Peat Formation in Rewetted Fens as Reflected by Saturated n-Alkyl Acid Concentrations and Patterns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Analytical Methods
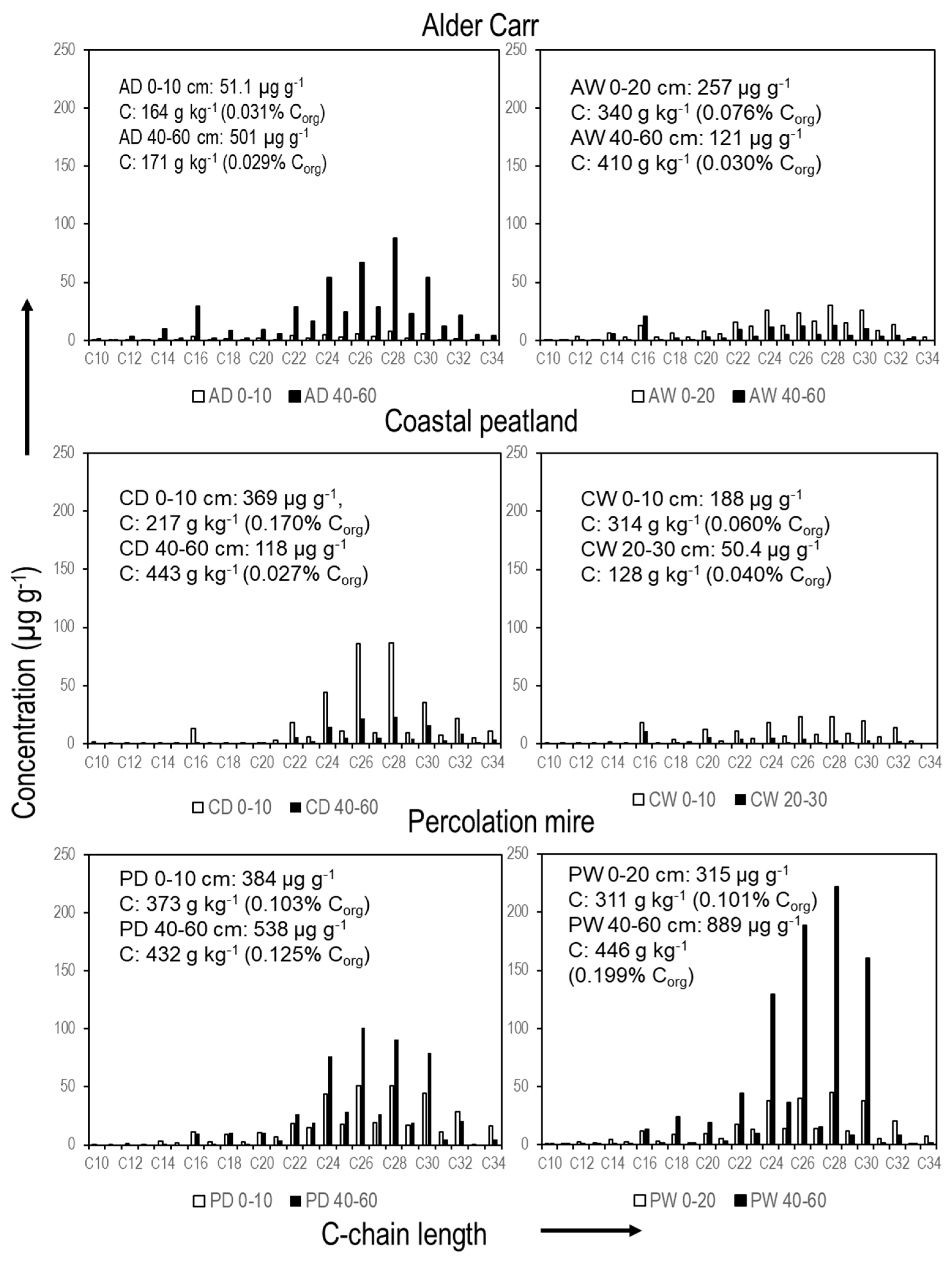
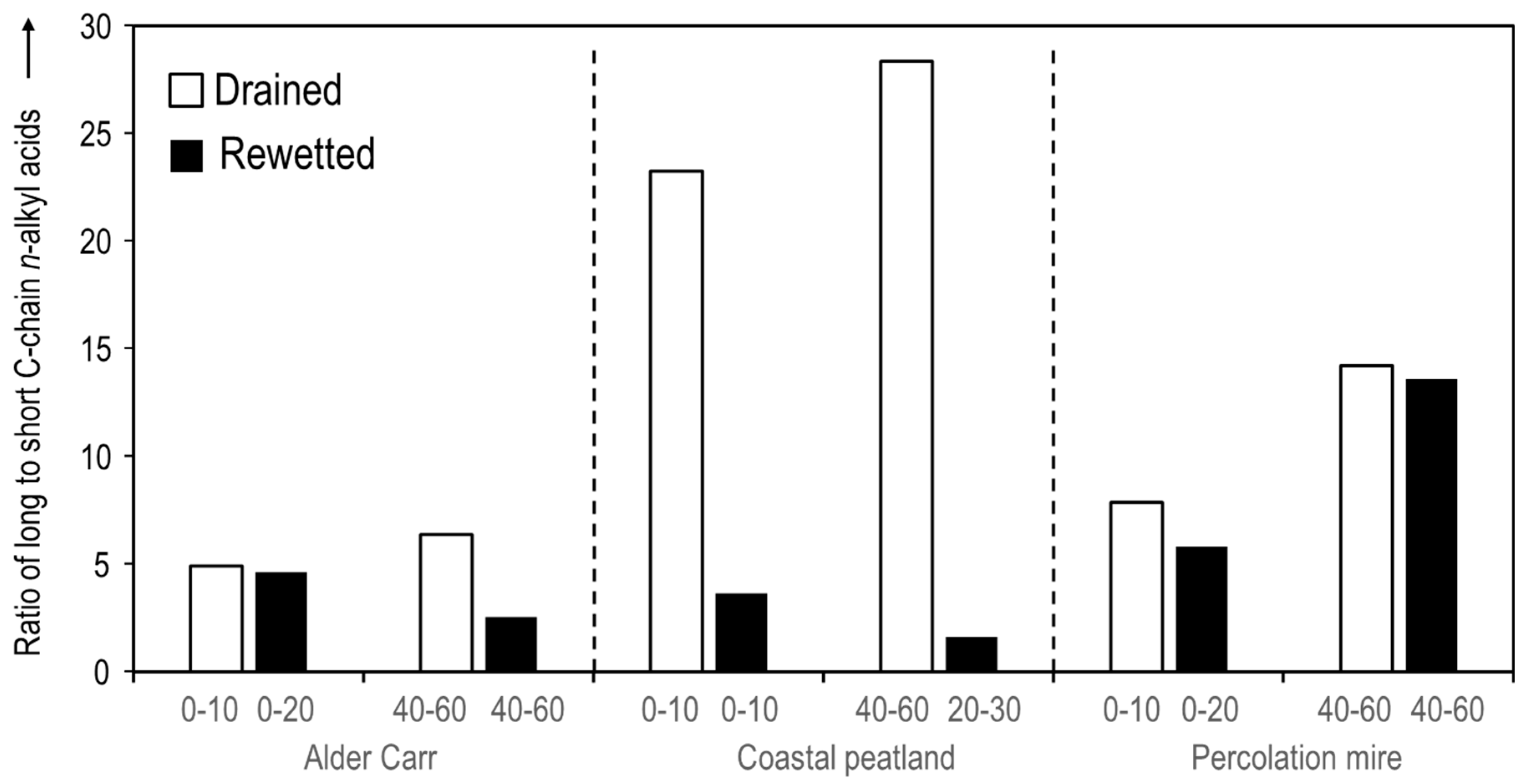
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands -Background and Principles Including a Framework for Decision-Making; International Mire Conservation Group and International Peat Society: Saarijärvi, Finland, 2002. [Google Scholar]
- Melton, J.R.; Wania, R.; Hodson, E.L.; Poulter, B.; Ringeval, B.; Spahni, R.; Bohn, T.; Avis, C.A.; Beerling, D.J.; Chen, G.; et al. Present state of global wetland extent and wetland methane modelling: Conclusions from a model inter-comparison project (WETCHIMP). Biogeosciences 2013, 10, 753–788. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; DeLaune, R.D. Biogeochemistry of Wetlands: Science and Applications; Tylor and Francis: New York, NY, USA, 2008. [Google Scholar]
- Chambers, F.M.; Beilman, D.W.; Yu, Z. Methods for determining peat humification and for quantifying peat bulk density, organic matter and carbon content for palaeostudies of climate and peatland carbon dynamics. Mires Peat 2011, 7, 1–10. [Google Scholar]
- Nahlik, A.M.; Fennessy, M.S. Carbon storage in US wetlands. Nat. Commun. 2016, 7, 13835. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Carbon management in agricultural soils. Mitig. Adapt. Strateg. Glob. Chang. 2007, 12, 303–322. [Google Scholar] [CrossRef]
- Verhoeven, J.T.A. Wetlands in Europe: Perspectives for restoration of a lost paradise. Ecol. Eng. 2014, 66, 6–9. [Google Scholar] [CrossRef]
- Lamers, L.P.M.; Vile, M.A.; Grootjans, A.P.; Acreman, M.C.; Diggelen, R.; Evans, M.G.; Smolders, A.J.P. Ecological restoration of rich fens in Europe and North America: From trial and error to an evidence-based approach. Biol. Rev. 2015, 90, 182–203. [Google Scholar] [CrossRef]
- Davies, M.A.; Blewett, J.; Naafs, B.D.A.; Finkelstein, S.A. Ecohydrological controls on apparent rates of peat carbon accumulation in a boreal bog record from the Hudson Bay Lowlands, northern Ontario, Canada. Quat. Res. 2021, 104, 14–27. [Google Scholar] [CrossRef]
- Klein, K.; Schellekens, J.; Groβ-Schmölders, M.; von Sengbusch, P.; Alewell, C.; Leifeld, J. Characterizing ecosystem-driven chemical composition differences in natural and drained Finnish bogs using pyrolysis-GC/MS. Org. Geochem. 2022, 165, 104351. [Google Scholar] [CrossRef]
- Schäfer, A. Moore und Euros–die vergessenen Millionen. Arch. Forstwes. Landschaftsökol. 2009, 43, 156–160. [Google Scholar]
- Joosten, H.; Tanneberger, F.; Moen, A. Mires and Peatlands of Europe: Status, Distribution and Conservation; Schweizerbart Science Publishers: Stuttgart, Germany, 2017. [Google Scholar]
- Säurich, A.; Tiemeyer, B.; Dettmann, U.; Fiedler, S.; Don, A. Substrate quality of drained organic soils—Implications for carbon dioxide fluxes. J. Plant Nutr. Soil Sci. 2021, 184, 543–555. [Google Scholar] [CrossRef]
- Ballantine, K.; Schneider, R. Fifty-five years of soil development in restored freshwater depressional wetlands. Ecol. Appl. 2009, 19, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Förster, J. Peatlands Restoration in Germany—A Potential Win-Win-Win Solution for Climate Protection, Biodiversity Conservation and Land Use. 2010. Available online: https://www.teebweb.org (accessed on 22 January 2018).
- Ahmad, S.; Liu, H.; Günther, A.; Couwenberg, J.; Lennartz, B. Long-term rewetting of degraded peatlands restores hydrological buffer function. Sci. Total Environ. 2020, 749, 141571. [Google Scholar] [CrossRef] [PubMed]
- Negassa, W.; Acksel, A.; Eckhardt, K.-U.; Regier, T.; Leinweber, P. Soil organic matter characteristics in different peatland types of northern Germany: Chemical and spectroscopic analyses. Geoderma 2019, 353, 468–481. [Google Scholar] [CrossRef]
- Negassa, W.; Baum, C.; Beyer, F.; Leinweber, P. Spatial variability of selected soil properties in long-term rewetted and drained peatlands. Front. Environ. Sci. 2022, 10, 804041. [Google Scholar] [CrossRef]
- Belyea, L.R.; Malmer, N. Carbon sequestration in peatland: Patterns and mechanisms of response to climate change. Glob. Chang. Biol. 2004, 10, 1043–1052. [Google Scholar] [CrossRef]
- Freeman, C.; Fenner, N.; Shirsat, A.H. Peatland geoengineering: An alternative approach to terrestrial carbon sequestration. Philos. Trans. R. Soc. A 2012, 370, 4404–4421. [Google Scholar] [CrossRef]
- Mrotzek, A.; Michaelis, D.; Günther, A.; Wrage-Mönnig, N.; Couwenberg, K. Mass balances of a drained and a rewetted peatland: On former losses and recent gains. Soil Syst. 2020, 4, 16. [Google Scholar] [CrossRef]
- Jandl, G.; Leinweber, P.; Schulten, H.-R.; Eusterhues, K. The concentrations of fatty acids in organo-mineral particle-size fractions of a Chernozem. Eur. J. Soil Sci. 2004, 55, 459–469. [Google Scholar] [CrossRef]
- Jandl, G.; Leinweber, P.; Schulten, H.-R. Origin and fate of soil lipids in a Phaeozem under rye and maize monoculture in Central Germany. Biol. Fertil. Soils 2007, 43, 321–332. [Google Scholar] [CrossRef]
- Jandl, G.; Baum, C.; Blumschein, A.; Leinweber, P. The impact of short rotation coppice on the concentrations of aliphatic soil lipids. Plant Soil 2012, 350, 163–177. [Google Scholar] [CrossRef]
- Jandl, G.; Acksel, A.; Baum, C.; Leinweber, P. Indicators for soil organic matter quality in no-till soils under perennial crops in Central Sweden. Soil Tillage Res. 2015, 148, 74–84. [Google Scholar] [CrossRef]
- Jandl, G.; Leinweber, P.; Schulten, H.-R.; Ekschmitt, K. Contribution of primary organic matter to the fatty acid pool in agricultural soils. Soil Biol. Biochem. 2005, 37, 1033–1041. [Google Scholar] [CrossRef]
- Strel’nikova, E.B.; Russkikh, I.V.; Preis, Y.I. n-Alkanes and n-alkan-2-ones as lipid biomarkers of high-moor peats and marsh plants in Western Siberia. Solid Fuel Chem. 2021, 55, 321–331. [Google Scholar] [CrossRef]
- Huang, X.; Xue, J.; Zhang, J.; Qin, Y.; Meyers, P.A.; Wang, H. Effect of different wetness conditions on Sphagnum lipid composition in the Erxianyan peatland, central China. Org. Geochem. 2012, 44, 1–7. [Google Scholar] [CrossRef]
- Jurasinski, G.; Ahmad, S.; Anadon-Rosell, A.; Berendt, J.; Beyer, F.; Bill, R.; Blume-Werry, G.; Couwenberg, J.; Günther, A.; Joosten, H.; et al. From understanding to sustainable use of peatlands: The WETSCAPES Approach. Soil Syst. 2020, 4, 14. [Google Scholar] [CrossRef]
- Weil, M.; Wang, H.; Bengtsson, M.; Günther, A.; Jurasinski, G.; Couwenberg, J.; Köhn, D.; Günther, A.; Negassa, W.; Zak, D.; et al. Rewetting of three drained peatlands drives congruent compositional changes in pro- and eukaryotic microbiomes through environmental filtering. Microorganisms 2020, 8, 550. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis. Part 3. Chemical Methods; SSSA Book Ser. 5; Sparks, D.L., Ed.; SSSA: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- van Bergen, P.F.; Bull, I.D.; Poulton, P.R.; Evershed, R.P. Organic geochemical studies of soils from the Rothamsted Classical Experiments—I. Total lipid extracts, solvent insoluble residues and humic acids from Broadbalk Wilderness. Org. Geochem. 1997, 26, 117–135. [Google Scholar] [CrossRef]
- Jandl, G.; Schulten, H.-R.; Leinweber, P. Quantification of long-chain fatty acids in dissolved organic matter and soils. J. Plant Nutr. Soil Sci. 2002, 165, 133–139. [Google Scholar] [CrossRef]
- Preston, M.D.; Basiliko, N. Carbon mineralization in peatlands: Does the soil microbial community composition matter? Geomicrobiol. J. 2015, 33, 151–162. [Google Scholar] [CrossRef]
- Amblès, A.; Colina-Tejada, A.; Jambu, P.; Lemee, L.; Parlanti, E. Experimental leaching of podzol soil lipids. Nature and biological origin of water soluble components. Agrochimica 1998, 42, 158–171. [Google Scholar]
- Bull, I.D.; van Bergen, P.F.; Poulton, P.R.; Evershed, R.P. Organic geochemical studies of soils from the Rothamsted Classical Experiments-II, Soils from the Hoosfield spring barley experiment treated with different quantities of manure. Org. Geochem. 1998, 28, 11–26. [Google Scholar] [CrossRef]
- Xie, S.; Nott, C.J.; Avsejs, L.A.; Maddy, D.; Chambers, F.M.; Evershed, R.P. Molecular and isotopic stratigraphy in an ombrotrophic mire for paleoclimate reconstruction. Geochim. Cosmochim. Acta 2004, 68, 2849–2862. [Google Scholar] [CrossRef]
- Lehtonen, K.; Ketola, M. Solvent-extractable lipids of Sphagnum, Carex, Bryales and Carex-Bryales peats: Content and compositional features vs peat humification. Org. Geochem. 1993, 20, 363–380. [Google Scholar] [CrossRef]
- Negassa, W.; Eckhardt, K.-U.; Regier, T.; Leinweber, P. Dissolved organic matter concentration, molecular composition, and functional groups in contrasting management practices of peatlands. J. Environ. Qual. 2021, 50, 1364–1380. [Google Scholar] [CrossRef] [PubMed]
- Negassa, W.; Michalik, D.; Klysubun, W.; Leinweber, P. Phosphorus speciation in long-term drained and rewetted peatlands of Northern Germany. Soil Syst. 2020, 4, 11. [Google Scholar] [CrossRef]
- Negassa, W.; Klysubun, W.; Leinweber, P. Sulfur speciation in drained and restored minerotrophic peatland types of northeastern Germany. J. Environ. Manag. 2022, 316, 115282. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.; Leinweber, P.; Schlichting, A. Effects of chemical conditions in re-wetted peats on temporal variation of microbial biomass and acid phosphatase activity within the growing season. Appl. Soil Ecol. 2003, 22, 167–174. [Google Scholar] [CrossRef]
- Nurulita, Y.; Adetutu, E.M.; Gunawan, H.; Zul, D.; Ball, A.S. Restoration of tropical peat soils: The application of soil microbiology for monitoring the success of the restoration process. Agric. Ecosyst. Environ. 2016, 216, 293–303. [Google Scholar] [CrossRef]
- Könönen, M.; Jauhiainen, J.; Straková, P.; Heinonsalo, J.; Laiho, R.; Kusin, K.; Limin, S.; Vasander, H. Deforested and drained tropical peatland sites show poorer peat substrate quality and lower microbial biomass and activity than unmanaged swamp forest. Soil Biol. Biochem. 2018, 123, 229–241. [Google Scholar] [CrossRef]
- Abbott, K.M.; Quirk, T.; Fultz, L.M. Soil microbial community development across a 32-year coastal wetland restoration time series and the relative importance of environmental factors. Sci. Total Environ. 2022, 821, 153359. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, M.; Xiao, R.; Cui, Y.; Yu, F. Changes in soil microbial biomass and community composition in coastal wetlands affected by restoration projects in a Chinese delta. Geoderma 2017, 289, 124–134. [Google Scholar] [CrossRef]
- Morales, M.; Afalo, C.; Bernard, O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Volkman, J.K. Lipids of Geochemical Interest in Microalgae. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate. Handbook of Hydrocarbon and Lipid Microbiology; Wilkes, H., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Beadle, J.M.; Brown, L.E.; Holden, J. Biodiversity and ecosystem functioning in natural bog pools and those created by rewetting schemes. WIREs Water 2015, 2, 65–84. [Google Scholar] [CrossRef]
| Peatland Types | Water Table (cm) | Depth (m) | Georeference (N, E) | Altitude (m) | Dominant Plant Species | Mean Temp. (°C) | Annual Rainfall (mm) |
|---|---|---|---|---|---|---|---|
| AD | −70 | 0.6 | 54.1349, 12.5127 | 39.00 | Alnus glatinosa, Fraxinus excelsior | 8.1 | 571 |
| AW | +15 | >1 | 54.1271, 12.4853 | 10.00 | Alnus glatinosa | 8.1 | 571 |
| CD | −70 | 0.7 | 54.1578, 13.3859 | 0.25 | Deschampsia cespitosa, Calamagrostis epigejos | 8.2 | 557 |
| CW | −5 | 0.3 | 54.1576, 13.3893 | 0.45 | Agrostis stolomifera, Elymus repens | 8.2 | 557 |
| PD | −70 | 6 | 54.1316, 12.6289 | 2.25 | Ranunculus repens, Deschampsia cespitosa, | 8.2 | 568 |
| PW | +5–10 | 6 | 54.1011, 12.7395 | 1.25 | Carex acutiformes, Carex rostrata | 8.2 | 522 |
| Site | Layer (cm) | Acronym | SOM (g kg−1) | C (g kg−1) | N (g kg−1) | C:N | pH Value |
|---|---|---|---|---|---|---|---|
| Alder Carr forest drained | 0–10 | AD 0–10 | 291 (27) | 164 (3) | 13 (0.27) | 13 (0.12) | 4.2 (0.23) |
| 40–60 | AD 40–60 | 338 (29) | 171 (67) | 13 (4.62) | 13 (0.46) | 4.9 (0.02) | |
| Alder Carr forest rewetted | 0–20 | AW 0–20 | 676 (17) | 340 (20) | 28 (1.47) | 12 (0.22) | 5.1 (0.16) |
| 40–60 | AW 40–60 | 762 (9) | 410 (9) | 27 (0.70) | 15 (0.64) | 5.1 (0.09) | |
| Coastal peatland drained | 0–10 | CD 0–10 | 415 (10) | 217 (27) | 16 (2.00) | 14 (0.03) | 3.8 (0.14) |
| 40–60 | CD 40–60 | 769 (67) | 443 (2) | 17 (0.31) | 26 (0.79) | 3.9 (0.05) | |
| Coastal peatland rewetted | 0–10 | CW 0–10 | 487 (72) | 314 (38) | 18 (1.39) | 18 (0.76) | 4.7 (0.08) |
| 20–30 | CW 20–30 | 224 (57) | 128 (41) | 7 (2.60) | 22 (3.61) | 6.0 (2.39) | |
| Percolation mire drained | 0–10 | PD 0–10 | 754 (8) | 373 (1.16) | 34 (0.14) | 11 (0.03) | 4.9 (0.12) |
| 40–60 | PD 40–60 | 863 (3) | 432 (30) | 29 (1.95) | 15 (2.00) | 5.2 (0.13) | |
| Percolation mire rewetted | 0–20 | PW 0–20 | 658 (34) | 311 (46) | 26 (3.48) | 12 (0.32) | 5.4 (0.19) |
| 40–60 | PW 40–60 | 866 (2) | 446 (21) | 29 (0.72) | 16 (0.39) | 5.4 (0.03) |
| Site, Sam- Pling Depth | n-C10:0 to n-C34:0 | Long C-Chain n-C21:0 to n-C34:0 | Short C-Chain n-C10:0 to n-C20:0 |
|---|---|---|---|
| AD 0–10 cm | 51.1 | 42.5 | 8.6 |
| AW 0–20 cm | 256.9 | 211.0 | 45.8 |
| AD 40–60 cm | 501.0 | 432.9 | 68.1 |
| AW 40–60 cm | 121.0 | 86.7 | 34.3 |
| CD 0–10 cm | 369.0 | 353.8 | 15.2 |
| CW 0–10 cm | 188.4 | 147.7 | 40.8 |
| CD 40–60 cm | 117.8 | 113.8 | 4.0 |
| CW 20–30 cm | 50.4 | 30.9 | 19.5 |
| PD 0–10 cm | 384.2 | 340.9 | 43.3 |
| PW 0–20 cm | 315.1 | 268.7 | 46.5 |
| PD 40–60 cm | 537.8 | 502.5 | 35.4 |
| PW 40–60 cm | 888.9 | 827.8 | 61.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jandl, G.; Negassa, W.; Eckhardt, K.-U.; Leinweber, P. Peat Formation in Rewetted Fens as Reflected by Saturated n-Alkyl Acid Concentrations and Patterns. Land 2023, 12, 1768. https://doi.org/10.3390/land12091768
Jandl G, Negassa W, Eckhardt K-U, Leinweber P. Peat Formation in Rewetted Fens as Reflected by Saturated n-Alkyl Acid Concentrations and Patterns. Land. 2023; 12(9):1768. https://doi.org/10.3390/land12091768
Chicago/Turabian StyleJandl, Gerald, Wakene Negassa, Kai-Uwe Eckhardt, and Peter Leinweber. 2023. "Peat Formation in Rewetted Fens as Reflected by Saturated n-Alkyl Acid Concentrations and Patterns" Land 12, no. 9: 1768. https://doi.org/10.3390/land12091768
APA StyleJandl, G., Negassa, W., Eckhardt, K.-U., & Leinweber, P. (2023). Peat Formation in Rewetted Fens as Reflected by Saturated n-Alkyl Acid Concentrations and Patterns. Land, 12(9), 1768. https://doi.org/10.3390/land12091768







