Rotational Grazing Strategies Minimally Impact Soil Microbial Communities and Carbon Dynamics—A Texas Case Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Descriptions
2.2. Vegetation and Soil Sampling
2.3. Soil Assessments
2.4. 16S rRNA Amplicon Sequencing
2.5. Statistical Analysis
3. Results and Discussion
3.1. Ranch Management Goals Were Reflected in Vegetation Data
3.2. Ranch Management Showed Minor but Significant Differences in Soil Biological Properties
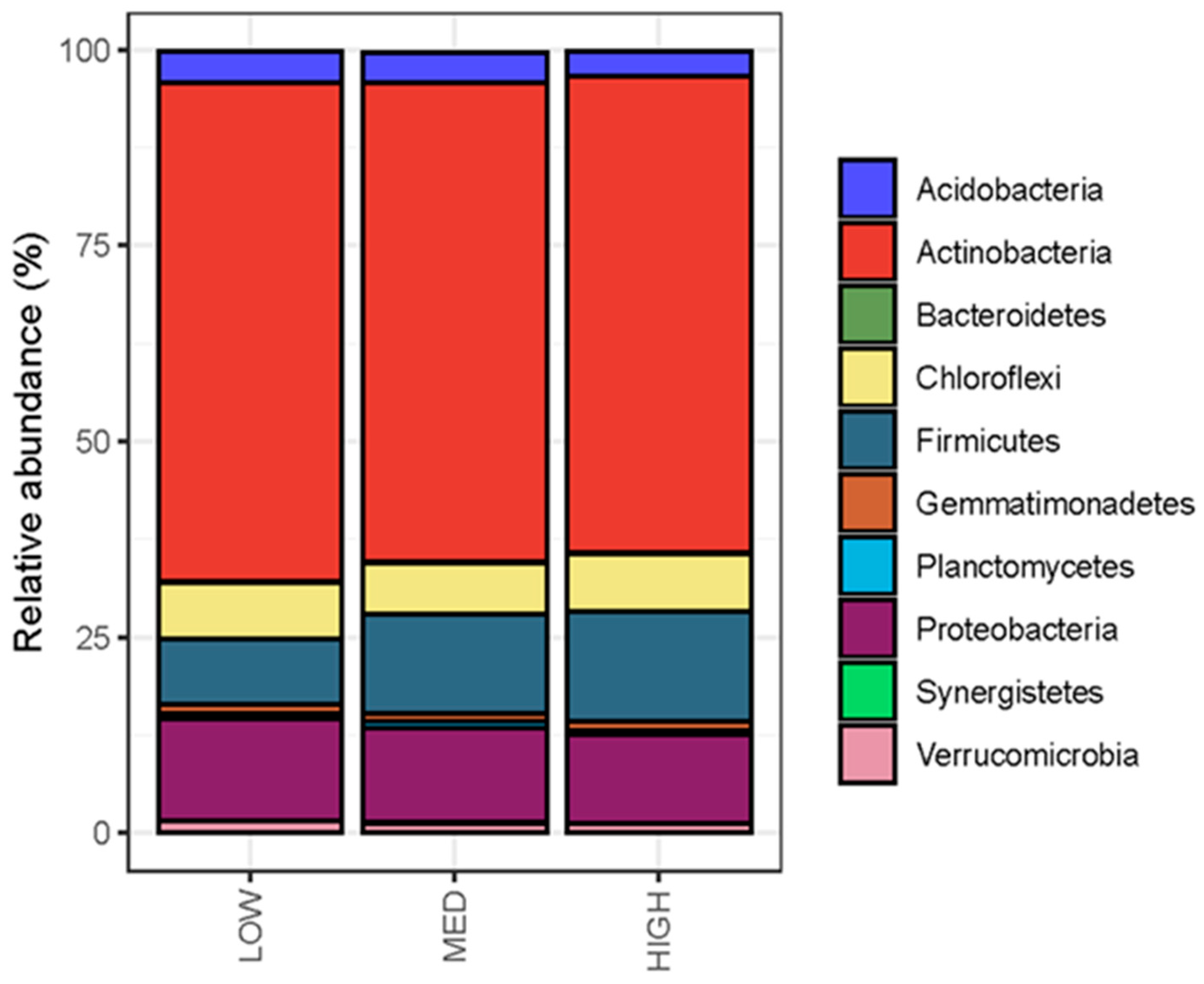
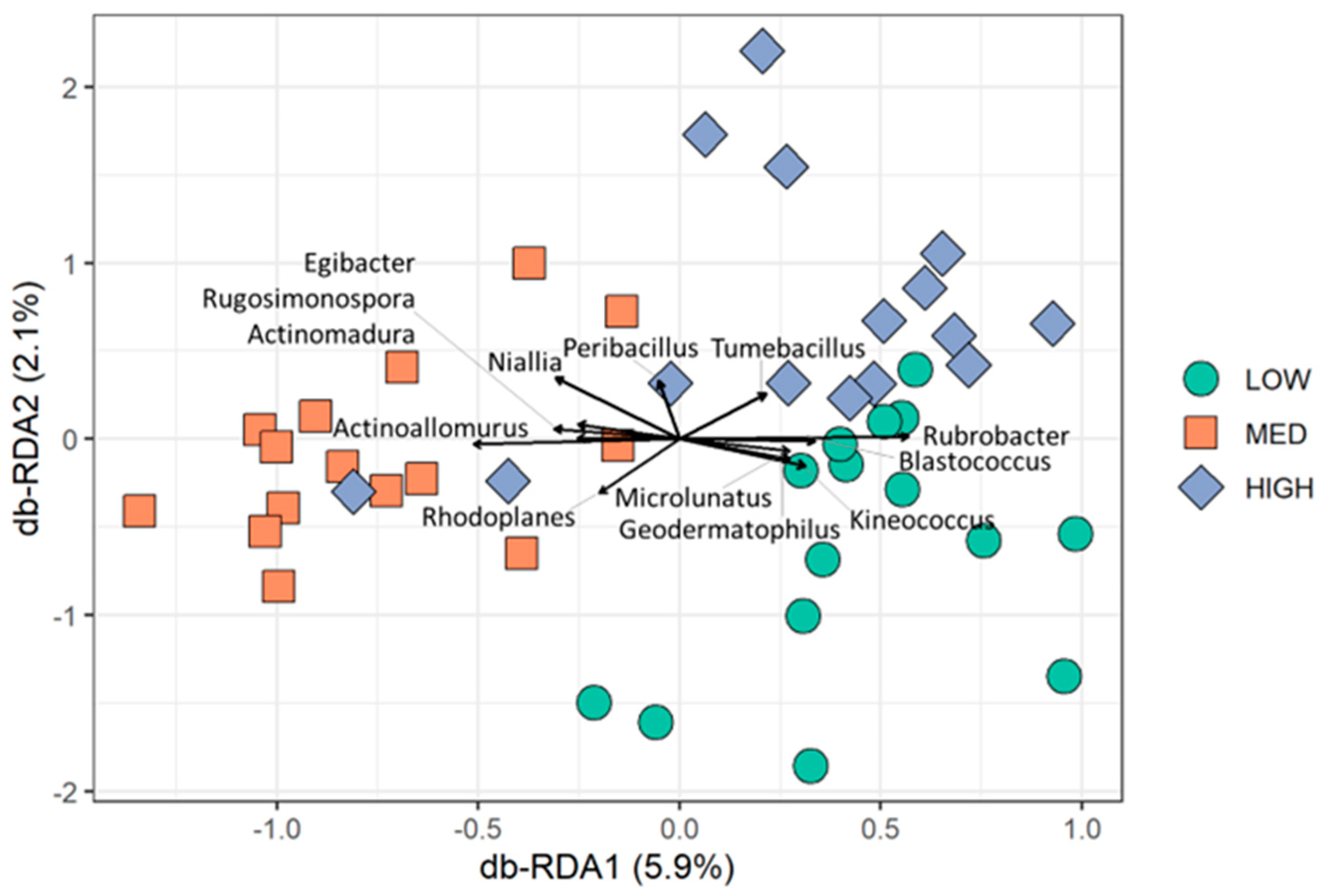
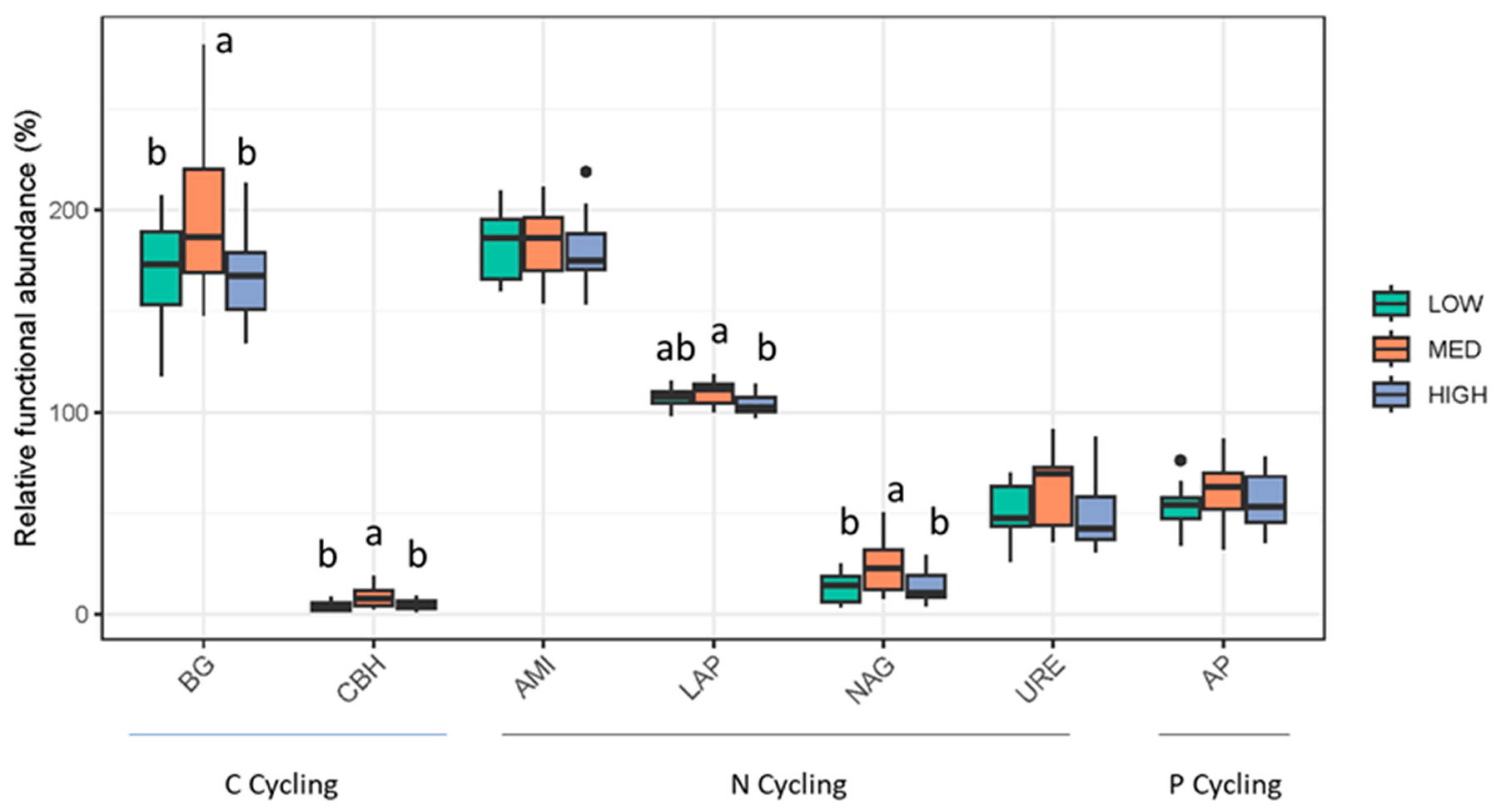
3.3. Rangeland Management Had No Effect on Microbial Diversity, but Management Differences Were Reflected in Microbial Community Composition and Enzymatic Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briske, D.D.; Richards, J.H. Plant responses to defoliation: A physiological, morphological, and demographic evaluation. In Wildland Plants: Physiological Ecology and Developmental Morphology; Bedunah, D.J., Sosebee, R.E., Eds.; Society for Range Management: Denver, CO, USA, 1995; pp. 635–710. [Google Scholar]
- Li, Y.; Dong, S.; Gao, Q.; Fan, C.; Fayiah, M.; Ganjurjav, H.; Hu, G.; Wang, X.; Yan, Y.; Gao, X.; et al. Grazing changed plant community composition and reduced stochasticity of soil microbial community assembly of alpine grasslands on the Qinghai-Tibetan plateau. Front. Plant Sci. 2022, 13, 864085. [Google Scholar] [CrossRef]
- Vetter, S. Rangelands at equilibrium and non-equilibrium: Recent developments in the debate. J. Arid Environ. 2005, 62, 321–341. [Google Scholar] [CrossRef]
- Briske, D.D. Conservation Benefits of Rangeland Practices: Assessment, Recommendations, and Knowledge Gaps; Allen Press: Lawrence, KS, USA, 2011. [Google Scholar]
- Pierson, F.B.; Spaeth, K.E.; Weltz, M.A.; Carlson, D.H. Hydrologic response of diverse western rangelands. Rangel. Ecol. Manag. 2002, 55, 558–570. [Google Scholar] [CrossRef]
- Blackburn, W.H. Impacts of grazing intensity and specialized grazing systems on watershed characteristics and responses. In Developing Strategies for Rangeland Management, 1st ed.; Gardner, B.D., Brothov, J.H., Eds.; CRC Press: Boca Raton, FL, USA, 1985; pp. 927–983. [Google Scholar]
- Teague, W.R.; Dowhower, S.L.; Baker, S.A.; Haile, N.; DeLaune, P.B.; Conover, D.M. Grazing management impacts on vegetation, soil biota and soil chemical, physical and hydrological properties in tall grass prairie. Agric. Ecosyst. Environ. 2011, 141, 310–322. [Google Scholar] [CrossRef]
- Briske, D.D.; Derner, J.D.; Brown, J.R.; Fuhlendorf, S.D.; Teague, W.R.; Havstad, K.M.; Gillen, R.L.; Ash, A.J.; Willms, W.D. Rotational grazing on rangelands: Reconciliation of perception and experimental evidence. Rangel. Ecol. Manag. 2008, 61, 3–17. [Google Scholar] [CrossRef]
- Byrnes, R.C.; Eastburn, D.J.; Tate, K.W.; Roche, L.M. A global meta-analysis of grazing impacts on soil health indicators. J. Environ. Qual. 2018, 47, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Teague, R.; Barnes, M. Grazing management that regenerates ecosystem function and grazingland livelihoods. Afr. J. Range Forage Sci. 2017, 34, 77–86. [Google Scholar] [CrossRef]
- Augustine, D.J.; Derner, J.D.; Fernández-Giménez, M.E.; Porensky, L.M.; Wilmer, H.; Briske, D.D. Adaptive, multipaddock rotational grazing management: A ranch-scale assessment of effects on vegetation and livestock performance in semiarid rangeland. Rangel. Ecol. Manag. 2020, 73, 796–810. [Google Scholar] [CrossRef]
- Roche, L.M.; Cutts, B.B.; Derner, J.D.; Lubell, M.N.; Tate, K.W. On-ranch grazing strategies: Context for the rotational grazing dilemma. Rangel. Ecol. Manag. 2015, 68, 248–256. [Google Scholar] [CrossRef]
- Witt, C.; Wallander, S. Rotational Grazing Adoption by Cow-Calf Operations; Economic Information Bulletin No. (EIB-243); USDA-Economic Research Service: Washington, DC, USA, 2022; 38p. [CrossRef]
- Derner, J.D.; Smart, A.J.; Toombs, T.P.; Larsen, D.; McCulley, R.L.; Goodwin, J.; Sims, S.; Roche, L.M. Soil health as a transformational change agent for us grazing lands management. Rangel. Ecol. Manag. 2018, 71, 403–408. [Google Scholar] [CrossRef]
- Porensky, L.M.; Augustine, D.J.; Derner, J.D.; Wilmer, H.; Lipke, M.N.; Fernández-Giménez, M.E.; Briske, D.D. Collaborative adaptive rangeland management, multipaddock rotational grazing, and the story of the regrazed grass plant. Rangel. Ecol. Manag. 2021, 78, 127–141. [Google Scholar] [CrossRef]
- Spaeth, K.; Weltz, M.; Briske, D.D.; Jolley, L.W.; Metz, L.J.; Rossi, C. Rangeland CEAP: An assessment of natural resources conservation service practices. Rangelands 2013, 35, 2–10. [Google Scholar] [CrossRef][Green Version]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fertil. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Manter, D.; Delgado, J.; Moore-Kucera, J. Integrated soil health management: A framework for soil health, conservation and regeneration. In Managing Soil Health for Sustainable Agriculture; Reicosky, D., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; Volume 1, pp. 87–106. [Google Scholar]
- Zhao, F.; Ren, C.; Shelton, S.; Wang, Z.; Pang, G.; Chen, J.; Wang, J. Grazing intensity influence soil microbial communities and their implications for soil respiration. Agric. Ecosyst. Environ. 2017, 249, 50–56. [Google Scholar] [CrossRef]
- Wilkins, R.; Neal, A.G.; Fitzsimons, B.G.; Stevener, B.M.; Skow, K.L.; Anderson, R.E.; Dube, A.M. Current Land Use Trends, Texas land Trends. 2009. Available online: https://tpwd.texas.gov/business/feedback/meetings/2009/0827/agenda/item_3/media/exhibitc.pdf (accessed on 3 March 2023).
- Natural Resources Conservation Service—USDA. Land Resource Regions and Major Land Resource Areas of the United States, the Caribbean, and the Pacific Basin; U.S. Department of Agriculture, Agriculture Handbook 296; USDA-NRCS: Washington, DC, USA, 2022.
- Herrick, J.E.; Van Zee, J.W.; McCord, S.E.; Courtright, E.M.; Karl, J.W.; Burkett, L.M. Monitoring Manual for Grassland, Shrubland, and Savanna Ecosystems, 2nd ed.; Volume I: Core Methods; USDA-ARS Jornada Experimental Range: Las Cruces, NM, USA, 2017. [Google Scholar]
- Schindelbeck, R.R.; Moebius-Clune, B.N.; Moebius-Clune, D.J.; Kurtz, K.S.; van Es, H.M. Cornell University Comprehensive Assessment of Soil Health Laboratory Standard Operating Procedures; Cornell University: Geneva, NY, USA, 2016. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis; Part 3; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Fine, A.K.; van Es, H.M.; Schindelbeck, R.R. Statistics, scoring functions, and regional analysis of a comprehensive soil health database. Soil Sci. Soc. Am. J. 2017, 81, 589–601. [Google Scholar] [CrossRef]
- Kettler, T.A.; Doran, J.W.; Gilbert, T.L. Simplified method for soil particle-size determination to accompany soil-quality analyses. Soil Sci. Soc. Am. J. 2001, 65, 849–852. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Leite-Mondin, M.; DiLegge, M.J.; Manter, D.K.; Weir, T.L.; Silva-Filho, M.C.; Vivanco, J.M. The gut microbiota composition of trichoplusia ni is altered by diet and may influence its polyphagous behavior. Sci. Rep. 2021, 11, 5786. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W.; et al. EMU: Species-level microbial community profiling of full-length 16S RNA Oxford Nanopore sequencing data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef]
- Barbera, P.; Kozlov, A.M.; Czech, L.; Morel, B.; Darriba, D.; Flouri, T.; Stamatakis, A. EPA-NG: Massively parallel evolutionary placement of genetic sequences. Syst. Biol. 2019, 68, 365–369. [Google Scholar] [CrossRef]
- Louca, S.; Doebeli, M. Efficient comparative phylogenetics on large trees. Bioinformatics 2018, 34, 1053–1055. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. LMERtest package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. Miroeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Liu, C.; Li, C.; Jiang, Y.; Zeng, R.J.; Yao, M.; Li, X. A guide for comparing microbial co-occurrence networks. iMeta 2023, 2, e71. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.-H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gao, Y.; Jia, X.; Wang, M.; Ding, J.; Cheng, L.; Bao, F.; Wu, B. Network analysis reveals the strengthening of microbial interaction in biological soil crust development in the Mu Us sandy land, northwestern China. Soil Biol. Biochem. 2020, 144, 107782. [Google Scholar] [CrossRef]
- Stinner, D.H.; Stinner, B.R.; Martsolf, E. Biodiversity as an organizing principle in agroecosystem management: Case studies of holistic resource management practitioners in the USA. Agric. Ecosyst. Environ. 1997, 62, 199–213. [Google Scholar] [CrossRef]
- Vermeire, L.T.; Gillen, R.L.; Bidwell, T.G. Ecology and Management of Western Ragweed on Rangeland; Cooperative Extension Service, Division of Agricultural Sciences and Natural Resources, Oklahoma State University: Stillwater, OK, USA, 2017. [Google Scholar]
- Skinner, R.H. High biomass removal limits carbon sequestration potential of mature temperate pastures. J. Environ. Qual. 2008, 37, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Natural Resources Conservation Service, United States Department of Agriculture. Soil Survey Geographic (SSURGO) Database. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 5 September 2018).
- Mosier, S.; Apfelbaum, S.; Byck, P.; Calderon, F.; Teague, R.; Thompson, R.; Cotrufo, M.F. Adaptive multi-paddock grazing enhances soil carbon and nitrogen stocks and stabilization through mineral association in southeastern U.S. grazing lands. J. Environ. Manag. 2021, 288, 112409. [Google Scholar] [CrossRef]
- Waters, C.M.; Orgill, S.E.; Melville, G.J.; Toole, I.D.; Smith, W.J. Management of grazing intensity in the semi-arid rangelands of southern Australia: Effects on soil and biodiversity. Land Degrad. Dev. 2017, 28, 1363–1375. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Carter, M.R.; Angers, D.A.; Monreal, C.M.; Ellert, B.H. Towards a minimum data set to assess soil organic matter quality in agricultural soils. Can. J. Soil Sci. 1994, 74, 367–385. [Google Scholar] [CrossRef]
- Magdoff, F.; Van Es, H.M. Building Soils for Better Crops: Sustainable Soil Management, 3rd ed.; Sustainable Agriculture Research and Education (SARE) Program; SARE: Fullerton CA, USA, 2009. [Google Scholar]
- Franzluebbers, A.J. Assessment and interpretation of soil-test biological activity. In Soil Health Series: Volume 2 Laboratory Methods for Soil Health Analysis; Karlen, D.L., Stott, E.E., Mikha, M.M., Eds.; ASA, CSSA, SSSA Books; Wiley: Hoboken, NJ, USA, 2021; pp. 126–151. [Google Scholar] [CrossRef]
- Culman, S.W.; Hurisso, T.T.; Wade, J. Permanganate oxidizable carbon. In Soil Health Series: Volume 2 Laboratory Methods for Soil Health Analysis; Karlen, D.L., Stott, E.E., Mikha, M.M., Eds.; ASA, CSSA, SSSA Books; Wiley: Hoboken, NJ, USA, 2021; pp. 152–175. [Google Scholar] [CrossRef]
- Xun, W.; Yan, R.; Ren, Y.; Jin, D.; Xiong, W.; Zhang, G.; Cui, Z.; Xin, X.; Zhang, R. Grazing-induced microbiome alterations drive soil organic carbon turnover and productivity in meadow steppe. Microbiome 2018, 6, 170. [Google Scholar] [CrossRef]
- Klumpp, K.; Fontaine, S.; Attard, E.; Le Roux, X.; Gleixner, G.; Soussana, J.-F. Grazing triggers soil carbon loss by altering plant roots and their control on soil microbial community. J. Ecol. 2009, 97, 876–885. [Google Scholar] [CrossRef]
- Su, J.; Ji, W.; Sun, X.; Wang, H.; Kang, Y.; Yao, B. Effects of different management practices on soil microbial community structure and function in alpine grassland. J. Environ. Manag. 2023, 327, 116859. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Hariharan, J.; Grewal, P.S.; Dick, W.A. Bacterial community dissimilarity in soils is driven by long-term land-use practices. Agrosyst. Geosci. Environ. 2020, 3, e20031. [Google Scholar] [CrossRef]
- Fierer, N.; Leff, J.W.; Adams, B.J.; Nielsen, U.N.; Bates, S.T.; Lauber, C.L.; Owens, S.; Gilbert, J.A.; Wall, D.H.; Caporaso, J.G. Cross-biome metagenomic analyses of soil microbial communities and their functional attributes. Proc. Natl. Acad. Sci. USA 2012, 109, 21390–21395. [Google Scholar] [CrossRef] [PubMed]
- Fanin, N.; Kardol, P.; Farrell, M.; Nilsson, M.-C.; Gundale, M.J.; Wardle, D.A. The ratio of Gram-positive to Gram-negative bacterial PLFA markers as an indicator of carbon availability in organic soils. Soil Biol. Biochem. 2019, 128, 111–114. [Google Scholar] [CrossRef]
- Kramer, C.; Gleixner, G. Soil organic matter in soil depth profiles: Distinct carbon preferences of microbial groups during carbon transformation. Soil Biol. Biochem. 2008, 40, 425–433. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Shao, X.; Li, H.; He, Y.; Sirimuji; Wang, B. Microbes require a relatively long time to recover in natural succession restoration of degraded grassland ecosystems. Ecol. Indic. 2021, 129, 107881. [Google Scholar] [CrossRef]
- Piao, Z.; Yang, L.; Zhao, L.; Yin, S. Actinobacterial community structure in soils receiving long-term organic and inorganic amendments. Appl. Environ. Microbiol. 2008, 74, 526–530. [Google Scholar] [CrossRef][Green Version]
- de Menezes Alexandre, B.; Prendergast-Miller Miranda, T.; Poonpatana, P.; Farrell, M.; Bissett, A.; Macdonald Lynne, M.; Toscas, P.; Richardson Alan, E.; Thrall Peter, H. C/N ratio drives soil actinobacterial cellobiohydrolase gene diversity. Appl. Environ. Microbiol. 2015, 81, 3016–3028. [Google Scholar] [CrossRef]
- Alessi, A.M.; Bird, S.M.; Bennett, J.P.; Oates, N.C.; Li, Y.; Dowle, A.A.; Polikarpov, I.; Young, J.P.W.; McQueen-Mason, S.J.; Bruce, N.C. Revealing the insoluble metasecretome of lignocellulose-degrading microbial communities. Sci. Rep. 2017, 7, 2356. [Google Scholar] [CrossRef]
- Lynd, L.R.; Welmer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamental and biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Travers, S.K.; Val, J.; Wang, J.-T.; Liu, H.; Singh, B.K.; Delgado-Baquerizo, M. Grazing regulates the spatial heterogeneity of soil microbial communities within ecological networks. Ecosystems 2020, 23, 932–942. [Google Scholar] [CrossRef]
- Röttjers, L.; Faust, K. From hairballs to hypotheses–biological insights from microbial networks. FEMS Microbiol. Rev. 2018, 42, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, P.; Qin, Y.; Tu, Q.; Yang, Y.; He, Z.; Schadt, C.W.; Zhou, J. Network succession reveals the importance of competition in response to emulsified vegetable oil amendment for uranium bioremediation. Environ. Microbiol. 2016, 18, 205–218. [Google Scholar] [CrossRef]
- Ghoul, M.; Mitri, S. The ecology and evolution of microbial competition. Trends Microbiol. 2016, 24, 833–845. [Google Scholar] [CrossRef]
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef]
- Paine, R.T. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar] [CrossRef]
- Dong, G.; Fan, J.; Shekhtman, L.M.; Shai, S.; Du, R.; Tian, L.; Chen, X.; Stanley, H.E.; Havlin, S. Resilience of networks with community structure behaves as if under an external field. Proc. Natl. Acad. Sci. USA 2018, 115, 6911–6915. [Google Scholar] [CrossRef]
- Almeida, R.F.; Naves, E.R.; Mota, R.P. Soil quality: Enzymatic activity of b-glucosidase. Glob. J. Agric. Res. Rev. 2017, 3, 146–150. [Google Scholar]




| Ranch | Ranch Size (ha) | Paddock Size (ha) | Animal Units 1 (AU) | Stocking Density (AU ha−1) | Grazing Period and Stocking Rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grazing Period (Days) | Rest Period (Days) | Season (Days) | Total Grazing (Days) | Total Grazing (Months) | Stocking Rate (AUM ha−1) | |||||
| HIGH | 5747 | 41 | 4000 | 97.4 | 0.25 | 52.5 | 365 | 2.5 | 0.08 | 8 |
| MED | 1358 | 388 | 772 | 2 | 10 | 60 | 365 | 31 | 1.7 | 3.4 |
| LOW | 4856 | 500 | 96 | 0.2 | 365 | 0 | 365 | 365 | 12 | 2 |
| Dependent Variable | F-Value | Pr (>F) | Mean (SD) | ||
|---|---|---|---|---|---|
| LOW | MED | HIGH | |||
| Introduced | 4.231 | 0.047 | 1.33 (0.58) a | 0.40 (0.55) a | 1.20 (0.50) a |
| Forbs | 12.97 | 0.002 | 2.33 (0.58) ab | 0.20 (0.45) b | 4.60 (2.16) a |
| Grass-like | 1.058 | 0.383 | 0.667 (0.58) a | 0.60 (0.55) a | 0.20 (0.50) a |
| Cool-season grasses | 4.231 | 0.047 | 1.67 (0.58) a | 0.60 (0.55) b | 0.80 (0.50) ab |
| Short grasses | 0.769 | 0.489 | 0 (0) a | 0.40 (0.55) a | 0.20 (0.50) a |
| Medium grasses | 3.123 | 0.088 | 1.67 (0.58) a | 4.20 (1.79) a | 2.80 (1.41) a |
| Tall grasses | 1.058 | 0.383 | 2.33 (1.15) a | 2.20 (1.30) a | 1.40 (0.58) a |
| Total | 0.742 | 0.501 | 10.0 (2.00) a | 8.60 (4.39) a | 11.2 (2.31) a |
| Dependent Variable | F-Value | Pr (>F) | Mean (SD) | ||
|---|---|---|---|---|---|
| LOW | MED | HIGH | |||
| Soil organic matter (%) * | 11.5 | <0.001 | 2.18 (0.32) b | 2.77 (0.48) a | 2.14 (0.41) b |
| Respiration (mg CO2 g−1 soil) | 6.256 | 0.004 | 0.45 (0.06) ab | 0.48 (0.08) a | 0.40 (0.05) b |
| Active C (ppm) * | 1.177 | 0.318 | 377 (75.3) a | 412 (75.1) a | 357 (86.6) a |
| pH | 3.59 | 0.036 | 6.32 (0.15) ab | 6.22 (0.19) b | 6.37 (0.12) a |
| Dependent Variable | F-Value | Pr (>F) | Mean (SD) | ||
|---|---|---|---|---|---|
| LOW | MED | HIGH | |||
| qPCR (log10 16S copies g−1 soil) | 1.74 | 0.189 | 8.79 (0.347) a | 8.96 (0.306) a | 8.73 (0.455) a |
| Species Richness | 0.661 | 0.522 | 183 (47.1) a | 196 (28.7) a | 198 (33.3) a |
| Shannon’s Diversity Index | 0.395 | 0.676 | 3.95 (0.323) a | 4.04 (0.246) a | 3.97 (0.231) a |
| Simpson’s Diversity Index | 0.148 | 0.863 | 19.9 (7.45) a | 20.9 (7.38) a | 18.9 (7.78) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, J.M.; Manter, D.K.; Maczko, K.A. Rotational Grazing Strategies Minimally Impact Soil Microbial Communities and Carbon Dynamics—A Texas Case Study. Land 2023, 12, 1517. https://doi.org/10.3390/land12081517
Moore JM, Manter DK, Maczko KA. Rotational Grazing Strategies Minimally Impact Soil Microbial Communities and Carbon Dynamics—A Texas Case Study. Land. 2023; 12(8):1517. https://doi.org/10.3390/land12081517
Chicago/Turabian StyleMoore, Jennifer M., Daniel K. Manter, and Kristie A. Maczko. 2023. "Rotational Grazing Strategies Minimally Impact Soil Microbial Communities and Carbon Dynamics—A Texas Case Study" Land 12, no. 8: 1517. https://doi.org/10.3390/land12081517
APA StyleMoore, J. M., Manter, D. K., & Maczko, K. A. (2023). Rotational Grazing Strategies Minimally Impact Soil Microbial Communities and Carbon Dynamics—A Texas Case Study. Land, 12(8), 1517. https://doi.org/10.3390/land12081517







