyuk, O.; Sementsova, K.; Inacio, M.; Barcelo, D. Russian-Ukrainian War Impacts on the Environment. Evidence from the Field on Soil Properties and Remote Sensing. Sci. Total Environ. 2023, 902, 166122. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.R.; Medhi, H.; Bhattacharyya, K.G.; Hussain, C.M. Severe Deterioration in Food-Energy-Ecosystem Nexus Due to Ongoing Russia-Ukraine War: A Critical Review. Sci. Total Environ. 2023, 902, 166131. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Kuemmerle, T. The Impacts of Warfare and Armed Conflict on Land Systems. J. Land Use Sci. 2016, 11, 672–688. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Stemberger, H.L.J.; Zolderdo, A.J.; Struthers, D.P.; Cooke, S.J. The Effects of Modern War and Military Activities on Biodiversity and the Environment. Environ. Rev. 2015, 23, 443–460. [Google Scholar] [CrossRef]
- Murillo-Sandoval, P.J.; Gjerdseth, E.; Correa-Ayram, C.; Wrathall, D.; Van Den Hoek, J.; Dávalos, L.M.; Kennedy, R. No Peace for the Forest: Rapid, Widespread Land Changes in the Andes-Amazon Region Following the Colombian Civil War. Glob. Environ. Chang. 2021, 69, 102283. [Google Scholar] [CrossRef]
- Broomandi, P.; Guney, M.; Kim, J.R.; Karaca, F. Soil Contamination in Areas Impacted by Military Activities: A Critical Review. Sustainability 2020, 12, 9002. [Google Scholar] [CrossRef]
- Baliuk, S.A.; Kucher, A.V.; Solokha, M.O.; Solovei, V.B.; Smirnova, K.B.; Momot, H.F.; Levin, A.Y. Impact of Armed Aggression and Hostilities on the Current State of the Soil Cover, Assessment of Damage and Losses, Restoration Measures: Scientific Report; Brovin: Kharkiv, Ukraine, 2022; 102p. [Google Scholar]
- Shumilova, O.; Tockner, K.; Sukhodolov, A.; Khilchevskyi, V.; De Meester, L.; Stepanenko, S.; Trokhymenko, G.; Hernández-Agüero, J.A.; Gleick, P. Impact of the Russia–Ukraine Armed Conflict on Water Resources and Water Infrastructure. Nat. Sustain. 2023, 6, 578–586. [Google Scholar] [CrossRef]
- Xenarios, S. Water at Time of War. Nat. Sustain. 2023, 6, 485–486. [Google Scholar] [CrossRef]
- Rawtani, D.; Gupta, G.; Khatri, N.; Rao, P.K.; Hussain, C.M. Environmental Damages Due to War in Ukraine: A Perspective. Sci. Total Environ. 2022, 850, 157932. [Google Scholar] [CrossRef] [PubMed]
- Solomon, N.; Birhane, E.; Gordon, C.; Haile, M.; Taheri, F.; Azadi, H.; Scheffran, J. Environmental Impacts and Causes of Conflict in the Horn of Africa: A Review. Earth-Sci. Rev. 2018, 177, 284–290. [Google Scholar] [CrossRef]
- Saxena, A. Deteriorating Environmental Quality with Special Reference to War and Its Impact on Climate Change. Natl. Acad. Sci. Lett. 2024, 47, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Brown, O.; Froggatt, A.; Gozak, N.; Katser-Buchkovska, N.; Lutsevych, O. The Consequences of Russia’s War on Ukraine for Climate Action, Food Supply and Energy Security; Royal Institute of International Affairs: London, UK, 2023. [Google Scholar]
- Appiah-Otoo, I.; Chen, X. Russian-Ukrainian War Degrades the Total Environment. Lett. Spat. Resour. Sci. 2023, 16, 32. [Google Scholar] [CrossRef]
- Hupy, J. The Environmental Footprint of War. Environ. Hist. 2008, 14, 405–421. [Google Scholar] [CrossRef]
- Jorgenson, A.K.; Clark, B.; Givens, J.E. The Environmental Impacts of Militarization in Comparative Perspective: An Overlooked Relationship. Nat. Cult. 2012, 7, 314–337. [Google Scholar] [CrossRef]
- Timeline of 20th and 21st Century Wars. Available online: https://www.iwm.org.uk/history/timeline-of-20th-and-21st-century-wars (accessed on 1 January 2024).
- Curchoe, C.L.; Chang, T.A.; Trolice, M.P.; Telfer, E.E.; Quaas, A.M.; Kearns, W.G.; Stern, J.E.; Albertini, D.F. Protecting Life in a Time of War. J. Assist. Reprod. Genet. 2022, 39, 555–557. [Google Scholar] [CrossRef]
- Pereira, P.; Zhao, W.; Symochko, L.; Inacio, M.; Bogunovic, I.; Barcelo, D. The Russian-Ukrainian Armed Conflict Will Push Back the Sustainable Development Goals. Geogr. Sustain. 2022, 3, 277–287. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils: Rome; FAO and ITPS; Status of the World’s Soil Resources (SWSR): Rome, Italy, 2015.
- Gomiero, T. Soil Degradation, Land Scarcity and Food Security: Reviewing a Complex Challenge. Sustainability 2016, 8, 281. [Google Scholar] [CrossRef]
- Lang, T.; McKee, M. The Reinvasion of Ukraine Threatens Global Food Supplies. BMJ 2022, 376, o676. [Google Scholar] [CrossRef]
- Kemmerling, B.; Schetter, C.; Wirkus, L. The Logics of War and Food (in)Security. Glob. Food Secur. 2022, 33, 100634. [Google Scholar] [CrossRef]
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Pichtel, J. Distribution and Fate of Military Explosives and Propellants in Soil: A Review. Appl. Environ. Soil Sci. 2012, 2012, 617236. [Google Scholar] [CrossRef]
- Shukla, S.; Mbingwa, G.; Khanna, S.; Dalal, J.; Sankhyan, D.; Malik, A.; Badhwar, N. Environment and Health Hazards Due to Military Metal Pollution: A Review. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100857. [Google Scholar] [CrossRef]
- Kucher, A. Methodology for assessing damages and losses caused by the armed aggression to the land fund and soils: Problems and directions of improvement. J. Innov. Sustain. 2022, 6, 10. [Google Scholar] [CrossRef]
- Zaitsev, Y.; Hryshchenko, O.; Romanova, S.; Zaitseva, I. Influence of Combat Actions on the Content of Gross Forms of Heavy Metals in the Soils of Sumy and Okhtyrka Districts of Sumy Region. Agroecol. J. 2022, 3, 136–149. [Google Scholar] [CrossRef]
- Splodytel, A.; Holubtsov, O.; Chumachenko, S.; Sorokina, L. The Impact of Russia’s War against Ukraine on the State of Ukrainian Soils. Kyiv: Public organization “Center for Environmental Initiatives “Ecoaction”. 2023. Available online: https://en.ecoaction.org.ua/wp-content/uploads/2023/05/impact-on-soil-russian-war.pdf (accessed on 1 January 2024).
- Bonchkovskyi, O.S.; Ostapenko, P.O.; Shvaiko, V.M.; Bonchkovskyi, A.S. Remote Sensing as a Key Tool for Assessing War-Induced Damage to Soil Cover in Ukraine (the Case Study of Kyinska Territorial Hromada). J. Geol. Geogr. Geoecol. 2023, 32, 474–487. [Google Scholar] [CrossRef]
- Kulish, I.M. A Mini-Review of the Problem of Pollution of the Territories of Ukraine as a Result of Hostilities. Mod. Concepts Dev. Agron. 2023, 13, 000812. [Google Scholar] [CrossRef]
- Stadler, T.; Temesi, Á.; Lakner, Z. Soil Chemical Pollution and Military Actions: A Bibliometric Analysis. Sustainability 2022, 14, 7138. [Google Scholar] [CrossRef]
- Francis, R.A.; Krishnamurthy, K. Human Conflict and Ecosystem Services: Finding the Environmental Price of Warfare. International Affairs. Int. Aff. 2014, 90, 853–869. [Google Scholar] [CrossRef]
- Darbyshire, E.; Weir, D. Environmental Dimensions during and after the Nagorno-Karabakh Conflict of 2020. Integr. Environ. Assess. Manag. 2023, 19, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Xiao, C.; Feng, Z. Impact of Armed Conflict on Land Use and Land Cover Changes in Global Border Areas. Land Degrad. Dev. 2023, 34, 873–884. [Google Scholar] [CrossRef]
- Alhasan, M.; Lakmes, A.; Alobaidy, M.G.; AlHaeek, S.; Assaf, M.; Dawson, L.; Pirrie, D.; Abdeldayem, Z.; Bridge, J. A Baseline Survey of Potentially Toxic Elements in the Soil of North-West Syria Following a Decade of Conflict. Environ. Sci. Adv. 2023, 2, 886–897. [Google Scholar] [CrossRef]
- Gorecki, S.; Nesslany, F.; Hubé, D.; Mullot, J.-U.; Vasseur, P.; Marchioni, E.; Camel, V.; Noël, L.; Le Bizec, B.; Guérin, T.; et al. Human Health Risks Related to the Consumption of Foodstuffs of Plant and Animal Origin Produced on a Site Polluted by Chemical Munitions of the First World War. Sci. Total Environ. 2017, 599–600, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Symochko, L.; Pereira, P.; Demyanyuk, O.; Coelho Pinheiro, M.N.; Barcelo, D. Resistome in a Changing Environment: Hotspots and Vectors of Spreading with a Focus on the Russian-Ukrainian War. Heliyon 2024, 10, e32716. [Google Scholar] [CrossRef]
- Perkins, D.B.; Haws, N.W.; Jawitz, J.W.; Das, B.S.; Rao, P.S.C. Soil Hydraulic Properties as Ecological Indicators in Forested Watersheds Impacted by Mechanized Military Training. Ecol. Indic. 2007, 7, 589–597. [Google Scholar] [CrossRef]
- Certini, G.; Scalenghe, R.; Woods, W.I. The Impact of Warfare on the Soil Environment. Earth-Sci. Rev. 2013, 127, 1–15. [Google Scholar] [CrossRef]
- Schwenk, M. Chemical Warfare Agents. Classes and Targets. Toxicol. Lett. 2018, 293, 253–263. [Google Scholar] [CrossRef]
- Fayiga, A.O. Remediation of Inorganic and Organic Contaminants in Military Ranges. Environ. Chem. 2019, 16, 81. [Google Scholar] [CrossRef]
- Harada, K.H.; Soleman, S.R.; Ang, J.S.M.; Trzcinski, A.P. Conflict-Related Environmental Damages on Health: Lessons Learned from the Past Wars and Ongoing Russian Invasion of Ukraine. Environ. Health Prev. Med. 2022, 27, 35. [Google Scholar] [CrossRef]
- Zwijnenburg, W.; Hochhauser, D.; Dewachi, O.; Sullivan, R.; Nguyen, V.-K. Solving the Jigsaw of Conflict-Related Environmental Damage: Utilizing Open-Source Analysis to Improve Research into Environmental Health Risks. J. Public Health 2020, 42, e352–e360. [Google Scholar] [CrossRef] [PubMed]
- Tešan Tomić, N.; Smiljanić, S.; Jović, M.; Gligorić, M.; Povrenović, D.; Došić, A. Examining the Effects of the Destroying Ammunition, Mines and Explosive Devices on the Presence of Heavy Metals in Soil of Open Detonation Pit; Part 2: Determination of Heavy Metal Fractions. Water Air Soil Pollut. 2018, 229, 303. [Google Scholar] [CrossRef]
- Fernandez-Lopez, C.; Posada-Baquero, R.; Ortega-Calvo, J.-J. Nature-Based Approaches to Reducing the Environmental Risk of Organic Contaminants Resulting from Military Activities. Sci. Total Environ. 2022, 843, 157007. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Chauhan, S.; D’Cruz, R.; Faruqi, S.; Singh, K.K.; Varma, S.; Singh, M.; Karthik, V. Chemical Warfare Agents. Environ. Toxicol. Pharmacol. 2008, 26, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Sánchez, E.; Hernández-Plata, I.; Martínez, M.S.; Valencia-Cuevas, L.; Galante, P.M. Heavy Metal Pollution as a Biodiversity Threat. In Heavy Metals; Saleh, H.E.-D.M., Aglan, R.F., Eds.; InTech: Houston, TX, USA, 2018; ISBN 978-1-78923-360-5. [Google Scholar]
- Skalny, A.V.; Aschner, M.; Bobrovnitsky, I.P.; Chen, P.; Tsatsakis, A.; Paoliello, M.M.B.; Buha Djordevic, A.; Tinkov, A.A. Environmental and Health Hazards of Military Metal Pollution. Environ. Res. 2021, 201, 111568. [Google Scholar] [CrossRef]
- Singh, R.; Ahirwar, N.K.; Tiwari, J.; Pathak, J. Review on Sources and Effect of Heavy Metal in Soil: Its Bioremediation. Int. J. Res. Appl. Nat. Soc. Sci. 2018, 8, 1–22. [Google Scholar]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in Soil pH and Mobility of Heavy Metals in Contaminated Soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Kumar, D.; Malik, S.; Rani, R.; Kumar, R.; Duhan, J.S. Behavior, Risk, and Bioremediation Potential of Heavy Metals/Metalloids in the Soil System. Rend. Lincei Sci. Fis. Nat. 2023, 34, 809–831. [Google Scholar] [CrossRef]
- Panz, K.; Miksch, K.; Sójka, T. Synergetic Toxic Effect of an Explosive Material Mixture in Soil. Bull. Environ. Contam. Toxicol. 2013, 91, 555–559. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Q.; Wu, J.; Zhou, J.; Zhou, L.; Wu, W. Field Study on the Soil Bacterial Associations to Combined Contamination with Heavy Metals and Organic Contaminants. Sci. Total Environ. 2021, 778, 146282. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Cai, A.; Han, J.; Che, R.; Hao, J.; Wang, F.; Ye, M.; Jiang, X. The Characteristics and Metabolic Potentials of the Soil Bacterial Community of Two Typical Military Demolition Ranges in China. Sci. Total Environ. 2023, 874, 162562. [Google Scholar] [CrossRef] [PubMed]
- Pal, Y.; Mayilraj, S.; Krishnamurthi, S. Exploring the Distinct Distribution of Archaeal Communities in Sites Contaminated with Explosives. Biomolecules 2022, 12, 489. [Google Scholar] [CrossRef] [PubMed]
- Elgh Dalgren, K.; Waara, S.; Düker, A.; Von Kronhelm, T.; Van Hees, P.A.W. Anaerobic Bioremediation of a Soil With Mixed Contaminants: Explosives Degradation and Influence on Heavy Metal Distribution, Monitored as Changes in Concentration and Toxicity. Water Air Soil Pollut. 2009, 202, 301–313. [Google Scholar] [CrossRef]
- Chatterjee, S.; Deb, U.; Datta, S.; Walther, C.; Gupta, D.K. Common Explosives (TNT, RDX, HMX) and Their Fate in the Environment: Emphasizing Bioremediation. Chemosphere 2017, 184, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Aguero, S.; Terreux, R. Degradation of High Energy Materials Using Biological Reduction: A Rational Way to Reach Bioremediation. Int. J. Mol. Sci. 2019, 20, 5556. [Google Scholar] [CrossRef] [PubMed]
- Rylott, E.L.; Bruce, N.C. Right on Target: Using Plants and Microbes to Remediate Explosives. Int. J. Phytoremediat. 2019, 21, 1051–1064. [Google Scholar] [CrossRef]
- Aamir Khan, M.; Sharma, A.; Yadav, S.; Celin, S.M.; Sharma, S. A Sketch of Microbiological Remediation of Explosives-Contaminated Soil Focused on State of Art and the Impact of Technological Advancement on Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) Degradation. Chemosphere 2022, 294, 133641. [Google Scholar] [CrossRef]
- Luo, J.; Li, Y.; Cao, H.; Zhu, Y.; Liu, X.; Li, H.; Liao, X. Variations of Microbiota in Three Types of Typical Military Contaminated Sites: Diversities, Structures, Influence Factors, and Co-Occurrence Patterns. J. Hazard. Mater. 2023, 443, 130290. [Google Scholar] [CrossRef]
- Gui, H.; Wang, J.; Hu, M.; Zhou, Z.; Wan, S. Impacts of Fire on Soil Respiration and Its Components: A Global Meta-Analysis. Agric. For. Meteorol. 2023, 336, 109496. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. Temperature-Associated Increases in the Global Soil Respiration Record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef]
- Hu, M.; Song, J.; Li, S.; Li, Z.; Hao, Y.; Di, M.; Wan, S. Understanding the Effects of Fire and Nitrogen Addition on Soil Respiration of a Field Study by Combining Observations with a Meta-Analysis. Agric. For. Meteorol. 2020, 292–293, 108106. [Google Scholar] [CrossRef]
- Krishnan, G.; Horst, G.L.; Shea, P.J. Differential Tolerance of Cool- and W Arm-Season Grasses to TNT -Contaminated Soil. Int. J. Phytoremediat. 2000, 2, 369–382. [Google Scholar] [CrossRef]
- Rylott, E.L.; Lorenz, A.; Bruce, N.C. Biodegradation and Biotransformation of Explosives. Curr. Opin. Biotechnol. 2011, 22, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Lorber-Pascal, S.; Laurent, F. Phytotoxicity to and Uptake of TNT by Rice. Environ. Geochem. Health 2008, 30, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Winfield, L.E.; Rodger, J.H.; D’surney, S.J. The Responses of Selected Terrestrial Plants to Short (<12 Days) and Long Term (2, 4 and 6 Weeks) Hexahydro-1,3,5-Trinitro-1,3,5-Triazine (RDX) Exposure. Part I: Growth and Developmental Effects. Ecotoxicology 2004, 13, 335–347. [Google Scholar] [CrossRef]
- Vila, M.; Mehier, S.; Lorber-Pascal, S.; Laurent, F. Phytotoxicity to and Uptake of RDX by Rice. Environ. Pollut. 2007, 145, 813–817. [Google Scholar] [CrossRef]
- Lachance, B.; Renoux, A.Y.; Sarrazin, M.; Hawari, J.; Sunahara, G.I. Toxicity and Bioaccumulation of Reduced TNT Metabolites in the Earthworm Eisenia Andrei Exposed to Amended Forest Soil. Chemosphere 2004, 55, 1339–1348. [Google Scholar] [CrossRef]
- Schäfer, R.; Achazi, R.K. The Toxicity of Soil Samples Containing TNT and Other Ammunition Derived Compounds in the Enchytraeid and Collembola-Biotest. Environ. Sci. Pollut. Res. 1999, 6, 213–219. [Google Scholar] [CrossRef]
- Best, E.P.H.; Geter, K.N.; Tatem, H.E.; Lane, B.K. Effects, Transfer, and Fate of RDX from Aged Soil in Plants and Worms. Chemosphere 2006, 62, 616–625. [Google Scholar] [CrossRef][Green Version]
- Robidoux, P.Y.; Hawari, J.; Bardai, G.; Paquet, L.; Ampleman, G.; Thiboutot, S.; Sunahara, G.I. TNT, RDX, and HMX Decrease Earthworm (Eisenia Andrei) Life-Cycle Responses in a Spiked Natural Forest Soil. Arch. Environ. Contam. Toxicol. 2002, 43, 379–388. [Google Scholar] [CrossRef]
- Simini, M.; Checkai, R.T.; Kuperman, R.G.; Phillips, C.T.; Kolakowski, J.E.; Kurnas, C.W.; Sunahara, G.I. Reproduction and Survival of Eisenia Fetida in a Sandy Loam Soil Amended with the Nitro-Heterocyclic Explosives RDX and HMX. Pedobiologia 2003, 47, 657–662. [Google Scholar] [CrossRef]
- Google Earth. Google (2024) Cardiff Bay. Available online: http://maps.google.co.uk (accessed on 1 January 2024).
- ISO 10381-1:2002; Soil Quality—Sampling—Part 1: Guidance on the Design of Sampling Programmes (DSTU ISO 10381-1:2004). ISO: Geneva, Switzerland, 2002.
- ISO 10381-2:2002; Soil Quality—Sampling—Part 2: Guidance on Sampling Techniques (DSTU ISO 10381-2:2004). ISO: Geneva, Switzerland, 2002.
- ISO 10381-5:2005; Soil Quality—Sampling—Part 5: Guidance on the Procedure for the Investigation of Urban and Industrial Sites with Regard to Soil Contamination (DSTU ISO 10381-5:2009). ISO: Geneva, Switzerland, 2005.
- DSTU 4362:2004; Soil Quality Fertility Indexes of Soils. Derzhspozhyvstandart: Kyiv, Ukraine, 2005.
- ISO 11464:2006; Soil Quality—Pretreatment of Samples for Physicochemical Analyses. (DSTU ISO 11464:2007). ISO: Geneva, Switzerland, 2006.
- DSTU 8346:2015; Soil Quality—Methods for Determining the Conductivity, pH and Solid Residue of a Water Extract. Derzhspozhyvstandart: Kyiv, Ukraine, 2017.
- DSTU 4289:2004; Soil Quality—Methods for Determination of Organic Matter. Derzhspozhyvstandart: Kyiv, Ukraine, 2004.
- DSTU 4114-2002; Soils. Determination of Mobile Compounds of Phosphorus and Potassium according to the Modified Machigin Method. Derzhspozhyvstandart: Kyiv, Ukraine, 2002.
- DSTU 8347:2015; Soil Quality—Determination of Mobile Sulfur in Modification of Nsc Issar Sokolovsky O.N. Derzhspozhyvstandart: Kyiv, Ukraine, 2015.
- ISO 18400-102:2017(E); Soil Quality—Sampling—Part 104: Selection and Application Sampling Techniques. ISO: Geneva, Switzerland, 2017.
- DSTU 4730:2007; Soil Quality—The Soil Granulometric Composition Analysis by Pipette Method in Modification of N.A. Kachinskiy. Derzhspozhyvstandart: Kyiv, Ukraine, 2007.
- Zvyagintsev, D.G. Methods of Soil Microbiology and Biochemistry; MSU Press: East Lansing, MI, USA, 1991. [Google Scholar]
- Strickland, M.S.; Rousk, J. Considering Fungal: Bacterial Dominance in Soils—Methods, Controls, and Ecosystem Implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Andreyuk, E.I.; Valagurova, E.V. Fundamentals of the Ecology of Soil Microorganisms; Naukova Dumka: Kyiv, Ukraine, 1992. [Google Scholar]
- Magurran, A.E. Diversity Indices and Species Abundance Models. In Ecological Diversity and Its Measurement; Springer: Dordrecht, The Netherlands, 1988; pp. 7–45. ISBN 978-94-015-7360-3. [Google Scholar]
- Anderson, J.P.E.; Domsch, K.H. A Physiological Method for the Quantitative Measurement of Microbial Biomass in Soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Blagodatsky, S.A.; Blagodatskaya, E.V.; Gorbenko, A.Y.; Panikov, N.S. Rehydration Method for Microbial Biomass Determination in Soil. Eurasian Soil Sci. 1987, 4, 64–71. [Google Scholar]
- Anderson, T.-H.; Domsch, K.H. Application of Eco-Physiological Quotients (qCO2 and qD) on Microbial Biomasses from Soils of Different Cropping Histories. Soil Biology and Biochemistry 1990, 22, 251–255. [Google Scholar] [CrossRef]
- Volkohon, V.V. Experimental Soil Microbiology; Agrarian Science: Kyiv, Ukraine, 2010. [Google Scholar]
- DSTU ISO 22030:2007; Soil Quality. Biological Methods. Chronic Toxicity to Higher Plants (ISO 22030:2005, IDT). Derzhspozhyvstandart: Kyiv, Ukraine, 2007.
- DSTU ISO 11268-1:2003; Soil Quality. Effects of Pollutants on Earthworms (Eisenia Fetida). Derzhspozhyvstandart: Kyiv, Ukraine, 2003.
- Russo-Ukrainian Warspotting. Available online: https://ukr.warspotting.net/uk/map/ (accessed on 1 January 2024).
- Bonds, E. Legitimating the Environmental Injustices of War: Toxic Exposures and Media Silence in Iraq and Afghanistan. Environ. Politics 2016, 25, 395–413. [Google Scholar] [CrossRef]
- Lengefeld, M.R.; Hooks, G.; Smith, C.L. War and the Environment. In Handbook of Environmental Sociology; Schaefer Caniglia, B., Jorgenson, A., Malin, S.A., Peek, L., Pellow, D.N., Huang, X., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 381–401. ISBN 978-3-030-77711-1. [Google Scholar]
- Jagtap, S.; Trollman, H.; Trollman, F.; Garcia-Garcia, G.; Parra-López, C.; Duong, L.; Martindale, W.; Munekata, P.E.S.; Lorenzo, J.M.; Hdaifeh, A.; et al. The Russia-Ukraine Conflict: Its Implications for the Global Food Supply Chains. Foods 2022, 11, 2098. [Google Scholar] [CrossRef]
- Yang, X.; Huan, Z.; Zhao, S.; Xi, H. Study on Environmental Pollution Behavior/Fate of Ammunition Soil and Microbial Remediation of TNT and Its Intermediates. J. Clean. Prod. 2023, 432, 139715. [Google Scholar] [CrossRef]
- Angurets, O.; Khazan, P.; Kolesnikova, K.; Kushch, M.; Černochova, M.; Havránek, M. Environmental Consequences of Russian War in Ukraine; Arnika: Prague, Czech Republic, 2023; ISBN 978-80-88508-05-2. [Google Scholar]
- Johnsen, A.R.; Boe, U.S.; Henriksen, P.; Malmquist, L.M.V.; Christensen, J.H. Full-scale bioremediation of diesel-polluted soil in an Arctic landfarm. Environ. Pollut. 2021, 280, 116946. [Google Scholar] [CrossRef]
- Decree of the Ministry of Health of Ukraine “On Approval of Hygienic Regulations for Permissible Content of Chemical Substances in Soil” Dated July 14, 2020, № 1595. Available online: https://zakon.rada.gov.ua/laws/show/z0722-20#Text (accessed on 1 January 2024).
- Sun, W.; Luo, X.; Fang, Y.; Shiga, Y.P.; Zhang, Y.; Fisher, J.B.; Keenan, T.F.; Michalak, A.M. Biome-Scale Temperature Sensitivity of Ecosystem Respiration Revealed by Atmospheric CO2 Observations. Nat. Ecol. Evol. 2023, 7, 1199–1210. [Google Scholar] [CrossRef]
- Hu, X.; Wang, J.; Lv, Y.; Liu, X.; Zhong, J.; Cui, X.; Zhang, M.; Ma, D.; Yan, X.; Zhu, X. Effects of Heavy Metals/Metalloids and Soil Properties on Microbial Communities in Farmland in the Vicinity of a Metals Smelter. Front. Microbiol. 2021, 12, 707786. [Google Scholar] [CrossRef] [PubMed]
- Scheidemann, P.; Klunk, A.; Sens, C.; Werner, D. Species Dependent Uptake and Tolerance of Nitroaromatic Compounds by Higher Plants. J. Plant Physiol. 1998, 152, 242–247. [Google Scholar] [CrossRef]
- Gong, P.; Wilke, B.-M.; Fleischmann, S. Soil-Based Phytotoxicity of 2,4,6-Trinitrotoluene (TNT) to Terrestrial Higher Plants. Arch. Environ. Contam. Toxicol. 1999, 36, 152–157. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guideline for the Testing of Chemicals №. 207. Earthworm, Acute Toxicity; OECD: Paris, France, 1984. [Google Scholar]
- Yadav, R.; Kumar, R.; Gupta, R.K.; Kaur, T.; Kiran; Kour, A.; Kaur, S.; Rajput, A. Heavy Metal Toxicity in Earthworms and Its Environmental Implications: A Review. Environ. Adv. 2023, 12, 100374. [Google Scholar] [CrossRef]
- Zheng, R.; Li, C. Effect of Lead on Survival, Locomotion and Sperm Morphology of Asian Earthworm, Pheretima Guillelmi. J. Environ. Sci. 2009, 21, 691–695. [Google Scholar] [CrossRef]
- Takacs, V.; Molnar, L.; Klimek, B.; Gałuszka, A.; Morgan, A.J.; Plytycz, B. Exposure of Eisenia Andrei (Oligochaeta; Lumbricidea) to Cadmium Polluted Soil Inhibits Earthworm Maturation and Reproduction but Not Restoration of Experimentally Depleted Coelomocytes or Regeneration of Amputated Segments. Folia Biol. 2016, 64, 275–284. [Google Scholar] [CrossRef][Green Version]
- Lukkari, T.; Taavitsainen, M.; Väisänen, A.; Haimi, J. Effects of Heavy Metals on Earthworms along Contamination Gradients in Organic Rich Soils. Ecotoxicol. Environ. Saf. 2004, 59, 340–348. [Google Scholar] [CrossRef]
- Sivakumar, S. Effects of Metals on Earthworm Life Cycles: A Review. Environ. Monit. Assess. 2015, 187, 530. [Google Scholar] [CrossRef]
- Homa, J.; Stürzenbaum, S.R.; Kolaczkowska, E. Metallothionein 2 and Heat Shock Protein 72 Protect Allolobophora Chlorotica from Cadmium But Not Nickel or Copper Exposure: Body Malformation and Coelomocyte Functioning. Arch. Environ. Contam. Toxicol. 2016, 71, 267–277. [Google Scholar] [CrossRef]
- Demuynck, S.; Succiu, I.R.; Grumiaux, F.; Douay, F.; Leprêtre, A. Effects of Field Metal-Contaminated Soils Submitted to Phytostabilisation and Fly Ash-Aided Phytostabilisation on the Avoidance Behaviour of the Earthworm Eisenia Fetida. Ecotoxicol. Environ. Saf. 2014, 107, 170–177. [Google Scholar] [CrossRef]
- Yang, G.; Chen, C.; Yu, Y.; Zhao, H.; Wang, W.; Wang, Y.; Cai, L.; He, Y.; Wang, X. Combined Effects of Four Pesticides and Heavy Metal Chromium (VI) on the Earthworm Using Avoidance Behavior as an Endpoint. Ecotoxicol. Environ. Saf. 2018, 157, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Aguzie, I.O.; Enekwe, K.D.; Emekekwue, I.J.; Asogwa, C.N.; Onyishi, G.C.; Oluah, N.S.; Ekeh, F.N.; Nwani, C.D. Behavioral and Oxidative Stress Responses of Earthworm, Nsukkadrilus Mbae (Segun 1976), Exposed to Lead and Cadmium: A Preliminary Investigation: Behavior and Oxidative Stress in Earthworm on Pb and Cd Exposure. Soil Sediment Contam. Int. J. 2021, 30, 569–589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).